ABSTRACT
The small protein ARPP19 plays a dual role during oocyte meiosis resumption. In Xenopus, ARPP19 phosphorylation at S109 by PKA is necessary for maintaining oocytes arrested in prophase of the first meiotic division. Progesterone downregulates PKA, leading to the dephosphorylation of ARPP19 at S109. This initiates a transduction pathway ending with the activation of the universal inducer of M-phase, the kinase Cdk1. This last step depends on ARPP19 phosphorylation at S67 by the kinase Greatwall. Hence, phosphorylated by PKA at S109, ARPP19 restrains Cdk1 activation while when phosphorylated by Greatwall at S67, ARPP19 becomes an inducer of Cdk1 activation. Here, we investigate the functional interplay between S109 and S67-phosphorylations of ARPP19. We show that both PKA and Gwl phosphorylate ARPP19 independently of each other and that Cdk1 is not directly involved in regulating the biological activity of ARPP19. We also show that the phosphorylation of ARPP19 at S67 that activates Cdk1, is dominant over the inhibitory S109 phosphorylation. Therefore our results highlight the importance of timely synchronizing ARPP19 phosphorylations at S109 and S67 to fully activate Cdk1.
KEYWORDS: ARPP19, Cdk1, greatwall, meiotic division, PKA, PP2A, Xenopus oocyte
Introduction
The endosulfine-α (ENSA) protein family includes well-conserved basic-, heat- and acid-stable monomers that regulate a plethora of biological processes including insulin secretion, quiescence upon nutrient deprivation in yeast, neurite outgrowth and cell cycle progression.1-7 In particular, ENSA and its paralog ARPP19 (cAMP-Regulated Phosphoprotein 19) are crucial regulators of cell division.7-9 Entry into mitosis and meiosis is orchestrated by the phosphorylation of hundred of mitotic substrates under the control of active Cdk1-Cyclin B complexes, namely MPF for M-phase promoting factor. To avoid futile cycles of phosphorylation/dephosphorylation, the specific Cdk1-antagonizing phosphatase, PP2A-B55δ, must be simultaneously inactivated.7-13 This process is achieved by the activation of the kinase Greatwall (Gwl), which phosphorylates ENSA/ARPP19 at S67 in Xenopus ARPP19 within the FDSGDY signature motif.8,9 Gwl-phosphorylated ENSA/ARPP19 then binds to and subsequently inactivates PP2A-B55δ to secure the phosphorylation state of mitotic substrates.8,9,14-17
Likewise, PP2A-B55δ inactivation is required for the complete activation of MPF by directly controlling the activity of kinases and phosphatases themselves regulated by Cdk1-dependent phosphorylations, establishing the “MPF auto-amplification” process.18 In G2, MPF is held inactive because Cdk1 is phosphorylated at T14 and Y15 by the Wee1/Myt1 kinase family.18 As the cell enters M-phase, the phosphatase Cdc25 is activated and dephosphorylates Cdk1 at T14 and Y15 to activate it.18-20 Cdk1 in turn phosphorylates its own regulators, thus enhancing Wee1/Myt1 inhibition and Cdc25 activation.18 This mechanism unleashes Cdk1 activation and constitutes the core part of the MPF auto-amplification loop that also involves other kinases, such as Polo, Aurora-A, or the Mos/MAPK module to regulate various players of the loop.18,20 This switch-like transition being counterbalanced by PP2A-B55δ activity, Cdk1 further activates the Gwl-ARPP19 module to ensure PP2A-B55δ inactivation.8,9,21-25
Besides Gwl, 2 other kinases phosphorylate ENSA/ARPP19 at additional sites: CDKs and the cAMP-dependent protein kinase, PKA.9,26,27 CDKs target the N-terminal region of many ENSA/ARPP family members in various species.26 While the Cdk1-dependent phosphorylation of ENSA has little effect on its ability to inactivate PP2A-B55δ in vitro,26 the phosphorylation of ARPP19 by Cdk1 is able to inhibit PP2A-B55δ independently of Gwl and is sufficient in vivo for meiosis resumption in starfish oocytes.28 PKA also phosphorylates ENSA and ARPP19 at a consensus RKP/SSLV motif conserved among most vertebrates.9,26,27,29 This PKA-dependent phosphorylation of ENSA has been proposed to antagonize PP2A-B55δ inhibition induced by its Cdk1-dependent phosphorylation in vitro.26 While the function of this phosphorylation on the mitotic cell cycle has not been yet formally proven, our recent results have shown that the PKA-dependent phosphorylation of ARPP19 is essential to arrest Xenopus oocytes in prophase of the first meiotic division.29
Oocytes are maintained in prophase by the activity of PKA that constitutes the universal limiting step for meiosis resumption in vertebrates. In Xenopus, oocytes re-enter the first meiotic division upon hormonal stimulation by progesterone. Progesterone induces a drop in the intracellular concentration of cAMP, followed by PKA inhibition within 30 min.30-35 This early event is necessary and sufficient for Cdk1 activation that takes place 3 to 4 hours later, inducing the first meiotic division of the cell. It has been recently proposed that the release of oocyte meiotic arrest in Xenopus would be independent of a reduction in either cAMP levels or PKA activity.36 This surprising result challenges a long- and well-established model validated by many worldwide publications and requires further confirmation. The several hours-long transduction pathway starting from PKA downregulation and ending with Cdk1 activation has not been fully elucidated yet. The drop in PKA activity indirectly controls the translation of 2 proteins, Cyclin B and the kinase Mos.34,37-44 The coordinated action of newly synthesized Cyclin B and Mos generates a threshold level of active Cdk1 that initiates the MPF auto-amplification loop.44-46 Similarly to mitosis, Cdk1 regulates Myt1 and Cdc25 activities45 and further inactivates PP2A-B55δ through the Gwl-ARPP19 module, both events being necessary for the auto-amplification process. Under physiological conditions, PKA activity remains low all along meiotic maturation.34,47
We recently identified the crucial PKA substrate that restrains the Cdk1 activation pathway in Xenopus prophase oocytes, as ARPP19.29 ARPP19 is phosphorylated by PKA at S109 in prophase and this residue is dephosphorylated upon progesterone stimulation within one hour to unlock the signaling pathway leading to Cdk1 activation. At the final step of the pathway, Cdk1 activates the Gwl-ARPP19 module and S67-phosphorylated ARPP19 becomes an essential player of the MPF auto-amplification loop by inhibiting PP2A-B55δ .13 Therefore, depending on its phosphorylation state at either S109 or S67, ARPP19 can either restrain or activate Cdk1 in Xenopus oocytes.
The underlying mechanism controlled by ARPP19 phosphorylation at S109 that prevents Cdk1 activation in prophase-arrested Xenopus oocytes remains unknown. This phosphorylation by PKA could impede the phosphorylation at S67 by Gwl or its ability to activate Cdk1 in vivo, when phosphorylated at S67, thus accounting for its inhibitory action toward Xenopus oocyte meiosis resumption. This study investigates the functional interplay between S109 and S67 phosphorylation of ARPP19. We show that PKA and Gwl can phosphorylate ARPP19 independently of each other and further exclude a role for S28 phosphorylation of ARPP19 by Cdk1 in Xenopus. Remarkably, the phosphorylation of ARPP19 at S67 overcomes the inhibitory effect of S109 phosphorylation on Cdk1 activation.
Results
Neither PKA nor S109-phosphorylation of ARPP19 impede the phosphorylation of ARPP19 by Gwl in vitro
We have previously reported that PKA-dependent phosphorylation of ARPP19 at S109 maintains prophase arrest in Xenopus oocytes.29 Upon progesterone stimulation, ARPP19 is dephosphorylated at S109 within one hour and is then phosphorylated at S67 by Gwl 3 to 4 hours later, at the time of GVBD.13,29 This latter event inhibits PP2A-B55δ and is required for full Cdk1 activation.13 To determine whether an antagonistic interplay takes place between S109 and S67 phosphorylation of ARPP19, we investigated whether PKA prevents Gwl phosphorylation of ARPP19 at S67 in vitro. Recombinant wild-type ARPP19 protein (WT-ARPP) was incubated with either recombinant PKA or Gwl, or both kinases in the presence of γS-ATP (Fig. 1A). The phosphorylation status of ARPP19 at either S109 or S67 was verified by western blot, using specific antibodies directed against either phospho-S109 or phospho-S67-ARPP19, as described previously13,29 (Fig. 1A). When PKA and Gwl were used separately, ARPP19 was efficiently phosphorylated at S109 or S67 respectively. When Gwl and PKA were incubated at the same time with ARPP19, both S109 and S67 residues were phosphorylated as well (Fig. 1A). When ARPP19 was incubated with PKA first and then with Gwl, or alternatively first with Gwl and then with PKA, phosphorylation occurred at S109 and S67, independently of the addition sequence of the 2 kinases (Fig. S1A). Therefore, neither PKA nor ARPP19 phosphorylation at S109 interfere with Gwl ability to phosphorylate ARPP19 at S67 in vitro, and reciprocally. Both PKA and Gwl phosphorylate ARPP19 independently of each other.
Figure 1.
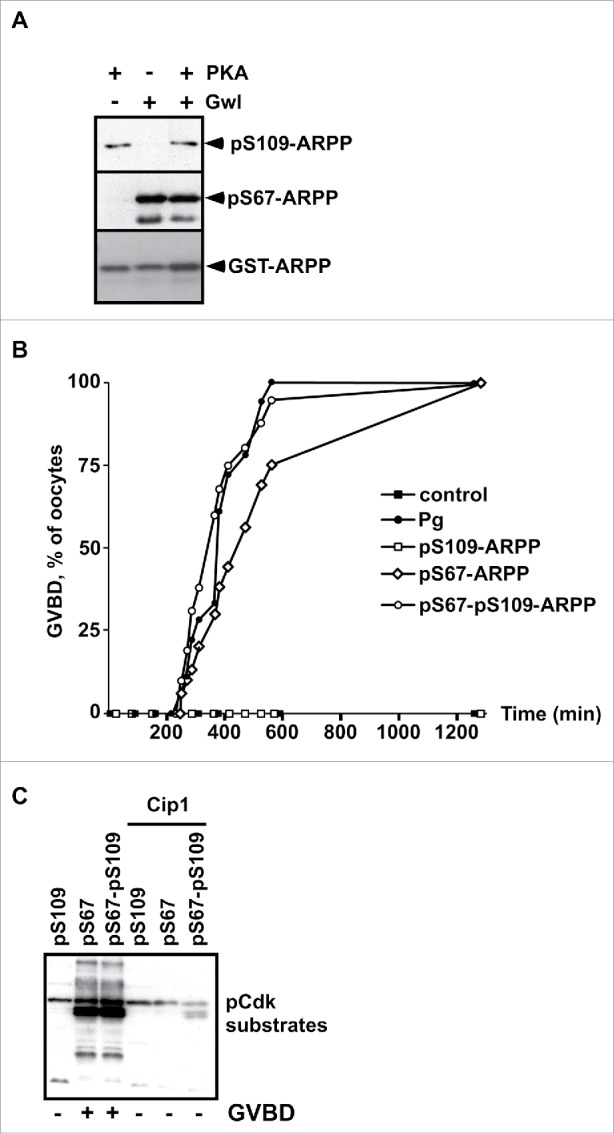
S109 phosphorylation of ARPP19 neither prevents its S67 phosphorylation by Gwl nor its ability to activate Cdk1. A. WT-ARPP was incubated with PKA, Gwl or both kinases in the presence of γS-ATP. The phosphorylation of WT-ARPP at S67 and at S109 was monitored by western blot using antibodies directed against S109-phosphorylated ARPP (pS109-ARPP) and S67-phosphorylated ARPP (pS67-ARPP). Total ARPP19 was immunoblotted with an anti-GST antibody (GST-ARPP). B. Prophase-arrested oocytes were stimulated with progesterone (Pg) or injected with in vitro thiophosphorylated ARPP19 at either S109 (pS109-ARPP), at S67 (pS67-ARPP) or both sites (pS67-pS109-ARPP). Meiosis resumption was followed by scoring the % of oocytes at GVBD as a function of time. C. Prophase-arrested oocytes were injected or not with p21Cip1 (Cip1), and then with in vitro thiophosphorylated ARPP19 at S109 (pS109), at S67 (pS67) or at both sites (pS67-pS109). Oocytes were collected at the time of GVBD and ARPP19 proteins were GST-pulled down. Cdk1 activation was monitored in supernatants by immunoblotting phosphorylated Cdk substrates (pCdk substrates).
ARPP19 phosphorylated at S67 promotes Cdk1 activation independently of its phosphorylation status at S109
To ascertain the activities of the various phosphorylated forms of ARPP19 on meiosis resumption, the double S109 and S67 thiophosphorylated form of ARPP19, termed pS67-pS109-ARPP, or the single phosphorylated forms, pS109-ARPP (S109 phosphorylation) and pS67-ARPP (S67 phosphorylation) were injected into prophase oocytes. Meiosis resumption was followed by scoring the percentage of GVBD as a function of time (Fig. 1B) and Cdk1 activation was monitored by western blotting phosphorylated Cdk substrates (Fig. 1C). In a control experiment, Cdk1 activation was prevented by injecting prophase-arrested oocytes with a specific Cdk inhibitor, the p21Cip1 protein.48 As already published,13 injecting pS109-ARPP neither induced meiosis resumption nor Cdk1 activation, whereas pS67-ARPP injection efficiently promoted GVBD and Cdk1 activation in the absence of progesterone (Fig. 1B-C). Interestingly, the double thiophosphorylated pS67-pS109-ARPP induced meiosis resumption and Cdk1 activation as efficiently as did the pS67-ARPP protein (Fig. 1B-C). Accordingly, the injection of pS67-pS109-ARPP, resulting from sequential thiophosphorylation by PKA and Gwl, also triggered GVBD and Cdk1 activation (Fig. S1B).
However, the products of the phosphorylation reaction combining PKA and Gwl could generate a mix of the following ARPP19 molecules: pS67-ARPP, pS109-ARPP and pS67-pS109-ARPP. As a result, meiosis resumption could be promoted by pS67-ARPP, and the effect of pS67-pS109-ARPP19 could thus be misinterpreted. To exclude this possibility, we performed in vitro thiophosphorylation assays using 2 ARPP19 proteins wherein the S109 residue was mutated either to an aspartic acid to mimic phosphorylation (S109D-ARPP), or to an alanine that cannot be phosphorylated (S109A-ARPP). As seen in Fig. 2A, recombinant WT-ARPP, S109A-ARPP and S109D-ARPP were efficiently thiophosphorylated in vitro by Gwl at S67, confirming that the constitutive phosphorylation of ARPP19 at S109 does not hamper Gwl to phosphorylate the same molecule at S67. These proteins were then injected into prophase oocytes. As previously reported,13,29 unphosphorylated WT-ARPP, S109A-ARPP and S109D-ARPP did not induce GVBD on their own whereas injecting pS67-ARPP promoted meiotic maturation (Fig. 2B). Interestingly, mutating S109 to either D or A did not interfere with the ability of the additional S67 phosphorylation to induce meiotic maturation, although GVBD occurrence was slightly delayed in oocytes injected with pS67-S109A-ARPP (Fig. 2B). Cdk1 activation was examined by immunoblotting for in vivo MAPK and Cdc27 phosphorylation, both being dependent on Cdk1 activity. As a control, Cdk1 activation was prevented by injecting the Cdk inhibitor, p21Cip1. Upon progesterone addition or following the injection of pS67-ARPP proteins, Cdc27 and MAPK were both phosphorylated (Fig. 2C). Therefore, the mutation of the S109 residue into either a non-phosphorylatable or a phosphomimic residue does not prevent S67-phosphorylated ARPP19 from triggering GVBD and activating Cdk1. Hence, ARPP19 phosphorylated at S67 is a strong inducer of meiosis resumption, independently of its phosphorylation at S109.
Figure 2.
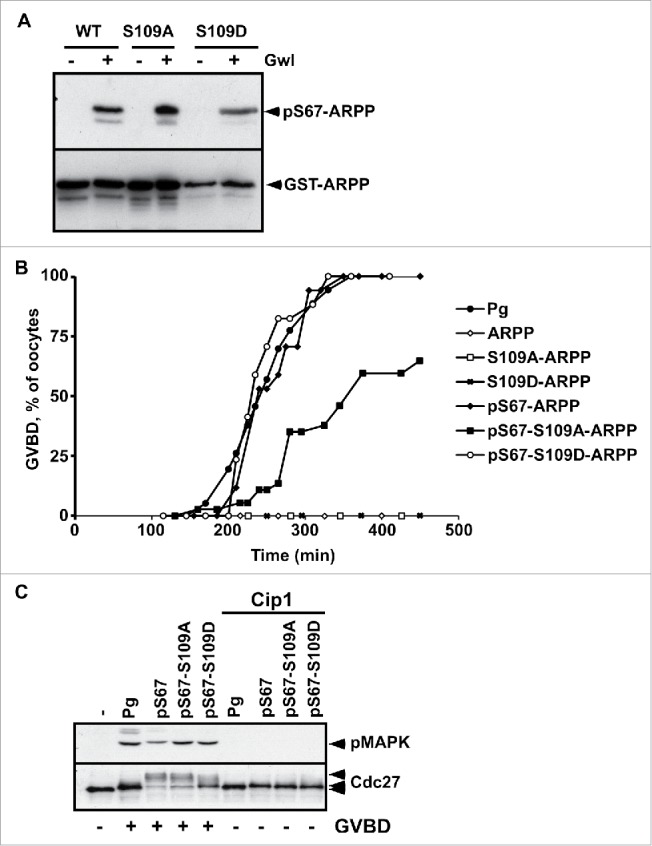
S109D mutation does not impair the ability of S67-phosphorylation of ARPP19 to activate Cdk1. A. WT-ARPP, S109A-ARPP and S109D-ARPP were incubated or not with recombinant Gwl in the presence of γS-ATP. The phosphorylation of WT-ARPP at S67 was visualized by western blot using an antibody directed against S67-phosphorylated ARPP (pS67-ARPP). Total ARPP19 was immunoblotted with an anti-GST antibody (GST-ARPP). B. Prophase-arrested oocytes were stimulated with progesterone (Pg) or injected with either unphosphorylated WT-ARPP (ARPP), S109A-ARPP and S109D-ARPP or S67-thiophosphorylated WT-ARPP, S109A-ARPP and S109D-ARPP (respectively pS67-ARPP, pS67-S109A-ARPP and pS67-S109D-ARPP). Meiosis resumption was followed by scoring the % of oocytes at GVBD as a function of time. C. Prophase-arrested oocytes were injected or not with p21Cip1 (Cip1) and then stimulated with progesterone (Pg) or by injecting S67-phosphorylated WT-ARPP, S109A-ARPP or S109D-ARPP (respectively pS67, pS67-S109A, pS67-S109D). Oocytes were collected at GVBD time. Cdk1 activation was monitored by western blotting phosphorylated MAPK (pMAPK) and Cdc27.
ARPP19 phosphorylated at both S109 and S67 still interacts with PP2A-B55δ
S67 phosphorylation converts ARPP19 into an inhibitor of PP2A-B55δ, which is necessary and sufficient for Cdk1 activation and thus, meiosis resumption.13 Since S67-phosphorylated ARPP19 activates Cdk1 in spite of its S109 phosphorylation, we wondered whether ARPP19 phosphorylated at both S109 and S67 still associates with PP2A-B55δ. If not, the double phosphorylated form of ARPP19 would promote Cdk1 activation independently of its ability to inactivate PP2A-B55δ. To address this question, prophase oocytes were injected with different forms of in vitro thiophosphorylated ARPP19, either pS109, pS67 and pS67-pS109-ARPP, or the S109 mutants of ARPP: pS67-S109A-ARPP and pS67-S109D-ARPP. Oocytes were collected at GVBD time and ARPP19 proteins were recovered by GST-pull down. The pulled down fractions were then immunoblotted with antibodies directed against PP2A catalytic and B55δ regulatory subunits. Neither pS109-ARPP nor S109D-ARPP19 or S109A-ARPP interacted with PP2A-B55δ (Fig. 3). In contrast, pS67-ARPP and pS67-pS109-ARPP as well as pS67-S109A-ARPP and pS67-S109D-ARPP efficiently associated with PP2A-B55δ (Fig. 3). Similarly, pS67-pS109-ARPP, resulting from a sequential thiophosphorylation by PKA and Gwl, also interacted with PP2A-B55δ (Fig. S1C). Therefore, the thiophosphorylation at S109 or the mutation of the S109 residue into a phosphomimic residue does not prevent S67-phosphorylated ARPP19 to bind PP2A-B55δ.
Figure 3.
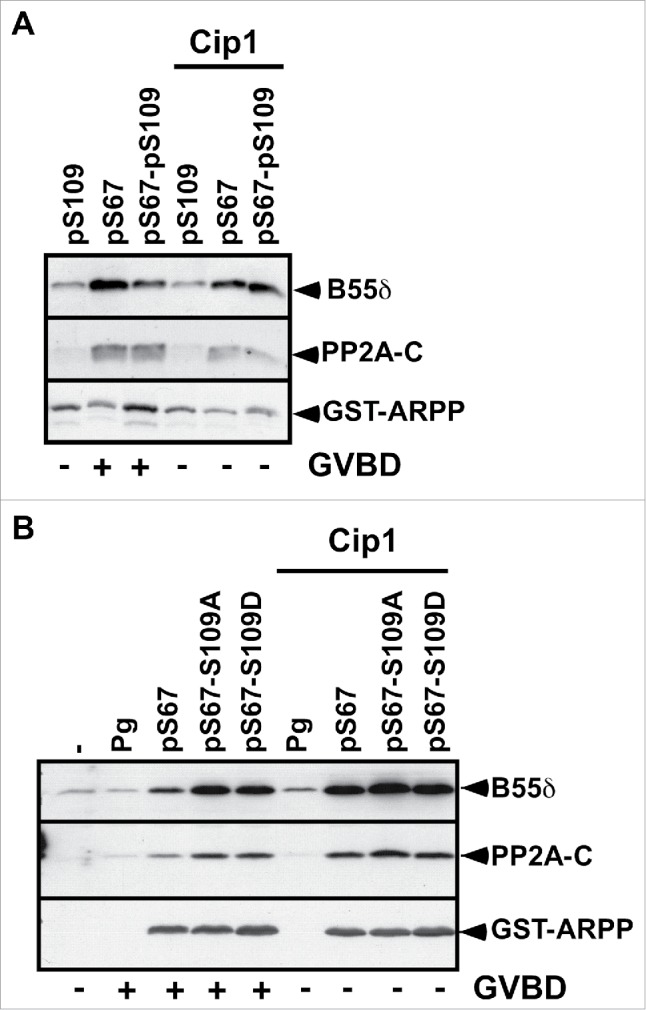
S67-phosphorylated ARPP19 interacts with PP2A-B55δ independently of its S109 phosphorylation. A. Prophase-arrested oocytes were injected or not with p21Cip1 (Cip1) and then injected with thiophosphorylated ARPP at either S109 (pS109), or S67 (pS67) or both residues (pS67-pS109). Oocytes were collected at the time of GVBD and ARPP19 proteins were GST-pulled down. GST-pulled down fractions were immunoblotted for PP2A-C ανδ B55δ subunits. Total ARPP19 was immunoblotted using a GST antibody (GST-ARPP). B. Prophase-arrested oocytes were induced to mature with progesterone (Pg) or by injecting S67-phosphorylated WT-, S109A- or S109D-ARPP (respectively pS67, pS67-S109A, pS67-S109D). Oocytes were collected at the time of GVBD and ARPP19 proteins were GST-pulled down. GST-pulled down fractions were immunoblotted for B55δ, PP2A-C subunits and total ARPP19 using a GST antibody (GST-ARPP).
Cdk1 is not required for PP2A-B55δ inhibition induced by S67-phosphorylated ARPP19
It has been reported in Xenopus and starfish that Cdk1 is able to phosphorylate ARPP19 and to regulate its binding to PP2A-B55δ.13,26,28 Injection of S67-phosphorylated ARPP19 activates Cdk1 in Xenopus oocytes, and thus could lead to Cdk1-dependent phosphorylation of ARPP19, enhancing its binding to PP2A-B55δ. To assess a possible role of Cdk1 in ARPP19 association with PP2A-B55δ, Cdk1 activation was prevented by injecting prophase-arrested oocytes with the Cdk inhibitor, the p21Cip1 protein.48 Oocytes were then injected with in vitro thiophosphorylated ARPP19 proteins, pS67-ARPP, pS109-ARPP or pS67-pS109-ARPP. The injection of p21Cip1 efficiently abolished GVBD induction and Cdk substrate phosphorylation induced by pS67-ARPP or pS67-pS109-ARPP (Fig. 1C). However, both pS67-ARPP and pS67-pS109-ARPP efficiently interacted with PP2A-B55δ, as revealed by immunoblotting the GST-pulled down fractions (Fig. 3A). We next performed the same experiment but using S109 mutated versions of ARPP19 previously thiophosphorylated at S67. The presence of p21Cip1 fully abolished Cdk1 activation, as seen by MAPK and Cdc27 phosphorylation levels (Fig. 2C). Under these conditions, PP2A subunits were also recovered in association with pS67-ARPP, pS67-S109A-ARPP and pS67-S109D-ARPP in the GST-pulled down fractions (Fig. 3B). Altogether, these experiments show that S67 phosphorylation of ARPP19 is sufficient for its interaction with PP2A-B55δ, independently of both Cdk1 activation and ARPP19 phosphorylation at S109.
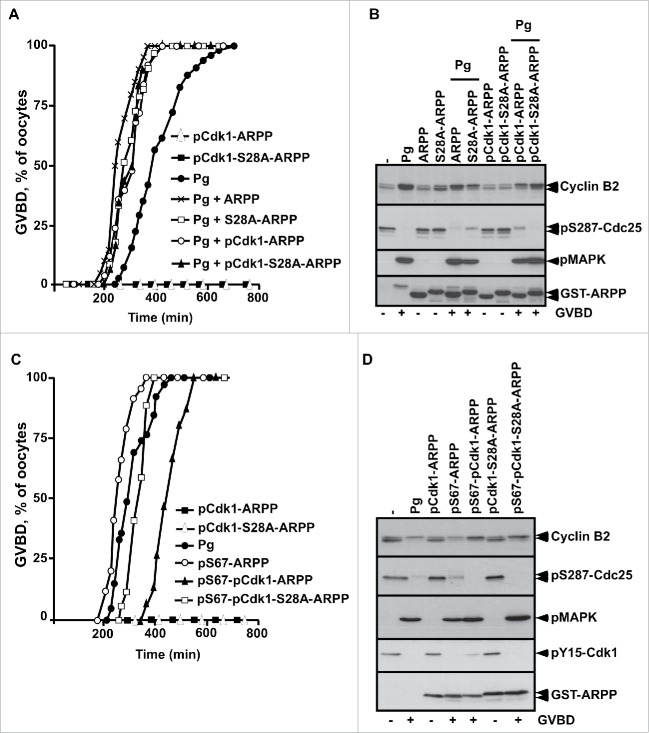
To directly evaluate the role of ARPP19 phosphorylation by Cdk1 toward meiosis resumption, the major putative Cdk1 phosphorylation site of Xenopus ARPP19, S28,9,26 was mutated into either a phosphomimic aspartic residue (S28D-ARPP), or a non-phosphorylatable alanine residue (S28A-ARPP). Prophase oocytes were injected with WT-ARPP, S28A-ARPP and S28D-ARPP and then induced to mature with progesterone (Fig. 4A). All these recombinant proteins slightly accelerated GVBD induced by progesterone in a similar manner (Fig. 4A), as already reported for exogenous WT-ARPP at this concentration (100 ng per oocyte).13 Accordingly, Cdk1 was activated as seen by Cdk1 dephosphorylation at Y15 and Cyclin B2 upshift (Fig. 4B). Note that the electrophoretic migration of the ARPP19 protein mutated at S28 is retarded when compared with the wild type protein, probably due to a conformational change introduced by the mutation (Fig. 4B). Notably, none of the ARPP19 proteins harboring a S28 mutation was able to trigger meiosis resumption on its own (Fig. 4). As mutating a serine into a glutamic acid can fail to mimic a constitutive phosphorylation, recombinant WT-ARPP19 and S28A-ARPP were incubated with active Cdk1 in the presence of γS-ATP to generate thiophosphorylated forms of ARPP19 at the Cdk1 phosphosites, including S28 (Fig. S2A).26 These Cdk1-phosphorylated forms of ARPP19 were injected in prophase oocytes. They did not induce GVBD nor Cdk1 activation on their own (Fig. 5A-B). In response to progesterone, GVBD was induced and Cdk1 was activated as seen by Cyclin B2 upshift, Cdc25 dephosphorylation at S287 and MAPK phosphorylation (Fig. 5A-B). To determine whether Cdk1 phosphorylation of ARPP19 could enhance the activity of the Gwl-phosphorylation at S67, WT-ARPP and the non-phosphorylatable S28A-ARPP19 protein were in vitro thiophosphorylated by both Gwl and Cdk1 (Fig. S2B). Note that other sites than S28 were phosphorylated by Cdk1 as thiophosphorylation was still detected in the S28A-ARPP19 mutant. Prophase oocytes were then injected with the double Gwl and Cdk1 phosphorylated forms of ARPP, either WT-ARPP (pS67-pCdk1-ARPP) or S28A-ARPP (pS67-pCdk1-S28A-ARPP). The single phosphorylated forms of ARPP by either Gwl (pS67-ARPP) or Cdk1 (pCdk1-ARPP and pCdk1-S28A-ARPP) were used as controls. Oocytes underwent GVBD and Cdk1 was activated in response to S67-thiophosphorylated ARPP19 whether S28 was phosphorylated or not (Fig. 5C-D). Therefore, Cdk1-dependent phosphorylation of ARPP19 at S28 or at other sites is dispensable for ARPP19 ability to promote meiosis resumption in Xenopus.
Figure 4.
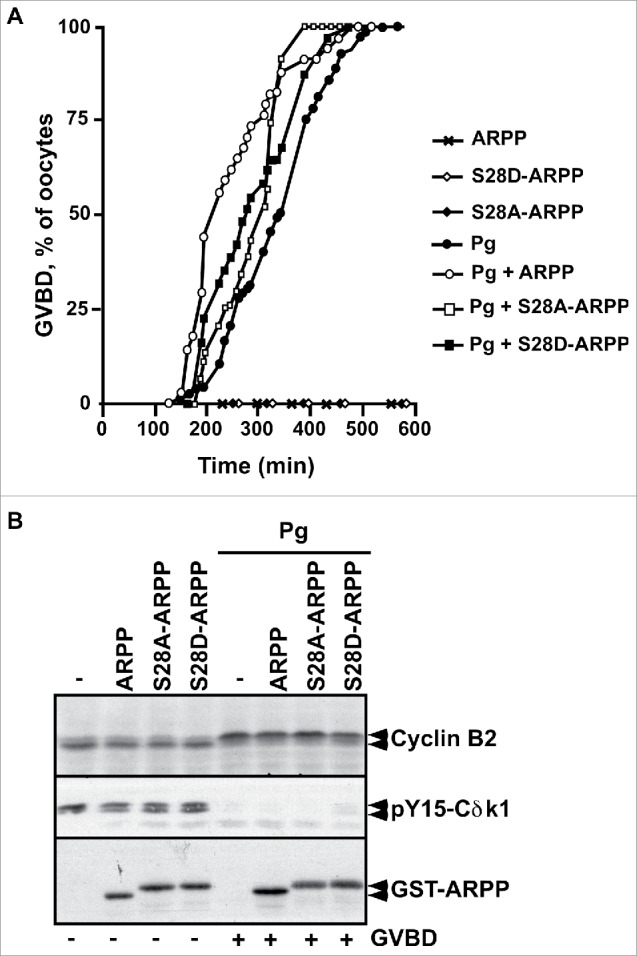
S28D mutation of ARPP19 does not induce meiosis resumption. A. Prophase-arrested oocytes were injected with 100 ng of either WT-ARPP19 (ARPP), S28A-ARPP or S28D-ARPP and then stimulated or not with progesterone (Pg). Meiosis resumption was followed by scoring the % of oocytes at GVBD as a function of time. B. Oocytes from panel (A) were collected at the time of GVBD and Cdk1 activation was analyzed by western blotting Cyclin B2, Y15-phosphorylated Cdk1 (pY15-Cdk1) and total ARPP19 (GST-ARPP).
Figure 5.
The phosphorylation of ARPP19 by Cdk1 is not essential for meiosis resumption. A. Prophase-arrested oocytes were injected or not with either unphosphorylated WT-ARPP19 (ARPP) or S28A-ARPP or Cdk1-thiophosphorylated forms of ARPP: WT-ARPP (pCdk1-ARPP) or S28A-ARPP (pCdk1-S28A-ARPP). Oocytes were then stimulated or not with progesterone (Pg). Meiosis resumption was followed by scoring the % of oocytes at GVBD as a function of time. B. Oocytes from panel (A) were collected at the time of GVBD and Cdk1 activation was analyzed by western blotting Cyclin B2, S287-phosphorylated Cdc25 (pS287-Cdc25), phosphorylated MAPK (pMAPK) and total ARPP19 (GST-ARPP). C. Prophase-arrested oocytes were injected with single S67-phosphorylated ARPP (pS67-ARPP), or single Cdk1-thiophosphorylated forms of either WT-ARPP19 (pCdk1-ARPP) or S28A-ARPP (pCdk1-S28A-ARPP) or with double Cdk1 and Gwl phosphorylated ARPP proteins: WT-ARPP19 (pS67-pCdk1-ARPP) or S28A-ARPP (pS67-pCdk1-S28A-ARPP). As controls, oocytes were stimulated with progesterone (Pg). Meiosis resumption was followed by scoring the % of oocytes at GVBD as a function of time. D. Oocytes from panel (C) were collected at the time of GVBD and Cdk1 activation was analyzed by western blotting Cyclin B2, S287-phosphorylated Cdc25 (pS287-Cdc25), phosphorylated MAPK (pMAPK), Y15-phosphorylated Cdk1 (pY15-Cdk1) and total ARPP19 (GST-ARPP).
S109-phosphorylation of ARPP19 does not impair meiosis resumption induced by PP2A-B55δ inhibition
After its initial dephosphorylation at S109 that occurs within less than one hour in response to progesterone, ARPP19 is rephosphorylated at S109 at GVBD time by an unknown kinase, concomitantly with its S67 phosphorylation.13,29 Whether S109 rephosphorylation of ARPP19 regulates the MPF auto-amplification loop that takes place at that moment is unknown and deserves investigation. To address this issue, we used pS67-ARPP that directly launches the MPF auto-amplification loop, hence bypassing the transduction pathway induced by progesterone. Indeed, pS67-ARPP directly activates Cdk1 by inhibiting PP2A-B55δ.13 To circumvent any effect of a possible intramolecular phosphorylation of the injected protein at S109, we injected the pS67-S109A-ARPP non-phosphorylatable mutant that induces both GVBD and Cdk1 activation (Fig. 6). On the other hand, to determine whether the S109 phosphorylation of ARPP19 can counteract the action of the S67-phosphorylation toward the MPF auto-amplification loop in trans, we used the double S67A-S109D-ARPP mutant. As already shown,29 the injection of S67A-S109D-ARPP inhibited meiosis resumption induced by progesterone in a dose-dependent manner, due to the S109D mutation (Fig. 6). Interestingly, this mutant neither prevented GVBD nor Cdk1 activation induced by pS67-S109A-ARPP, even at the highest concentration (300 ng/oocyte) (Fig. 6). Therefore, pS67-S109A-ARPP triggers the MPF auto-amplification loop even in the presence of S67A-S109D-ARPP. Moreover, high levels of S109A-ARPP did not rescue meiosis resumption in oocytes blocked by the injection of S109D-ARPP (Fig. S3), demonstrating that S109A does not act as a dominant negative over the S109D mutation. Altogether, these results show that the phosphorylation of S109 does not counterbalance the ability of S67-phosphorylated ARPP to initiate the MPF auto-amplification loop through PP2A-B55δ inactivation.
Figure 6.
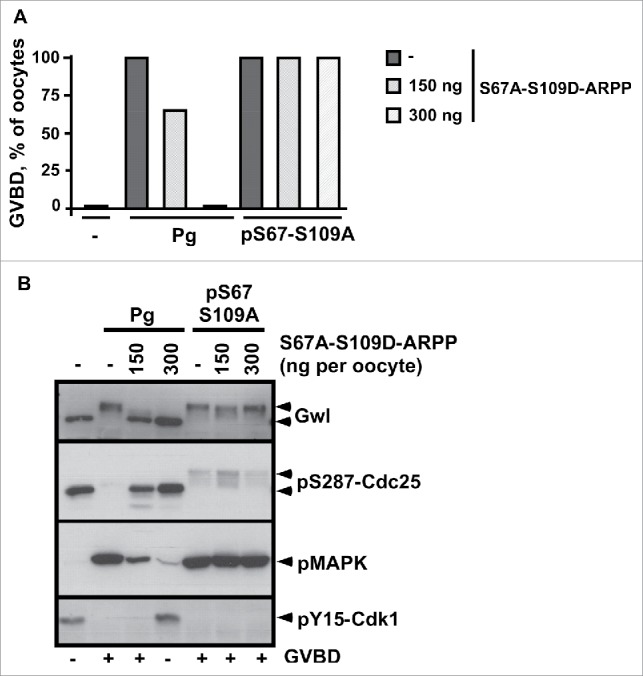
ARPP19 phosphorylation at S109 does not impair meiosis resumption triggered by S67-phosphorylated ARPP and the subsequent inhibition of PP2A-B55δ. A. Prophase-arrested oocytes were injected or not with 150 ng or 300 ng of S67A-S109D-ARPP and then stimulated either with progesterone (Pg) or by injecting S67-thiophosphorylated S109A-ARPP (pS67-S109A-ARPP, 150 ng per oocyte). Meiosis resumption was followed by scoring the % of GVBD 18 hours after hormonal stimulation or pS67-S109A-ARPP injection. B. Cdk1 activation was analyzed in oocytes from panel (A) by western blotting Gwl, S287-phosphorylated Cdc25 (pS287-Cdc25), phosphorylated MAPK (pMAPK) and Y15-phosphorylated Cdk1 (pY15-Cdk1).
Discussion
The meiosis resumption of oocyte offers powerful features to study the molecular network that governs G2/M transition and MPF activation. In Xenopus oocytes, Cdk1 activation obeys a 2-step mechanism: a starter amount of active Cdk1 is first generated and consequently launches the MPF auto-amplification loop, leading to full Cdk1 activation. Upon progesterone stimulation, PKA is downregulated and ARPP19 is dephosphorylated at S109, this event being necessary for the subsequent initial activation of Cdk1. Then, the MPF amplification loop operates independently of PKA and ARPP19 phosphorylation at S109.13,29,34,37-43,49,50 The independency of the MPF auto-amplification loop from PKA relies on PP2A-B55δ inactivation, under the control of ARPP19 phosphorylation at S67 by Gwl.13 Hence, ARPP19 has a dual function during meiosis resumption. As a PKA substrate, it negatively controls the initial step of the pathway leading to Cdk1 activation while, when phosphorylated by Gwl, it participates in MPF auto-amplification by inhibiting PP2A-B55δ. The phosphorylation of ARPP19 by PKA and Gwl being temporally dissociated, the first taking place during the prophase arrest while the second occurring at the time of MPF activation, we hypothesized that an interplay could functionally connect ARPP19 dephosphorylation at S109 and its phosphorylation at S67 in vivo. A similar process has been recently reported in the striatum, where the inhibition of PP2A depends on the phosphorylation of ARPP16, another member of the ENSA family, at S46 (equivalent residue of S67 in ARPP19) by MAST3 kinase (that has highest similarity to Xenopus Gwl), this mechanism being counterbalanced by PKA.51
We show here that Gwl phosphorylates ARPP19 in vitro, even when the protein is already phosphorylated by PKA at S109 or when this serine is mutated into a glutamic acid (S109D). Moreover, Gwl and PKA are able to phosphorylate ARPP19 at their respective sites without interfering with each other. Hence, neither PKA nor the phosphorylation of ARPP19 at S109 interferes with Gwl ability to phosphorylate ARPP19 (Fig. 7), in agreement with.26 We investigated the effects of ARPP19 phosphorylation at S109 on both PP2A-B55δ inhibition and Cdk1 activation specifically induced by S67-phosphorylation of ARPP19. Since the double phosphorylated form of ARPP19 at S109 and S67 still interacts with PP2A-B55δ and promotes meiosis resumption, the PKA-dependent phosphorylation of ARPP19 does not antagonize the biological ability of S67-phosphorylated ARPP19. Most importantly, the effect of S67-phosphorylation is dominant over the negative function of S109-phosphorylation. Studies conducted in Xenopus cell-free extracts shed light on another possible interplay between ARPP19 phosphorylations: rather than impairing the interaction between PP2A-B55δ and S67-phosphorylated ENSA, the PKA-dependent phosphorylation of ENSA would abolish PP2A-B55δ inhibition induced by its Cdk1-dependent phosphorylation as demonstrated in vitro.26 Moreover, the Cdk1-dependent phosphorylation of ARPP19 enables PP2A-B55δ inactivation independently of Gwl in starfish oocyte.28 It was therefore important to clarify whether Cdk1 and the resulting phosphorylation of ARPP19 at S28, the main phosphorylation site for Cdk1, contributes to the activity of ARPP19 during Xenopus oocyte meiosis resumption. We demonstrate here that neither Cdk1 activation nor ARPP19 phosphorylation by Cdk1 are required for PP2A-B55δ inactivation induced by S67-phosphorylated ARPP19, regardless its phosphorylation at S109. Hence, in contrast to starfish oocytes,28 the phosphorylation of ARPP19 by Gwl is the major event necessary and sufficient for ARPP19 interaction with PP2A-B55δ and for its biological activity as a potent activator of Cdk1. Importantly, the single S109 phosphorylation of ARPP19 (S109D-ARPP19) does not prevent MPF auto-amplification triggered by either cytoplasm transfer, overexpression of an active form of Gwl, or the injection of S67-phosphorylated ARPP1929. Altogether, our results demonstrate that the Gwl-phosphorylation of ARPP19 at S67 overcomes the inhibitory effects of ARPP19 phosphorylation at S109, whether or not these phosphorylations occur within the same ARPP molecule. Likewise, the inhibition of PP2A-B55δ resulting from S67 phosphorylation of ARPP19 activates Cdk1 independently of the PKA-ARPP19 pathway, thus arguing for the Gwl-dependent phosphorylation of ARPP19 being involved in the MPF auto-amplification loop as already suggested.13,40,41,49,50
Figure 7.
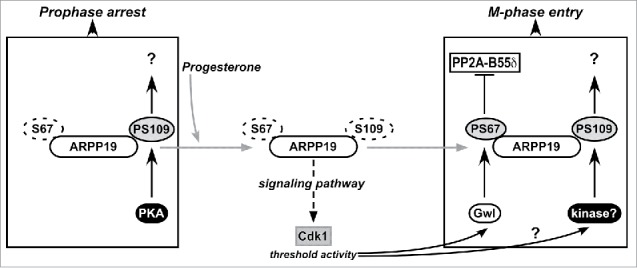
Schematic representation of the regulation of meiosis resumption by ARPP19 in Xenopus oocytes. ARPP19 is phosphorylated by PKA at S109 in prophase-arrested oocytes. The molecular targets of the single S109-phosphorylated form of ARPP19 that are responsible for the prophase arrest are unknown. Our study shows that S109 phosphorylation does not prevent ARPP19 phosphorylation at S67 by Gwl. In response to progesterone, PKA activity drops down and consequently, ARPP19 is dephosphorylated at S109. This event unlocks a signaling pathway that generates a threshold activity of Cdk1. Once this starter amount of Cdk1 is formed, it induces Gwl activation that in turn phosphorylates ARPP19 at S67. As a consequence, PP2A-B55δ is inhibited and the MPF auto-amplification loop is launched. Moreover, ARPP19 is also re-phosphorylated at S109 by an unknown kinase distinct of PKA. This phosphorylation contributes to M-phase entry. Hence, the active form of ARPP19 that sustains Cdk1 activation does not only rely on phosphorylation at S67 as previously thought, but also on its concomitant phosphorylation at S109.
Under physiological circumstances, PKA activity is decreased within 10 to 30 min after progesterone stimulation and remains low thereafter.34,47 These results have been recently challenged.36 However, the methods used in this study could have failed to detect these variations and the study needs to be confirmed. Given the dozen of articles since 1978 documenting the drop in cAMP and PKA activity that are induced by progesterone and showing that PKA activity remains low during meiotic maturation, PKA unlikely interferes with the Cdk1 auto-amplification loop under normal conditions. However, ARPP19 is partially rephosphorylated at S109 at the time of MPF activation, by a kinase distinct from PKA.29 Our results, showing that S67 phosphorylation overcomes the inhibitory effect of S109 phosphorylation, explain why the S109 rephosphorylation of ARPP19 does not impede MPF activation at the time of GVBD. Altogether, our results highlight the importance of timely synchronizing ARPP19 phosphorylations at S109 and S67 to fully activate Cdk1. Upon progesterone stimulation, the inhibition of PKA licenses the formation of the starter amount of active Cdk1 through ARPP19 dephosphorylation at S109. ARPP19 is then re-phosphorylated at S109 by another kinase, hence neither preventing S67 phosphorylation of ARPP19 by Gwl nor its conversion into a PP2A-B55δ inhibitor. Thus, MPF is properly activated (Fig. 7).
Interestingly, ARPP19 phosphorylated at both S109 and S67 could activate Cdk1, not only because S67 phosphorylation blocks PP2A-B55δ and overcomes the negative effect of phosphorylated S109, but also because S109 phosphorylation would confer new properties to ARPP19, contributing to Cdk1 activation in a context where S67 is already phosphorylated (Fig. 7). This hypothesis is supported by our present finding that S67-phosphorylated ARPP19 is less efficient in activating Cdk1 when S109 is mutated in a non-phosphorylatable residue. For example, double S109-S67 phosphorylated ARPP19 could regulate phosphatases other than PP2A-B55δ. Such hypothesis deserves further investigation. It would also be of interest to identify the kinase responsible for ARPP19 phosphorylation at S109 at the time of MPF activation.
Materials and methods
Materials
Xenopus laevis adult females (Centre de Ressources Biologiques Xenopes, CNRS, France) were bred and maintained under laboratory conditions (Animal Facility Agreement: #B75–05–13). Reagents, unless otherwise specified, were from Sigma.
Preparation and handlings of xenopus oocytes
Fully grown Xenopus prophase oocytes were obtained as described.29 The usual microinjected volume was 50 nl per oocyte. Progesterone (Pg) was used in the external medium at 2 µM. Oocytes were referred to as GVBD when the first pigment rearrangement was detected at the animal pole. Oocytes were homogenized at 4°C in 10 volumes of Extraction Buffer (EB: 80 mM β-glycerophosphate pH 7.3, 20 mM EGTA, 15 mM MgCl2), centrifuged at 15 000 g for 10 min at 4°C and supernatants were used for further analysis.
Antibodies and western blot
An equivalent of 0.5 oocyte was loaded on 12% SDS polyacrylamide gels and immunoblotted as described.29 To visualize PP2A subunits, the equivalent of 1.5 oocytes was loaded on 10% SDS polyacrylamide gels. The antibodies directed against the following proteins were used: phosphorylated MAP kinase (1/1000, Cell Signaling 9106), Cyclin B2 (1/1000, Abcam ab18250), Cdc27 (1/500, BD Transduction Laboratory 610454), Y15-phosphorylated Cdk1 (1/1000, Cell Signaling 9111), S287-phosphorylated Cdc25 (pS287-Cdc25, 1/1000, Cell Signaling 4901), phosphorylated CDK substrates (1/1000, Cell Signaling 2324), anti-alkylated thiophosphates (1/3000, Abcam ab92570) and GST (1/10000, Sigma A-7340). Rabbit B55δ antibody was a kind gift from Dr. S. Mochida.9 Antibodies directed against the following proteins were used: Gwl,13 PP2A-C subunit,29,52 S109-phosphorylated ARPP1929 and S67-phosphorylated ARPP19.13 Appropriate horseradish peroxidase-labeled secondary antibodies (Jackson Immunoresearch) were revealed by chemiluminescence (Pierce).
Cloning of recombinant GST-ARPP proteins
DNA encoding GST-tagged wild-type ARPP19 or harboring either S109A or S109D point mutations or double S67A and S109D mutations were described previously.29 DNA encoding GST-ARPP with point mutations at S28, either S28A or S28D, were generated using the Quick Change site mutagenesis kit (Stratagene) and mutations were ascertained by DNA sequencing (MWG Eurofins).
Expression and purification of recombinant proteins
Recombinant GST-ARPP and GST-p21Cip1 were produced in E. coli by autoinduction 52 and purified respectively as described in.13,29,48 Fractions containing purified recombinant proteins were dialyzed overnight against PBS (Phosphate Buffered Saline pH 7.4, 13.7 mM NaCl, 2.7 mM KCl, 4.3 mM KH2PO4, 1.4 mM Na2HPO4) and stored at −80°C.
In vitro thiophosphorylation of recombinant GST-ARPP proteins by Gwl, PKA and Cdk1
Active Gwl was obtained by injecting prophase oocytes with mRNA encoding Histidine-tagged Xenopus K71M-Gwl.13 Oocytes were collected at metaphase II and K71M-Gwl was recovered by incubating oocyte lysates with Nickel beads (Qiagen) in the presence of 1 µM okadaic acid (Enzo Life Sciences). Nickel beads were washed in kinase buffer (20 mM Hepes pH 7.4, 2 mM 2-Mercaptoethanol) and 500 µg of recombinant ARPP19 proteins were added in the presence of 1mM γS-ATP. The reaction was performed in a final volume of 40 µl for 90 min at 30°C. For PKA thiophosphorylation, Histidine-tagged recombinant PKA (a gift from Susan Taylor, Addgene plasmid #14921) was bacterially expressed by autoinduction,53 purified on Nickel beads and incubated with 500 µg of recombinant ARPP19 proteins in the presence of 1mM γS-ATP for 90 min at 30°C. The double thiophosphorylation of ARPP19 at S67 and S109 was performed as described above in the presence of both Gwl and PKA or by adding sequentially either PKA and then Gwl or Gwl and then PKA. For Cdk1 thiophosphorylation, Cdk1 was affinity-purified from oocytes arrested at metaphase II on p13-agarose beads.54 P13-beads were incubated with 500 µg of recombinant ARPP19 proteins in the presence of 1mM γS-ATP for 90 min at 30°C. ARPP19 proteins were then recovered by centrifugation and dialyzed against PBS. ARPP19 thiophosphorylation by Cdk1 was ascertained by incubating ARPP19 proteins for 60 min at room temperature with p-nitrobenzyl mesylate (Abcam 138910) to alkylate incorporated thiophosphates and then western blotting the proteins with an antibody directed against alkylated thiophosphates. The double thiophosphorylation of ARPP19 by Cdk1 and Gwl was performed as described above by mixing equal amounts of p13-coupled Cdk1 purified from metaphase II oocytes and Nickel-beads coupled to Gwl. Proteins were injected at the final concentration of 3 µM in oocytes (corresponding to 150 ng per oocyte), a concentration sufficient to induce meiosis resumption with WT-ARPP19 in vitro phosphorylated by Gwl.13 In some experiments, proteins were injected at the final concentration of 4 µM, 6 µM or 8 µM (corresponding to 200 ng, 300 ng or 400 ng per oocyte), as indicated.
GST Pull Down
50 µl of oocyte lysates were incubated for 1 hour at 4°C with 30 µl of GST magnetic beads (Promega) equilibrated in EB. GST beads were recovered, washed extensively in EB and resuspended in an equal volume of loading buffer for western blot analysis.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all members of our laboratory for helpful discussions. We are grateful to Dr S. Mochida for providing the anti-B55δ antibody.
Funding
This work was supported by CNRS, University Pierre and Marie Curie (UPMC), and Agence Nationale de la Recherche (ANR-13-BSV2–0008–01 to CJ).
Author contributions
AID, CJ and OH conceived the original idea, designed and planned the experiments, analyzed the data and wrote the paper. AD and OH performed experiments.
References
- [1].Bontron S, Jaquenoud M, Vaga S, Talarek N, Bodenmiller B, Aebersold R, De Virgilio C. Yeast endosulfines control entry into quiescence and chronological life span by inhibiting protein phosphatase 2A. Cell Reports 2013; 3:16-22; PMID:23273919; https://doi.org/ 10.1016/j.celrep.2012.11.025 [DOI] [PubMed] [Google Scholar]
- [2].Talarek N, Bontron S, De Virgilio C. Quantification of mRNA stability of stress-responsive yeast genes following conditional excision of open reading frames. RNA Biol 2013; 10:1299-308; PMID:23792549; https://doi.org/ 10.4161/rna.25355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Talarek N, Cameroni E, Jaquenoud M, Luo X, Bontron S, Lippman S, Devgan G, Snyder M, Broach JR, De Virgilio C. Initiation of the TORC1-regulated G0 program requires Igo1/2, which license specific mRNAs to evade degradation via the 5′-3′ mRNA decay pathway. Mol Cell 2010; 38:345-55; PMID:20471941; https://doi.org/ 10.1016/j.molcel.2010.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Juanes MA, Khoueiry R, Kupka T, Castro A, Mudrak I, Ogris E, Lorca T, Piatti S. Budding yeast greatwall and endosulfines control activity and spatial regulation of PP2A(Cdc55) for timely mitotic progression. PLoS Genet 2013; 9:e1003575; PMID:23861665; https://doi.org/ 10.1371/journal.pgen.1003575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].White RE, Giffard RG. MicroRNA-320 induces neurite outgrowth by targeting ARPP-19. Neuroreport 2012; 23:590-5; PMID:22617447; https://doi.org/ 10.1097/WNR.0b013e3283540394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jiang T, Zhao B, Li X, Wan J. ARPP-19 promotes proliferation and metastasis of human glioma. Neuroreport 2016; 27:960-6; PMID:27380244; https://doi.org/ 10.1097/WNR.0000000000000638 [DOI] [PubMed] [Google Scholar]
- [7].Vigneron S, Robert P, Hached K, Sundermann L, Charrasse S, Labbe JC, Castro A, Lorca T. The master Greatwall kinase, a critical regulator of mitosis and meiosis. Int J Dev Biol 2016; 60:245-54; PMID:27759153; https://doi.org/ 10.1387/ijdb.160155tl [DOI] [PubMed] [Google Scholar]
- [8].Gharbi-Ayachi A, Labbe J-C, Burgess A, Vigneron S, Strub J-M, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T. The Substrate of Greatwall Kinase, Arpp19, Controls Mitosis by Inhibiting Protein Phosphatase 2A. Science 2010; 330:1673-7; PMID:21164014; https://doi.org/ 10.1126/science.1197048 [DOI] [PubMed] [Google Scholar]
- [9].Mochida S, Maslen SL, Skehel M, Hunt T. Greatwall Phosphorylates an Inhibitor of Protein Phosphatase 2A That Is Essential for Mitosis. Science 2010; 330:1670-3; PMID:21164013; https://doi.org/ 10.1126/science.1195689 [DOI] [PubMed] [Google Scholar]
- [10].Yu J, Fleming SL, Williams B, Williams EV, Li Z, Somma P, Rieder CL, Goldberg ML. Greatwall kinase: a nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J Cell Biol 2004; 164:487-92; PMID:14970188; https://doi.org/ 10.1083/jcb.200310059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Castilho PV, Williams BC, Mochida S, Zhao Y, Goldberg ML. The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites. Mol Biol Cell 2009; 20:4777-89; PMID:19793917; https://doi.org/ 10.1091/mbc.E09-07-0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mochida S, Ikeo S, Gannon J, Hunt T. Regulated activity of PP2A-B55delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J 2009; 28(18):2777-85; PMID:19696736; https://doi.org/ 10.1038/emboj.2009.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dupre A, Buffin E, Roustan C, Nairn AC, Jessus C, Haccard O. The phosphorylation of ARPP19 by Greatwall renders the auto-amplification of MPF independently of PKA in Xenopus oocytes. J Cell Sci 2013; 126:3916-26; PMID:23781026; https://doi.org/ 10.1242/jcs.126599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rangone H, Wegel E, Gatt MK, Yeung E, Flowers A, Debski J, Dadlez M, Janssens V, Carpenter AT, Glover DM. Suppression of scant identifies Endos as a substrate of greatwall kinase and a negative regulator of protein phosphatase 2A in mitosis. PLoS Genet 2011; 7:e1002225; PMID:21852956; https://doi.org/ 10.1371/journal.pgen.1002225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim MY, Bucciarelli E, Morton DG, Williams BC, Blake-Hodek K, Pellacani C, Von Stetina JR, Hu X, Somma MP, Drummond-Barbosa D, et al.. Bypassing the Greatwall-Endosulfine pathway: plasticity of a pivotal cell-cycle regulatory module in Drosophila melanogaster and Caenorhabditis elegans. Genetics 2012; 191:1181-97; PMID:22649080; https://doi.org/ 10.1534/genetics.112.140574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Labandera AM, Vahab AR, Chaudhuri S, Kerk D, Moorhead GB. The mitotic PP2A regulator ENSA/ARPP-19 is remarkably conserved across plants and most eukaryotes. Biochem Biophys Res Commun 2015; 458:739-44; PMID:25666948; https://doi.org/ 10.1016/j.bbrc.2015.01.123 [DOI] [PubMed] [Google Scholar]
- [17].Williams BC, Filter JJ, Blake-Hodek KA, Wadzinski BE, Fuda NJ, Shalloway D, Goldberg ML. Greatwall-phosphorylated Endosulfine is both an inhibitor and a substrate of PP2A-B55 heterotrimers. eLife 2014; 3:e01695; PMID:24618897; https://doi.org/ 10.7554/eLife.01695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kishimoto T. Entry into mitosis: a solution to the decades-long enigma of MPF. Chromosoma 2015; 124:417-28; PMID:25712366; https://doi.org/ 10.1007/s00412-015-0508-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lindqvist A, Rodriguez-Bravo V, Medema RH. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol 2009; 185:193-202; PMID:19364923; https://doi.org/ 10.1083/jcb.200812045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hegarat N, Rata S, Hochegger H. Bistability of mitotic entry and exit switches during open mitosis in mammalian cells. BioEssays 2016; 38:627-43; PMID:27231150; https://doi.org/ 10.1002/bies.201600057 [DOI] [PubMed] [Google Scholar]
- [21].Yu J, Zhao Y, Li Z, Galas S, Goldberg ML. Greatwall kinase participates in the CDC2 autoregulatory loop in Xenopus egg extracts. Mol Cell 2006; 22:83-91; PMID:16600872; https://doi.org/ 10.1016/j.molcel.2006.02.022 [DOI] [PubMed] [Google Scholar]
- [22].Vigneron S, Brioudes E, Burgess A, Labbe JC, Lorca T, Castro A. Greatwall maintains mitosis through regulation of PP2A. EMBO J 2009; 28(18):2786-93; PMID:19680222; https://doi.org/ 10.1038/emboj.2009.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Burgess A, Vigneron S, Brioudes E, Labbe JC, Lorca T, Castro A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci U S A 2010; 107:12564-9; PMID:20538976; https://doi.org/ 10.1073/pnas.0914191107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lorca T, Bernis C, Vigneron S, Burgess A, Brioudes E, Labbe JC, Castro A. Constant regulation of both the MPF amplification loop and the Greatwall-PP2A pathway is required for metaphase II arrest and correct entry into the first embryonic cell cycle. J Cell Sci 2010; 123:2281-91; PMID:20554897; https://doi.org/ 10.1242/jcs.064527 [DOI] [PubMed] [Google Scholar]
- [25].Schmitz MH, Held M, Janssens V, Hutchins JR, Hudecz O, Ivanova E, Goris J, Trinkle-Mulcahy L, Lamond AI, Poser I, et al.. Live-cell imaging RNAi screen identifies PP2A-B55alpha and importin-beta1 as key mitotic exit regulators in human cells. Nat Cell Biol 2010; 12:886-93; PMID:20711181; https://doi.org/ 10.1038/ncb2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mochida S. Regulation of alpha-endosulfine, an inhibitor of protein phosphatase 2A, by multisite phosphorylation. FEBS J 2014; 281:1159-69; PMID:24354984; https://doi.org/ 10.1111/febs.12685 [DOI] [PubMed] [Google Scholar]
- [27].Dulubova I, Horiuchi A, Snyder GL, Girault JA, Czernik AJ, Shao L, Ramabhadran R, Greengard P, Nairn AC. ARPP-16/ARPP-19: a highly conserved family of cAMP-regulated phosphoproteins. J Neurochem 2001; 77:229-38; PMID:11279279; https://doi.org/ 10.1046/j.1471-4159.2001.t01-1-00191.x [DOI] [PubMed] [Google Scholar]
- [28].Okumura E, Morita A, Wakai M, Mochida S, Hara M, Kishimoto T. Cyclin B-Cdk1 inhibits protein phosphatase PP2A-B55 via a Greatwall kinase-independent mechanism. J Cell Biol 2014; 204:881-9; PMID:24616226; https://doi.org/ 10.1083/jcb.201307160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dupre A, Daldello EM, Nairn AC, Jessus C, Haccard O. Phosphorylation of ARPP19 by protein kinase A prevents meiosis resumption in Xenopus oocytes. Nat commun 2014; 5:3318; PMID:24525567; https://doi.org/ 10.1038/ncomms4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mulner O, Huchon D, Thibier C, Ozon R. Cyclic AMP synthesis in Xenopus laevis oocytes: inhibition by progesterone. Biochim Biophys Acta 1979; 582:179-84; PMID:83880; https://doi.org/ 10.1016/0304-4165(79)90301-5 [DOI] [PubMed] [Google Scholar]
- [31].Ozon R, Belle R, Huchon D, Mulner O. Roles of cyclic AMP and calcium in maturation of Xenopus laevis oocytes. J Steroid Biochem 1979; 11:709-13; PMID:226799; https://doi.org/ 10.1016/0022-4731(79)90004-9 [DOI] [PubMed] [Google Scholar]
- [32].Ozon R, Marot J, Huchon D. Progesterone stimulated meiotic maturation in Xenopus laevis: inhibition by methylxanthines. Res Steroids 1979; 8:259-63. [Google Scholar]
- [33].Smith LD. The induction of oocyte maturation: transmembrane signalling events and regulation of the cell cycle. Development 1989; 107:685-99; PMID:2698799 [DOI] [PubMed] [Google Scholar]
- [34].Wang J, Liu XJ. Progesterone inhibits protein kinase A (PKA) in Xenopus oocytes: demonstration of endogenous PKA activities using an expressed substrate. J Cell Sci 2004; 117:5107-16; PMID:15456849; https://doi.org/ 10.1242/jcs.01383 [DOI] [PubMed] [Google Scholar]
- [35].Maller JL, Butcher FR, Krebs EG. Early effect of progesterone on levels of cyclic adenosine 3′:5′- monophosphate in Xenopus oocytes. J Biol Chem 1979; 254:579-82; PMID:216678 [PubMed] [Google Scholar]
- [36].Nader N, Courjaret R, Dib M, Kulkarni RP, Machaca K. Release from Xenopus oocyte prophase I meiotic arrest is independent of a decrease in cAMP levels or PKA activity. Development 2016; 143:1926-36; PMID:27122173; https://doi.org/ 10.1242/dev.136168 [DOI] [PubMed] [Google Scholar]
- [37].Maller JL, Krebs EG. Progesterone-stimulated meiotic cell division in Xenopus oocytes. Induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem 1977; 252:1712-8; PMID:190238 [PubMed] [Google Scholar]
- [38].Huchon D, Ozon R, Fischer EH, Demaille JG. The pure inhibitor of cAMP-dependent protein kinase initiates Xenopus laevis meiotic maturation. A 4-step scheme for meiotic maturation. Mol Cell Endocrinol 1981; 22:211-22; PMID:7016632; https://doi.org/ 10.1016/0303-7207(81)90092-7 [DOI] [PubMed] [Google Scholar]
- [39].Rime H, Haccard O, Ozon R. Activation of p34cdc2 kinase by cyclin is negatively regulated by cyclic amp-dependent protein kinase in Xenopus oocytes. Dev Biol 1992; 151:105-10; PMID:1533599; https://doi.org/ 10.1016/0012-1606(92)90217-5 [DOI] [PubMed] [Google Scholar]
- [40].Eyers PA, Liu J, Hayashi NR, Lewellyn AL, Gautier J, Maller JL. Regulation of the G2/M transition in Xenopus oocytes by the cAMP-dependent protein kinase. J Biol Chem 2005; 280(26):24339–46; PMID:15860459; https://doi.org/ 10.1074/jbc.M412442200 [DOI] [PubMed] [Google Scholar]
- [41].Matten W, Daar I, Vande Woude GF. Protein kinase A acts at multiple points to inhibit Xenopus oocyte maturation. Mol Cell Biol 1994; 14:4419-26; PMID:8007949; https://doi.org/ 10.1128/MCB.14.7.4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Daar I, Yew N, Vande Woude GF. Inhibition of mos-induced oocyte maturation by protein kinase A. J Cell Biol 1993; 120:1197-202; PMID:8436591; https://doi.org/ 10.1083/jcb.120.5.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Duckworth BC, Weaver JS, Ruderman JV. G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc Natl Acad Sci USA 2002; 99:16794-9; PMID:12477927; https://doi.org/ 10.1073/pnas.222661299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Haccard O, Jessus C. Oocyte maturation, Mos and cyclins-a matter of synthesis: two functionally redundant ways to induce meiotic maturation. Cell Cycle 2006; 5:1152-9; PMID:16760654; https://doi.org/ 10.4161/cc.5.11.2800 [DOI] [PubMed] [Google Scholar]
- [45].Gaffre M, Martoriati A, Belhachemi N, Chambon JP, Houliston E, Jessus C, Karaiskou A. A critical balance between Cyclin B synthesis and Myt1 activity controls meiosis entry in Xenopus oocytes. Development 2011; 138:3735-44; PMID:21795279; https://doi.org/ 10.1242/dev.063974 [DOI] [PubMed] [Google Scholar]
- [46].De Smedt V, Poulhe R, Cayla X, Dessauge F, Karaiskou A, Jessus C, Ozon R. Thr-161 Phosphorylation of Monomeric Cdc2. Regulation by protein phosphatase 2C in Xenopus oocyte. J Biol Chem 2002; 277:28592-600; PMID:12036957; https://doi.org/ 10.1074/jbc.M202742200 [DOI] [PubMed] [Google Scholar]
- [47].Wang J, Cao WL, Liu XJ. Protein kinase A(PKA)-restrictive and PKA-permissive phases of oocyte maturation. Cell Cycle 2006; 5:213-7; PMID:16397412; https://doi.org/ 10.4161/cc.5.2.2365 [DOI] [PubMed] [Google Scholar]
- [48].Frank-Vaillant M, Jessus C, Ozon R, Maller JL, Haccard O. Two distinct mechanisms control the accumulation of cyclin B1 and mos in Xenopus oocytes in response to progesterone. Mol Biol Cell 1999; 10:3279-88; PMID:10512866; https://doi.org/ 10.1091/mbc.10.10.3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Goris J, Hermann J, Hendrix P, Ozon R, Merlevede W. Okadaic acid, a specific protein phosphatase inhibitor, induces maturation and MPF formation in Xenopus laevis oocytes. FEBS Lett 1989; 245:91-4; PMID:2538367; https://doi.org/ 10.1016/0014-5793(89)80198-X [DOI] [PubMed] [Google Scholar]
- [50].Huchon D, Ozon R. Effects of 3-isobutyl-methylxanthine (IBMX) and of cholera toxin on maturation induced by the injection of maturation promoting factor (MPF) in oocyte of Xenopus laevis. Biol Cell 1979; 35:15-20. [Google Scholar]
- [51].Andrade EC, Musante V, Horiuchi A, Matsuzaki H, Brody AH, Wu T, Greengard P, Taylor JR, Nairn AC. ARPP-16 is a striatal-enriched inhibitor of protein phosphatase 2A regulated by microtubule-associated serine/threonine kinase 3 (Mast 3 kinase). J Neurosci 2017; 37:2709-22; PMID:28167675; https://doi.org/ 10.1523/JNEUROSCI.4559-15.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bosch M, Cayla X, Van Hoof C, Hemmings BA, Ozon R, Merlevede W, Goris J. The PR55 and PR65 subunits of protein phosphatase 2A from Xenopus laevis molecular cloning and developmental regulation of expression. Eur J Biochem 1995; 230:1037-45; PMID:7601134; https://doi.org/ 10.1111/j.1432-1033.1995.tb20653.x [DOI] [PubMed] [Google Scholar]
- [53].Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 2005; 41:207-34; PMID:15915565; https://doi.org/ 10.1016/j.pep.2005.01.016 [DOI] [PubMed] [Google Scholar]
- [54].Daldello EM, Le T, Poulhe R, Jessus C, Haccard O, Dupre A. Fine-tuning of Cdc6 accumulation by Cdk1 and MAP kinase is essential for completion of oocyte meiotic divisions. J Cell Sci 2015; 128:2482-96; PMID:26092930; https://doi.org/ 10.1242/jcs.166553 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.