Abstract
The right-handed (B) conformation of poly[d(G-C)] in 7.5 mM sodium cacodylate and 25% ethylene glycol can be readily converted to the left-handed (Z) conformation by the addition of 250 microM MnCl2 and this transition can be reversed by chelation of the Mn ions with EDTA or by addition of NaCl. This ability to obtain such reversible transitions in solvent and solute conditions which allow DNA-protein interactions and their assessment by c.d. permitted an analysis of the effect of purified histones, polyamino acids, protamine and polyamines on these transitions. Individual core histones H3, H4, H2a and H2b or protamine stabilised the Mn-induced Z form and prevented the transition to B DNA normally observed after chelation with EDTA or on dialysis to physiological salt concentrations. A similar suppression of Z leads to B transition was also achieved with poly-L-arginine (but not with poly-L-lysine). In contrast, histones H1 and H5 promoted the Z leads to B transition. Polyamines (spermine and spermidine) converted the B form to another right-handed (A) form which transformed to the Z form after the addition of EDTA and this Z form was restored to the B conformation on the addition of NaCl. These results suggest that sequence-dependent variations in the conformation of natural DNA may be modulated by interaction with histones and other basic cellular components and may provide a conformational basis for nucleosome formation and possibly for the control of gene expression.
Full text
PDF
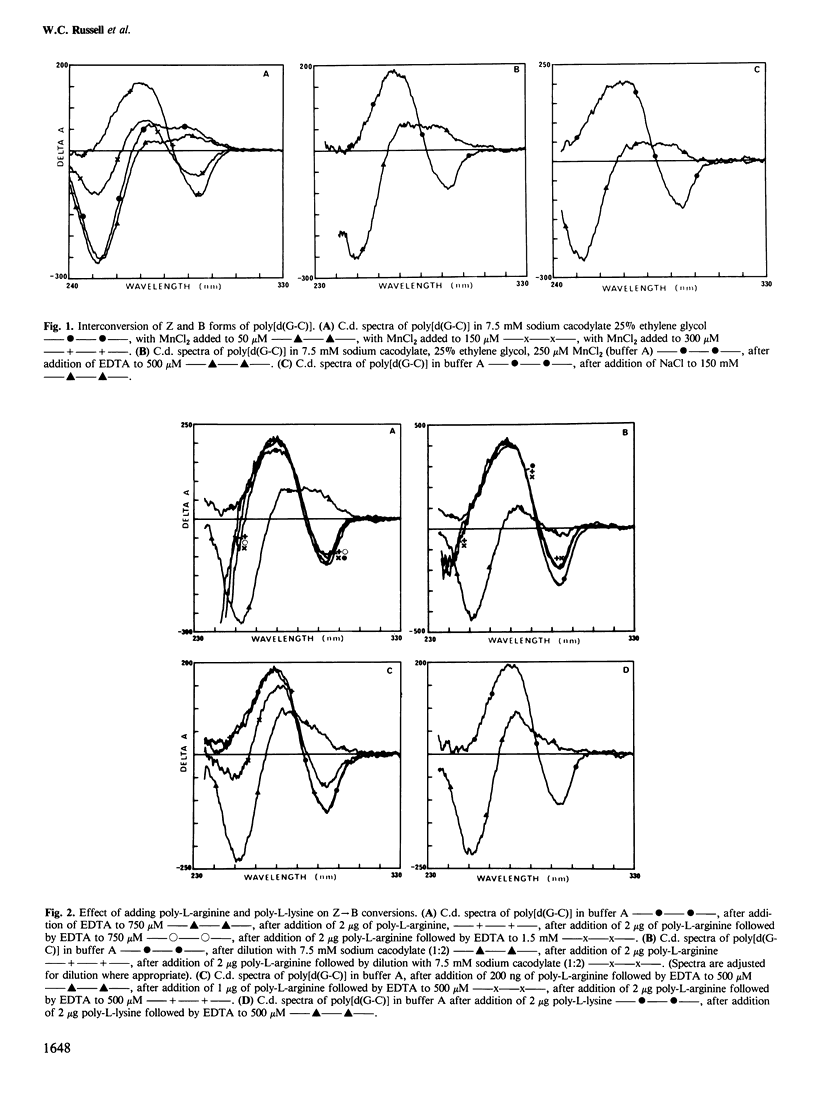
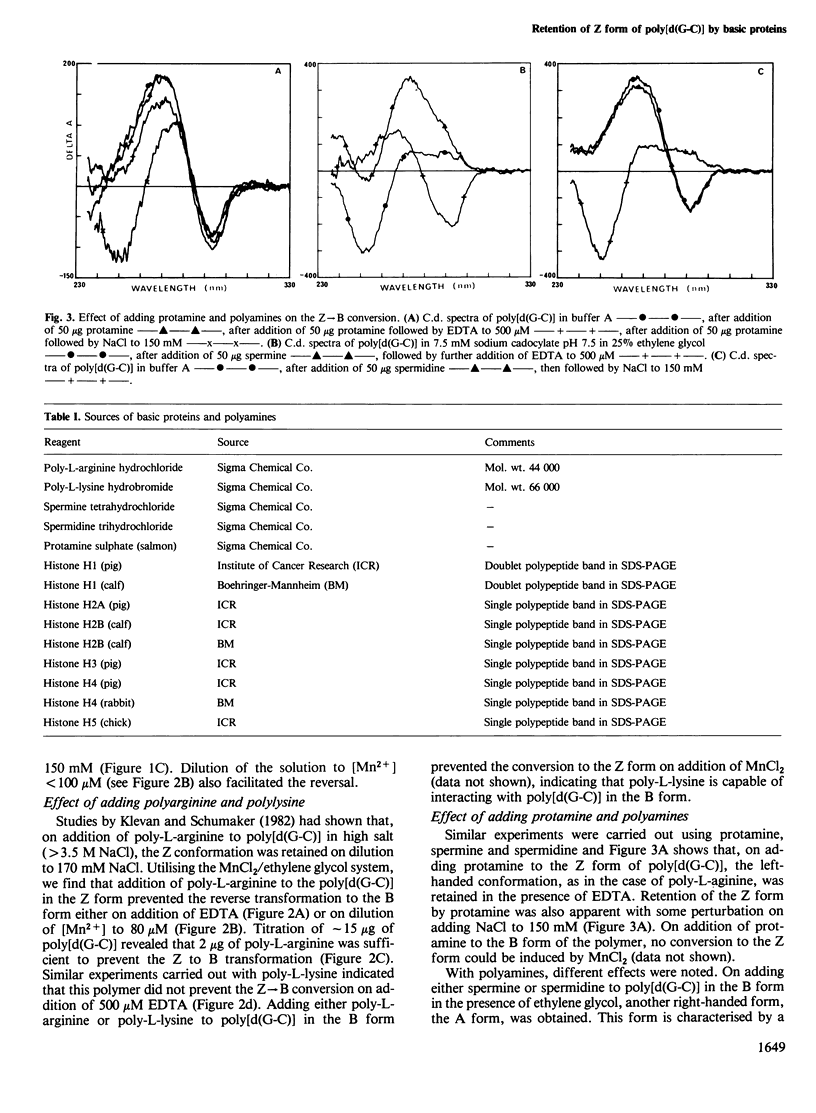
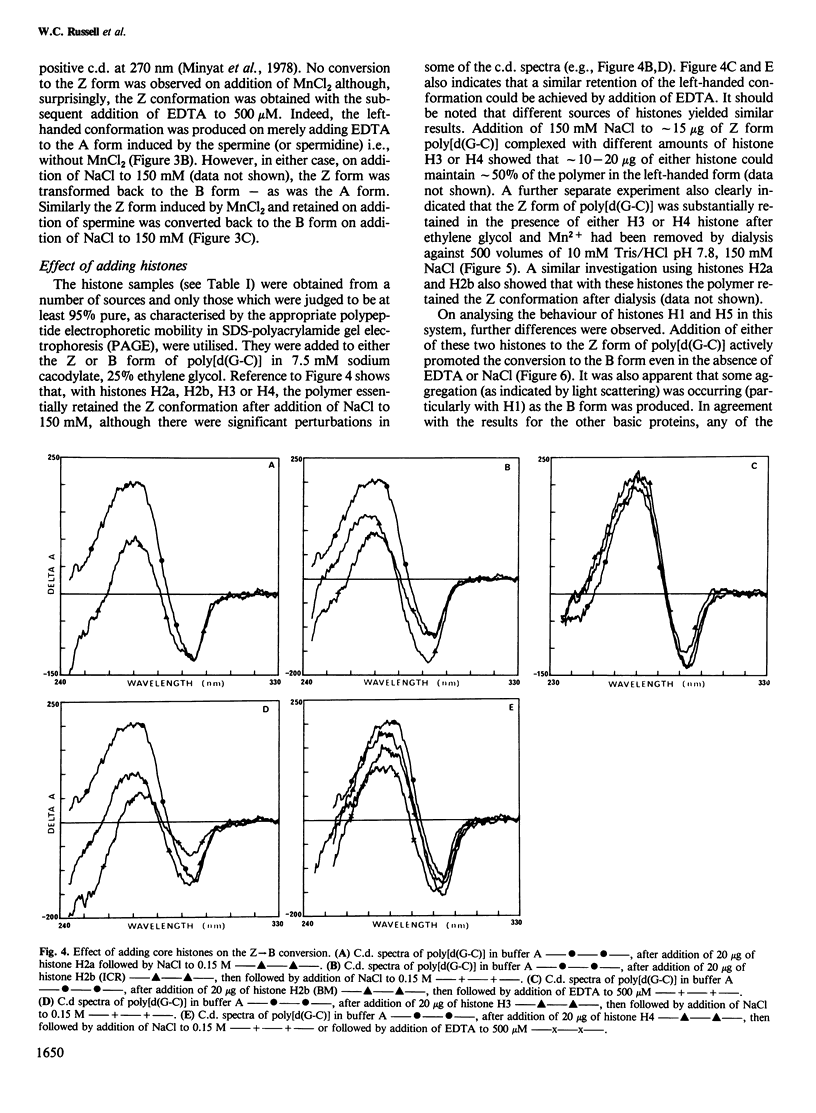
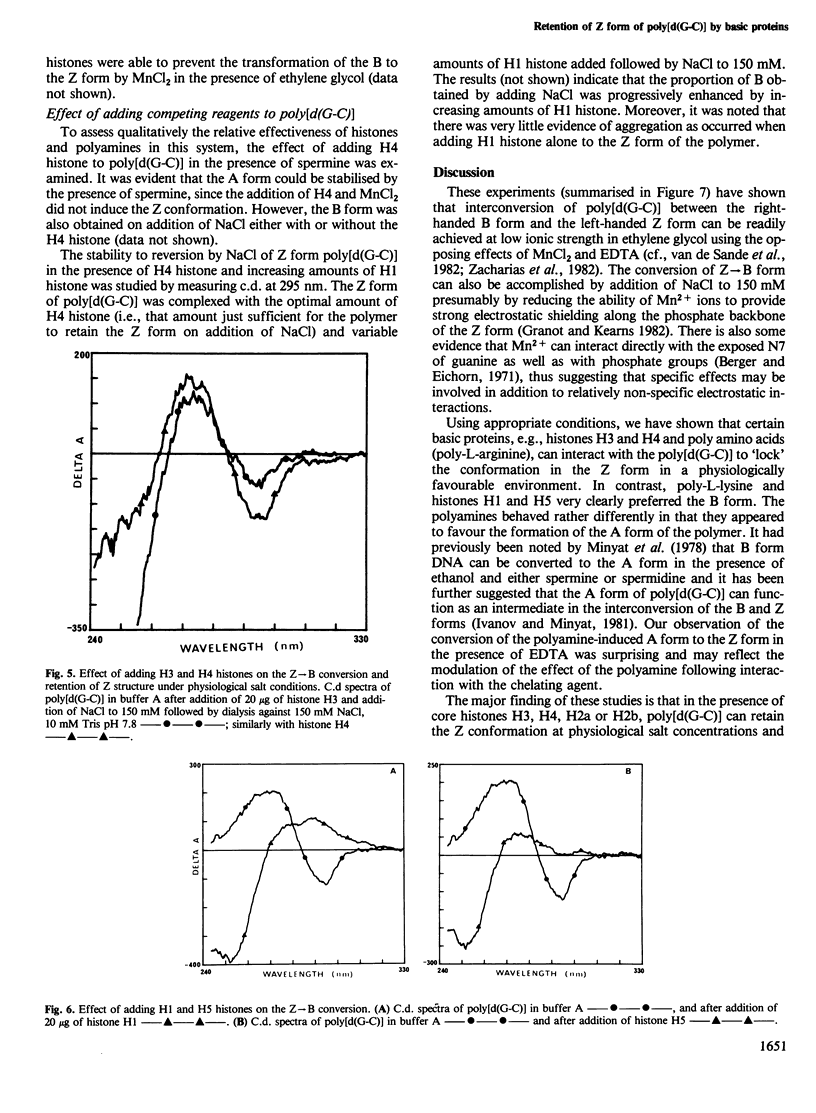


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger N. A., Eichhorn G. L. Interaction of metal ions with polynucleotides and related compounds. XV. Nuclear magnetic resonance studies of the binding of copper(II) to nucleotides and polynucleotides. Biochemistry. 1971 May 11;10(10):1857–1864. doi: 10.1021/bi00786a020. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M. Conformations and flexibilities of histones and high mobility group (HMG) proteins in chromatin structure and function. Ciba Found Symp. 1983;93:246–270. doi: 10.1002/9780470720752.ch14. [DOI] [PubMed] [Google Scholar]
- Cantor C. R. DNA choreography. Cell. 1981 Aug;25(2):293–295. doi: 10.1016/0092-8674(81)90045-3. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R., Conner B. N., Wing R. M., Fratini A. V., Kopka M. L. The anatomy of A-, B-, and Z-DNA. Science. 1982 Apr 30;216(4545):475–485. doi: 10.1126/science.7071593. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R. Structure of a B-DNA dodecamer. II. Influence of base sequence on helix structure. J Mol Biol. 1981 Jul 15;149(4):761–786. doi: 10.1016/0022-2836(81)90357-0. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E., Itakura K. A salt-induced conformational change in crystals of the synthetic DNA tetramer d(CpGpCpG). J Mol Biol. 1978 Nov 15;125(4):535–543. doi: 10.1016/0022-2836(78)90315-7. [DOI] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Goodwin G. H., Johns E. W. The isolation and purification of the high mobility group (HMG) nonhistone chromosomal proteins. Methods Cell Biol. 1977;16:257–267. doi: 10.1016/s0091-679x(08)60104-1. [DOI] [PubMed] [Google Scholar]
- Granot J., Kearns D. R. Interactions of DNA with divalent metal ions. III. Extent of metal binding: experiment and theory. Biopolymers. 1982 Jan;21(1):219–232. doi: 10.1002/bip.360210117. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minyat E. E. The transitions between left- and right-handed forms of poly(dG-dC). Nucleic Acids Res. 1981 Sep 25;9(18):4783–4798. doi: 10.1093/nar/9.18.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns E. W. The isolation and purification of histones. Methods Cell Biol. 1977;16:183–203. doi: 10.1016/s0091-679x(08)60100-4. [DOI] [PubMed] [Google Scholar]
- Klevan L., Schumaker V. N. Stabilization of Z-DNA by polyarginine near physiological ionic strength. Nucleic Acids Res. 1982 Nov 11;10(21):6809–6817. doi: 10.1093/nar/10.21.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A., Jack A., Viswamitra M. A., Kennard O., Shakked Z., Steitz T. A. A hypothesis on a specific sequence-dependent conformation of DNA and its relation to the binding of the lac-repressor protein. J Mol Biol. 1979 Jul 15;131(4):669–680. doi: 10.1016/0022-2836(79)90196-7. [DOI] [PubMed] [Google Scholar]
- Kornberg R. The location of nucleosomes in chromatin: specific or statistical. Nature. 1981 Aug 13;292(5824):579–580. doi: 10.1038/292579a0. [DOI] [PubMed] [Google Scholar]
- Kłysik J., Stirdivant S. M., Larson J. E., Hart P. A., Wells R. D. Left-handed DNA in restriction fragments and a recombinant plasmid. Nature. 1981 Apr 23;290(5808):672–677. doi: 10.1038/290672a0. [DOI] [PubMed] [Google Scholar]
- Lomonossoff G. P., Butler P. J., Klug A. Sequence-dependent variation in the conformation of DNA. J Mol Biol. 1981 Jul 15;149(4):745–760. doi: 10.1016/0022-2836(81)90356-9. [DOI] [PubMed] [Google Scholar]
- Miller F. D., Rattner J. B., van de Sande J. H. Nucleosome-core assembly on B and Z forms of poly[d(G-m5C)]. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):571–575. doi: 10.1101/sqb.1983.047.01.067. [DOI] [PubMed] [Google Scholar]
- Minyat E. E., Ivanov V. I., Kritzyn A. M., Minchenkova L. E., Schyolkina A. K. Spermine and spermidine-induced B to A transition of DNA in solution. J Mol Biol. 1979 Mar 5;128(3):397–409. doi: 10.1016/0022-2836(79)90094-9. [DOI] [PubMed] [Google Scholar]
- Nickol J., Behe M., Felsenfeld G. Effect of the B--Z transition in poly(dG-m5dC) . poly(dG-m5dC) on nucleosome formation. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1771–1775. doi: 10.1073/pnas.79.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Lafer E. M., Peck L. J., Wang J. C., Stollar B. D., Rich A. Negatively supercoiled plasmids contain left-handed Z-DNA segments as detected by specific antibody binding. Cell. 1982 Dec;31(2 Pt 1):309–318. doi: 10.1016/0092-8674(82)90124-6. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Pardue M. L., Lafer E. M., Möller A., Stollar B. D., Rich A. Antibodies to left-handed Z-DNA bind to interband regions of Drosophila polytene chromosomes. Nature. 1981 Dec 3;294(5840):417–422. doi: 10.1038/294417a0. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Blair G. E. Polypeptide phosphorylation in adenovirus-infected cells. J Gen Virol. 1977 Jan;34(1):19–35. doi: 10.1099/0022-1317-34-1-19. [DOI] [PubMed] [Google Scholar]
- Seeman N. C., Rosenberg J. M., Rich A. Sequence-specific recognition of double helical nucleic acids by proteins. Proc Natl Acad Sci U S A. 1976 Mar;73(3):804–808. doi: 10.1073/pnas.73.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T., Künzler P. Cromatin and core particles formed from the inner histones and synthetic polydeoxyribonucleotides of defined sequence. Nucleic Acids Res. 1979 Apr;6(4):1387–1415. doi: 10.1093/nar/6.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T., Shindo H. Conformation of 145 base pair length poly (dG-dC) . poly (dG-dC) in solution and in association with histones. Nucleic Acids Res. 1980 May 10;8(9):2093–2103. doi: 10.1093/nar/8.9.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton C. K., Klysik J., Stirdivant S. M., Wells R. D. Left-handed Z-DNA is induced by supercoiling in physiological ionic conditions. Nature. 1982 Sep 23;299(5881):312–316. doi: 10.1038/299312a0. [DOI] [PubMed] [Google Scholar]
- Tomasz M., Barton J. K., Magliozzo C. C., Tucker D., Lafer E. M., Stollar B. D. Lack of Z-DNA conformation in mitomycin-modified polynucleotides having inverted circular dichroism. Proc Natl Acad Sci U S A. 1983 May;80(10):2874–2878. doi: 10.1073/pnas.80.10.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Zacharias W., Larson J. E., Klysik J., Stirdivant S. M., Wells R. D. Conditions which cause the right-handed to left-handed DNA conformational transitions. Evidence for several types of left-handed DNA structures in solution. J Biol Chem. 1982 Mar 25;257(6):2775–2782. [PubMed] [Google Scholar]
- Zachau H. G., Igo-Kemenes T. Face to phase with nucleosomes. Cell. 1981 Jun;24(3):597–598. doi: 10.1016/0092-8674(81)90084-2. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Jovin T. M. Z* DNA, the left-handed helical form of poly[d(G-C)] in MgCl2-ethanol, is biologically active. EMBO J. 1982;1(1):115–120. doi: 10.1002/j.1460-2075.1982.tb01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Sande J. H., McIntosh L. P., Jovin T. M. Mn2+ and other transition metals at low concentration induce the right-to-left helical transformation of poly[d(G-C)]. EMBO J. 1982;1(7):777–782. doi: 10.1002/j.1460-2075.1982.tb01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Holt C., Brandt W. F. Fractionation of histones on molecular sieve matrices. Methods Cell Biol. 1977;16:205–225. doi: 10.1016/s0091-679x(08)60101-6. [DOI] [PubMed] [Google Scholar]



