Abstract
Background
The purpose of this study was to investigate the impact of the modified Glasgow Prognostic Score (GPS) at the time of recurrence on post-recurrence survival (PRS) in non-small cell lung cancer (NSCLC) patients after surgical resection.
Material/Methods
The clinicopathologic characteristics and outcome data of 266 patients with recurrent NSCLC were collected and reviewed retrospectively. The prognostic impact of mGPS at recurrence in patients with recurrent NSCLC was investigated in univariate and multivariate analyses.
Results
A total of 266 patients were analyzed. The mGPS at the time of recurrence of 0, 1, and 2 was assigned to 60.9%, 33.1%, and 6.0% of total patients, respectively. In univariate analyses, the median post-recurrence survival times for those with mGPS 0, 1, and 2 were 19, 14, and 4 months, respectively (log-rank test; P=0.005). No statistically significant difference in post-recurrence survival was observed among the patients with different mGPS before surgery (log-rank test; P=0.064). Age at surgery, histological type, C-reactive protein (CRP), albumin, and mGPS at recurrence significantly predicted PRS. After adjusting for confounding variables in the model, age (hazard ratio 1.59, P=0.003) as well as disease-free interval (DFI) (hazard ratio 1.40, P=0.023), and mGPS at recurrence (hazard ratio 1.47, P=0.002) remained independent predictors of PRS.
Conclusions
mGPS at the time of recurrence might be an independent adverse prognostic factor in recurrent NSCLC.
MeSH Keywords: Carcinoma, Non-Small-Cell Lung; Patients; Recurrence
Background
Lung cancer is the leading cause of cancer-related death worldwide [1,2], and non-small cell lung cancer (NSCLC) represents approximately 80% of all lung cancer cases. Although surgery offers the greatest curative potential for patients with stage I/II disease and selected patients with stage III disease [3], the recurrence rate after surgical treatment of NSCLC varies (30~75%); the prognosis remains elusive, and survival characteristics of patients who have recurrent NSCLC after surgical resection are not well-understood [4,5]. Therefore, identifying predictors of post-recurrence survival (PRS) is crucial for the evaluation of mortality risk at recurrence. The prognostic factors in patients with recurrent lung cancer have been investigated in several studies [6–8]. Decreased disease-free interval (DFI) from initial resection to recurrence, symptoms at recurrence, liver recurrence, initial lung cancer stage of IIB or worse, and multiple recurrences have been reported as significant predictors of shorter survival 5,9–11].
A few studies have investigated the relationship between systemic inflammatory response and progression many types of common solid tumors, including lung cancer [12–15]. Accumulating evidence suggests that the Glasgow prognostic score (GPS) system, based on inflammation criteria and including only serum C-reactive protein (CRP) and albumin, is a reliable and practical scoring system for predicting outcome in lung cancer [16]. Recently, Shafique et al. showed that modified GPS (mGPS), which is superior to GPS, is a powerful prognostic factor, independent of tumor site, in cancer patients [17]. They also demonstrated that GPS predicts overall survival in lung cancer patients, but the patients enrolled in their study included only those with inoperable disease [16,18–20]. To the best of our knowledge, the prognostic significance of mGPS at the time of NSCLC recurrence has never been investigated. Because CRP and serum may be a highly practical method for predicting survival in patients with recurrent NSCLC.
Therefore, we hypothesized that mGPS is a predictor of poor prognosis in patients with recurrent NSCLC, which may be due to more aggressive tumor biology. To test this hypothesis, the clinical data, including CRP and serum albumin either before surgery or at disease recurrence, of 266 NSCLC patients were collected and analyzed, and the prognostic significance of mGPS in patients with recurrent NSCLC was evaluated.
Material and Methods
Patients
Using electronic medical records, 1311 patients who underwent surgical resection for primary NSCLC at Yantai Yuhuangding Hospital from January 2006 through January 2010 were identified. Eligibility criteria for this study were: (1) histologically proven primary NSCLC; (2) postoperative recurrence occurred before Nov 2014; (3) recurrent NSCLC was diagnosed at our institution (recurrence was defined as the presence of disease after a more than 3 months DFI after resection); (4) received systemic chemotherapy for recurrence in our hospital; and (5) records of blood tests were complete. Exclusion criteria were: (1) evidence of acute or chronic infection identified within 14 days before crucial blood tests; (2) presented with other types of carcinoma within 5 years; (3) received neoadjuvant chemotherapy or radiotherapy prior to surgery; and (4) had active infection prior to surgery. Of the 311 patients who were considered for inclusion in this retrospective study, 45 patients were excluded and 266 were included. The study protocol was approved by the Ethics Committees of the Yantai Yuhuangding Hospital, and all participants provided written informed consent.
Definition of mGPS
The mGPS was calculated with CRP and albumin values as follows: patients with elevated CRP levels (>1.0 mg/dl) and hypoalbuminemia (<3.5 g/dl) were allocated a score of 2; patients with elevated CRP levels (>1.0 mg/dl) only were allocated a score of 1; and patients with normal CRP levels (≤1.0 mg/dl) and any albumin concentration were allocated a score of 0 [17] (Supplementary Table 1).
Evaluated variables and process of treatment
Blood samples were obtained at 2 time points for CRP and serum albumin, and mGPS was calculated on the basis of obtained results. Data, including age at surgery, sex, preoperative smoking status, histological type, pathological stage, adjuvant treatment, surgical procedures, DFI, recurrent site, number of recurrent foci, and PRS, were collected and evaluated.
Histological typing was based on the WHO classification, and tumor staging was determined according to the TNM classification of the International System for Staging Lung Cancer [21]. All patients had been preoperatively staged based on findings of contrast CT, FDG-PET, and magnetic resonance imaging (MRI) of the brain. As a general rule in surgical procedures, lobectomy or pneumonectomy with systematic or selective mediastinal lymph node dissection is performed. Neoadjuvant chemotherapy is not routinely administrated in patients. All patients with stage II–III disease received adjuvant platinum-based chemotherapy and/or postoperative radiotherapy (PORT).
Recurrent NSCLC was diagnosed based on the results of a physical examination and diagnostic imaging. Histopathology of the diagnosis was confirmed only when clinically required. Follow-up information was collected directly from the outpatient clinic records or from family contacts. Data regarding the date of recurrence diagnosis, site and number of recurrent tumors, treatment of recurrence, date of death or last visit, and cause of death were recorded. The DFI referred to the period from the date of surgical treatment until the date of recurrence diagnosis.
All patients in our cohort underwent systemic chemotherapy for recurrence, so the possible confounding effect of treatment after recurrence could be partly avoided. Among all 266 participants, some patients also received local therapy, endothelial growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), or a vascular endothelial growth factor inhibitor. PRS was defined as the time between the detection of recurrence and the date of death or the last follow-up.
Ethics statement
The study was approved by the Research Ethics Committee of Yantai Yuhuangding Hospital. Informed consent was obtained either from the patients or from the patient’s family before participating in the study.
Statistical analysis
Continuous data such as age and DFI were described using mean±SD or median and range. Frequency counts and proportions were calculated for categorical data such as sex and stage data, and chi-square tests or the Fisher exact tests were used to compare between groups. Spearman rank correlation coefficient was calculated to assess the relationship between the patients’ mGPS at 2 time points. Median PRS was determined by the Kaplan-Meier method and compared among different groups using the log-rank test. Furthermore, we performed Cox proportional hazards regression analysis with stepwise variable selection to identify significant independent prognostic factors for PRS. Hazard ratios (HR) and 95% confidence intervals (CI) were generated. All P values were two-sided, and P<0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS version 20.
Results
Patient characteristics and treatment
The clinicopathological characteristics of the 266 patients included in the study are shown in Table 1. The mean age of the patients was 59.6 years. Of the 266 patients, 161 (60.5%) patients had stage IA to stage IIB disease, 104 (39.1%) had stage IIIA disease, and 1 had stage IIIB disease. Histologically, 110 patients (41.4%) had squamous cell carcinoma, 134 patients (50.4%) had adenocarcinoma, 17 patients (6.4%) had adenosquamous carcinoma, 1 (0.4%) had large cell lung cancer, and the other 4 cases (1.5%) had sarcoma of the lung.
Table 1.
The association between characteristics and mGPS at recurrence in 266 patients.
| Variables | All patients (N=235) | mGPS at recurrence | χ2 | P value | ||
|---|---|---|---|---|---|---|
| 0 (N=25) | 1 (N=210) | 2 (N=25) | ||||
| Clinical factors | ||||||
| Age at surgery (years) | ||||||
| Mean ±SD | 59.56±9.66 | |||||
| Range | 35–82 | |||||
| <65 | 179 (67.3) | 107 (59.8) | 59 (33.0) | 13 (7.3) | 1.532 | 0.465 |
| ≥65 | 87 (32.7) | 55 (63.2) | 29 (33.3) | 3 (3.4) | ||
| Gender | ||||||
| Male | 186 (69.9) | 109 (58.6) | 63 (33.9) | 14 (7.5) | 0.244* | |
| Female | 80 (30.1) | 53 (66.3) | 25 (31.3) | 2 (2.5) | ||
| Preoperative smoking status | ||||||
| Non-/Light smoker | 164 (61.7) | 99 (60.4) | 57 (34.8) | 8 (4.9) | 1.301 | 0.522 |
| Heavy smoker | 102 (38.3) | 63 (61.8) | 31 (30.4) | 8 (7.8) | ||
| Preoperative mGPS | ||||||
| 0 | 176 (66.2) | 118 (67.0) | 52 (29.5) | 6 (3.4) | <0.001* | |
| 1 | 77 (28.9) | 36 (46.8) | 35 (45.5) | 6 (7.8) | ||
| 2 | 13 (4.9) | 8 (61.6) | 1 (7.7) | 4 (30.8) | ||
| Pathological factors | ||||||
| Histological type | ||||||
| A or AS | 110 (41.4) | 72 (65.5) | 34 (30.9) | 4 (3.6) | 1.736 | 0.420 |
| SCC | 151 (56.8) | 89 (58.9) | 52 (34.4) | 10 (6.6) | ||
| Pathological stage | ||||||
| IA to IIB | 161 (60.5) | 97 (60.2) | 56 (34.8) | 8 (5.0) | 1.127 | 0.596 |
| IIIA/IIIB | 105 (39.5) | 65 (61.9) | 32 (30.5) | 8 (7.6) | ||
| Adjuvant treatment | ||||||
| Chemotherapy | 130 (48.9) | 81 (62.3) | 41 (31.5) | 8 (6.2) | 0.971* | |
| PORT | 14 (5.3) | 9 (64.3) | 4 (28.6) | 1 (7.1) | ||
| Chemoradiotherapy | 73 (27.4) | 45 (61.6) | 24 (32.9) | 4 (5.5) | ||
| None | 49 (18.4) | 27 (55.1) | 19 (38.8) | 3 (6.1) | ||
| Factors related to recurrence | ||||||
| Disease-free interval (months) | ||||||
| Median | 15.5 | |||||
| Range | 3–71 | |||||
| ≤12 | 160 (60.2) | 93 (58.1) | 57 (35.6) | 10 (6.3) | 1.330 | 0.514 |
| >12 | 106 (39.8) | 69 (65.1) | 31 (29.2) | 6 (5.7) | ||
| Recurrent site | ||||||
| Local (intrathoracic) | 68 (25.6) | 37 (54.4) | 27 (39.7) | 4 (5.9) | 0.346* | |
| Distant (extrathoracic) | 138 (51.9) | 90 (65.2) | 42 (30.4) | 6 (4.3) | ||
| Both | 60 (22.6) | 35 (58.3) | 19 (31.7) | 6 (10.0) | ||
| No. of recurrent foci | ||||||
| Single | 178 (66.9) | 111 (68.0) | 57 (69.5) | 10 (5.6) | 0.511 | 0.774 |
| Multiple | 88 (33.1) | 51 (58.0) | 64 (35.2) | 6 (6.8) | ||
| All | 266 (100) | 162 (60.9) | 88 (33.1) | 16 (6.0) | ||
A – adenocarcinoma; AS – adenosquamous carcinoma; SCC – squamous cell carcinoma; LSLC – large cell lung cancer; PORT – post operation radiotherapy; Light smoker – smoking index ≤40 pack-years; Heavy smoker – smoking index >40 pack-years; numbers in parentheses are percentages.
Fisher’s exact probability test P values. P values <0.05 in bold.
Among all the patients, 49 underwent surgical resection as the only therapy in the initial treatment, 203 patients underwent surgical resection and received 3~6 cycles of adjuvant chemotherapy of TP/DP/GP/NP regimen [TP: paclitaxel 135–175 mg/m2 on day 1 and carboplatin area under the ROC curve (AUC)=5 on day 1; DP: docetaxel 75 mg/m2 on days 1 and 8 and cisplatin 75 mg/m2 on day 1; GP: gemcitabine 1000 mg/m2 on days 1 and 8 and cisplatin 75 mg/m2 on day 1; NP: vinorelbine 25 mg/m2 on days 1 and 8 and cisplatin 75 mg/m2 on day 1], 87 patients received PORT at a dose of 50–62 Gy, and 73 patients received chemoradiotherapy.
We observed 236 recurrences (88.7%) within 3 years of the primary surgery. The median DFI was 15.5 months. The recurrence was intrathoracic in 68 (25.6%) patients, extrathoracic in 138 (51.9%), and a combination of intrathoracic and extrathoracic in 60 (22.6%). Eighty-eight (33.1%) patients developed recurrences in multiple organs.
All of the 266 patients received systemic salvage chemotherapy after recurrence. There were 222 patients who received at least 4 cycles of cisplatin-based doublet chemotherapy as mono-therapy after recurrence. The regimens mainly included TP, DP, GP, NP, and pemetrexed plus cisplatin (pemetrexed 500 mg/m2 on day 1 and cisplatin 75 mg/m2 on day 1, for non-squamous carcinoma). Further, 38 cases received local therapy before salvage chemotherapy (thoracic radiation for recurrent lesions in 23 patients, stereotactic radiosurgery for brain metastases in 14 patients, and re-resection in 1 patient), 43 patients also received EGFR-TKIs (gefitinib, erlotinib, or icotinib) for at least 1 month, and 5 patients were treated with a vascular endothelial growth factor inhibitor (bevacizumab) in combination with chemotherapy.
Patient characteristics
As shown in Table 1, of a total of 266 patients, 90 (33.8%) showed an elevated CRP level (>1.0 g/dl) and 27 (10.2%) had hypoalbuminemia (<3.5 g/dl) before surgery; 106 (39.8%) showed an elevated CRP level (>1.0 g/dl) and 31 (11.3%) had hypoalbuminemia (<3.5 g/dl) at recurrence. A total of 162 (60.9%) patients were allocated an mGPS of 0, 88 (33.1%) were allocated an mGPS of 1, and 16 (6.0%) were allocated an mGPS of 2 at the time of recurrence. The association between baseline characteristics and mGPS at recurrence is also shown in Table 1.
In a comparison among the 3 groups, there were no significant differences observed in age (χ2=0.052, P=0.820), sex (χ2=0.905, P=0.341), preoperative mGPS (P=0.092), histological type (P=0.148), pathological stage (χ2=0.993, P=0.319), adjuvant treatment (χ2=0.740, P=0.864), DFI (χ2=3.156, P=0.076), recurrent site (χ2=0.022, P=0.989), or number of recurrent foci (χ2=0.024, P=0.876). We also examined the relationship between the mGPS at the time of recurrence diagnosis and preoperative mGPS. A significant correlation was demonstrated between the mGPS at the 2 time points (Spearman rank correlation coefficient, r=0.298; P<0.001).
The median post-recurrence follow-up for the entire cohort was 17 months (range, 1 to 90), and the 1- and 2-year PRS rates were 58.4% and 32.6%, respectively. The median PRS time after recurrence was 13 months (range, 1 to 81).
Univariate analysis
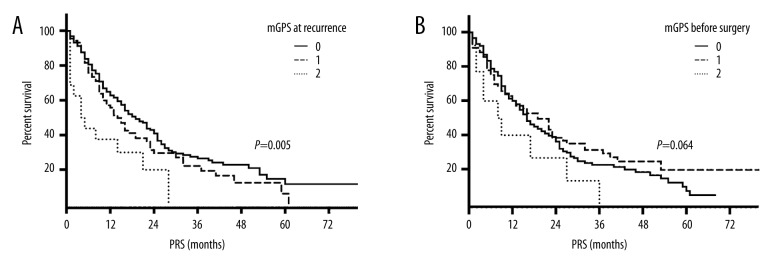
To investigate the prognostic significance of the mGPS in patients with recurrent NSCLC, we performed univariate analyses. A statistically significant difference in PRS was observed between patients with different mGPS at recurrence (Figure 1A); the median PRS times for those with mGPS 0, 1, and 2 was 19, 14, and 4 months, respectively (log-rank test; P=0.005). No statistically significant difference in PRS was observed among the patients with different mGPS before surgery (log-rank test; P=0.064) (Figure 1B).
Figure 1.
Percent survival. (A) mGPS at recurrence. (B) mGPS before surgery.
PRS was also analyzed with respect to other clinical factors (age, sex, and preoperative smoking status), pathological factors (histological type and pathological stage), adjuvant treatment, factors related to recurrence (DFI, site, and type of recurrence), and biochemical characteristics (CRP and albumin). In our study population, age at surgery, histological type, CRP, albumin, and mGPS at recurrence were significantly correlated with prognosis (Table 2).
Table 2.
Univariate analysis of factors affect post-recurrence survival.
| Variables | Median post-recurrence survival (months) | χ2 | P value |
|---|---|---|---|
| Age at surgery (years) | |||
| <65 | 17 | 6.702 | 0.010 |
| ≥65 | 11 | ||
| Gender | |||
| Male | 15 | 1.842 | 0.175 |
| Female | 22 | ||
| Preoperative smoking status | |||
| Non-/Light smoker | 15 | 0.856 | 0.355 |
| Heavy smoker | 17 | ||
| Preoperative CRP | |||
| ≤1.0 g/dl | 16 | 0.126 | 0.723 |
| >1.0 g/dl | 17 | ||
| CRP at recurrence | |||
| ≤1.0 g/dl | 19 | 4.931 | 0.026 |
| >1.0 g/dl | 13 | ||
| Albumin at recurrence | |||
| ≥3.5 g/dl | 17 | 7.517 | 0.006 |
| <3.5 g/dl | 5 | ||
| Preoperative mGPS | |||
| 0 | 16 | 5.492 | 0.064 |
| 1 | 19 | ||
| 2 | 8 | ||
| mGPS at recurrence | |||
| 0 | 19 | 10.711 | 0.005 |
| 1 | 14 | ||
| 2 | 4 | ||
| Histological type | |||
| A or AS | 17 | 29.581 | <0.001 |
| SCC | 16 | ||
| LCLC/Sarcoma | 4 | ||
| Pathological stage | |||
| IA to IIB | 19 | 2.657 | 0.103 |
| IIIA/IIIB | 14 | ||
| Adjuvant treatment | |||
| Chemotherapy | 15 | 2.835 | 0.418 |
| PORT | 25 | ||
| Chemoradiotherapy | 15 | ||
| None | 15 | ||
| Disease-free interval (months) | |||
| <12 | 14 | 3.556 | 0.059 |
| ≥12 | 19 | ||
| Recurrent site | |||
| Local (intrathoracic) | 17 | 1.177 | 0.555 |
| Distant (extrathoracic) | 15 | ||
| Both | 22 | ||
| No. of recurrent foci | |||
| Single | 18 | 0.067 | 0.796 |
| Multiple | 11 | ||
A – adenocarcinoma; AS – adenosquamous carcinoma; SCC – squamous cell carcinoma; LSLC – large cell lung cancer; PORT – post operation radiotherapy; Light smoker – smoking index ≤40 pack-years; Heavy smoker – smoking index >40 pack-years. P values <0.05 in bold.
Multivariate analysis
Factors influencing either the survival status or the mGPS at recurrence (age, preoperative smoking status, histological type, pathological stage, DFI) were estimated with the Cox proportional hazards regression model (backward stepwise procedure) to adjust for the effects of covariates. As preoperative mGPS was correlated with mGPS at recurrence, it was not analyzed in the model. After adjusting for confounding variables in the model, mGPS at recurrence (HR 1.47; 95% CI, 1.15–1.88; P=0.002), age (HR 1.59; 95% CI 1.17–2.14; P=0.003) as well as DFI (HR 1.40; 95% CI 1.05–1.88; P=0.023) remained independent predictors of PRS (Table 3).
Table 3.
Multivariate Cox-proportional hazards regression analysis of prognostic factors for post-recurrence survival.
| Variables | HR (95%CI) | P value |
|---|---|---|
| Age at surgery (years) | ||
| <65 | ||
| ≥65 | 1.59 (1.17–2.14) | 0.003 |
| mGPS at recurrence | ||
| 0 | ||
| 1 | ||
| 2 | 1.47 (1.15–1.88) | 0.002 |
| Disease-free interval (months) | ||
| <12 | ||
| ≥12 | 1.40 (1.05–1.88) | 0.023 |
HR – hazard ratio; CI – confidence interval. P values <0.05 in bold.
Discussion
Relational studies of chronic inflammation and cancer have been carried out extensively. Chronic inflammation may be in association with the development of cancer, showing enhancing cell proliferation and then inducing DNA damage [22,23]. Balkwill et al. assumed that many cancers occurred originally in sites of chronic inflammation [24], and Coussens et al. indicated that inflammation contributed to cancer progression [25]. On the contrary, some other studies showed that the growth of cancer resulted in tissue damage, which disrupted the microenvironment and then incited the related inflammation [26].
From the similarities of HR across different tumors, countries, and clinical scenarios in the present review, GPS/mGPS appears to be a reliable independent prognostic factor in cancer patients. It also has been shown that mGPS is a reliable predictor for poor survival in patients with operable colorectal, gastro-esophageal, and pancreatic cancers [27–37]. Only 2 studies were in patients with inoperable lung cancer, and it was reported the GPS/mGPS possessed prognostic value independent of tumor stage, treatment, and other measures of the systemic inflammatory response. Over these 2 studies, the weighted average HR for an incremental increase in the GPS/mGPS was 2.0 [20].
Consistent with the previous reports, our study shows that an elevated mGPS is associated with survival and HR when the increase in the mGPS was 1.47. Our novel finding is that mGPS at the time of recurrence diagnosis was an independent predictor of PRS in NSCLC patients, even after adjusting for possible confounders. Besides mGPS at recurrence, older age and shorter DFI of the primary cancer were also indicators of short survival in patients with recurrent NSCLC, which coincides with the previous study [38].
Moreover, we found that, in our study population, the prognostic significance of mGPS in advanced-stage NSCLC was stronger than in the early stage, thus we may draw the conclusion that mGPS at recurrence could be a better predictor of PRS compared with the preoperative value. The possible explanation is that mGPS at recurrence could reflect the biological activity of the tumor at the time of recurrence diagnosis more accurately, while the predictive significance of preoperative mGPS was strongly affected by many confounders such as surgical procedure, whether adjuvant chemotherapy was used, and which regimen.
As the main approach for recurrent NSCLC in current clinical practice, chemotherapy is routinely administered to unresectable advanced NSCLC based on the recommended regimen. Hitherto, few studies have evaluated the prognostic factors and treatments especially for recurrent NSCLC after curative resection. Our study proposes one possible prognostic factor that had been paid little attention in previous studies. The results of our research indicate that increased mGPS at recurrence is associated with a higher risk of unfavorable prognosis in NSCLC. In other words, despite current intensive salvage treatment for recurrent disease, most patients with an mGPS of 2 at recurrence would derive no clinical benefit (their median PRS was 8 months), indicating that current conventional salvage treatments are not effective in this patient population. On the other hand, although tests of CRP and serum albumin are normally used in clinical practice, it is surprising that few studies have examined the role of mGPS profiles as prognostic markers in resectable NSCLC. Studies were limited to pretreatment values only and typically did not include mGPS during the whole course of disease for recurrent NSCLC. Our findings provide the potential to identify a high-risk patient group by performing simple tests that are almost universally available and add no additional cost to routine laboratory measurements, and could be beneficial for the development of a novel treatment strategy for this type of aggressive recurrent tumor.
As for methodology, this retrospective cohort study was appropriately designed. The cases were categorized in accordance with uniform inclusion criteria, exclusion criteria, and strict screening, thereby strengthening the evidence provided by the present study. Additionally, we chose patients who underwent systemic platinum-based chemotherapy for recurrence as our study population to minimize the possibility that the outcomes could have been affected by the varies therapies administered after recurrence. However, the influence of mGPS in the subgroup of recurrent NSCLC patients who underwent targeted therapy or palliative care as the only therapy was not evaluated in this study. Therefore, to further elucidate the etiology of elevated mGPS in recurrent NSCLC and to establish treatment strategies, large-scale, multicenter, prospective studies are needed.
Conclusions
In summary, this study has demonstrated for the first time that mGPS at recurrence has strong negative predictive value in PRS for NSCLC patients after surgical resection. Preoperative mGPS, however, cannot predict PRS, indicating that the prognosis of recurrent NSCLC could be much better predicted by mGPS at recurrence. We can conclude that mGPS at the time of recurrence should be included in the assessment of PRS and be used to identify high-risk patients in recurrent NSCLC.
Supplementary Table
Supplementary Table 1.
Definitions of Modified Glasgow Prognostic Score (mGPS).
| mGPS | Score |
|---|---|
| CRP ≤1.0 mg/dl and any albumin concentration | 0 |
| CRP >1.0 mg/dl and albumin ≥3.5 g/dl | 1 |
| CRP >1.0 mg/dl and albumin <3.5 g/dl | 2 |
CRP – C-reactive protein.
Footnotes
Source of support: Departmental sources
Referrences
- 1.Chen W, Zheng R, Zhang S, et al. Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Res. 2013;25(1):10–21. doi: 10.3978/j.issn.1000-9604.2012.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Pearson FG. Non-small cell lung cancer: Role of surgery for stages I–III. Chest. 1999;116(6 Suppl):500S–3S. doi: 10.1378/chest.116.suppl_3.500s. [DOI] [PubMed] [Google Scholar]
- 4.Rothschild SI, Lardinois D, Bremerich J, et al. Follow-up in non-small-cell lung cancer. memo – Magazine of European Medical Oncology. 2014;7(2):97–101. [Google Scholar]
- 5.Saisho S, Yasuda K, Maeda A, et al. Post-recurrence survival of patients with non-small-cell lung cancer after curative resection with or without induction/adjuvant chemotherapy. Interactive cardiovascular and thoracic surgery. 2013;16(2):166–72. doi: 10.1093/icvts/ivs450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong J, Mao Y, Li J, He J. Stair-climbing test predicts postoperative cardiopulmonary complications and hospital stay in patients with non-small cell lung cancer. Med Sci Monit. 2017;23:1436–41. doi: 10.12659/MSM.900631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun L, Chen Y, Su Q, et al. Increased plasma miRNA-30a as a biomarker for non-small cell lung cancer. Med Sci Monit. 2016;22:647–55. doi: 10.12659/MSM.897330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Shao X. Isoflurane promotes non-small cell lung cancer malignancy by activating the Akt-mammalian target of rapamycin (mTOR) signaling pathway. Med Sci Monit. 2016;22:4644–50. doi: 10.12659/MSM.898434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi Y, Horio H, Hato T, et al. Predictors of post-recurrence survival in patients with non-small-cell lung cancer initially completely resected. Interact Cardiovasc Thorac Surg. 2015;21(1):14–20. doi: 10.1093/icvts/ivv085. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki H, Suzuki A, Tatematsu T, et al. Prognosis of recurrent non-small cell lung cancer following complete resection. Oncol Lett. 2014;7(4):1300–4. doi: 10.3892/ol.2014.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimada Y, Saji H, Yoshida K, et al. Prognostic factors and the significance of treatment after recurrence in completely resected stage I non-small cell lung cancer. Chest. 2013;143(6):1626–34. doi: 10.1378/chest.12-1717. [DOI] [PubMed] [Google Scholar]
- 12.Farhan-Alanie OM, McMahon J, McMillan DC. Systemic inflammatory response and survival in patients undergoing curative resection of oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2015;53(2):126–31. doi: 10.1016/j.bjoms.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Li QQ, Lu ZH, Yang L, et al. Neutrophil count and the inflammation-based glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev. 2014;15(2):945–50. doi: 10.7314/apjcp.2014.15.2.945. [DOI] [PubMed] [Google Scholar]
- 14.Grose D, Devereux G, Brown L, et al. Simple and objective prediction of survival in patients with lung cancer: Staging the host systemic inflammatory response. Lung Cancer Int. 2014;2014:731925. doi: 10.1155/2014/731925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannousi Z, Gioulbasanis I, Pallis AG, et al. Nutritional status, acute phase response and depression in metastatic lung cancer patients: Correlations and association prognosis. Support Care Cancer. 2012;20(8):1823–29. doi: 10.1007/s00520-011-1282-x. [DOI] [PubMed] [Google Scholar]
- 16.Forrest LM, McMillan DC, McArdle CS, et al. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer. 2004;90(9):1704–6. doi: 10.1038/sj.bjc.6601789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shafique K, Proctor MJ, McMillan DC, et al. The modified Glasgow prognostic score in prostate cancer: Results from a retrospective clinical series of 744 patients. BMC Cancer. 2013;13:292. doi: 10.1186/1471-2407-13-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gioulbasanis I, Pallis A, Vlachostergios PJ, et al. The Glasgow Prognostic Score (GPS) predicts toxicity and efficacy in platinum-based treated patients with metastatic lung cancer. Lung Cancer. 2012;77(2):383–88. doi: 10.1016/j.lungcan.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Meek CL, Wallace AM, Forrest LM, McMillan DC. The relationship between the insulin-like growth factor-1 axis, weight loss, an inflammation-based score and survival in patients with inoperable non-small cell lung cancer. Clin Nutr. 2010;29(2):206–9. doi: 10.1016/j.clnu.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Leung EY, Scott HR, McMillan DC. Clinical utility of the pretreatment glasgow prognostic score in patients with advanced inoperable non-small cell lung cancer. J Thorac Oncol. 2012;7(4):655–62. doi: 10.1097/JTO.0b013e318244ffe1. [DOI] [PubMed] [Google Scholar]
- 21.Mountain CF. Revisions in the International System for staging lung cancer. Chest. 1997;111(6):1710–17. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 22.Sayar I, Isik A, Akbas EM, et al. Bone marrow metaplasia in multinodular goiter with primary hyperparathyroidism. Am J Med Sci. 2014;348(6):530–31. doi: 10.1097/MAJ.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 23.Isik A, Gursul C, Peker K, et al. Metalloproteinases and their inhibitors in patients with inguinal hernia. World J Surg. 2017;41(5):1259–66. doi: 10.1007/s00268-016-3858-6. [DOI] [PubMed] [Google Scholar]
- 24.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 25.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–67. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang EC, Hwang IS, Yu HS, et al. Utility of inflammation-based prognostic scoring in patients given systemic chemotherapy first-line for advanced inoperable bladder cancer. Jpn J Clin Oncol. 2012;42(10):955–60. doi: 10.1093/jjco/hys124. [DOI] [PubMed] [Google Scholar]
- 27.Richards CH, Roxburgh CS, MacMillan MT, et al. The relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancer. PLoS One. 2012;7(8):e41883. doi: 10.1371/journal.pone.0041883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansouri D, Powell AG, Park JH, et al. Long-term follow-up of patients undergoing resection of TNM Stage I colorectal cancer: An analysis of tumour and host determinants of outcome. World J Surg. 2016;40(6):1485–91. doi: 10.1007/s00268-016-3443-z. [DOI] [PubMed] [Google Scholar]
- 29.Park JH, Powell AG, Roxburgh CS, et al. Mismatch repair status in patients with primary operable colorectal cancer: Associations with the local and systemic tumour environment. Br J Cancer. 2016;114(5):562–70. doi: 10.1038/bjc.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue Y, Iwata T, Okugawa Y, et al. Prognostic significance of a systemic inflammatory response in patients undergoing multimodality therapy for advanced colorectal cancer. Oncology. 2013;84(2):100–7. doi: 10.1159/000343822. [DOI] [PubMed] [Google Scholar]
- 31.Guthrie GJK. Comparison of the prognostic value of longitudinal measurements of systemic inflammation in patients undergoing curative resection of colorectal cancer. Br J Cancer. 2013;109(1):24–28. doi: 10.1038/bjc.2013.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishizuka M, Nagata H, Takagi K, et al. Inflammation-based prognostic system predicts postoperative survival of colorectal cancer patients with a normal preoperative serum level of carcinoembryonic antigen. Ann Surg Oncol. 2012;19(11):3422–31. doi: 10.1245/s10434-012-2384-5. [DOI] [PubMed] [Google Scholar]
- 33.Dutta S, Going JJ, Crumley ABC, et al. The relationship between tumour necrosis, tumour proliferation, local and systemic inflammation, microvessel density and survival in patients undergoing potentially curative resection of oesophageal adenocarcinoma. Br J Cancer. 2012;106(4):702–10. doi: 10.1038/bjc.2011.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dutta S, Crumley AB, Fullarton GM, et al. Comparison of the prognostic value of tumour and patient related factors in patients undergoing potentially curative resection of gastric cancer. Am J Surg. 2012;204(3):294–99. doi: 10.1016/j.amjsurg.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 35.Jiang X, Hiki N, Nunobe S, et al. Prognostic importance of the inflammation-based Glasgow prognostic score in patients with gastric cancer. Br J Cancer. 2012;107(2):275–79. doi: 10.1038/bjc.2012.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.La Torre M, Nigri G, Cavallini M, et al. The glasgow prognostic score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19(9):2917–23. doi: 10.1245/s10434-012-2348-9. [DOI] [PubMed] [Google Scholar]
- 37.Jamieson NB, Mohamed M, Oien KA, et al. The relationship between tumor inflammatory cell infiltrate and outcome in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2012;19(11):3581–90. doi: 10.1245/s10434-012-2370-y. [DOI] [PubMed] [Google Scholar]
- 38.Yoshino I, Yohena T, Kitajima M, et al. Survival of non-small cell lung cancer patients with postoperative recurrence at distant organs. Ann Thorac Cardiovasc Surg. 2001;7(4):204–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Definitions of Modified Glasgow Prognostic Score (mGPS).
| mGPS | Score |
|---|---|
| CRP ≤1.0 mg/dl and any albumin concentration | 0 |
| CRP >1.0 mg/dl and albumin ≥3.5 g/dl | 1 |
| CRP >1.0 mg/dl and albumin <3.5 g/dl | 2 |
CRP – C-reactive protein.