Abstract
Background
Although HSV-2 is the major cause of genital lesions, HSV-1 accounts for half of new cases in developed countries.
Methods
Three healthy SHIV-SF162P3 infected Indian rhesus macaques were inoculated with 4x108 pfu of HSV-1 twice, with the second inoculation performed after the vaginal mucosa was gently abraded with a cytobrush.
Results
HSV-1 DNA was detected in vaginal swabs 5 days after the 2nd, but not the 1st inoculation in all 3 macaques. An increase in inflammatory cytokines was detected in the vaginal fluids of the animals with no or intermittent shedding. Higher frequency of blood α4β7high CD4+ T cells was measured in the animals with consistent and intermitted shedding, while a decrease in the frequency of CD69+ CD4+ T cells was present in all animals.
Conclusions
This macaque model of genital HSV-1 could be useful to study the impact of the growing epidemic of genital HSV-1 on HIV infection.
Keywords: HSV-1, HSV-2, HIV, SIV, SHIV-SF162P3, transmission
Introduction
Herpes simplex viruses are members of the Alphaherpesvirinae subfamily within the Herpesviridae virus family. There are two serotypes of herpes simplex virus (HSV): HSV type 1 (HSV-1), which is more frequently found in the oral mucosa and ocular areas, and HSV type 2 (HSV-2), which is most commonly encountered as the causative agent of genital tract HSV infections (1). HSV is a ubiquitous human pathogen, with worldwide prevalence rates approaching 90 % for HSV-1 and up to 25 % for HSV-2, depending on socioeconomic class (2, 3). The virus contains a large, linear double-stranded DNA (dsDNA) genome of 150 kbp and it is approximately 83 % homologous between HSV-1 and HSV-2 in the coding regions (4). HSV-1, which is usually transmitted in childhood through nonsexual contact, is the primary cause of orolabial herpes, but it can also cause genital infection. HSV-2, which is usually sexually transmitted, is an uncommon cause of orolabial herpes. Among Americans who are 14 to 19 years of age, the seroprevalence of HSV-1 has decreased by 30% over the past 30 years; thus, an increasing proportion of adolescents lack protective HSV-1 antibodies when they become sexually active (5, 6). This lack of HSV-1 antibodies has led to an increased frequency of HSV-1 genital herpes acquired from oral–genital sex practices (7). In some populations (especially young heterosexual women who are 18 to 22 years of age, non-Hispanic whites, and men who have sex with men (MSM)), HSV-1 is a more common cause of initial episodes of genital herpes than HSV-2 (8, 9). Young women and MSM are also at high risk of acquiring HIV infection (10, 11) and, although genital HSV-2 infection is associated with a three fold increase in the risk of acquiring HIV infection (12, 13), nothing is know about the risk of HIV acquisition in the context of genital HSV-1 infection.
HSV-2 genital infection increases the risk of HIV infection even in absence of detectable HSV-2 replication, lesions or inflammation (14). It was reported that the vaginal mucosa of HSV-2 infected women retains an increased number of CCR5+ CD4+ T cells long after HSV-2 replication abates. Likewise, plasmacytoid and myeloid dendritic cells (DCs), which infiltrate areas of skin infected with HSV-2, persist after lesion healing even in the context of acyclovir therapy (15, 16). In order to study the mechanisms driving the increase in HIV infection due to HSV-2, we developed a model of HSV-2 infection in rhesus macaques (17). This model was used to describe how HSV-2 infection can lead to sustained increase in local and systemic inflammatory mediators and increased availability of highly susceptible HIV target cells long after the primary infection abates (>1 year) (18–20).
Primary HSV infection usually starts with virion attachment and replication into epithelial cells and keratinocytes at mucocutaneous sites including the mouth, eyes, and genitalia. HSV subsequently accesses the peripheral nervous system (PNS) where it can either initiate productive replication, which ultimately leads to destruction of the neuron, or to the establishment of latent infection (21). Although humans are the only natural hosts for HSV-1 and HSV-2, mice have been used as preferred models to study the mechanisms of HSV infection and immune responses to the virus also because the mouse models mimic several aspects of the human disease (22). Ocular infection in squirrel monkeys (Saimiri sciureus) and intracerebral infection in owl monkeys (Aotus trivirgatus) and common marmoset (Callithrix jacchus) are the only documented experimental HSV-1 infection models in non-human primates to date (23–25). However, human-to-nonhuman zoonotic infections have been reported for Old World primates, including gorillas, chimpanzees, bonobos and white-handed gibbons, New World monkeys, and prosimians (26). Notably, while human-borne HSV-1 infections of Old World primates remain localized and cause only mild mucocutaneous lesions, New World monkeys, including marmosets and tamarin species, and prosimians are highly susceptible for this zoonotic infection, resulting in severe, often fatal disease (27, 28).
Experimental infection of rhesus macaques with simian immunodeficiency viruses (SIV) or chimeras encoding the HIV envelope or reverse transcriptase (SHIV, or RT-SHIV) are among the most widely used animal models of HIV infection. Thus, to investigate the potential impact of genital HSV-1 infection on HIV infection, a model of genital HSV-1 infection in macaques is highly desirable. Herein, we describe the results of experimental genital inoculation of three SHIV-infected rhesus macaques of Indian origin with the HSV-1 strain McKrae, a strain more neurovirulent in animal models than other strains of HSV-1 (29–31). HSV-1 shedding was detected in the vaginal swabs after the second inoculation, which was preceded by gentle cytobrushing. The impact of HSV-1 on SHIV viral loads, soluble factors and cell subsets highly susceptible to HIV infection were monitored.
Methods
Humane Care Guidelines
Three adult female Indian rhesus macaques (Macaca mulatta) with a mean age of: 8 years, ranging from 6 to 10 years; and a mean weight of 8.5kg, ranging from 7.2–10.85 kg) were enrolled for these studies. Animals had previously tested negative by serology for Herpes B and been infected intra-rectally with SHIV-SF162P3 ~three months before the start of this study as part of a different study and protocol. The animals were healthy and had undetectable SHIV-SF162P3 plasma viral load at the start of this study. All animal care complied with the regulations under the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals. All treatments were approved by the Institutional Animal Care and Use committee (IACUC) of the Tulane National Primate Research Center (OLAW assurance #A4499-01) (TNPRC, Covington, LA). The TNPRC is accredited by the Association for Accreditation of Laboratory Animal Care (AAALAC#000594). Animals were socially housed indoors in climate-controlled conditions with a 12/12-light/dark cycle. Animals were closely monitored twice daily by veterinarians and technicians for all their daily healthcare needs. Any abnormalities are reported and carefully recorded, including loss of appetite, changes in stool, or other behaviors. Animals are fed twice daily with commercially prepared monkey chow. As part of the TNPRC environmental enrichment program, supplemental foods such as fruits, vegetables, or foraging treats are provided. Water is continuously supplied by an automated system. The TNPRC environment enrichment program is reviewed and approved by IACUC semiannually. To minimize pain, discomfort and distress from procedures the veterinarians at the TNPRC Division of Veterinary Medicine comply by the recommendations of the Weatherall Report. Macaques were anesthetized with ketamine-HCl (10mg/kg) or tiletamine/zolazepam (6mg/kg) before any procedures or blood collections. Buprenorphine (0.01mg/kg) was administered for procedures that may cause more than temporary pain or distress to provide preemptive and post-procedural analgesia.
Macaque Treatment
Three healthy SHIV-SF162P3 infected Indian rhesus macaques with undetectable SIV viral load were vaginally challenged twice using 4x108 pfu of HSV-1 strain McKrae. The second inoculation was performed two days after a vaginal biopsy and right after the vaginal mucosa was gently abraded with a cytobrush. Blood and vaginal swabs were collected prior to the first HSV-1 inoculation and according to the schedule shown in Figure 1A. Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA blood using Ficoll-Hypaque density gradient centrifugation (GE Healthcare Biosciences, Uppsala, Sweden). Vaginal swabs were collected using a Merocel eye spear pre-moistened in 1ml PBS and inserted in the vaginal cavity for 5 minutes to absorb liquid and rotated to brush the vaginal epithelia before being returned to the tube containing PBS. Vaginal tissues (one 3mm x 3mm biopsy) were biopsied two days before the second HSV-1 inoculation and 20 days after. Blood, swabs, and tissues were shipped to the Population Council offices in New York overnight and were processed immediately upon arrival.
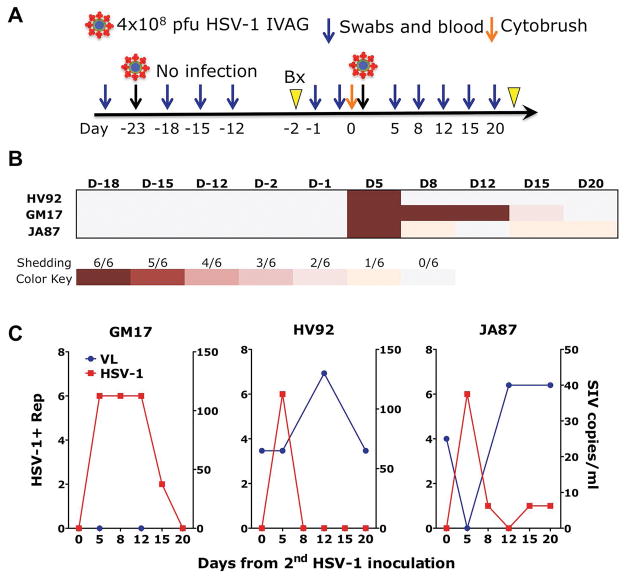
Figure 1. HSV-1 genital infection of rhesus macaques may require gentle cytobrushing before challenge.
A) A schematic of the study design is depicted B) A heat map representing the frequency of positive replicates detected by HSV-1-specific nested PCR in DNA extracted from complete vaginal swabs is shown for every time point that was analyzed after the first (day −23) and second (day 0) HSV-1 inoculation. C) The frequency of HSV-1 replicates detected after the second HSV-1 inoculation is plotted on the left Y-axis (in red) against the SHIV-SF162P3 plasma VL on the right Y axis (in blue).
Virus Stock
HSV-1 strain McKrae stock was supplied by Dr. Moriah Szpara and was derived from a stock recently characterized and genome-sequenced by Dr. Lynda Morrison (29, 31). Vero cells were used to expand the stock by inoculating for 3 hours with 0.6 MOI HSV-1 McKrae in Dulbecco’s modified Eagle medium (DMEM) and then cultured overnight in DMEM with 2% fetal bovine serum (FBS). Vero cell monolayers were then scraped with a cell scraper, and the cell/supernatant mixture collected into 50ml tubes and spun down. All of the cell pellets were combined into one tube, spun, and supernatant aspirated. The cell pellet was resuspended in 3ml of saved supernatant and cells cracked in a dry ice/ethanol bath two times, sonicated for 1 minute, and then cleared by centrifugation. Virus aliquots were stored in −80C. The Vero cell plaque assay was used to determine the titer as follows: Several dilutions of the McKrae virus were incubated on Vero cells for 1 hour, overlayed with methylcellulose, and incubated for 48 hours. Methylcellulose was then aspirated, and the Vero cell layer was fixed with 10% formalin, washed with water, stained with 1% crystal violet, washed again with water, and air dried by inverting the plates overnight. Plaques were then counted to determine the titer. The HSV-1 strain McKrae titer used in vivo was 4 x 109 pfu/ml.
HSV-1 detection
Total vaginal swabs, including both mucosal cells and fluid, were shipped overnight, cleared by centrifugation, aliquoted and frozen as previously described (17). DNA was extracted from the total swab using the DNeasy Blood and Tissue kit (Qiagen) according to manufacturer’s instructions. HSV-1 infection was monitored by nested PCR in the gD gene of HSV-1 using the following primers. External Primers - FW: 5′-ATC ACG GTA GCC CGG CCG TGT GAC A, RV: 5′ - CAT ACC GGA ACG CAC CAC ACA A. Internal Primers - FW: 5′-CCA TAC CGA CCA CAC CGA CGA, RV: 5′ – GGT AGT TGG TCG TTC GCG CTG AA. HSV-1 (MacIntyre Strain) Quantitated Viral Load Control (Advanced Biotechnologies) was used as a positive control. The product of the external and internal PCR of two samples was sequenced to confirm specificity (Genewiz). A heat map was generated based on the number of positive replicates, out of 6 total replicates, assigned grades of color intensity to indicate higher frequency of positive reactions.
Luminex
Cytokine and chemokine soluble factors were measured using a Novex® Monkey Cytokine Magnetic 29-Plex Panel kit (Life Technologies) on a MAGPIX ® system (Luminex XMAP Technology) with Luminex xPOPNENT software. Luminex was performed on clarified vaginal fluid supernatants from BL, day 5 and 20 days post HSV-1 infection. Vaginal fluid supernatant was thawed from −80C, spun, and used as neat in assay, with the kit setup diluting 1:3 according to manufactures instructions. The soluble factors measured were as follows. FGF, IL-B, G-CSF, IL-10, IL-6, IL-12, CCL5, CCL11, IL-17, MIP-17agr;, GM-CSF, MIP-1β, MCP-1, IL-15, EGF, IL-5, HGF, VEGF, IFN-γ, CCL22, I-TAC, MIF, IL-1RA, TNFα, IL-2, IP-10, MIG, IL-4, and IL-8. Values graphed using Prism Graphpad. (GraphPad Prism version 5.02 for Windows, GraphPad Software, San Diego, CA)
SHIV-SF162P3 detection
Plasma and cell-associated viral loads were monitored at baseline (BL) and at day 5, 12 and 20 post-HSV-1 infection. SIV plasma was isolated from EDTA blood by centrifugation and stored −80C. Plasma viral RNA copies were measured by quantitative RT-qPCR for SIV gag, as previously described (by Leidos Biomedical Research Inc (32). The lower limit of quantification of the assay is 15 RNA copies/ml.
SIVgag qPCR was used to detect the presence of virus in DNA extracted from snap frozen vaginal biopsies. The DNeasy Blood and Tissue kit (Qiagen) was used to extract the DNA according to the manufacturer’s instructions. DNA was eluted using DNAse/RNAse free water and quantified on a Nanodrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE). TaqMan master mix (Life technologies) was used for the qPCR on a ViiA-7 Real-Time PCR system (ThermoFisher). SIV copies were normalized on albumin copies. Primers were SIVgag667 FW: 5′ - GGT TGC ACC CCC TAT GAC AT; SIVgag731REV: 5′ - TGC ATA GCC GCT TGA TGG T; SIVgag 688 PROBE: 5′ -/56 FAM/ATT CAG ATG TTA AAT TGT GTG; RhAlb FW: 5′ – ATT TTC AGC TTC GCG TCT TTT; RhAlb RV: 5′ – TTC TCG CTT ACT GGC GTT TTC T; RhAlb PROBE: 5′ - /56-FAM/CCT GTT CTT TAG CTG TCC GTG
Flow cytometry
Fresh PBMCs were phenotyped at base-line (BL) and day 5 after the 2nd inoculation. PBMCs were isolated using Ficoll-Hypaque density gradient centrifugation. Cells were stained with the LIVE/DEAD Aqua dye (Invitrogen), and the antibodies listed in Table 1. Unlabeled anti-CD195 from the NIH AIDS repository was directly conjugated with AF647H-labeling kit (Innova Bioscences). At least 200,000 events were acquired in the lymphocyte live-cells gate using the BD LSRII Flow Cytometer. Data were acquired using Diva software and analyzed using FlowJo software.
Table 1. Antibodies used to phenotype PBMC.
List of antibodies used for phenotyping blood CD4+ T cells by flow cytometry.
| mAb | Color | Clone | Company |
|---|---|---|---|
| CD3 | V450 | SP34-2 | BD Bioscience |
| CD4 | BUV395 | L200 | BD Bioscience |
| CD95 | PerCP-EFluor710 | DX2 | eBioscience |
| CCR6 | PE-CF594 | 11A9 | BD Bioscience |
| α4β7 | PE | Act-1 | Mass Biologics |
| CCR5 | AF647H | 3A9 | NIH AIDS Repository |
| CD69 | AF700 | FN50 | BD Bioscience |
| CCR7 | APC-Cy7 | 3D12 | BD Bioscience |
| CXCR3 | AF488 | 1C6 | BD Bioscience |
| PD-1 | BV605 | EH12 | BD Bioscience |
| FoxP3 | PE-Cy7 | PCH101 | eBioscience |
Results
Gentle cytobrushing before HSV-1 inoculation may be needed for genital HSV-1 infection of rhesus macaques
Three healthy, SHIV-SF162P3 infected female rhesus macaques of Indian origin with undetectable plasma viral load (VL) at the start of the study, were inoculated vaginally atraumatically with 4x108 pfu of HSV-1 McKrae. Vaginal swabs were collected after 5, 8 and 11 days, but no HSV-1 DNA was detected in any of the three animals at any time point (Figure 1A and B). HSV-1 detection in vaginal swabs was carried out by HSV-1 nested PCR similar to the HSV-2 nested PCR described in (17, 33) and the PCR product sequenced to confirm specificity. Vaginal biopsies were taken after 21 days to determine if virus could be found in the vaginal tissues and stimulate viral reactivation if any latent infection was present. No HSV-1 was detected even after the biopsy and the macaques were inoculated again two days later with 4x108 pfu of HSV-1 McKrae right after a gentle abrasion was performed at the site of inoculation with a cytobrush. Vaginal swabs were collected at day 5, 8, 12, 15 and 20 and blood at day 5, 12 and 20 after the second inoculation. HSV-1 DNA was detected in the vaginal swabs of all three animals at day 5 post-inoculation and, since residual inoculum was not detected at any time point after the first inoculation, all three animals were considered infected with the second inoculation. HSV-1 was detected in vaginal swabs also at day 8, 12, and 15 - post-HSV-1 infection in GM17 and at day 8, 15 and 20 in JA87 (Figure 1B and C). The animals were released from the study after day 20.
Impact of genital HSV-1 infection on SHIV-SF162P3 loads
Blood was collected at baseline (BL; day of the second inoculation/ HSV-1 infection) and day 5, 15 and 20 post-HSV-1 infection. Plasma SIV load was monitored by an RT-qPCR with a sensitivity of 15 copies/ml. The plasma VL of GM17, which had undetectable VL at base-line, remained undetectable throughout the monitoring phase, while both HV92 and JA87 had increased VL at day 12 compared to BL (Figure 1C). SHIV-SF162P3 was also undetectable in the vaginal biopsy of GM17 from both before and after HSV-1 infection (Figure 2), while HV92 had similar amount of SHIV-SF162P3 DNA in tissue before and after HSV-1 infection. In contrast, JA87, which had intermittent HSV-1 shedding, had increased vaginal tissue SHIV-SF162P3 load on day 20 compared with BL (Figure 2).
Figure 2. Impact of genital HSV-1 infection on vaginal tissue SHIV-SF162P3 loads.
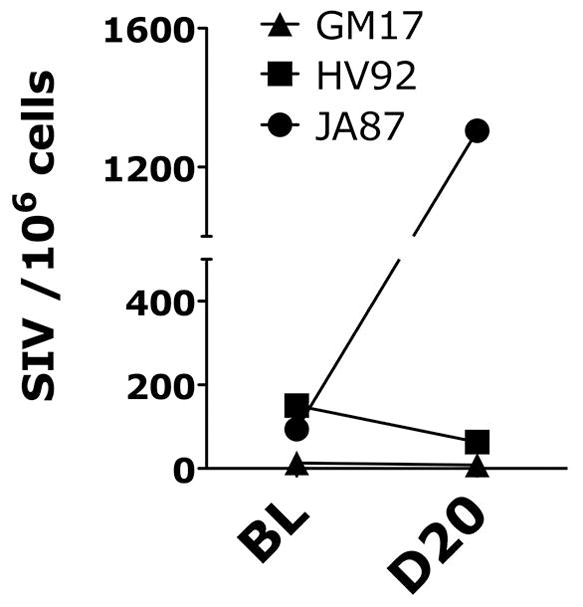
The number of copies of SIV-gag normalized on total cell number (albumin copies) detected in the vaginal biopsies 2 days before and 20 days after HSV-2 infection are shown for all three animals.
Impact of genital HSV-1 infection on cytokines and chemokines in vaginal fluids
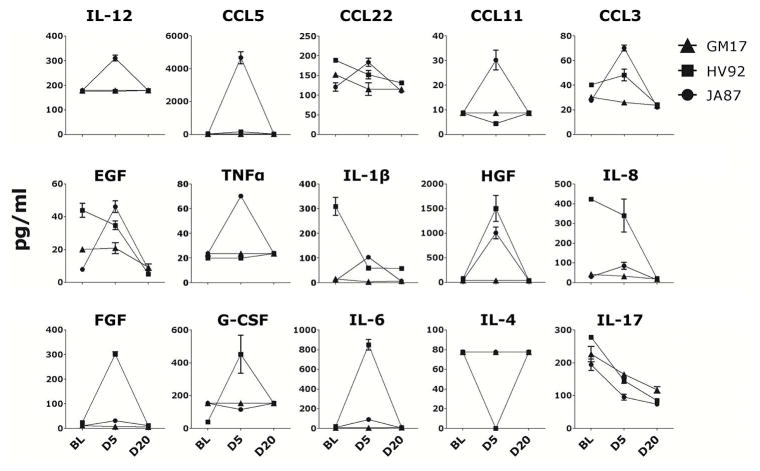
Cytokines and chemokines (CC/CK) from all three animals were monitored in clarified vaginal swabs by 29-plex Luminex at BL, day 5 and day 20 post-HSV-1 infection. JA87, which had increased SHIV-SF162P3 load in the vaginal tissue, had also increased inflammatory CC/CK in the vaginal swabs at day 5 post-infection compared to BL and day 20 (Figure 3, top two rows). In contrast, GM17, which had undetectable SHIV-SF162P3 VL in plasma and vaginal tissue, had very low levels of most CC/CK at BL and levels were mostly unchanged by HSV-1 infection. HV92 had only increased levels of factors involved in wound healing (such as HGF, FGF and G-CSF) and IL-6, also critical factor in skin wound healing (34) at day 5 post-HSV-1 infection compared with BL and day 20. Of note, HSV-1 was detected only at day 5 post-HSV-1 infection in HV92, which indicates that this animal may have cleared HSV-1 infection very rapidly. Interestingly, all three animals had decreased levels of vaginal IL-17 at day 5 and 20 post-HSV-1 infection compared to BL.
Figure 3. Impact of genital HSV-1 infection on soluble factors in vaginal fluids.
The concentration of soluble factors (Mean ± SEM of duplicates) for each animal present in clarified vaginal swabs of the three animals 2 days before (BL) and 5 and 20 days after HSV-1 infection are shown (only factors with at least one animal per time point with a concentration greater than the lowest standard are shown).
Impact of genital HSV-1 infection on the frequency of certain CD4+ T cells subsets in blood
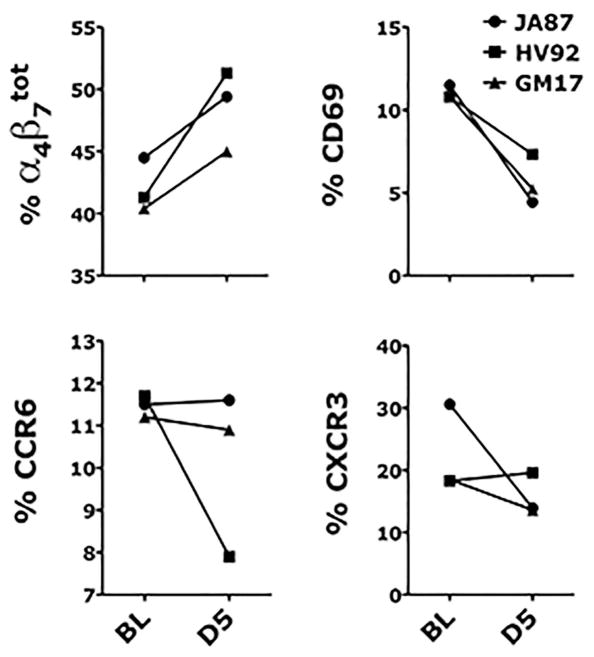
PBMCs from all 3 animals were isolated at BL and day 5 post-HSV-1 infection and stained with the antibodies listed in Table 1. Consistent changes in all three animals were seen only in the frequency of total α4β7+ CD4+ T cells, which increased at day 5 compared to BL and in the frequencies of CD69+ CD4+ T cells and CCR6+ CD4+ T cells, which decreased at day 5 compared to BL (Figure 4). The frequency of α4β7high CD4+ T cells increased in GM17 and JA87, while a decrease was detected in HV92 (not shown). No changes were detected in the frequencies of FoxP3+ and PD1+ CD4+ T cells (not shown), while a decrease in the frequency of CXCR3+ CD4+ T cells was present in GM17 and JA87, but no change was detected in HV92 (Figure 4).
Figure 4. Impact of genital HSV-1 infection on blood CD4+ T cell subsets.
The frequency of live (Live-dead Aqua negative) CD3+ CD4+ cells expressing α4β7, CD69, CCR6 and CXCR3 in blood the day of (BL) and 5 days after HSV-1 infection are shown.
Discussion
There are ~140 million people living with genital HSV-1 infections and a growing number of young women and MSM are at high risk of acquiring genital HSV-1 in both developed and developing countries (3). The impact of genital HSV-1 infection on HIV acquisition and pathogenesis is unknown and models to study HSV-1/HIV interplay are urgently needed. Herein we describe the first reported, successful HSV-1 genital inoculation in three SHIV-SF162P3 infected animals with undetectable SHIV-SF162P3 plasma VL. HSV-1 infection was carried out with the neurovirulent McKrae strain and viral shedding was detected only when inoculation was preceded by a gentle abrasion of the vaginal mucosa with a cytobrush. We do not know if other HSV-1 strains would be able to establish genital infection in macaques and what role, if any, played the vaginal biopsies performed two days before the second inoculation. HSV-1 infection appeared to be transient. However, the animals were followed only for three weeks and more work will be needed to investigate the ability of genital HSV-1 infection to spread to sensory ganglia and to measure local and systemic immune responses to the virus. HSV-1 shedding seemed to be independent, if not inversely correlated with SHIV-SF162P3 plasma VL. Therefore, we anticipate that the same results could be obtained in naïve rhesus macaques. Interestingly, we did not detect SHIV-SF162P3 reactivation in the animal (GM17) that had undetectable VL in both plasma and tissue. However, GM17 may have cleared SHIV-SF162P3 infection completely before the initiation of the HSV-1 study. In contrast, we detected a very small increase in plasma SHIV-SF162P3 VL in the other two animals. Thus, absence of SHIV-SF162P3 detection was associated with lower inflammatory response in GM17, but higher and prolonged HSV-1 shedding. In contrast, in the animals with detectable SHIV-SF162P3, HSV-1 infection had two opposite outcomes. HV92 cleared HSV-1 infection very rapidly with no detectable inflammatory response, but release of wound-healing associated factors and no local increase in SHIV-SF162P3 replication. JA87, which had prolonged intermittent HSV-1 shedding, had higher vaginal tissue SHIV-SF162P3 load and an increase in pro-inflammatory CC/CK in vaginal fluids, which may have contributed to increase SHIV-SF162P3 replication in the vaginal tissue. Interestingly, we detected a consistent decrease in the levels of IL-17 secreted in the intravaginal compartment. This may be due to Th1-responses being more critical to protection against HSV than Th17 or other T cell polarization types (35−37). Interestingly, however, HV92, which cleared HSV-1 infection more rapidly than the other two macaques, had a more profound decrease in the frequency of CCR6+ CD4+ T cells, but no decrease in CXCR3+ CD4+ T cells in blood, while the opposite occurred in the animals with more prolonged HSV-1 shedding. These data may reflect redistribution of the CD4+ T cell subsets at the site of infection, with a more profound redistribution of Th1 CD4+ T cells required in the macaques with prolonged HSV-1 replication. More difficult to explain is the consistent increase in the frequency of cells expressing α4β7, since those include both naïve and memory CD4+ T cells (38). Using the genital and rectal HSV-2 models in macaques, we have shown that HSV-2 increases the frequency of α4β7high CD4+ T cells (19, 33). This memory cell subset was increased in the two animals with prolonged shedding, but decreased in HV92, perhaps reflecting the earlier mobilization of α4β7high CD4+ T cells and CCR6+ Th-17 like T cells in this animal, which cleared the virus more rapidly, in contrast to the mobilization of Th1 subsets in the other two animals. The decrease in the frequency of activated CD69+ CD4+ T cells was expected and most likely due to their redistribution at the site of HSV-1 infection.
In conclusion, we describe the first reported experimental vaginal infection of rhesus macaques with HSV-1. More work is needed to investigate the innate and adaptive immune responses elicited by HSV-1 in this species locally and systemically and how similar they are to the immune responses in humans. Importantly, the ability of HSV-1 to establish latent infection in the dorsal root ganglia of macaques needs to be clarified. However, even if latent infection is not established, a model of primary HSV-1 infection in macaques could be useful to investigate the impact of active HSV-1 infection on SIV acquisition and to study the potential impact of genital HSV-1 infection on HIV-1 epidemiology and pathogenesis.
Acknowledgments
Support: This work was supported by NIH grants R01 AI098456-04 and OD011104.
References
- 1.Groves MJ. Genital Herpes: A Review. American family physician. 2016;93(11):928–34. Epub 2016/06/10. [PubMed] [Google Scholar]
- 2.Pellett PERB. Fields Virology. 6. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. Herpesviridae; pp. 1802–22. [Google Scholar]
- 3.Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Gottlieb SL, et al. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PloS one. 2015;10(10):e0140765. doi: 10.1371/journal.pone.0140765. Epub 2015/10/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu F, Zhou ZH. Comparative virion structures of human herpesviruses. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, et al., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press; 2007. [PubMed] [Google Scholar]
- 5.Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2--United States, 1999–2010. The Journal of infectious diseases. 2014;209(3):325–33. doi: 10.1093/infdis/jit458. Epub 2013/10/19. [DOI] [PubMed] [Google Scholar]
- 6.Xu F, Lee FK, Morrow RA, Sternberg MR, Luther KE, Dubin G, et al. Seroprevalence of herpes simplex virus type 1 in children in the United States. The Journal of pediatrics. 2007;151(4):374–7. doi: 10.1016/j.jpeds.2007.04.065. Epub 2007/09/25. [DOI] [PubMed] [Google Scholar]
- 7.Gnann JW, Jr, Whitley RJ. CLINICAL PRACTICE. Genital Herpes. The New England journal of medicine. 2016;375(7):666–74. doi: 10.1056/NEJMcp1603178. Epub 2016/08/18. [DOI] [PubMed] [Google Scholar]
- 8.Ryder N, Jin F, McNulty AM, Grulich AE, Donovan B. Increasing role of herpes simplex virus type 1 in first-episode anogenital herpes in heterosexual women and younger men who have sex with men, 1992–2006. Sexually transmitted infections. 2009;85(6):416–9. doi: 10.1136/sti.2008.033902. Epub 2009/03/11. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein DI, Bellamy AR, Hook EW, 3rd, Levin MJ, Wald A, Ewell MG, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;56(3):344–51. doi: 10.1093/cid/cis891. Epub 2012/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenton KA, Imrie J. Increasing rates of sexually transmitted diseases in homosexual men in Western europe and the United States: why? Infectious disease clinics of North America. 2005;19(2):311–31. doi: 10.1016/j.idc.2005.04.004. Epub 2005/06/21. [DOI] [PubMed] [Google Scholar]
- 11.Harrison A, Colvin CJ, Kuo C, Swartz A, Lurie M. Sustained High HIV Incidence in Young Women in Southern Africa: Social, Behavioral, and Structural Factors and Emerging Intervention Approaches. Current HIV/AIDS reports. 2015;12(2):207–15. doi: 10.1007/s11904-015-0261-0. Epub 2015/04/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corey L. Synergistic copathogens--HIV-1 and HSV-2. The New England journal of medicine. 2007;356(8):854–6. doi: 10.1056/NEJMe068302. [DOI] [PubMed] [Google Scholar]
- 13.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Aids. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 14.Barnabas RV, Wasserheit JN, Huang Y, Janes H, Morrow R, Fuchs J, et al. Impact of herpes simplex virus type 2 on HIV-1 acquisition and progression in an HIV vaccine trial (the Step study) Journal of acquired immune deficiency syndromes (1999) 2011;57(3):238–44. doi: 10.1097/QAI.0b013e31821acb5. Epub 2011/08/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nature medicine. 2009;15(8):886–92. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362(5):427–39. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crostarosa F, Aravantinou M, Akpogheneta OJ, Jasny E, Shaw A, Kenney J, et al. A macaque model to study vaginal HSV-2/immunodeficiency virus co-infection and the impact of HSV-2 on microbicide efficacy. PloS one. 2009;4(11):e8060. doi: 10.1371/journal.pone.0008060. Epub 2009/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinelli E, Veglia F, Goode D, Guerra-Perez N, Aravantinou M, Arthos J, et al. The frequency of alpha4beta7high memory CD4+ T cells correlates with susceptibility to rectal SIV infection. Journal of acquired immune deficiency syndromes. 1999:2013. doi: 10.1097/QAI.0b013e31829f6e1a. Epub 2013/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goode D, Truong R, Villegas G, Calenda G, Guerra-Perez N, Piatak M, et al. HSV-2-driven increase in the expression of alpha4beta7 correlates with increased susceptibility to vaginal SHIV(SF162P3) infection. PLoS pathogens. 2014;10(12):e1004567. doi: 10.1371/journal.ppat.1004567. Epub 2014/12/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerra-Perez N, Aravantinou M, Veglia F, Goode D, Truong R, Derby N, et al. Rectal HSV-2 Infection May Increase Rectal SIV Acquisition Even in the Context of SIVDeltanef Vaccination. PloS one. 2016;11(2):e0149491. doi: 10.1371/journal.pone.0149491. Epub 2016/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith G. Herpesvirus transport to the nervous system and back again. Annual review of microbiology. 2012;66:153–76. doi: 10.1146/annurev-micro-092611-150051. Epub 2012/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kollias CM, Huneke RB, Wigdahl B, Jennings SR. Animal models of herpes simplex virus immunity and pathogenesis. Journal of neurovirology. 2015;21(1):8–23. doi: 10.1007/s13365-014-0302-2. Epub 2014/11/13. [DOI] [PubMed] [Google Scholar]
- 23.Varnell ED, Kaufman HE, Hill JM, Wolf RH. A primate model for acute and recurrent herpetic keratitis. Current eye research. 1987;6(1):277–9. doi: 10.3109/02713688709020105. Epub 1987/01/01. [DOI] [PubMed] [Google Scholar]
- 24.Rootman DS, Haruta Y, Hill JM. Reactivation of HSV-1 in primates by transcorneal iontophoresis of adrenergic agents. Investigative ophthalmology & visual science. 1990;31(3):597–600. Epub 1990/03/01. [PubMed] [Google Scholar]
- 25.Deisboeck TS, Wakimoto H, Nestler U, Louis DN, Sehgal PK, Simon M, et al. Development of a novel non-human primate model for preclinical gene vector safety studies. Determining the effects of intracerebral HSV-1 inoculation in the common marmoset: a comparative study. Gene therapy. 2003;10(15):1225–33. doi: 10.1038/sj.gt.3302003. Epub 2003/07/15. [DOI] [PubMed] [Google Scholar]
- 26.Sekulin K, Jankova J, Kolodziejek J, Huemer HP, Gruber A, Meyer J, et al. Natural zoonotic infections of two marmosets and one domestic rabbit with herpes simplex virus type 1 did not reveal a correlation with a certain gG-, gI- or gE genotype. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2010;16(11):1669–72. doi: 10.1111/j.1469-0691.2010.03163.x. Epub 2010/02/04. [DOI] [PubMed] [Google Scholar]
- 27.Schrenzel MD, Osborn KG, Shima A, Klieforth RB, Maalouf GA. Naturally occurring fatal herpes simplex virus 1 infection in a family of white-faced saki monkeys (Pithecia pithecia pithecia) Journal of medical primatology. 2003;32(1):7–14. doi: 10.1034/j.1600-0684.2003.01040.x. Epub 2003/05/08. [DOI] [PubMed] [Google Scholar]
- 28.Huemer HP, Larcher C, Czedik-Eysenberg T, Nowotny N, Reifinger M. Fatal infection of a pet monkey with Human herpesvirus. Emerging infectious diseases. 2002;8(6):639–42. doi: 10.3201/eid0806.010341. Epub 2002/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macdonald SJ, Mostafa HH, Morrison LA, Davido DJ. Genome sequence of herpes simplex virus 1 strain McKrae. Journal of virology. 2012;86(17):9540–1. doi: 10.1128/jvi.01469-12. Epub 2012/08/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dix RD, McKendall RR, Baringer JR. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infection and immunity. 1983;40(1):103–12. doi: 10.1128/iai.40.1.103-112.1983. Epub 1983/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Davido DJ, Morrison LA. HSV-1 strain McKrae is more neuroinvasive than HSV-1 KOS after corneal or vaginal inoculation in mice. Virus research. 2013;173(2):436–40. doi: 10.1016/j.virusres.2013.01.001. Epub 2013/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cline AN, Bess JW, Piatak M, Jr, Lifson JD. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. Journal of medical primatology. 2005;34(5–6):303–12. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 33.Martinelli E, Tharinger H, Frank I, Arthos J, Piatak M, Jr, Lifson JD, et al. HSV-2 infection of dendritic cells amplifies a highly susceptible HIV-1 cell target. PLoS pathogens. 2011;7(6):e1002109. doi: 10.1371/journal.ppat.1002109. Epub 2011/07/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. Journal of leukocyte biology. 2003;73(6):713–21. doi: 10.1189/jlb.0802397. Epub 2003/05/30. [DOI] [PubMed] [Google Scholar]
- 35.Shin H, Iwasaki A. Generating protective immunity against genital herpes. Trends in immunology. 2013;34(10):487–94. doi: 10.1016/j.it.2013.08.001. Epub 2013/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462(7272):510–3. doi: 10.1038/nature08511. Epub 2009/11/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandgren KJ, Bertram K, Cunningham AL. Understanding natural herpes simplex virus immunity to inform next-generation vaccine design. Clinical & translational immunology. 2016;5(7):e94. doi: 10.1038/cti.2016.44. Epub 2016/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, et al. Development and homeostasis of T cell memory in rhesus macaque. Journal of immunology. 2002;168(1):29–43. doi: 10.4049/jimmunol.168.1.29. Epub 2001/12/26. [DOI] [PubMed] [Google Scholar]