Abstract
Inflammatory reactivity to acute laboratory stress is thought to reflect individual differences in responsivity to environmental stressors and may confer future health risk. To characterize this response, we conducted a meta-analysis of 34 studies that measured circulating inflammatory markers and 15 studies that measured stimulated production of inflammatory markers before and after exposure to laboratory challenge. Results showed significant stress-related increases in circulating interleukin (IL)-1β (d = 0.66, p < .001), IL-6 (d = 0.35, p < .001), IL-10 (d = 0.69, p < .001), and tumor necrosis factor(TNF)-α (d = 0.28, p < .001), but not IL-1ra, IL-2, interferon-γ, or C-reactive protein. There were sufficient data to assess the time course of IL-6, IL-1β, and TNF-α reactivity. IL-6 increased from baseline to measures taken 40–50, 60–75, 90, and 120 min following stress, with the largest effect at 90min post-stress (d = 0.70, p < .001). IL-1β increased from baseline to 20–30, 40–50, and 60–70 min following stress, with the largest effect between 40–50 min post-stress (d = .73, p = .02). For TNF-α, there was a significant increase from baseline to 31–50 min post stress (d = 0.44, p = .01), but not at later times. There was no difference in magnitude of IL-6 reactivity as a function of type of stress (social-evaluative versus other). For stimulated inflammatory markers, results showed stress-related increases in IL-1β when measured 20–120 min post-stress (d = 1.09, p < .001), and in IL-4 and interferon-γ when measured 0–10 min post stressor (d = −0.42, p < .001 and d = 0.47, p < .001). These results extend findings from a prior meta-analysis (Steptoe, Hamer, & Chida, 2007) to show reliable increases in circulating IL-6, IL-1β, IL-10 and TNF-α and stimulated IL-1β, IL-4 and interferon-γ in response to acute stress. It is possible that these responses contribute to associations between exposure to life challenges and vulnerability to inflammatory disease.
Keywords: Inflammation, acute psychological stress, systematic review, inflammatory cytokines
Introduction
Considerable literature documents an association between naturalistic psychological stress, markers of inflammation, and future disease risk (Segerstrom & Miller, 2004; Rohleder, 2014, Steptoe et al., 2007). Brief naturalistic stressors such as taking examinations, life event stressors such as loss of a spouse or natural disaster, and more chronic stressors such as caregiving for an ill loved one, all associate with elevated markers of inflammation in peripheral circulation (Marshall et al., 1998; Kiecolt-Glaser, McGuire, Robles, & Glaser, 2002). These proinflammatory markers, including concentrations of cytokines and the acute phase protein C-reactive protein (CRP), predict risk for incident cardiovascular disease (CVD; Pearson et al., 2003), as well as accelerated progression of diseases that involve inflammatory pathophysiology (e.g. cancer, HIV, asthma, and rheumatoid arthritis (Choy, 2012; Deeks et al., 2013; Elinav et al., 2013; Fu et al., 2013; McInnes & Schett, 2011; Naugler & Karin, 2008). These associations raise the possibility that psychological stress may increase disease risk and shape disease course through an inflammatory pathway.
A growing number of studies have further explored the impact of psychological stress on inflammation using experimental protocols to examine changes in mediators of inflammation in response to short-term laboratory stressors designed to characterize transient stresses of daily life. Early results suggest that acute stress induces reliable changes in both enumerative and functional aspects of immunity, including increases in both circulating and stimulated markers of inflammation (Marsland, Bachen, Cohen, Rabin & Manuck, 2002; Steptoe, Hamer, & Chida, 2007). In addition to main effects of stress on immune function, individuals differ substantially in the magnitude of their immunologic reactivity to stress, with some individuals exhibiting large responses and others little or no response (Black, 2003; Marsland et al., 2002). Further evidence, including our own, has shown that individual differences in the magnitude of immune reactivity are relatively stable across both time and task, possibly having implications for susceptibility to disease (Marsland et al., 2002).
Initial evidence supports the possibility that individual differences in the magnitude of stress-related changes in inflammatory mediators are clinically significant. For example, in one longitudinal study the magnitude of interleukin-6 (IL-6) response to acute laboratory stress predicted ambulatory blood pressure after a 3-year follow-up (Brydon & Steptoe, 2005). Similarly, acute stress-evoked increases in fibrinogen and tumor necrosis factor alpha (TNF-α) predicted increasing carotid artery stiffness over time (Ellins et al., 2008). It is also known that individual differences in immune responses to acute psychological stress parallel magnitude of cardiovascular reactivity (Manuck, Cohen, Rabin, Muldoon & Bachen, 1991), a known risk factor for CVD (Chida & Steptoe, 2010). Finally, recent evidence suggests that individuals who are predisposed to larger increases in inflammatory mediators in the laboratory may be prone to higher levels of systemic inflammation, possibly conferring increased risk for inflammatory disease (Lockwood, Marsland, Cohen, Gianaros, 2016). Thus, it is conceivable that magnitude of stress-related increases in inflammatory mediators may form a physiological basis for vulnerability to inflammatory disease at times of naturalistic stress.
In light of our growing understanding of the clinical significance of concentrations of inflammatory mediators, the purpose of the current work was to update a meta-analytic review of studies examining circulating and in vitro stimulated inflammatory responses to acute laboratory stressors that was conducted in 2007 by Steptoe, Hamer, & Chida. The 2007 review identified 18 studies that measured circulating markers of inflammation (IL-6, IL-1β, TNF-α, and CRP). Results showed modest increases in IL-6 and IL-1β and marginally significant increases in CRP from before to after acute psychological challenge. In addition, the authors identified 13 studies that measured stimulated production of inflammatory mediators, a functional measure of the ability of immune cells to produce inflammatory cytokines. Here, results showed increased stimulated concentrations of IL-1β from pre- to post-acute laboratory stress, but no significant stress-related change in IL-6 or TNF-α. At the time of the 2007 meta-analysis it was not possible to reliably characterize time course of acute stress-induced inflammatory reactivity; for this reason, the researchers collapsed post-stress measures over time to calculate the mean effect. Although the authors reflected on the possibility that demographic characteristics, health status, and psychosocial factors may moderate magnitude of stress-related changes in inflammatory mediators, there were insufficient data to explore this possibility.
Since this meta-analysis was published, the body of work examining changes in circulating and stimulated concentrations of inflammatory mediators following acute psychological stress has steadily grown. The current project aimed to update and extend the earlier review by incorporating results from more recently published studies that examine a wider range of inflammatory markers. This larger literature also permitted a novel examination of the temporal dynamics of the circulating IL-6, TNF-α, and IL-1β response, as well as an examination of study and sample characteristics that may moderate magnitude of response. When possible, we also explored whether age, sex, type of stress task (social evaluative threat versus other stressor) and “health status” moderated inflammatory responses. For the purposes of this review, health was defined as absence of a diagnosed chronic physical or mental health condition.
Methods
Literature Search Strategy
The review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Moher et al., 2009). First, articles included in the most recent review of this literature by Steptoe et al. (2007) were obtained. To identify articles published since the earlier review, three researchers [NJ-H, KL, CW] conducted separate systematic searches using PubMed between February and May, 2015. The searches used combinations of the following key terms: social, laboratory, psychological, brief, Trier Social Stress Test, acute, stress, IL-1β, TNF-α, CRP, IFN-γ, IL-6, Fibrinogen, inflammation, and cytokines. In addition, the reference lists of all identified papers were scrutinized to identify other potentially relevant manuscripts.
Inclusion/Exclusion Criteria
Identified studies were screened by three authors [NJ-H, KL, CW] and selected forV inclusion using the following criteria: (1) peer-reviewed article published in an English language journal, (2) described changes in concentration of inflammatory mediators in response to an acute psychological laboratory stressor and evaluated at least one circulating or stimulated marker of inflammation in peripheral blood samples. Inclusion criteria matched those used by Steptoe et al (2007). Studies that examined the effects of acute physical exercise and/or local cytokine production at the site of wounds were excluded. Given a recent meta-analytic review of the effect of acute psychological stress on salivary inflammatory markers (Slavish, Graham-Engeland, Smyth, Engeland, 2015), the current review also excluded studies examining markers of inflammation in saliva. Consensus for inclusion/exclusion of articles was obtained through discussion among all authors.
Data abstraction and Study Coding
Articles were examined for independent studies, and descriptions of “unique samples” within these studies. “Unique samples” refers to subgroups of subjects examined within a study. For example, a number of studies presented data separately for subjects with a health condition and a healthy control group. Each of these groups was labelled as a “unique sample” within the study. Information gathered included age and sex of participants, stressor type, the length of time between insertion of the indwelling catheter and the baseline measurement, the length of time between the end of the task and post-task blood draws, and the dependent inflammatory measures. In addition, biological or psychological moderators of the acute inflammatory response were recorded. Sample groups were coded as unhealthy if they were described as having a chronic physical or mental health diagnosis. Sex was coded as percent male. Stressor type was coded as a “social threat” if it was specified that participants completed the Trier Social Stress Test (Kirschbaum et al., 1993) or a speech task that involved social evaluative threat. The stressor type was coded as “other” for all other tasks. For studies investigating stimulated cytokine responses, the type of stimulant was coded as “LPS” for lipopolysaccharide or “PHA” for phytohaemagglutinin.
Three authors [NJ-H, KL, CW] extracted mean values for individual markers of inflammation reported for each unique sample at each time point. When data could not be obtained from the original article, the corresponding author was contacted by email, and a follow-up email was sent after one month if there was no response. If the corresponding author did not reply, the first author or senior author was contacted by email as an alternative. If no data could be obtained, the study was not included in the analyses.
Study Selection
The initial search yielded 4,416 potentially relevant studies, 4,353 of which were excluded based on the title or abstract alone. For circulating markers of inflammation, 42 articles were subjected to full text review and a further 4 were excluded on the basis of the inclusion criteria. We were not able to obtain data for nine of the selected studies, resulting in a final sample of 33 studies (See Figure S1). For stimulated markers of inflammation, 21 articles were subjected to full text review and 6 were excluded due to insufficient data, leaving a final sample of 15 studies in the quantitative analysis (See Figure S2).
Calculation of Effect Sizes
Data were analyzed and aggregated using Comprehensive Meta-Analysis (CMA) software, version 2.0 (M. Borenstein, Rothstein, & Cohen, 2005). Effect sizes were calculated using standardized mean differences (Cohen’s d) derived from mean (+/− SD) difference in concentration of each inflammatory marker from pre- to post-stress. Standardized differences in means can be calculated using fixed or random-effects models. Given the variation in effect sizes and populations across the identified studies and in order to permit generalization of the results, we used a random-effects model in the calculation of aggregate effect sizes, subgroup analyses, and meta-regression. Random-effects models provide a more conservative estimate of the effect sizes than fixed-effects models. The sign of the effect was calculated so that positive effect sizes reflected an increase in the immune marker in response to psychological stress. Using Cohen’s criteria, effect sizes were interpreted as small (0.2), moderate (0.5) or large (0.8) (Cohen, 1988).
Heterogeneity among study effect sizes was assessed by calculating the Cochrane’s Q test and I2. The Q statistic provides an estimate of whether variability across studies is sufficiently large to reject the hypothesis that they are from the same population. P < .10 was considered significant. I2 represents the percentage of variability in the effect estimate that is due to true, study-level differences rather than random sampling error (M. H. Borenstein et al., 2009). As recommended by Higgins (Higgins, Thompson, Deeks, & Altman, 2003), I2 < 25% was interpreted as low, 50% as moderate, and 75% as high heterogeneity. Low levels of I2 reflect homogeneity, indicating that the dispersion of effect sizes around their mean is not greater than what would be expected from sampling error alone. Subgroup analyses based on sample or task characteristics were only conducted if there was evidence of significant heterogeneity.
When indicated, subgroup moderation analyses were conducted to examine if effect sizes differed by type of stressor (“social threat” versus “other”) or whether the population was “healthy” or “unhealthy.” These analyses compared effect sizes across subgroups using the between-group heterogeneity (QB) test, which provides an estimate of the between-groups variance. Effects were aggregated according to the categories within the moderator variable of interest. Continuous moderators (age and percentage of each sample that was male) were evaluated using meta-regression.
Publication bias was examined by computing a fail-safe N (the number of studies with a null result that would be required to eliminate the significant effect) for each of the aggregated effect sizes. In addition, funnel plots were constructed. A funnel plot displays the effect size on the X-axis and the standard error for each study on the Y-axis. In the absence of publication bias, the scatter is attributable to sampling variation and the plot should resemble a symmetrical inverted funnel. Possible bias is represented by an asymmetrical distribution with an overrepresentation of positive results in the literature.
Results
The studies included in the meta-analyses are summarized in Table S1 (circulating markers) and Table S2 (stimulated markers). Among articles that examined circulating markers of inflammation, 33 unique studies met inclusion/exclusion criteria (Figure S1; Table S1). Of these, 24 studies recruited only healthy samples and 9 studies recruited samples with rheumatoid arthritis, psoriasis, multiple sclerosis, coronary artery disease, major depressive disorder, bipolar disorder or a past history of breast cancer, which were coded as unhealthy. From these studies, a total of 35 unique healthy samples and 11 unique unhealthy samples were used in the quantitative analysis of effect size. The average age of participants in these groups was 48.7 years, although the range in age across studies was substantial (17 – 82 years). On average, males comprised 45% of the 44 unique samples that recorded sex. Overall, 20 studies (26 unique samples) exposed participants to a social evaluative stressor and 13 (20 unique samples) to a different laboratory task, including the Stroop task, mirror tracing, mental arithmetic, auditory learning and recall tasks, anger and frustration recall, and capsaicin injection.
Among studies reporting on stimulated markers of inflammation, 14 articles met inclusion/exclusion criteria, describing 15 unique studies that were included in the meta-analysis (Figure S2; Table S1). Among these, 8 studies reported on healthy samples only, and 7 included samples that were coded as unhealthy, including breast cancer survivors and participants with breast cancer, rheumatoid arthritis, psoriasis, multiple sclerosis, depression, chronic fatigue syndrome, or systemic lupus erythematosus. From these studies, a total of 17 unique healthy samples and 9 unique unhealthy samples were used in the quantitative analysis. The average age of participants was 41.3 years, although the range in age across studies was substantial (18 – 71 years). On average, males comprised 30.08% of the 26 unique samples that were examined. Overall, 13 studies exposed participants to a social evaluative stressor, while 2 studies used different laboratory tasks, a mock job interview plus difficult puzzle task and a mental arithmetic task.
Identified studies measured a wide range of circulating (IL-1, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IFN-γ, TNF-α, Fibrinogen, and CRP) and stimulated (IL-1, IL-2, IL-4, IL-6, IL-6R, IL-10, TNF-α, and IFN-γ) markers of inflammation. However, in many cases, there were too few replications to meaningfully combine findings by meta-analysis. When markers were assessed in 4 or fewer unique samples, we provide only a qualitative description of results. We were able to calculate mean effects for 8 circulating (IL-6, TNF-α, CRP, IL-1β, IL-1ra, IFN-γ, IL-2, & IL-10) and 7 stimulated (IL-6, TNFα, IL-1β, IFNγ, IL-10, IL-2, IL-4) measures. IL-6 and TNF-α were the most commonly assessed cytokines. Concentrations of cytokines were measured at various times following acute laboratory stress, ranging from immediately following the task to 2 hours later. A majority of studies reported the length of time between insertion of the indwelling catheter and the baseline measurement to be 30 mins (19 studies for circulating, 8 for stimulated), with other common times falling between 10 and 25 mins (10 studies for circulating, 4 for stimulated), and the remaining studies reporting times greater than 30 mins (4 studies for circulating, 3 for stimulated).
Circulating Inflammatory Markers
IL-6
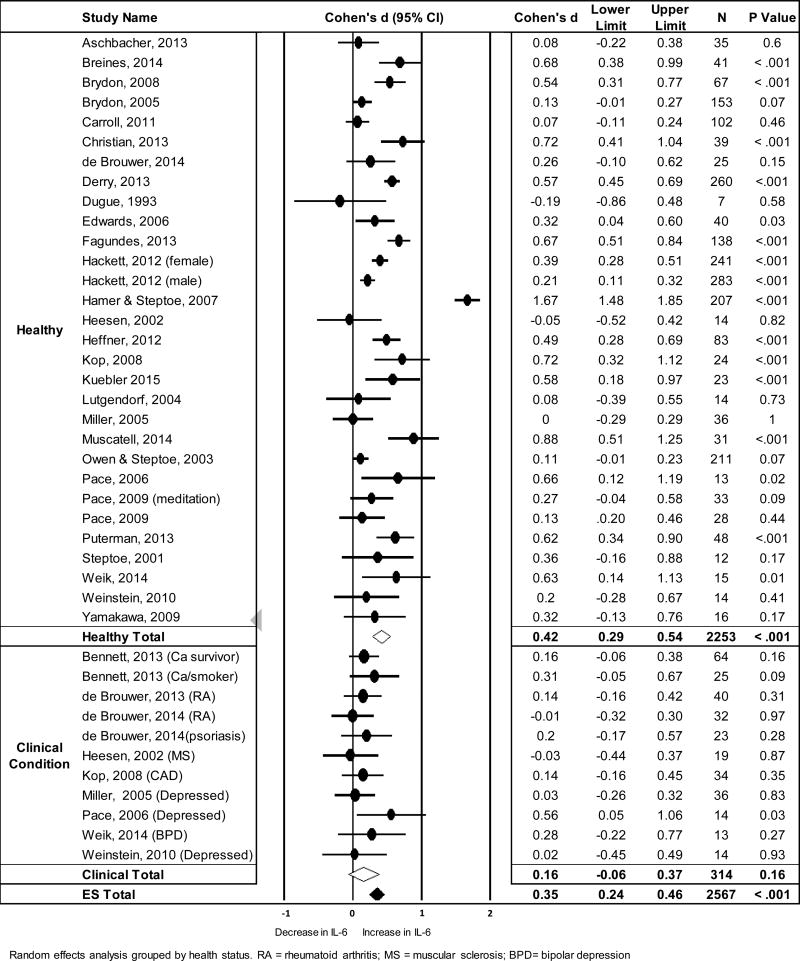
IL-6 was the most frequently measured circulating marker of inflammation and the analysis represented data from 2567 subjects within 41 unique samples taken from 29 studies. There was no change in IL-6 from pre- to 0–10 mins post-stress, but an increase when evaluated across 11–120 mins following acute stress (See Table 1). Figure 1 shows a forest plot of effect sizes, confidence intervals, and sample sizes for mean changes in IL-6 from pre- to 11–120 mins post stress grouped by health status, as well as the pooled effect estimates. Significant heterogeneity was observed across samples, with the I2 suggesting that 87.91% of the variation in IL-6 response was due to true study-level differences in effect size rather than sampling error alone (Table 1).
Table 1.
Summary of results of meta-analyses examining circulating concentrations of immune mediators
|
|
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 – 10min
|
all other collapsed time points
|
||||||||||||
| Cytokine | n | da [95% CI] | Z | Q(df) | I2 | τ | n | TPb | da[95 % CI] | I2 | Q(df) | I2 | τ |
|
|
|
|
|||||||||||
| IL-6 | 14 | 0.07 [−0.001, 0.14] | 1.93 | 14.68(13) | 11.45 | 0.045 | 41 | 11–120min | 0.35 [0.24, 0.46] | 6.14** | 330.81(40)** | 87.91 | 0.3 |
| IL-1β | — | — | — | — | — | — | 8 | 20–120min | 0.66 [0.21, 1.12] | 2.84* | 80.44(7)** | 91.30 | 0.62 |
| IL-1ra | — | — | — | — | — | — | 4 | 20–120min | 0.46 [−0.07, 0.98] | 1.71τ | 40.67 (3)** | 92.62 | 0.5 |
| TNF-α | 7 | 0.37 [−0.19, 0.92] | 1.3 | 71.87(6)** | 91.65 | 0.71 | 17 | 11–120min | 0.28 [0.04, 0.52] | 2.38** | 149.68(16)** | 89.31 | 0.44 |
| IFN-γ | — | — | — | — | — | — | 4 | 20–90min | 0.20 [−0.63, 1.03] | 0.47 | 57.81(3)** | 94.81 | 0.83 |
| CRP | 7 | 0.21 [−0.21, 0.62] | 0.99 | 66.67(6)** | 91.00 | 0.53 | 8 | 20–120min | 0.04 [−0.10, 0.17] | 0.53 | 0.50 (7) | 0 | 0 |
| IL-2 | — | — | — | — | — | — | 7 | 20–90min | 0.22 [−0.33, 0.77] | 0.77 | 65.16(6)** | 90.79 | 0.66 |
| IL-10 | — | — | — | — | — | — | 6 | 20–120min | 0.69 [0.03, 1.35] | 2.05* | 98.58(5)** | 94.93 | 0.8 |
|
|
|
|
|||||||||||
Notes: Includes both healthy and unhealthy samples;
indicates effect size as calculated by standardized mean difference;
time points included in the analysis
p < .05;
p < .001,
p = .09
Figure 1.
Forest plot showing standardized mean differences (Cohen’s d) and the pooled effect estimate for changes in circulating IL-6 from pre- to average concentration between 11–120 mins post stress
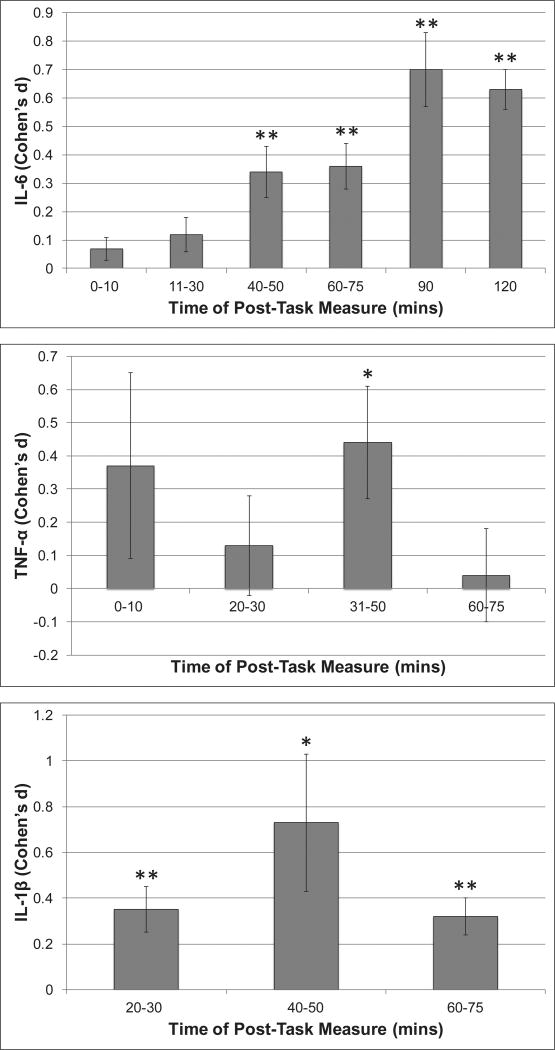
Next, we examined whether magnitude of circulating IL-6 reactivity varied as a function of time following the stressor (See Figure 2). From 11 – 120 mins post-stressor the magnitude of stress-related increases in IL-6 was significant at all times, with effect sizes ranging from small at 11–30 and 40–50 mins post stress (N = 22, d = 0.12, CI [−0.001, 0.24], p = .05; N = 20, d = 0.34, CI [0.16, 0.51], p < .001, respectively) to moderate/large at 90 and 120 mins post-stress (N = 8, d = 0.7, CI [0.45, 0.95], p < .001; N = 8, d = 0.63, CI [0.49, 0.76], p < .001, respectively). At all times, significant heterogeneity was observed (Q’s = 15.43–281.64, p’s = .03 to < .001).
Figure 2.
Standardized Mean difference (Cohen’s d) in Circulating Concentrations of IL-6, TNF-α and IL-β from pre- to various times post-acute stress (* p < .05; ** p < .005)
Next, we examined the possibility that the effect size varied as a function of health status. When averaged across measures taken 11–120 mins post stress, healthy samples showed a significant increase in IL-6 from pre-to post-stress (d = .42, CI [0.29, 0.54], p < .001), whereas the effect for unhealthy samples was not significant (d = .16, CI [−0.06, 0.37], p = .16). Mixed-effects moderation analyses confirmed a larger mean effect among “healthy” than “unhealthy” samples (QB = 4.01, p = .045). Contrary to expectations, the assumption of homogeneity was met for the “unhealthy” group (Q (df) = 6.29 (10), p = .71; I2 = 0), but not for the “healthy” group (Q (df) = 302.51 (29), p < .001).
We also assessed whether IL-6 reactivity varied as a function of task type (social threat vs. other stressor). When averaged across 11–120 mins post stress, a significant increase in IL-6 was observed in response to both social threat (N = 27, d = 0.34, CI [0.25, 0.44], p < .001) and other types of stress task (N = 13, d = 0.25, CI [0.12, 0.39], p < .001), with no between group difference in the magnitude of this effect (QB = 1.15, p = 0.28).
Exploratory analyses were conducted to see if IL-6 reactivity varied as a function of assay methods. The majority of studies employed R&D Systems ELISA kits to assess IL-6 (N = 18), with the remaining 12 studies using a variety of methods (See Table S3). Magnitude of change in IL-6 across 11–120 mins post stress did not differ significantly between these groups.
Finally, we employed meta-regression to evaluate the association of magnitude of IL-6 response with age and percentage of the sample that was male. These analyses revealed no significant relationships of age or percentage male with mean stress-related increase in IL-6 (Q = 0.48, p =.49 and Q = 0.11, p = .74, respectively; see Figures S4 and S5). Among the 14 studies that examined female only samples, a significant stress-related increase in IL-6 was observed (d = 0.34, CI [0.20, 0.48], p < .001.
TNF-α
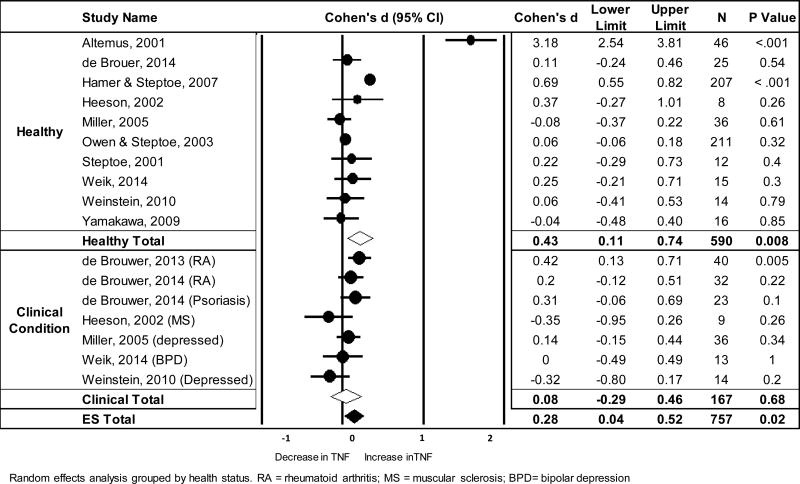
The meta-analysis of stress-related changes in circulating TNF-α included 17 unique samples from 11 studies (total = 757 subjects). Results showed no significant change in TNF-α from pre- to 0–10 mins post-stress, but a small increase when averaged across 20–120 mins post-stress (see Table 1). Figure 3 shows a forest plot of the pre to 20–120min post stress effect sizes and the pooled effect estimate grouped by health status. Significant heterogeneity was observed across trials (see Table 1), with 89% of the total variation in TNF-α response estimated to be due to true variability between study effect sizes rather than sampling error alone.
Figure 3.
Forest plot of standardized mean differences (Cohen’s d) and the pooled effect estimate for changes in circulating TNF-α from pre- to average concentrations between 20–120 mins post stress
The magnitude of the TNF-α effect varied as a function of time following the stressor (see Figure 2). No significant change in TNF-α concentration was observed until 31–50 mins post-stress when a moderate increase was seen (N = 10; d = .44 CI [0.10, 0.78], p =.01). This effect was no longer significant at 60–75mins post-stress (N = 7; d = .04, p = .78). At all times significant heterogeneity was observed (Q’s = 19.39–139.39, p’s = .004 to < .001).
Ten TNF-α samples were classified as “healthy” and 7 as “unhealthy.” The magnitude of effect did not vary as a function of health status when assessed from pre- to 20–120 mins post-task (QB = 1.93, p = .16) or from pre- to 31–50 mins post-task (QB = .97, p = .33). Out of the 17 samples that assessed TNF-α, 12 employed a social threat task and 5 a different stressor. When averaged across measures taken 20–120 mins post-stressor, a significant increase in TNF-α was observed in response to social threat (d = 0.34, CI [0.04, 0.64], p = .026), but not other types of stress (d = 0.16, CI [−0.29, 0.61], p = .47). However, mixed-effects moderation analyses showed no significant between group differences in the magnitude of this effect (QB = 0.40, p = .53). These results suggest that type of stressor did not significantly influence TNF-α response. Results of meta-regression showed no significant moderation of mean effect size by age (Q (df) = 1.13 (1), p = .29) or sex (Q (df) = 2.27 (1), p = .13).
CRP
Six studies, including results from 9 unique samples and 266 participants measured changes in circulating CRP in response to stress. There was no evidence for a significant change in CRP from pre- to 0–10, 20–30 (N = 8, d = .04, p = .89), or 20–120 mins post stress (See Table 1). Although there was evidence for heterogeneity of effects measured 0–10 mins after stress, effect sizes were homogeneous when assessed across 20–120 mins after stress. There were too few studies to systematically examine health status, type of stress, or demographic factors.
IL-1β
Six studies, including results from 8 unique samples and a total of 229 subjects, assessed circulating IL-1β reactivity. Only 1 study reported cytokine concentrations at 0–10 mins post-stressor. When averaged across 20–120 mins post-stress, an increase in IL-1β concentration was observed, with significant heterogeneity between groups (See Table 1). Significant increases in IL-1β were observed from pre- to 20–30 (N = 5, d = .35, p < .001), 40–50 (N = 6, d = .73, p = .02) and 60–75 (N = 5, d = .32, p < .001) mins post-stress (see Figure 2). There was no significant difference in the magnitude of the effect between “healthy (N = 5)” and “unhealthy (N = 3)” samples (QB = .74, p = .39). All but one study employed a social threat task and there was insufficient data to reliably assess moderation by age or sex.
IL-2
Seven unique samples from 4 studies (total N = 164) examined IL-2 reactivity. When averaged across samples collected 20–120 mins following stress, there was no significant stress-related change in IL-2 concentration (see Table 1). However, there was an increase in IL-2 from pre- to 40–50 mins post-stress (N = 6, d = .40, p < .001), with a similar trend at 20–30 (N = 5, d = .24, p = .15) and 80–90 (N = 5, d = .25, p = .09) mins following stress. Effect sizes were homogeneous at 40–50 mins following stress (Q (df) = 1.19 (5), p = .95), but heterogeneous at other time points (Q = 16.26 (4), p = .003 at 20–30mins and Q = 12.88 (4), p = .01 at 80–90 mins).
IL-10
Four studies representing 6 unique samples and a total of 191 subjects assessed circulating concentration of IL-10. Results showed a significant increase in circulating IL-10 from pre- to 20–120 mins post-stressor, with significant heterogeneity in magnitude of effect (See Table 1). Significant increases in IL-10 were observed from pre- to 20–30 (N = 4, d = .18, p = .03) and 40–50 (N = 5, d = .24, p = .003) min post-task, with a similar tendency at 80–90 mins post-task (N = 5, d = .11, p =.14). There were too few studies to examine health status and all 4 studies employed a social threat task.
IFN-γ
Three studies (5 unique samples including a total of 136 subjects) examined stress-related changes in circulating IFN-γ. There was no evidence for a significant change in circulating concentration of this cytokine in response to acute stress (See Table 1).
Cytokines Assessed in Fewer than 5 Independent Samples
A number of cytokines were assessed in too few studies to reliably determine mean effect sizes. Preliminary standardized mean differences from pre- to post-stress were calculated to inform future research. Findings showed a possible stress-related increase in IL-4 (2 studies; N = 4 unique samples; d = 0.23, p = .04) and decrease in IL-8 (2 studies; N = 4 unique samples; d = −1.2, p = .03) and IL-12 (N = 1, d = −.71, p = .004). There was no preliminary support (p’s >.10) for stress-related changes in IL-1ra (N = 4; d = .46), IL-5 (N = 4, d = .15), IL-7 (N = 4, d = .03), IL-6r (N = 2, d = −.08), or fibrinogen (N = 1, d = .10).
Biological and Psychological Moderators of Circulating Inflammatory Response
There were too few studies to systematically review individual moderators of the magnitude of stress-related changes in circulating inflammatory mediators. Results of studies that examine moderators of stress reactivity are described in Table S4. In general, biobehavioral (e.g., smoking, lower physical fitness, obesity, poor sleep), demographic (African American race, lower socioeconomic status), and psychosocial (e.g., negative affective responses to the task, loneliness and high effort-reward imbalance) health risk factors associated with larger stress-induced increases in circulating markers of inflammation. Increased circulating inflammatory mediators also accompanied lower cortisol (Kunz-Ebrechet et al., 2003) and higher norepinephrine reactivity (Kop et al., 2008). In contrast, psychosocial factors that are widely considered health-protective (e.g., positive affect, social support, self-compassion, compassionate meditation) tended to associate with less stress related change in inflammatory mediators.
Stimulated Inflammatory Markers
IL-6
IL-6 was the most frequent stimulated measure of inflammation, with 24 unique samples taken from 11 studies (724 subjects). IL-6 production was stimulated with LPS in 20 samples and with PHA in the remaining 4 samples. Results showed no mean change in LPS-stimulated IL-6 production from pre- to 0–10 mins post-stress (See Table 2). However, there was a tendency for LPS-stimulated IL-6 production to increase when averaged across 15–120 mins after stress (N = 20, d = .23, p = .06), with significant heterogeneity between samples (See Table 2). Preliminary evidence showed a larger effect for samples stimulated with LPS than PHA (QB = 4.14, p = .04), but this should be interpreted with caution because only 3 samples measured PHA-stimulated samples beyond 10 mins after stress. For samples stimulated with LPS, the largest effects were observed for measures taken 11–30 mins after stress (N = 11; d = .44, CI [−0.02 to 0.90], p = .06; Q (df) = 125.8 (10), p < .001). Measures taken 40–90 mins after stress showed no significant difference from pre-stress concentration (N = 10, d = −0.02, CI [−0.28, .0.24], p = .89). The magnitude of effect size was similar for “healthy (N = 15)” and “unhealthy (N = 9)” samples, regardless of assessment time (QB = 1.11, p = 0.29). Effect sizes were also similar in response to social threat (N = 18) and other (N = 6) stressors (QB = 1.13, p = 0.29). Only 29% of the total stimulated IL-6 sample was male and there was insufficient data to reliably assess moderation by age or sex.
Table 2.
Summary of results of meta-analyses examining stimulated concentrations of immune mediators
|
|
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 – 10min
|
all other collapsed time points
|
||||||||||||
| Cytokine (stimulant) |
n | da [95% CI] | Z | Q(df) | I2 | τ | n | TPb | da [95% CI] | Z | Q(df) | I2 | τ |
|
|
|
|
|||||||||||
| IL-6 (LPS) | 13 | 0.062 [−0.18, 0.30] | 0.50 | 63.97(12)** | 81.24 | 0.39 | 20 | 15–120min | 0.23 [−0.01, 0.47] | 1.87τ | 171.01 (19)** | 88.89 | 0.49 |
| IL-6 (PHA) | — | — | — | — | — | — | 3 | 60–75min | −0.53 [ −1.23, 0 . 16] | −1.51 | 1.81(2) | 0 | 0 |
| IL-1β (LPS) | 4 | 0.64 [−0.12, 1.40] | 1.65 | 45.4(3)** | 93.39 | 0.75 | 7 | 20–120min | 1.09 [0.42, 1.75] | 3.20** | 116.06 (6)** | 94.83 | 0.85 |
| TNF-α (LPS) | 10 | 0.02 [−0.35, 0.39] | 0.10 | 100.93(9)** | 91.08 | 0.56 | 14 | 20–120min | 0.16 [−0.17, 0.49] | 0.96 | 198.27 (13)** | 93.44 | 0.58 |
| IFN-γ (PHA) | 5 | 0.47 [0.24, 0.69] | 4.06** | 8.41(4) | 52.43 | 0.19 | 4 | 20–75min | −0.12 [−0.49, 0.26] | −0.59 | 12.83 (3)* | 76.62 | 0.34 |
| IL-2 (PHA) | 3 | −0.38 [−0.91, 0.41] | −1.42 | 14.72(2)** | 86.42 | 0.43 | 5 | 20–75min | 0.17 [−0.16, 0.50] | 1.02 | 7.90 (4) | 49.36 | 0.26 |
| IL-4 (PHA) | 5 | −0.42 [−0.79, −0.05] | −2.20* | 22.91(4)** | 82.54 | 0.38 | 4 | 20–75min | −0.10 [−0.28, 0.08] | −1.11 | 0.55 (3) | 0 | 0 |
| IL-10 (LPS) | — | — | — | — | — | — | 3 | 60–75 min | 0.01[−0.37, 0.39] | 0.06 | 3.25 (2) | 38.46 | 0.26 |
| IL-10 (PHA) | 3 | −0.82 [−1.85, 0.21] | −1.56 | 41.54(2)** | 95.19 | 0.88 | — | — | — | — | — | — | — |
|
|
|
|
|||||||||||
Notes: both healthy and unhealthy samples included;
indicates effect size as calculated by standardized mean difference;
time points included in the analysis
p < .05;
p < .001;
p=06
TNF-α
Nine studies examined stress-related changes in stimulated TNF-α. Mean effect sizes were calculated for the 8 studies that employed LPS (14 unique samples; 611 total subjects). Of the 14 samples, 8 examined “healthy” and 6 “unhealthy” samples, and 12 employed a social threat stressor. Results revealed a significant increase in stimulated TNF-α from pre- to 20–30 mins post stress (N = 8; d = 0.57, CI [0.08, 1.06], p = 0.02), with significant heterogeneity between samples (Q (df) = 116.8 (8), p < .001; τ = 0.72; I2 = 93). Effects were similar for “healthy” and “unhealthy” samples (QB = 2.22, p = .14). There were no significant effects of acute stress on stimulated TNF-α when assessed 0–10, 40–75 (N = 8; d = −.24, CI [0.17, −0.05], p = 0.16), or across 20–120 mins post-stress (See Table 2). There was insufficient data to reliably examine moderation by sex or age.
IL-1β
Seven unique samples (3 healthy and 4 unhealthy) from 4 studies (281 subjects) examined LPS-stimulated production of IL-1β in response social threat stress. Results showed a significant increase in IL-1β production from pre to 20–50 mins post social threat (N = 7; d = 1.04, CI [0.34, 1.70], p = .002), with significant heterogeneity between samples (Q (df) = 117.2 (6), p < .001, τ = 0.85, I2 = 94.9). A significant mean effect was also observed when measures were averaged across 20–120 mins following stress (See Table 2).
IFN-γ
Three studies with 5 unique samples (3 healthy and 2 unhealthy) and a total of 153 subjects examined PHA-stimulated production of IFN-γ in response to social threat stress. Here, results revealed a significant mean increase in IFN-γ from pre- to 0–10 min, with homogeneous effect sizes (Table 2). There was no evidence of a stress-related change in stimulated IFN-γ at later time points or when averaged across 20–75 mins after stress (Table 2).
IL-4
The IL-4 analysis included 153 subjects from 3 studies (5 unique samples) All used PHA and employed a social threat stressor. Results revealed a significant decrease in IL-4 production from pre-to 0–10 mins post stress, with significant heterogeneity across samples (See Table 2). Preliminary results from the 4 samples that examined IL-4 production at later time points were not significant (See Table 2).
IL-2
Three studies (6 unique samples) including 124 subjects examined changes in PHA-stimulated IL-2 production in response to social threat stress. Results showed no significant stress-related change in IL-2 (See Table 2).
IL-10
Three studies (6 unique samples; 127 subjects) assessed changes in stimulated IL-10 in response to social evaluative threat. Preliminary findings showed no significant stress-related change in LPS or PHA stimulated IL-10 production (See Table 2).
Biological and Psychological Moderators of Stimulated Inflammatory Response
Results of studies that examined moderators of stimulated inflammatory response are described in Table S5. Few moderators were examined more than once. The majority of these studies show an association of psychosocial/biological health risk factors (e.g., postmenopausal status, BMI, loneliness, negative affect, stress appraisal, fatigue) with increased stimulated production of inflammatory cytokines in response to acute laboratory challenge.
Testing for Publication Bias
Figure S5 displays funnel plots for a subset of cytokines that were significantly changed by acute psychological stress. The Egger’s regression intercepts shown in Table S6 reflect the degree to which the intercept deviates from 0, with larger numbers reflecting more pronounced asymmetry. If the p-value of the intercept is < 0.1 then publication bias is possible. Results suggest potential publication bias for studies examining changes in concentration of circulating IL-10 and IL-1β and stimulated concentration of IL-1β, TNF-α, and IL-4.
Discussion
Summary of Changes in Circulating Concentration of Inflammatory Mediators
The current review partially confirms and extends findings from an earlier meta-analysis of studies examining changes in inflammatory mediators in response to acute laboratory stress (Steptoe et al., 2007). The 2007 review assessed 18 studies that measured circulating concentrations of IL-6, IL-1β, TNF-α, and CRP; here, we expand this review to 33 studies (11 included in both reviews) and a wider range of inflammatory markers (IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IFN-γ, TNF-α, Fibrinogen, and CRP). Consistent with earlier findings, we found robust stress-related increases in IL-6 and IL-1β of moderate to large effect size, although the IL-1β findings should be interpreted with caution due to risk of publication bias. The current review also revealed small to moderate increases in TNF-α, IL-2 and IL-10 following acute challenge, although publication bias was a concern for IL-10. We did not confirm the marginally significant increase in CRP that was observed by Steptoe et al (2007), with no current evidence of stress-related change in this marker. We also found no evidence of stress-related increase in IL-1ra or IFN-γ. Although there were too few studies for findings to be considered reliable, initial evidence suggests an increase in IL-4, and a decrease in IL-8 and IL-12 following stress.
Dynamics of the observed responses differed by cytokine, with no significant increases in circulating IL-6, TNF-α, IL-1β, IL-2 or IL-10 within the first 10 mins, but increases in all of these cytokines evident by 40–50 mins after stress. For IL-6, the response was of greater magnitude and more prolonged, with moderate to large increases seen at 90 and 120 mins following stress. It is possible these different response patterns reflect the half-lives of the respective cytokines or differences in clearance mechanisms (Oliver et al., 1993). Research following subjects for more than 2 hours is needed to determine when peak and recovery occur for IL-6.
Considerable heterogeneity of effect sizes across studies was observed on analysis of all circulating cytokines. This variance may relate to the different populations that were examined across studies. In this regard, consistent with the findings of Steptoe et al (2007), we observed larger stress-related increases in circulating IL-6 among healthy samples than among varied clinical populations. However, no differences in the magnitude of effects between healthy and clinical groups were observed on analysis of the other cytokines. Effect sizes were also similar when responses to social evaluative threat were compared with responses to other psychological challenges. Additionally, there were no systematic differences in magnitude of increase in IL-6 or TNF-α as a function of sex or age of the participants. Finally, results of individual studies suggest that physical and psychosocial factors may moderate magnitude of cytokine responses to stress, with biobehavioral and psychosocial health risk factors associating with larger stress-induced increases in circulating markers of inflammation. Future work should continue to explore individual differences that may account for heterogeneity in effect sizes across studies.
Summary of Changes in Stimulated Production of Inflammatory Mediators
The current review included results from 15 studies that examined inflammatory cytokine production by white blood cells stimulated in vitro, 8 of which were included in the Steptoe et al (2007) review. Recent studies examined a broader range of cytokines than the earlier review, permitting an examination of acute stress-related changes in stimulated production of IL-6, TNFα, IL-1β, IFN-γ, IL-10, IL-2, IL-4. The most commonly measured cytokines were IL-6 and TNF-α. In contrast to earlier findings (Steptoe et al., 2007), the current review revealed a moderate increase in both stimulated IL-6 and TNF-α production that peaked between 10 and 30 mins after acute stress. This effect was larger in response to LPS than PHA. Consistent with Steptoe et al (2007), we observed a robust increase in LPS-stimulated production of IL-1β across 20 to 50 mins following stress. We also extended earlier findings to show an increase in PHA-stimulated production of IFN-γ and a decrease in PHA-stimulated production of IL-4 in the first 10 mins following stress. Findings did not support stress-related changes in IL-2 or IL-10 production. We found no evidence that magnitude of any of the observed effects differed as a function of health status of the sample or the type of stress task. Similar to the circulating results, there was considerable heterogeneity of effect sizes across studies for all of the stimulated cytokine measures. There was also evidence of possible publication bias for stimulated TNF-α, IL-1β and IL-4, raising concerns regarding whether the published literature accurately reflects the full range of findings.
Mechanisms of change in inflammatory mediators in response to acute stress
Results of this review show increased circulating and stimulated concentration of proinflammatory cytokines (IL-6, IL-1β, TNF-α) following exposure to brief laboratory stress. Interestingly, stimulated responses were observed earlier than circulating responses, raising the possibility that stress-related increases in cytokine production by immune cells contributes to increased circulating concentration. The primary immune cells responsible for production of these pro-inflammatory cytokines are monocytes/macrophages. During infection, microbial products (e.g. LPS) bind to toll-like receptors (TLRs) on these cells promoting the release of TNF-α and IL-1β, which, in turn, stimulate the release of IL-6 (Akira & Takeda, 2004; Mosser & Edwards, 2008). These cytokines mediate local and systemic inflammatory responses (Dantzer et al., 1998).
A diverse set of regulatory mechanisms function to constrain inflammation both locally and systemically. Locally, cytokines are regulated by their short half-lives (range 1–4 hours), the presence of receptor antagonists, and the production of anti-inflammatory cytokines (e.g. IL-10 and IL-4; Dinarello, 2000; Roitt & Delves, 2001). Systemically, IL-6 activates the hypothalamic-pituitary-adrenal (HPA) axis leading to the release of cortisol (Papanicolau, Wilder, Manolagas, Chrousos, 1998), which functions via glucocorticoid receptors on immune cells to inhibit production of proinflammatory cytokines and downregulate inflammation (Miller et al., 2002; Eisenberger & Cole, 2012).
Acute psychological stress activates biological pathways that contribute to the regulation of IL-6, TNF-α and IL-1β production by immune cells (Sanders & Kavaleers, 2007; Eisenberger & Cole, 2012). These pathways include the sympathetic nervous system (SNS), which activates immediately following stress exposure, and the HPA axis, which activates more slowly taking 20–30 mins from stressor onset for cortisol to reach peak levels and an hour or more to return to pre-stress levels (Dickerson & Kemeny, 2004; Sapolsky, Romero, Munck, 2000). It is proposed that these pathways serve an adaptive role, functioning together to regulate peripheral inflammatory responses and promote survival of the organism (Dhabhar, 2009; Dhabhar, 2014; Sapolsky et al., 2000). In this regard, activation of the SNS is associated with release of catecholamines that activate nuclear factor κB (NF-κB), a transcription factor that triggers expression of proinflammatory genes and production of IL-6, TNF-α, and IL-1β by immune cells (Bierhaus et al., 2003). This proinflammatory response may have evolved to protect the organism from immediate injury or infection (Dhabhar & McEwen, 1997; Segerstom & Miller, 2004). However, there are metabolic and health costs to prolonged activation of inflammatory processes (Dhabhar, 2014; Sapolsky et al., 2000). Thus, it is proposed that the delayed cortisol response evolved to shut down the inflammatory response, with glucocorticoid receptors functioning to down-regulate NFκB activity and decrease cytokine production (Lieberman, 2007).
In support of this stress response system, rodent and in vitro findings show that sympathoadrenal activation drives stress-related increases in circulating markers of inflammation (Bierhaus et al., 2003; Johnson et al., 2005; Szabo et al., 1997). Studies in humans show similar results with activation of the SNS in response to acute stress predicting increased expression of proinflammatory genes and circulating concentrations of inflammatory cytokines (Brydon et al., 2005; Kop et al., 2008). It is also possible that stress-related decreases in activation of the parasympathetic nervous system (PNS) contribute to increased inflammation. The PNS acts through acetylcholine receptors in immune cells to down-regulate pro-inflammatory cytokine production (Borovikova et al., 2000; Marsland, Gianaros, Prather, Manuck, 2007) and circulating markers of inflammation (Sloan et al., 2007; Pavlov & Tracey, 2005). Interestingly, the magnitude of cortisol and sympathetic responses to acute stress are positively related (Cacioppo et al., 1998; Cohen et al., 2000), supporting the possibility that they are part of a coordinated control mechanism. Stress-induced increases in cortisol associate with decreased activation of NF-kB (Wolf, Rohleder, Bierhaus, Nawroth, & Kirschbaum, 2009) and attenuated increases in circulating concentration of IL-6 and IL-1ra (Kunz-Ebrecht et al, 2003). Thus, the HPA axis may contribute to the down-regulation of the inflammatory response to stress.
It is also possible that acute stress-related increases in circulating concentration of cytokines derive from sources other than increased transcription of pro-inflammatory genes. An older literature shows changes in the numbers of circulating immune cell subtypes in response to acute stress (Dhabhar & McEwen, 1997; Dhabhar, Miller, McEwen, & Spencer, 1995; Manuck et al., 1991). This redistribution of cell populations is driven by sympathoadrenal activation (Manuck et al., 1991; Bachen et al., 1992), possibly via changes in expression of adhesion molecules on the surface of lymphocytes, leading to their release from the marginal pools of blood vessels into general circulation (Goebel & Mills, 2000). Stress-related changes in circulating cell subtypes favors an increase in cells, such as natural killer cells, that may contribute to pro-inflammatory cytokine production.
Clinical Implications
Individuals vary markedly in the magnitude of change in inflammatory mediators in response to acute stress and there is some evidence that this variability is relatively stable across time (Von Kanel 2006; Breines et al., 2014; Lockwood, John-Henderson, Marsland, 2016), and may even increase in magnitude in response to repeat testing (Rohleder, 2014; McInnis et al., 2015). Thus, it is conceivable that there is a meaningful distribution of differences in stress reactivity that may form a physiological basis for differences in susceptibility to disease. Individuals who mount larger increase in inflammatory mediators in response to the stresses of everyday life may be less susceptible to acute infections, but more vulnerable to chronic systemic inflammation and inflammatory disease, particularly when exposed to recurrent naturalistic stress.
It remains to be determined whether individuals who mount larger increases in inflammatory mediators in response to acute stress are protected from infection in the short-term. However, there is some evidence linking magnitude of reactivity to risk for chronic inflammatory disease. For example, larger increases in circulating IL-6 in response to laboratory stress predict systolic blood pressure 3 years later (Brydon & Steptoe, 2005). Similarly, acute stress-related increases in circulating TNF-α, but not IL-6, associate with increased future arterial stiffness (Ellins et al., 2008). In our work, we have also shown a positive association between magnitude of IL-6 reactivity to acute stress and circulating concentration of CRP among males (Lockwood et al., 2016). This raises the possibility that individuals who mount larger increases in inflammatory mediators in response to acute stress have higher tonic levels of systemic inflammation that are known to predict future risk for cardiovascular disease (Danesh et al, 2004; Hennekens, Buring, Rifai, 2000; Ridker, Rifai, Stampfer, Hennekens, 2000; Ridker et al., 2000). Taken together, these findings raise the possibility that stressor-evoked inflammatory mediator reactivity has implications for long-term health.
Limitations and Future Directions
Although the current meta-analysis included a larger body of literature than the review conducted by Steptoe et al. in 2007, recent studies were subject to many of the same limitations. As noted in the earlier review, few studies include a control condition and it remains possible that diurnal variation in cytokine concentrations (Opp et al., 2007) or local responses to the intravenous catheter (Haack, 2002) contribute to observed changes in circulating cytokine levels. However, results from studies that do include a control group suggest that these factors do not fully account for observed stress-related effects (Steptoe et al., 2007 Brydon et al., 2004; Kunz-Ebrecht et al., 2003). Another limitation of the literature involves failure to routinely consider the role of sex and age. Although we found no significant relationship of the percentage of the sample that was male or age with stress-related increases in circulating markers of inflammation, many of the studies were small, focused on only one sex, or included unequal numbers of males and females. This is a particular concern on analysis of stimulated markers of inflammation where only 30.08% of the sample was male. Thus, the findings should be interpreted with caution, especially given evidence in support of sex differences in magnitude of changes in inflammatory mediators (e.g., Edwards et al., 2006; Hackett et al., 2012; Lockwood et al., in press; Prather et al., 2009; Rohleder et al., 2003). Future work is needed to determine whether sex and age moderate changes in inflammatory mediators that accompany acute stress.
In regard to stimulated cytokine responses, studies varied considerably in the methods that were used, with differences in (a) how cells were prepared, using whole blood (e.g. Heesen 2005) versus isolated peripheral blood mononuclear cells (e.g., Buske-Kirshbaum 2007), (b) the concentration of stimulant that was used, with concentrations of LPS ranging from .0001 ug/mL (e.g. Bower et al., 2007) to 2.5ug/mL (e.g. Prather et al., 2009), and (c) the incubation time, ranging from 3 hours (e.g. Goebel et al., 2000) to 48 hours (e.g. Buske-Kirschbaum et al., 2007). It is likely that these differences contribute to observed heterogeneity of findings across studies. For example, length of incubation is likely to influence observed responses given the dynamics of cytokine responses to stimulation and the possible confound of secondary response to cell death among samples incubated for longer periods (De Groote et al., 1992). In the future, standardization of methods across studies is warranted to improve clarity of results.
To summarize, it is now well established that acute laboratory stress is associated with an increase in circulating and stimulated concentrations of pro-inflammatory mediators. It remains to be determined whether these responses generalize from laboratory assessment to immune measurement coincident with the naturally occurring stressors of daily life and whether individuals vary reliably and predictably in the magnitude of these effects. Longitudinal studies are needed to evaluate the clinical significance of these stress-induced changes. These studies should consider the possibility that increased concentrations of proinflammatory mediators are health protective in the short term, but may increase risk for chronic inflammatory diseases over time.
Supplementary Material
Highlights.
A meta-analysis of cytokine responses to acute psychological stress was conducted.
Results showed stress-related increases in circulating markers of inflammation.
Reliable increases in concentrations of IL6, IL1β, IL10 and TNFα were observed.
Stress effects on circulating cytokines peaked between 31 and 90 min post stress.
Stress also associated with increased stimulated production of IL1β, IL4 and IFNγ
Acknowledgments
This work was supported by National Institute of Health T32 grant HL07560 (NJ-H and CW) and National Science Foundation Graduate Research Fellowship Program DGE-1247842 (KL)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
*: Asterisk indicates studies that were included in meta-analysis.
- *.Ackerman KD, Martino M, Heyman R, Moyna NM, Rabin BS. Stressor-induced alteration of cytokine production in multiple sclerosis patients and controls. Psychosom. Med. 1998;60:484–491. doi: 10.1097/00006842-199807000-00016. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nature reviews: Immunology. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- *.Altemus M, Rao B, Dhabar FS, Ding W, Granstein RD. Stress indicued changes in skin barrier function in healthy women. J. Invest. Dermatol. 2001;117:309–317. doi: 10.1046/j.1523-1747.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- *.Aschbacher K, Epel E, Wolkowitz OM, Prather AA, Puterman E, Dhabar FS. Maintenance of a positive outlook during acute stress protects against pro-inflammatory reactivity and future depressive symptoms. Brain Behav. Immun. 2012;26:346–352. doi: 10.1016/j.bbi.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachen EA, Manuck SB, Marsland AL, Cohen S, Malkoff SB, Muldoon MF, Rabin BS. Lymphocyte subset and cellular immune responses to a brief experimental stressor. Psychosom. Med. 1992;54:673–679. doi: 10.1097/00006842-199211000-00007. [DOI] [PubMed] [Google Scholar]
- *.Bellingrath S, Rohleder N, Kudielka BM. Healthy working school teachers with high effort-reward imbalance and overcommitment show increased pro-inflammatory immune activity and a dampened innate immune defence. Brain Behav. Immun. 2010;24:1332–1339. doi: 10.1016/j.bbi.2010.06.011. [DOI] [PubMed] [Google Scholar]
- *.Bennett JM, Glaser R, Andridge RR, Peng J, Malarkey WB, Kiecolt-Glaser JK. Long lasting effects of smoking: Breast cancer survivors’ inflammatory responses to acute stress differ by smoking history. Psychoneuroendocrino. 2013;38(2):179–187. doi: 10.1016/j.psyneuen.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Nat. Acad. Sci. 2003;100(4):1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PG. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav. Immun. 2003;17:350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analnsts John Wiley & Sons. Ltd, Chichester, UK 2009 [Google Scholar]
- Borenstein M, Rothstein D, Cohen J. Comprehensive Meta- Analysis (Version 2) [Computer software] Englewood, NJ: Biostat; 2005. [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response. Nature. 2000;405(5785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- *.Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW. Inflammatory responses to psychological stress in fatigued breast cancer survivors: relationship to glucocorticoids. Brain Behav. Immun. 2007;21:251–258. doi: 10.1016/j.bbi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- *.Breines JG, Thoma M, Gianferante D, Hanlin L, Chen X, Rohleder N. Self-compassion as a predictor of interleukin-6 response to acute psychosocial stress. Brain Behav. Immun. 2014;37:109–114. doi: 10.1016/j.bbi.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Edwards S, Jia H, Mohamed-Ali V, Zachary I, Martin JF, Steptoe A. Psychological stress activates interleukin-1 gene expression in human mononuclear cells. Brain Behav. Immun. 2005;19:540–546. doi: 10.1016/j.bbi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- *.Brydon L, Steptoe A. Stress induced increases in interleukin-6 and fibrinogen predict ambulatory blood pressure at 3-year follow up. J. Hypertens. 2005;23(5):1001–1007. doi: 10.1097/01.hjh.0000166841.57474.d0. [DOI] [PubMed] [Google Scholar]
- *.Brydon L, Wright CE, O’Donnell K, Zachary I, Wardle J, Steptoe A. Stress-induced cytokine responses and central adiposity in young women. Int. J. Obesity. 2008;32:443–450. doi: 10.1038/sj.ijo.0803767. [DOI] [PubMed] [Google Scholar]
- *.Buske-Kirschbaum A, Kern S, Ebrecht M, Hellhammer DH. Altered distribution of leukocyte subsets and cytokine production in response to acute psychosocial stress in patients with psoriasis vulgaris. Brain Behav. Immun. 2007;21:92–99. doi: 10.1016/j.bbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Malarkey WB, Kiecolt-Glaser JK, Sheridan JF, Poehlmann KM, Burleson MH, Ernst JM, Hawkley LC, Glaser R. Autonomic, neuroendocrine, and immune responses to psychological stress: The reactivity hypothesis. Ann NY Acad. Sci. 1998;840:664–673. doi: 10.1111/j.1749-6632.1998.tb09605.x. [DOI] [PubMed] [Google Scholar]
- *.Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross Ross DC, Marsland AL. Brain Behav. Immun. 2011;25(2):232–238. doi: 10.1016/j.bbi.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertens. 2010;55(4):1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology. 2012;51(Suppl 5):3–11. doi: 10.1093/rheumatology/kes113. [DOI] [PubMed] [Google Scholar]
- Christian LM, Glaser R, Porter K, Iams JD. Stress-induced inflammatory responses in women: effects of race and pregnancy. Psychosom. Med. 2013;75(7):658–669. doi: 10.1097/PSY.0b013e31829bbc89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Cohen S, Hamrick N, Rodriguez MS, Feldman PJ, Rabin BS, Manuck SB. The stability of and intercorrelations among cardiovascular, immune, endocrine, and psychological reactivity. Ann. Behav. Med. 2000;22:171–197. doi: 10.1007/BF02895111. [DOI] [PubMed] [Google Scholar]
- Danesh MB, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Gudnason V. C-reative protein and other circulating markers of inflammation in the prediction of coronary heart disease. N. Engl. J. Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Laye S, Bret-dibat JL, Parnet P, Kelley KW. Cytokines and sickness behavior. Ann NY Acad Sci. 1998;840:586–590. doi: 10.1111/j.1749-6632.1998.tb09597.x. [DOI] [PubMed] [Google Scholar]
- *.de Bruower SJM, van Middendorp H, Kraaimaat FW, Radstake TRDJ, Joosten I, Donders ART, Evers AWM. Immune responses to stress after stress management training in patients with rheumatoid arthritis. Arthritis Research and Therapy. 2013;15:R200. doi: 10.1186/ar4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groote D, Zangerle PF, Gevaert Y, Fassotte MF, Beguin Y, Noizat-Pirenne F, Franchimont P. Direct stimulation of cytokines (IL-lβ,TNF-α, IL-6, IL-2, IFN-γ AND GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. 1992 doi: 10.1016/1043-4666(92)90062-v. [DOI] [PubMed] [Google Scholar]
- Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39(4):633–645. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Derry HM, Fagundes CP, Andridge R, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Lower subjective social status exaggerates interleukin-6 responses to a laboratory stressor. Psychoneuroendo. 2013;38:2676–2685. doi: 10.1016/j.psyneuen.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS. A hassle a day may keep the pathogens away: the fight-or-flight stress response and the augmentation of immune function. Integr Comp Biol. 2009;49:215–36. doi: 10.1093/icb/icp045. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Effects of stress on immune function, the good, the bad and the beautiful. Immunol Res. 2014;58:193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses immune function in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;711:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Effects of stress on immune cell distribution—dynamics and hormonal mechanisms. J Immunol. 1995;154:5511–27. [PubMed] [Google Scholar]
- *.Dickerson SS, Gable SL, Irwin MR, Aziz N, Kemeny ME. Social evaluative threat and proinflammatory cytokine regulation: An experimental laboratory investigation. Psychol. Sci. 2009;20(10):1237–1244. doi: 10.1111/j.1467-9280.2009.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130(3):355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Proinflammatory cytokines. Chest. 2000;118(2):503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- *.Dugue B, Leppanen EA, Teppo AM, Fyhrquist F, Grasbeck R. Effects of psychological stress on plasma interleukins-1beta and 6, C-reactive protein, tumour necrosis factor alpha, anti-diuretic hormone and serum cortisol. Scand. J. Clin. Lab. Invest. 1993;53:555–561. [PubMed] [Google Scholar]
- *.Edwards KM, Burns VE, Ring C, Carroll D. Sex differences in the interleukin-6 response to acute psychological stress. Biol. Psychol. 2006;71:236–239. doi: 10.1016/j.biopsycho.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanismims linking social ties with physical health. Nat Neurosc. 2012;15(5):669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- Elinav E, Nowarksi R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and miroorganisms. Nat. Rev. Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- Ellins E, Halcox J, Donald A, Field B, Brydon L, Deanfield J, Steptoe A. Arterial stiffness and inflammatory response to psychophysiological stress. Brain Behav. Immun. 2008;22(6):941–948. doi: 10.1016/j.bbi.2008.01.009. [DOI] [PubMed] [Google Scholar]
- *.Fagundes CP, Glaser R, Hwang BS, Malarkey WB, Kiecolt-Glaser JK. Depressive symptoms enhance stress-induced inflammatory responses. Brain Behav. Immun. 2013;31:172–176. doi: 10.1016/j.bbi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Baines KJ, Wood LG, Gibson PG. Systemic inflammation is associated with differential gene expression and airway neutrophilia in asthma. Omics. 2013;17(4):187–199. doi: 10.1089/omi.2012.0104. [DOI] [PubMed] [Google Scholar]
- *.Gaab J, Rohleder N, Heitz V, Engert V, Schad T, Schurmeyer TH, Ehlert U. Stress-induced changes in LPS-induced pro- inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrino. 2005;30:188–198. doi: 10.1016/j.psyneuen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Goebel MU, Mills PJ. Acute psychological stress and exercise and changes in peripheral leukocyte adhesion molecule expression and identity. Psychosom Med. 2000;62(5):664–670. doi: 10.1097/00006842-200009000-00010. [DOI] [PubMed] [Google Scholar]
- Haack M, Reichenberg A, Kraus T, Schuld A, Yirmiya R, Pollmacher T. Effects of an intravenous catheter on the local production of cytokines and soluble cytokine receptors in healthy men. Cytokine. 2000;12:694–698. doi: 10.1006/cyto.1999.0665. [DOI] [PubMed] [Google Scholar]
- *.Hackett RA, Hamer M, Endrighi R, Brydon L, Steptoe A. Loneliness and stress-related inflammatory and neuoroendocrine responses in older men and women. Psychoneuroendo. 2012;37:1801–1809. doi: 10.1016/j.psyneuen.2012.03.016. [DOI] [PubMed] [Google Scholar]
- *.Hamer M, Steptoe A. Association between physical fitness, parasympathetic control and proinflammatory responses to mental stress. Psychosom. Med. 2007;69:660–666. doi: 10.1097/PSY.0b013e318148c4c0. [DOI] [PubMed] [Google Scholar]
- *.Hamer M, Williams E, Vuonovirta R, Giacobazzi P, Gibson EL, Steptoe A. The effects of effort-reward imbalance on inflammatory and cardiovascular responses to mental stress. Psychosom. Med. 2006;68:408–413. doi: 10.1097/01.psy.0000221227.02975.a0. [DOI] [PubMed] [Google Scholar]
- *.Heesen C, Schulz H, Schmidt M, Gold S, Tessmer W, Schulz KH. Endocrine and cytokine responses to acute psychological stress in multiple sclerosis. Brain Behav. Immun. 2002;16:282–287. doi: 10.1006/brbi.2001.0628. [DOI] [PubMed] [Google Scholar]
- *.Heffner KL, Ng HM, Suhr JA, France CR, Marshall GD, Pigeon WR, Moynihan JA. Sleep disturbance and older adults’ inflammatory responses to acute stress. Am J. Geriat. Psychiat. 2012;20(9):744–752. doi: 10.1097/JGP.0b013e31824361de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Jacobs R, Pawlak CR, Mikeska E, Meyer-Olson D, Martin M, Heijnen CJ, Schedlowski M, Schmidt RE. Systemic lupus erythematosus and rheumatoid arthritis patients differ from healthy controls in their cytokine pattern after stress exposure. Rheumatology. 2001;40:868–875. doi: 10.1093/rheumatology/40.8.868. [DOI] [PubMed] [Google Scholar]
- *.Jaremka LM, Fagundes CP, Peng J, Bennett JM, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Loneliness promotes inflammation during acute stress. Psychol. Sci. 2013;24(7):1089–1097. doi: 10.1177/0956797612464059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, et al. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology: Psychological influences on immune function and health. J. Consult. Clin. Psychol. 2002;70(3):537–547. doi: 10.1037//0022-006x.70.3.537. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, Hellhammer DH. The “Trier Social Stress Test”—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- *.Kop WJ, Weissman NJ, Zhu J, Bonsall RW, Doyle M, Stretch MR, Tracy RP. Effects of acute mental stress and exercise on inflammatory markers in patients with coronary artery disease and healthy controls. Am. J. Cardiol. 2008;101:767–773. doi: 10.1016/j.amjcard.2007.11.006. [DOI] [PubMed] [Google Scholar]
- *.Kuebler U, Zuccarella-Hackly C, Arpagaus A, Wolf JM, Farahmand F, von Kanel R, Wirts PH. Stress-induced modulation of Nf-kB activation, inflammation-associated gene expression and cytokine levels in blood of healthy men. Brain Behav. Immun. 2015;46:87–95. doi: 10.1016/j.bbi.2014.12.024. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Mohamed-Ali V, Feldman PJ, Kirschbaum C, Steptoe A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain Behav. Immun. 2003;17:373–383. doi: 10.1016/s0889-1591(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: A review of core processes. Ann Rev Psychol. 2007;59:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lockwood KG, Marsland AL, Cohen S, Gianaros PJ. Sex differences in the association between stressor-evoked interleukin-6 reactivity and C-reactive protein. Brain Behav. Immun. 2016;58:173–180. doi: 10.1016/j.bbi.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lutgendorf SK, Logan H, Costanzo E, Lubaroff D. Effects of acute stress, relaxation, and a neurogenic inflammatory stimulus on interleukin-6 in humans. Brain Behav. Immun. 2004;18:55–64. doi: 10.1016/s0889-1591(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Cohen S, Rabin BS, Muldoon MF, Bachen EA. Individual differences in cellular immune responses. Psychol. Sci. 1991;2:111–115. [Google Scholar]
- Marshall GD, Jr, Agarwal SK, Lloyd C, Cohen L, Henninger EM, Morris GJ. Cytokine dysregulation associated with exam stress in healthy medical students. Brain Behav. Immun. 1998;12(4):297–307. doi: 10.1006/brbi.1998.0537. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Bachen EA, Cohen S, Rabin B, Manuck SB. Stress, immune reactivity and susceptibility to infectious disease. Phys. Behav. 2002;77(4–5):711–716. doi: 10.1016/s0031-9384(02)00923-x. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Prather AA, Manuck SB. Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosom. Med. 2007;69(8):709–716. doi: 10.1097/PSY.0b013e3181576118. [DOI] [PubMed] [Google Scholar]
- Mcinnes IB, Schett G. The pathogenesis of rheumatoid arthiritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- McInnis CM, Wang D, Gianferante Rohleder N. Response and habituation of pro and anti inflammatory gene expression to repeated acute stress. Brain Behav. Immun. 2015;46:237–248. doi: 10.1016/j.bbi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom. Med. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid resistance model. Health Psychol. 2002;2:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. J Clin Epidemiol. 2009;62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature Reviews: Immunology. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Muscatell KA, Dedovic K, Slavich G, Jarcho MR, Breen EC, Bower JE, Irwin MR, Eisenberger NI. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav. Immun. 2015;43:46–53. doi: 10.1016/j.bbi.2014.06.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol. Med. 2008;14(3):109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Oliver JC, Bland LA, Oettinger CW, Arduino MJ, McAllister SK, Aguero SM, Favero MS. Cytokine kinetics in an in vitro whole blood model following an endotoxin challenge. Lymphokine Cytokine Res. 1993;12(2):115–120. [PubMed] [Google Scholar]
- Opp M, Born J, Irwin M. Sleep and the immune system. In: Ader R, editor. Psychoneuroimmunology. Elsevier Academic Press; Burlington, MA: 2007. pp. 579–618. [Google Scholar]
- *.Owen N, Steptoe A. Natural killer cell and proinflammatory cytokine responses to mental stress: associations with heart rate and heart rate variability. Biol. Psychol. 2003;63:101–115. doi: 10.1016/s0301-0511(03)00023-1. [DOI] [PubMed] [Google Scholar]
- *.Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiat. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- *.Pace TWW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD, Issa MJ, Raison CL. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34(1):87–98. doi: 10.1016/j.psyneuen.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann. Intern. Med. 1998;128(2):127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav. Immun. 2005;19(6):493–499. doi: 10.1016/j.bbi.2005.03.015. Ra. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- *.Peters ML, Godaert GL, Ballieux RE, Brosschot JF, Sweep FC, Swinkels LM, van Vliet M, Heijnen CJ. Immune responses to experimental stress: effects of mental effort and uncontrollability. Psychosom. Med. 1999;61:513–524. doi: 10.1097/00006842-199907000-00016. [DOI] [PubMed] [Google Scholar]
- *.Prather AA, Caroll JE, Fury JM, McDade KK, Ross D, Marsland AL. Gender differences in stimulated cytokine production following acute psychological stress. Brain Behav. Immun. 2009;23:622–628. doi: 10.1016/j.bbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Puterman E, Epel ES, O’Donovan A, Prather AA, Aschbacher K, Dhabhar FS. Int. J. Behav. Med. 2013;21(6):936–945. doi: 10.1007/s12529-013-9368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101(18):2149–2153. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101(15):1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. 2014;76(3):181–189. doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- *.Rohleder N, Wolf JM, Herpfer I, Fiebich BL, Kirschbaum C, Lieb K. No response of plasma substance P, but delayed increase of interleukin-1 receptor antagonist to acute psychosocial stress. Life Sci. 2006;78:3082–3089. doi: 10.1016/j.lfs.2005.12.016. [DOI] [PubMed] [Google Scholar]
- *.Rohleder N, Wolf JM, Piel M, Kirschbaum C. Impact of oral contraceptive use on glucocorticoid sensitivity of pro-inflammatory cytokine production after psychosocial stress. Psychoneuroendocrino. 2003;28:261–273. doi: 10.1016/s0306-4530(02)00019-7. [DOI] [PubMed] [Google Scholar]
- Roitt IM, Delves PJ. Roitt’s Essential immunology. 10. Malden, MA: Blackwell science; 2001. [Google Scholar]
- Sanders VM, Kavelaars A. Adrenergic regulation of immunity. Psychoneuroimmunology. 2007;1:63–83. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavish DC, Graham-Engeland JE, Smyth JM, Engeland CG. Salivary markers of inflammation in response to acute stress. Brain Behav. Immun. 2015;0:253–269. doi: 10.1016/j.bbi.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol. Med. 2007;13(3–4):178–184. doi: 10.2119/2006-00112.Sloan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav. Immun. 2007;21(7):901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- *.Steptoe A, Willemsen G, Owen N, Flower L, Mohamed-Ali V. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin. Sci. 2001;101:185–192. [PubMed] [Google Scholar]
- Szabo C, Hasko G, Zingarelli B, Nemeth ZH, Salzman AL, Kveta V, McCarthy Pastores S, Vizi ES. Isoproterenol regulates tumor necrosis factor, interleukin-10, interleukin-6 and nitric oxide production and protects against the development of vascular hyporeactivity in endotoxaemia. Immunology. 1997;90:95–100. doi: 10.1046/j.1365-2567.1997.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Kanel R, Kudielka BM, Preckel D, Hanebuth D, Fischer JE. Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain Behav. Immun. 2006;20(1):40–48. doi: 10.1016/j.bbi.2005.03.013. [DOI] [PubMed] [Google Scholar]
- *.Weik U, Herforth A, Kolb-Bachofen V, Deinzer R. Acute stress induces proinflammatory signaling at chronic inflammation sites. Psychosom. Med. 2008;70:906–912. doi: 10.1097/PSY.0b013e3181835bf3. [DOI] [PubMed] [Google Scholar]
- *.Weinstein AA, Deuster PA, Francis JL, Bonsall RW, Tracy RP, Kop WJ. Neurohormonal and inflammatory hyper-responsiveness to acute mental stress in depression. Biol. Psychol. 2010;84:228–234. doi: 10.1016/j.biopsycho.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Wirtz PH, von Kanel R, Emini L, Suter T, Fontana A, Ehlert U. Variations in anticipatory cognitive stress appraisal and differential proinflammatory cytokine expression in response to acute stress. Brain Behav. Immun. 2007;21:851–859. doi: 10.1016/j.bbi.2007.02.003. [DOI] [PubMed] [Google Scholar]
- *.Wolf JM, Rohleder N, Bierhaus A, Petrov D, Nawroth PP, Kirschbaum C. Determinants of the NF-kappaB response to acute psychosocial stress in humans. Brain Behav. Immun. 2009;23(6):742–749. doi: 10.1016/j.bbi.2008.09.009. [DOI] [PubMed] [Google Scholar]
- *.Yamakawa K, Matsunaga M, Isowa T, Kimura K, Kasugai K, Yoneda M, Kaneko H, Ohira H. Transient repsonses of inflammatory cytokines in acute stress. Biol. Psychol. 2009 doi: 10.1016/j.biopsycho.2009.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.