Abstract
Mutant alleles at the maternal effect locus dorsal cause a dorsalization of the Drosophila embryo. In extreme mutants, the embryos develop exclusively structures which derive from the dorsal-most region in normal eggs, in less strong phenotypes in addition to dorsal structures, structures normally derived from a dorso-lateral to lateral egg region are formed. Injection of cytoplasm from wild-type embryos into mutant embryos partially restores the dorso-ventral pattern in that injected embryos develop additional structures never formed in uninjected control embryos or embryos injected with mutant cytoplasm. The phenotype of injected embryos resembles that of weaker alleles at the dorsal locus indicating that the wild-type cytoplasm partially rescues the mutant phenotype. The response of the mutant embryos is restricted to the site of injection and occurs only when cytoplasm is injected into the ventral and not into the dorsal side of mutant embryos. The rescuing activity appears to be equally distributed in cleavage stage wild-type embryos, whereas, in syncytial blastoderm embryos, cytoplasm from the ventral side is about twice as effective as that taken from the dorsal side.
Keywords: pattern formation, dorsal, cytoplasmic rescue, maternal effect gene
Full text
PDF

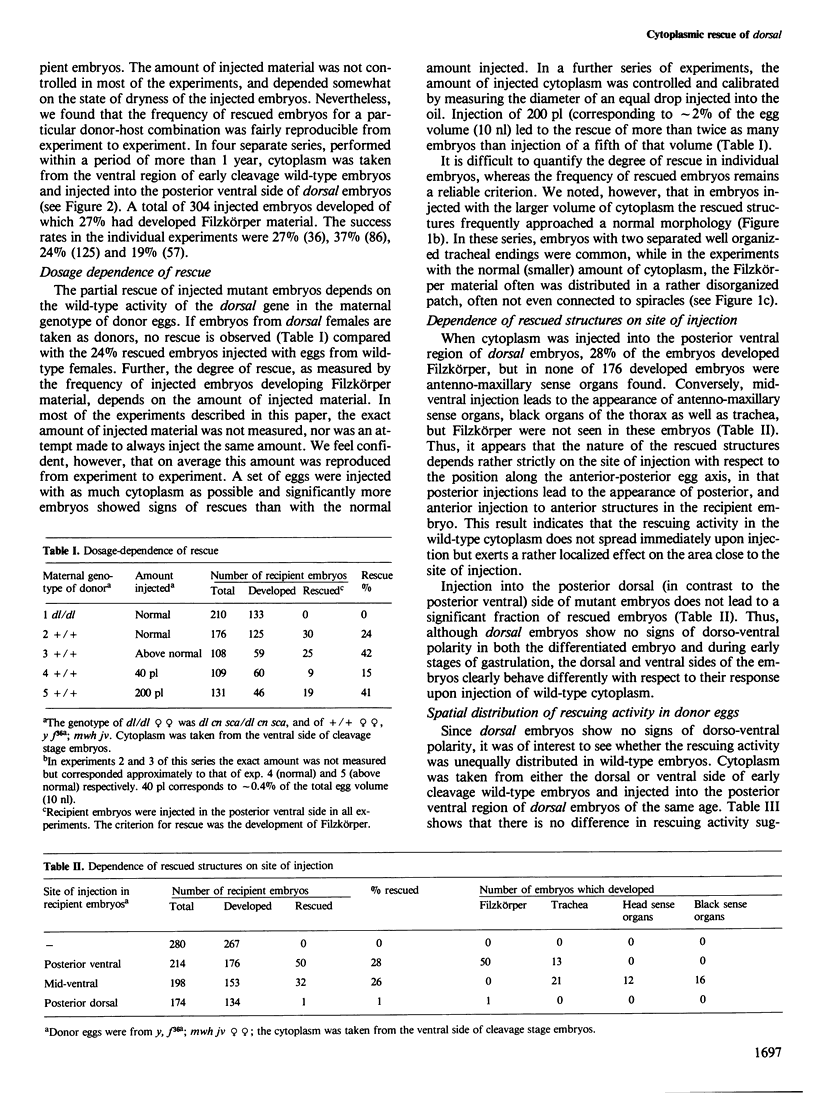


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs R., Cassens G. Accumulation in the oöcyte nucleus of a gene product essential for embryonic development beyond gastrulation. Proc Natl Acad Sci U S A. 1966 May;55(5):1103–1109. doi: 10.1073/pnas.55.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garen A., Gehring W. Repair of the lethal developmental defect in deep orange embryos of Drosophila by injection of normal egg cytoplasm. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2982–2985. doi: 10.1073/pnas.69.10.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohs-Schardin M., Cremer C., Nüsslein-Volhard C. A fate map for the larval epidermis of Drosophila melanogaster: localized cuticle defects following irradiation of the blastoderm with an ultraviolet laser microbeam. Dev Biol. 1979 Dec;73(2):239–255. doi: 10.1016/0012-1606(79)90065-4. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Lohs-Schardin M., Sander K., Cremer C. A dorso-ventral shift of embryonic primordia in a new maternal-effect mutant of Drosophila. Nature. 1980 Jan 31;283(5746):474–476. doi: 10.1038/283474a0. [DOI] [PubMed] [Google Scholar]
- Okada M., Kleinman I. A., Schneiderman H. A. Repair of a genetically-caused defect in oogenesis in Drosophila melanogaster by transplantation of cytoplasm from wild-type eggs and by injection of pyrimidine nucleosides. Dev Biol. 1974 Mar;37(1):55–62. doi: 10.1016/0012-1606(74)90169-9. [DOI] [PubMed] [Google Scholar]
- Underwood E. M., Turner F. R., Mahowald A. P. Analysis of cell movements and fate mapping during early embryogenesis in Drosophila melanogaster. Dev Biol. 1980 Feb;74(2):286–301. doi: 10.1016/0012-1606(80)90431-5. [DOI] [PubMed] [Google Scholar]
- Zalokar M., Audit C., Erk I. Developmental defects of female-sterile mutants of Drosophila melanogaster. Dev Biol. 1975 Dec;47(2):419–432. doi: 10.1016/0012-1606(75)90295-x. [DOI] [PubMed] [Google Scholar]




