Abstract
Dextran, the α-1,6-linked glucose polymer widely used in biology and medicine, promises new applications. Linear dextran applied as a blood plasma substitute demonstrates a high rate of biocompatibility. Dextran is present in foods, drugs, and vaccines and in most cases is applied as a biologically inert substance. In this review we analyze dextran’s cellular uptake principles, receptor specificity and, therefore, its ability to interfere with pathogen–lectin interactions: a promising basis for new antimicrobial strategies. Dextran-binding receptors in humans include the DC-SIGN (dendritic cell–specific intercellular adhesion molecule 3-grabbing nonintegrin) family receptors: DC-SIGN (CD209) and L-SIGN (the liver and lymphatic endothelium homologue of DC-SIGN), the mannose receptor (CD206), and langerin. These receptors take part in the uptake of pathogens by dendritic cells and macrophages and may also participate in the modulation of immune responses, mostly shown to be beneficial for pathogens per se rather than host(s). It is logical to predict that owing to receptor-specific interactions, dextran or its derivatives can interfere with these immune responses and improve infection outcome. Recent data support this hypothesis. We consider dextran a promising molecule for the development of lectin–glycan interaction-blocking molecules (such as DC-SIGN inhibitors) that could be applied in the treatment of diseases including tuberculosis, influenza, hepatitis B and C, human immunodeficiency virus infection and AIDS, etc. Dextran derivatives indeed change the pathology of infections dependent on DC-SIGN and mannose receptors. Complete knowledge of specific dextran–lectin interactions may also be important for development of future dextran applications in biological research and medicine.
INTRODUCTION
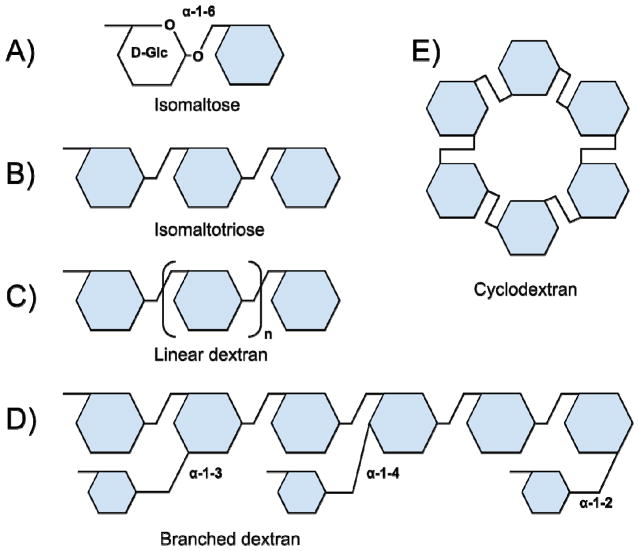
Dextran is a glucose polymer with a prevalence ofα-1,6-linked units and is usually linear (Figure 1). Dextran is a component of vaccines, cosmetics, foods, and drugs. In addition, it is one of the most widely used blood plasma substitutes. Dextran-based molecules (e.g., fluorescent markers) play an important role in biomedical research. Dextran’s properties provide various advantages including adjustable molecular size and viscosity; chemical stability and simplicity of modification; ability to target certain cell types and cellular compartments; relative biological inertness. We are the first to highlight that dextran shares specific receptors with many pathogens. According to recent studies, this commonality lends dextran the capability to have antimicrobial properties.
Figure 1.
Types of α-1,6 glucosides. A) Isomaltose (two glucose molecules with α-1-6 linkage). B) Isomaltotriose. C) Linear dextrans. D) Branched dextrans (schematically). e) α-Cyclodextran.
Detailed publications on dextran have been written for medical professionals (1), biochemists, pharmacists, and biotechnology specialists (2–4). However complex work is lacking on dextran’s fate at the cellular level. Topics that must be addressed include types of cells that take up dextran, its receptors and interference with infectious processes. Dextran’s biological inertness is implied in many of its applications: it is often used as a nonfunctional biocompatible core molecule conjugated with the functional groups (fluorescent dyes, drugs, charged or hydrophobic groups). However, dextran-binding receptors that belong to the family of C-type lectins, namely mannose receptors (MRs), dendritic cell (DCs)-specific intercellular adhesion molecule-3 (ICAM-3)-grabbing nonintegrin (DC-SIGN), L-SIGN (the liver and lymphatic endothelium homologue of DC-SIGN), and langerin, are involved in the immune recognition and uptake of numerous pathogens such as human immunodeficiency virus (HIV) and Mycobacterium tuberculosis (5).
In HIV infection, DC-SIGN binding to gp120 is considered to be a critical phase in the entry of HIV-1. DC-SIGN antibodies (6), short hairpin RNAs suppressing DC-SIGN gene expression (7) and carbohydrate-binding agents (8) have been touted to inhibit DC-SIGN binding of the HIV-1 envelope complex to DCs and to prevent viral transmission. We have successfully reported inhibition of DC-SIGN and gp120 interaction by screening known inhibitors and carbohydrate-binding agents by devising a novel target-specific high-throughput screening assay (9). We also found that DC-SIGN plays a critical role in infection through human T-lymphotropic virus-1 (HTLV-1) envelope glycoprotein binding and DCs to T-cell transmission (10, 11). Overall, in these studies blocking of DC-SIGN was shown to prevent the binding and transmission of human retroviruses, indicating the suitability of the dextran-binding receptor, DC-SIGN, as an antiretroviral drug target.
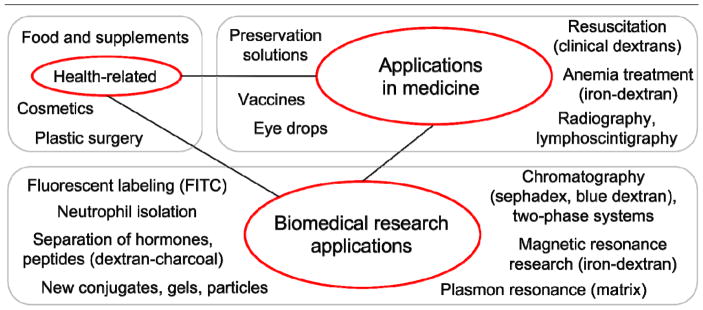
Hepatitis B and C viruses, influenza, and various fungi and protozoa are also associated with uptake via C-type lectins, specifically the dextran-binding receptors. These receptors take part in uptake of the pathogens by DCs and macrophages and also participate in the modulation of intracellular signaling and immune responses. In many cases such modulation is beneficial for pathogens (5). Pathogens’ interactions with MR and DC-SIGN suppress T-helper type 1 (Th1) immune responses which are crucial for defense against intracellular pathogens (12). Dextran unlike the surface molecules of pathogens is an inert ligand of mannose receptor and DC-SIGN that does not induce production of cytokines suppressing Th1 response (13). Therefore we suggest that dextran owing to receptor-specific interactions might interfere with an unfavorable immune response and give preference to Th1-inducing pathogen-Toll-like receptor signaling. Moreover dextran could prevent binding and uptake of many viruses via its receptors. To indicate all areas that show potential promise for future applications of dextran as a receptor-specific molecule, we point towards its existing medical and research applications (Figure 2). At last, the paradigm of “biologically inert” dextran can be revised, as this molecule affects the infectious process, most likely owing to the lectin-glycan interaction mechanism.
Figure 2.
Dextran applications. Many dextran applications, especially medical and biological, can benefit from taking into account the receptor specificity of dextran. FITC = fluorescein isothiocyanate.
DEXTRAN-BINDING RECEPTORS
Mannose receptor
Macrophage mannose receptor (MR, CD206) is a carbohydrate receptor from the superfamily of C-type lectins (14, 15). It is expressed in liver and spleen endothelial cells, in macrophages, and to a lesser extent, in DCs (16). Its main role in mammals is the metabolism of glycoproteins taking place predominantly in the liver (17, 18). MR is also responsible for recognition and phagocytosis of pathogens and allergens, promotion of Th2 immune responses, and antigen presentation (13, 15). Moreover, the uptake of dextran via MR has been proven before (19). A list of all the cell types expressing MR that are able to take up dextran is depicted in Table 1.
Table 1.
Expression of mannose receptor, LSECtin, langerin, and DC-SIGN family receptors correlates with dextran uptake capacity
| Organ | Receptor expression | Dextran uptake |
|---|---|---|
| Liver | 1) MR: Kupffer cells, LSEC (16) | 1) Dextran uptake is present in Kupffer cells (39) and in LSEC (40) |
| 2) L-SIGN, LSECtin: LSEC (31) | 2) Dextran uptake is present in LSEC (40); dextran uptake is present in liver DCs (41) | |
| Spleen | 1) MR: splenic macrophages, endothelial cells (16) | 1) Dextran uptake is present in phagocytes (39) and can be presumed according to dextran uptake along capillaries in endothelial cells (42) |
| 2) SIGN-R1: spleen macrophages (35) DC-SIGN: spleen DCs (26) |
2) SIGN-R1-dependent dextran uptake is present in spleen macrophages (35); dextran uptake is present in spleen phagocytes (39) and in spleen DCs (41) | |
| Lung | 1) MR: alveolar macrophages (16) | 1, 2) Dextran uptake is present in alveolar macrophages (43) |
| 2) DC-SIGN: alveolar macrophages (25) | ||
| Kidney | MR: macrophages, glomerular mesangial cells (16) | Dextran uptake is present in phagocytes (39) and in mesangial cells (44) |
| Heart muscles | MR: macrophages (16) | Dextran uptake is present in phagocytes (39) |
| Brain | MR: retinal microglia cells (45) | Dextran uptake is present (45) |
| Skin | MR: dermal microvascular endothelial cells (46) | Dextran uptake is present (46) |
| Lymphatic system | 1) MR: endothelial cells of the lymph ducts (47) | 1) Dextran uptake (or at least binding) seems to be present in lymphatic endothelial cells due to dextran use in visualization of lymph vessels (49–51) |
| 2) L-SIGN and LSECtin: endothelial cells of the lymph ducts and lymph nodes (31, 48); LSECtin: peripheral blood and thymic DCs (31) | 2) Dextran uptake or binding seems to be present in lymphatic endothelial cells due to dextran use in visualization of lymph vessels (49, 51) | |
| APC | 1) MR: APCs in skin, muscles, salivary gland, thyroid, pancreas (52) | 1, 2, 3) Dextran uptake is present in human immature MDDCs and Langerhans cells (53), plasmacytoid DCs (54), activated B cells (55) |
| 2) DC-SIGN: human immature MDDCs, mucosal DCs, immature DCs on periphery (skin, tonsils), and mature DCs in lymphoid organs (26); plasmacytoid DC precursors (25); activated B cells (23) | ||
| 3) Langerin: Langerhans cells |
APC, antigen-presenting cell; DC-SIGN, dendritic cell–specific intercellular adhesion molecule (ICAM) 3-grabbing nonintegrin; L-SIGN, liver/lymph node-specific intercellular adhesion molecule (ICAM)-3-grabbing nonintegrin; LSEC, liver sinusoidal endothelial cell; MDDC, monocyte-derived dendritic cell; MR, mannose receptor.
DC-SIGN family receptors
DC-SIGN is a receptor expressed by monocyte-derived dendritic cells (MDDCs) in vitro and in vivo (20), and by dermal/intestinal/genital mucosae dendritic cells in vivo (21, 22). It is also expressed on activated B cells (23), wound-healing (IL-4-activated) and alternative (M-CSF-activated) monocyte-derived macrophages, tumor-associated macrophages (24), certain tissue macrophages such as in the alveoli and lung (25). This receptor is responsible for the interactions of DCs with T cells (26), vascular and lymphatic endothelial cells (27), including umbilical vein (28) as well as blood-brain barrier endothelial cells (D. Sagar and P. Jain, unpublished results), and also pathogens (12) and allergens (29) (providing their uptake and/or intracellular signaling). Signaling via DC-SIGN limits Th1 responses influencing Toll-like receptor dependent pathways through Raf1 kinase (30). DC-SIGN is involved in the reception of pathogens of bacterial, viral, fungal, and protozoan origin, as well as those from multicellular parasites. This group of pathogens recognized by DC-SIGN includes mycobacteria, Helicobacter pylori, the worm Schistosoma mansoni, HIV-1, Ebola virus, cytomegalovirus, and Leishmania. Antigenic interaction with DC-SIGN shifts the T helper type1/T helper type 2 balance, causing a chronic infection (12). DC-SIGN receptor in humans has one homologue, L-SIGN (liver/lymph node-specific intercellular adhesion molecule (ICAM)-3-grabbing nonintegrin), expressed mainly in the liver (31); there are eight orthologues in mice, including SIGN-R1 to SIGN-R8 (32). Uptake of dextran via DC-SIGN family receptors (DFRs) DC-SIGN, L-SIGN, SIGN-R1, and SIGN-R3 is proven (33–36). Cells that express these receptors are able to take up dextran (Table 1).
Langerin and LSECtin
Langerin is a receptor specific to Langerhans cells of the skin (37) and uptake of dextran via langerin is proven (36). Human and mouse liver and lymph node sinusoidal endothelial C-type lectin receptors (LSECtins) are expressed mainly by liver endothelial sinusoidal cells and lymph endothelium (38). Although these receptors are not proven to bind dextran, it seems probable because of specificity similar to other dextran-binding receptors. Cells expressing these receptors take up dextran (Table 1).
RECEPTOR-DEPENDENT AND INDEPENDENT ENDOCYTOSIS OF DEXTRAN
In the context of possible antimicrobial application of dextran, it is important to note that this molecule can be taken up into the cells. Clinical dextrans (linear molecules with molecular masses 35,000–80,000 that can curculate in the bloodstream from hours to days) are more potent to be taken up into the cells compared to oligodextrans (linear oligomers of α-1,6-linked glucose) (36). The rate of endocytosis is critical for the development of new applications: bigger molecules provide prolonged action and delivery into the cells, while smaller molecules do not provide the receptor clustering and are more potent as the entry inhibitors because they do not induce receptor-dependent endocytosis by themselves.
Dextran is recognized and taken up by macrophages, DCs, LSECs and some other cell types prefferedly via specific receptors (33–36). However dextran can also be taken up via mechanisms of nonspecific fluid-phase endocytosis (FPE). Table 2 specifies the mechanisms of dextran internalization associated with certain cell types and receptors. MR (14) and DC-SIGN (56) participate in the clathrin-mediated endocytosis (CME) mechanism. MRs and DFRs are necessary and sufficient for receptor-mediated dextran uptake in human immature MDDCs (33, 57).
Table 2.
Dextran endocytosis
| Endocytosis | Characteristics of dextran uptake |
|---|---|
| CME, receptor-dependent uptake | 1) MR-dependent CME of fluorescent dextran in:
|
| Macropino-cytosis or FPE | FPE of fluorescent dextran in:
|
| Phagocytosis | Use of this term is misleading for dextran particles <0.5 μm in diameter |
CME, clathrin-mediated endocytosis; DFR, DC-SIGN (dendritic cell–specific intercellular adhesion molecule [ICAM]-3-grabbing nonintegrin) family receptors; FPE, fluid-phase endocytosis; L-SIGN, liver/lymph node-specific intercellular adhesion molecule (ICAM)-3-grabbing nonintegrin; MDDC, monocyte-derived dendritic cell; MR, mannose receptor.
Use of dextran as a marker for different endocytosis processes requires the discrimination between CME, phagocytosis, and FPE. In CME the uptake of dextran can be dependent on receptors including MR, DC-SIGN (human), L-SIGN (human), SIGN-R1 (mouse), SIGN-R3 (mouse), and langerin. CME is available for particles up to 200 nm (72). Uptake of small particles via CME (and other endocytosis mechanisms) is sometimes called phagocytosis. This term has specific implications. Phagocytosis indeed uses the machinery of different types of endocytosis at the initial stage. However, owing to the initiation of additional mechanisms, it allows uptake of much bigger particles of 500 to 2000 nm or more in diameter. Phagocytosis of dextran-based or dextran-covered particles can be dependent on the same receptors as CME (MRs, DFRs, langerin). Dextrans dissolved in media can be taken up by FPE mechanisms independent of ligand recognition. In the case of FPE, potential mechanisms include macropinocytosis or cdc42-dependent—so-called CLIC/GEEC—pinocytosis. The main molecules participating in this process are clathrin-independent carriers (CLICs) and glycosylphosphatidylinositol-enriched endocytic compartments (GEECs). Different endocytosis mechanisms may be activated simultaneously.
Fluorescently labeled dextrans became quite popular in endocytosis studies when Schröder et al. first developed fluorescently labeled dextran (fluorescein isothiocyanate, FITC-dextran) in 1976 (73). Ohkuma and Poole published their classical work on lysosomal acidification control using FITC-dextran in 1978 (74). In recent decades the labeled dextrans have been used extensively as lysosomal markers (75). They were used to evaluate FPE (76), endocytic activity in general (77), phagocytosis (78, 79), macropinocytosis (80), and macropinocytosis plus MR-mediated uptake (19). They were also applied as the ligands of MR (81), SIGN-R1 (35), and as the ligand of MR and DC-SIGN simultaneously (57). All the terms clathrin-mediated endocytosis, phagocytosis, fluid-phase endocytosis, and macropinocytosis applied to dextran (or dextran-containing particles) as an endocytotic or lysosomal marker are applicable, but in different cases: dependent on cell types and phenotypes.
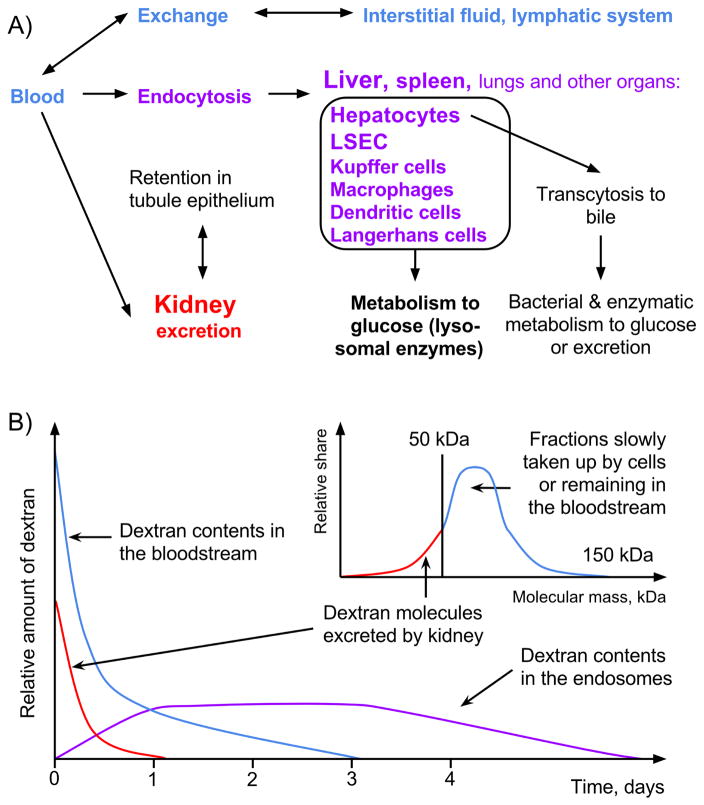
When clinical dextran is injected into the bloodstream, one part is taken up by cells, another part is excreted by the kidney and a third part is retained in the bloodstream. Ratio of these parts depends on the molecular weight and the dose (for more specific data see (39, 82, 83)). The main organs of dextran uptake are liver, spleen, lung, and kidney. From the blood, dextran can enter into interstitial fluid, then the lymph, and then back to the bloodstream. Hepatocytes are able to transport small amounts of dextran to the bile (39, 84–87). Kidney filtration of dextran is dependent on the molecular mass/size: molecules smaller than ~50 kDa are excreted quickly, whereas larger ones stay in the blood longer (Figure 3A and B) (85, 88). Cells that take up dextran are able to metabolize it slowly into glucose by acid and neutral α-glucosidases expressed in all cell types (89–92). These glucose molecules participate in glucose metabolism and can yield dextran-derived exhaled carbon dioxide (93, 94).
Figure 3.
A) Dextran metabolism and excretion pathways. Dextran from the blood circulates in the interstitial fluid and lymph ducts and interacts with most cell types. The main organs of active dextran uptake are the liver, spleen, and lungs. Kidney cells take up dextran via pinocytosis and do not metabolize it, providing only temporarily retention. B) Time dependence of clinical dextran excretion and metabolism. After dextran injection, kidneys excrete the fractions with low molecular mass. Heavier fractions circulate in the body fluids or are taken up into the endosomes. Endosomal compartment volume is limited and some injected dextran may remain in the circulation. In the endosomes, dextrans are metabolized to glucose or excreted by transcytosis. Owing to metabolism, new endosomal volume becomes available and can be filled with dextran molecules from the blood. Thus the dextran endosomal pool depletes when dextran concentration in the blood does not provide its renewal. LSEC, liver sinusoidal endothelial cells.
DEXTRAN DERIVATIVES IN TUBERCULOSIS, CANDIDIASIS, AND INFLUENZA MODELS
Dextran has shown to be inert to DC cytokine reactions while the ligands of pathogens binding to MR and DFRs restrict Th1 response (12, 13). The studies of dextran or dextran-drug conjugates in models of bacterial, fungal, and viral infections that are dependent on dextran-binding receptors (Table 3) are of great interest. In such models, the dextran core is able to interfere with pathogen macrophage and pathogen-DC interaction. Possible inhibition of pathogen uptake or changes in immune response by dextran should influence infection outcomes and several studies confirm this notion.
Table 3.
Dextran-binding receptors: roles in infections
| Receptor | Pathogens | Receptor role in infection |
|---|---|---|
| Mannose receptor | 1. Mycobacterium tuberculosis; M. kansasii, M. phlei, and M. smegmatis | 1. Uptake of bacteria (95), inhibition of phagosomal-lysosomal fusion (96) and restriction of Th1 response (13); uptake (97) |
| 2. Retroviridae (HIV-1; Visna/Maedi virus; lentivirus) | 2. Uptake of virus (98), induction of IFN-γ (99), increase of sexual transmission efficiency (100); virus uptake, in sheep (101); increased organ damage (102) | |
| 3. Candida albicans | 3. Impaired killing (103), uptake (104) | |
| 4. Orthomyxoviridae (influenza viruses) | 4. Uptake of virus (105) | |
| 5. Flaviviridae (Dengue virus) | 5. Uptake of virus (106) | |
| 6. Rhabdoviridae (vesicular stomatitis virus) | 6. Induction of IFN-γ (99) | |
| 7. Herpetoviridae (herpes simplex virus) | 7. Induction of IFN-γ (99) | |
| 8. Hepadnaviridae (hepatitis B virus) | 8. Uptake of virus (107) | |
| 9. Schistosoma mansoni | 9. Induction of Th2 phenotype (108) | |
| 10. Bunyaviridae (Rift Valley fever virus, Toscana virus, Uukuniemi virus) | 10. Uptake of virus (109) | |
| 11. Paramyxoviridae (measles virus) | 11. Virus attachment, DCs and T cells infection (110) | |
| 12. Francisella tularensis | 12. Bacteria uptake (111) | |
| 13. Yersinia pestis | 13. Bacteria uptake (112) | |
| 14. Leishmania spp. | 14. Uptake of the pathogen, modulation of immune response (113, 114) | |
| DC-SIGN | 1. M. tuberculosis | 1. Uptake of mycobacteria by DCs (115), restriction of Th1 response (12) |
| 2. Retroviridae (HIV-1; human T-lymphotropic virus 1) | 2. Uptake of virus and transinfection of other cells (6); cross-talk with Nef-1 signaling and decrease of IL-6 production (116); binding (11), uptake of virus, infection and transinfection (10) | |
| 3. Candida albicans | 3. Uptake of fungi (117) | |
| 4. Orthomyxoviridae (influenza viruses) | 4. Uptake of virus and transinfection of other cells (118); improved viral replication (119) | |
| 5. Coronaviridae (SARS; infectious bronchitis virus) | 5. Uptake of virus (120); uptake of virus (121) | |
| 6. Arenaviridae (Lassa virus, Junin virus) | 6. Uptake of virus (122); uptake of virus (123) | |
| 7. Flaviviridae (hepatitis C virus; Dengue virus; West Nile virus, Tick-borne encephalitis virus) | 7. Uptake of virus (124); uptake of virus (125), platelet activation (126); uptake of virus (127); predisposition to severe forms of encephalitis (128) | |
| 8. Paramyxoviridae (human respiratory syncytial virus) | 8. Modulation of immune response (129) | |
| 9. Herpesviridae (cytomegalovirus, herpesvirus 8) | 9. Uptake of virus and transinfection of other cells (130), virus uptake (131, 132) | |
| 10. Filoviridae (Ebola virus; Marburg virus) | 10. Uptake of virus, transinfection (120, 133) | |
| 11. Helicobacter pylori | 11. Uptake of bacteria, modulation of immune response (134) | |
| 12. Leishmania sp. | 12. Uptake of the pathogen, modulation of immune response (114, 134–136) | |
| 13. S. mansoni | 13. Binding of the surface molecule to the host cells, modulation of immune response (137) | |
| 14. Togaviridae (Sindbis virus) | 14. Uptake of virus (138) | |
| 15. Escherichia coli | 15. Support of phagocytosis (139) | |
| 16. Klebsiella pneumoniae lipopolysaccharide serotype O3 | 16. Binding of bacteria (134) | |
| 17. Bacteroides fragilis | 17. Processing and presentation to T cells (140) | |
| SIGN-R1 | 1. M. tuberculosis | 1. Binding of bacteria, modulation of immune response (141) |
| 2. Candida albicans | 2. Uptake of fungi (142) | |
| 3. Streptococcus pneumoniae | 3. SIGN-R1 plays a defensive role (143), being important in development of IgM response (144) | |
| SIGN-R3 | 1. M. tuberculosis | 1. Binding, modulation of immune response (145) |
| 2. Leishmania spp. | 2. Binding and uptake of bacteria, modulation of immune response (136) | |
| L-SIGN | 1. M. tuberculosis | 1. Binding, modulation of immune response (141) |
| 2. Retroviridae (HIV-1, HIV-2; SIV) | 2. Uptake of virus and transinfection of other cells (48, 146) | |
| 3. Coronaviridae (infectious bronchitis virus) | 3. Uptake of virus (121) | |
| 4. Arenaviridae (Lassa virus, Junin virus) | 4. Uptake of virus (123) | |
| 5. Flaviviridae (hepatitis C virus; West Nile virus) | 5. Uptake of virus (124, 147); uptake of virus (127) | |
| 6. S. mansoni | 6. Binding of the pathogen (148) | |
| 7. Filoviridae (Ebola virus; Marburg virus) | 7. Uptake of virus and transinfection of other cells (133, 149); uptake of virus (120) | |
| 8. Coronaviridae (SARS coronavirus) | 8. Uptake of virus (120) | |
| 9. Togaviridae (Sindbis virus) | 9. Uptake of virus (138) | |
| 10. Leishmania infantum | 10. Uptake of bacteria (135) | |
| Langerin | 1. Mycobacterium leprae | 1. Uptake and antigen presentation (150) |
| 2. Retroviridae (HIV-1) | 2. Uptake of virus and its degradation (151) | |
| 3. Candida spp. (including C. albicans), Saccharomyces species, and Malassezia furfur | 3. Binding and phagocytosis of fungi (152) | |
| 4. Paramyxoviridae (measles virus) | 4. Uptake of virus (153) | |
| LSECtin (probable dextran-binding receptor) | 1. Hepadnaviridae (hepatitis B virus) | 1. LSECtin downregulates inflammation but prolongs the time of virus liver clearance (154) |
| 2. Filoviridae (Ebola virus) | 2. Binding of the virus, infection enchancement (155, 156) | |
| 3. Coronaviridae (SARS coronavirus, SARS) | 3. Binding, infection enchancement (155) | |
| 4. Flaviviridae (hepatitis C virus) | 4. Virus binding (157) | |
| 5. Arenaviridae (Lassa virus) | 5. Virus binding (158) |
DC, dendritic cell; DC-SIGN, dendritic cell specific intercellular adhesion molecule (ICAM)-3-grabbing nonintegrin; IFN, interferon; L-SIGN, liver/lymph node-specific intercellular adhesion molecule (ICAM)-3-grabbing nonintegrin; SARS, severe acute respiratory syndrome; SIV, simian immunodeficiency virus.
Dextran-isoniazid has shown interesting results in a model of tuberculosis-like granulomatosis induced by Bacillus Calmette–Guérin (BCG) injection. The intensity of fibrotic lesions in this model after treatment with dextran conjugate was compared with free isoniazid treatment. Fibrosis of the lung decreased 30%, of the spleen 3.5-fold, and of the liver more than fourfold. Hepatotoxicity decreased 2.2-fold, and the development of necrosis into granulomas decreased 10-fold (159). Decreased lung remodeling may be beneficial for prevention of caviation and subsequent transmission (160) of tuberculosis, and could also help drugs reach the mycobacteria inside granulomas, that is itself an important problem (161).
Dextran influences the phagosomal-lysosomal fusion and the death rate of mycobacteria BCG inside mouse peritoneal macrophages. The control rate of death inside macrophages was 33%, and with dextran (22 μg/ml) it was 39%. Isoniazid treatment (7 μg/ml) yielded a bacterial death rate of 43%, while the conjugate of dextran with isoniazid (25 μg/ml, same isoniazid content) yielded a 53% death rate.
The latter result may be explained by targeted delivery of dextran into the phagosomes and lysosomes where the pathogen is taken up (162). An increase in phagocytic activity after dextran uptake is probably connected with NADPH oxidase 2 upregulation which is responsible for antimicrobial activity (163). In the systemic candidiasis model the dextran-amphotericin B conjugate given 10 days after infection decreased the number of granulomas in the liver by fourfold (164). In experiments on dextran–rimantadine this conjugate has shown to have a significantly better defencive effect in the chicken embryo and mouse models for influenza A and B virus and in the mouse model of tick-borne encephalitis (165). It remained unclear whether dextran alone could cause similar effects in the treatment of infections.
Regularly infused in mice in a model of BCG-induced granulomatosis, oxidized dextran (OD; in these studies-the molecule of clinical dextran containing less than 3% of glucose units oxidized with formation of aldehyde groups) reduced the number and size of granulomas in the organs; increased numbers of fibroblasts (with reduced activity) in the granulomas; decreased destructive and necrotic changes in the liver; and decreased fibrosis in the liver and lungs (166). In a mouse influenza model, OD decreased fatality by 3.3-fold and significantly decreased lung fibrosis (167). In a model of systemic candidiasis, the number of granulomas in the brain decreased eightfold after OD treatment compared with antifungal amphotericin B. While the control group of mice died, 60% of OD-treated mice survived (168).
The mechanism of OD action is still undiscovered; however, this form of dextran has been shown to increase the degree of adhesion of peritoneal cells, which may indicate increased activity of macrophages (169). OD reduces the viability of these cells, but conversely it stimulates metabolic and oxidative processes (169). In vitro dextran, and to a greater extent OD, are able to stimulate macrophage production of granulocyte-macrophage colony-stimulating factor (169), which supports the differentiation and activation of antigen-presenting cells (170). OD causes a shift in the balance of activities between nitric oxide synthase and arginase towards increasing nitric oxide production by macrophages (171). Another effect is increased macrophage ROS production (172).
Chemical differences between dextran and OD are not significant; it is unknown whether oxidation played a role in in vivo results. Probably specific binding of MR and DFRs by dextran modulates pathogen-induced T helper responses (Figure 4) (173, 174). Thus antifibrotic action of dextran in BCG model (159, 166) could be linked to restricted Th2 reaction contributing to tissue remodelling. If this hypothesis is true, dextran could also modulate the immune response to Th2 overreaction-inducing allergens dependent on MR (175) and DC-SIGN (176, 177).
Figure 4.
Dextran and glycan-lectin interactions. This simplified scheme shows that if dextran decrease the availability of MR and DC-SIGN for the pathogens, this may influence immune responses. It is known that DC-SIGN ligands prevent binding and entry of pathogens, interfere with trans-infection of T cells by DCs, skew the myeloid cells activation phenotypes and influence immune response.
Preliminary results are available concerning the in vivo action of nonmodified dextran in models of infections dependent on dextran-binding receptors. Dextran introduced intranasally simultaneously with heat-killed M. tuberculosis H37Rv decreased lung concentrations of both IFN-γ and IL-10, while the IFN-γ/IL-10 ratio decreased 2.5-fold, a result that rather illustrates suppression of Th1 response (178).
Dextran introduced intranasally simultaneously or a day before infection with 10 LD50 of the H5N1 influenza virus saved or prolonged lives of mice (179). These experiments do not provide evidence on dextran’s mechanisms of action, a question that will be addressed in future works. They show, however, that dextran may be a promising molecule to add to the long list of treatments against infections dependent on dextran-binding receptors (Table 3).
DEXTRAN IN PREVENTING HIV INFECTION AND TRANSMISSION
Sexual transmission of HIV is the most prevalent route for infection (180, 181). DCs of intestinal and genital mucosae express DC-SIGN (21). They can be productively infected with HIV and have high capacity to transinfect the T cells—the main HIV targets. DC-SIGN itself is an important player in the formation of DC-T cell infectious synapses (182, 183); signaling via DC-SIGN promotes increased viral uptake (184) and productive infection (185), and also influences DCs regulatory roles (30). HIV entry inhibitors are commonly used antiretrovirals (186), but there are still no inhibitors of HIV-DC-SIGN interaction introduced into the clinics, in spite of proven importance of receptor in myeloid cells infection and trans-infection of T cells.
Dextran 60 given before and after infection provides significant decrease of the HIV-1 viral RNA inside the B-THP-1/DC-SIGN cells. Dextran oligomers also inhibit infection (S. Pustylnikov and P. Jain, unpublished results) and indeed carbohydrate-binding domain of DC-SIGN binds to ~3 carbohydrate units (187). This suggests dextran is an effective inhibitor of HIV-DC-SIGN interaction. It was shown that dextran decreases the mortality rate of HIV-infected human monocyte-derived macrophages from 84% to 48% (188). This could be a result of the inhibition of the minor HIV-DC-SIGN binding (189), as well as a result of the inhibition of HIV-MR interaction shown in macrophage infection and viral transmission (98).
We suggest that dextran as a DC-SIGN and MR ligand could not only decrease the rates of HIV infection and trans-infection in myeloid cells, but could also serve to deliver the antiretrovirals or vaccines to DCs. Anti-HIV gel formulations have proven their efficiency in clinical trials (190); use of viral entry inhibitors in gel formultions can provide full protection in vivo (191). If dextran proves to be an HIV entry inhibitor, it could be used as a gel formulation.
CONCLUSIONS
The combination of dextran properties is unique. Dextran is a hydrophilic, nonionic molecule with adjustable molecular mass distribution (Figure 2) and viscosity/density in solutions. Dextran’s lack (or near lack) of toxic effects, pyrogenic or allergic reactions and accumulation in the body; its thermal and chemical stability allowing sterilization and obtaining the derivatives; its applicability in mass production at comparably low costs (82, 192): all make dextran an appealing biopolymer for multiple applications.
Antimicrobial strategies that could exploit dextran is a speculative topic due to the lack of data. However currently dextran is already used in a great amount of diverse aplications in fields of research and medicine which can benefit from our analysis of the dextran-binding receptors (Figure 2). Dextran is a popular component of conjugates and nano-particles. Numerous works on drug-dextran conjugates show interesting results in vitro and in vivo and provide arguments for improved pharmaceutical properties of such compounds (reviewed in (193–198). Our analysis suggests that concept of targeted delivery—the conjugation of dextran with antimicrobials to reach the pathogens inside the specific cells that take up dextran (liver cells, macrophages and DCs)—being itslef not a new idea, can benefit from knowledge of dextran-binding receptors and their roles in a number of infections.
Dextran’s influences on infections has not been studied comprehensively to date and only minor influences are known. Dextran-binding MR, DC-SIGN (in human)/SIGN-R1/SIGN-R3 (in mice), L-SIGN, and langerin play large roles in infectious diseases (Table 3). Besides regulation of immune cell interplay, these receptors participate in binding, recognition, and uptake of different pathogens. Targeting of dextran-binding receptors (e.g., MR and DC-SIGN) is a popular concept. In recent years studies devoted to the development of DC-SIGN therapeutic ligands have yielded new data in cell biology (203), immunology (204), and biochemistry (205, 206). The concept of therapeutic DC-SIGN antagonists/inhibitors is promising and in need of further development (9, 207). Targeting the MR is suggested for vaccine development (201), for delivery of cargo into macrophages (202) or liver cells (195). Dextran can play a role in the prevention of pathogen binding, entry and signaling in MR-expressing myeloid cells wich participate in blood-brain barrier disruption in neuroinvasive infections (208): this was probably the case in prevention of C. albicans infection in the brain (168). Skewing the T helper responses could be a mechanism that allowed dextran derivatives to decrease tissue remodelling in the BCG infection model (159, 166) (Figure 4). Dextran has been recently used as a backbone for the nucleic acids delivery conjugate and our analysis could help in the development of this field (199). We also note that dextran could be of use in the glycosilation of adenoviruses used for gene transfer (200), possibly improving the biocompatibility and providing predictable uptake by certain cell types and receptors.
Further, the route of delivery of dextran and its derivatives require to be taken into consideration. Infusion will result in primary uptake in the liver, which is not a target of respiratory or mucosal infections. Dextran-based sprays or gels are an option, but they are not helpful in generalized infections. Clinical dextrans with molecular weights in the range 35,000 to 80,000 cannot reach a systemic infection if given orally, but smaller molecules such as dextran with an average molecular weight of 1,000 probably can. Dextrans with high molecular weights induce active endocytosis, while smaller molecules do not (36). They may not only decrease the amount of available dextran-binding receptors on the cell surface but also prevent endocytosis and following recycling of receptors (shown for both MR (209) and DC-SIGN (210)) and keep the cells’ endocytic capacity at its initial level.
Medical and biological applications of dextran can be considered in a new way via the prism of receptor-specific interactions. This can be an instrument to interpret the data on dextran conjugates and derivatives. If antimicrobial properties of dextran can be applied in humans, dextran might become an approved, specific, nontoxic, cheap, and accessible immunomodulatory drug. These qualities are extremely important in the case of deadly infections that affect resource-limited populations. Dextran may possess antimicrobial and antiallergic effects owing to binding to MR, DFRs, and langerin. This review suggests a primary aim for future studies: testing of the ability of dextran to act against a panel of pathogens exploiting dextran-binding receptors to enter the cells and to modulate the immune responses.
Acknowledgments
This work was supported in part by Novosibirsk Tuberculosis Research Institute, Novosibirsk, Russia and Scientific Center for Clinical and Experimental Medicine, Novosibirsk, Russia. We thank Stefan Martin from University of Freiburg, Germany, for help in preparing the manuscript and helpful comments. All authors have no potential conflicts of interest to declare.
List of abbreviations
- APC
antigen-presenting cell
- BCG
Bacillus Calmette Guérin
- cdc42
cell division control protein 42 homolog
- CLIC
clathrin-independent carriers
- CME
clathrin-mediated endocytosis
- DC
dendritic cell
- DC-SIGN
dendritic cell specific ICAM-3-grabbing nonintegrin
- DFRs
DC-SIGN family receptors
- FITC
fluorescein isothiocyanate
- FPE
fluid-phase endocytosis
- GEEC
glycosylphosphatidylinositol-enriched endocytic compartments
- gp120
HIV envelope glycoprotein
- HIV
human immunodeficiency virus
- HIV-1
HIV type 1
- HTLV-1
Human T-lymphotropic virus 1
- ICAM-3
intercellular adhesion molecule-3
- IFN
interferon
- IL-4
interleukin 4
- LD50
median lethal dose
- LSEC
liver sinusoidal endothelial cells
- L-SIGN
liver/lymph node-specific ICAM-3-grabbing nonintegrin
- MDDCs
monocyte-derived dendritic cells
- MR
mannose receptor
- M-CSF
macrophage colony-stimulating factor
- NADPH
nicotinamide adenine dinucleotide phosphate
- OD
oxidized dextran
- Raf1
proto-oncogene serine/threonine-protein kinase
- SARS
severe acute respiratory syndrome
- SIGN-R1 (-R2
…-R8), murine homologues of DC-SIGN
- SIV
simian immunodeficiency virus
- Th1 (2)
Type 1 (2) T helper cell
- TLRs
toll-like receptors
References
- 1.Atik M. Dextrans, their use in surgery and medicine: With emphasis on the low molecular weight fractions. Anesthesiology. 1966;27:425–38. doi: 10.1097/00000542-196607000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Sidebotham RL. Dextrans. Advances in carbohydrate chemistry and biochemistry. 1974;30:371–444. doi: 10.1016/s0065-2318(08)60268-1. [DOI] [PubMed] [Google Scholar]
- 3.Belder AN. Amersham Biosciences. 2003. Dextran, Handbook. [Google Scholar]
- 4.Heinze T, Liebert T, Heublein B, Hornig S, Klemm D. Polysaccharides II. Springer; Berlin Heidelberg: 2006. Functional Polymers Based on Dextran; pp. 199–291. [Google Scholar]
- 5.van Kooyk Y, Geijtenbeek TBH. DC-SIGN: escape mechanism for pathogens. Nature Reviews Immunology. 2003;3(9):697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 6.Geijtenbeek TBH, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GCF, Middel J, et al. DC-SIGN, a Dendritic Cell-Specific HIV-1-Binding Protein that Enhances trans-Infection of T Cells. Cell. 2000;100(5):587–97. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 7.Arrighi J-Fo, Pion M, Wiznerowicz M, Geijtenbeek TB, Garcia E, Abraham S, et al. Lentivirus-mediated RNA interference of DC-SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells. Journal of virology. 2004;78(20):10848–55. doi: 10.1128/JVI.78.20.10848-10855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balzarini J, Van Herrewege Y, Vermeire K, Vanham G, Schols D. Carbohydrate-binding agents efficiently prevent dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)-directed HIV-1 transmission to T lymphocytes. Molecular pharmacology. 2007;71(1):3–11. doi: 10.1124/mol.106.030155. [DOI] [PubMed] [Google Scholar]
- 9.Tran TH, El Baz R, Cuconati A, Arthos J, Jain P, Khan ZK. A Novel High-Throughput Screening Assay to Identify Inhibitors of HIV-1 gp120 Protein Interaction with DC-SIGN. Journal of antivirals & antiretrovirals. 2011;3:49. doi: 10.4172/jaa.1000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain P, Manuel SL, Khan ZK, Ahuja J, Quann K, Wigdahl B. DC-SIGN mediates cell-free infection and transmission of human T-cell lymphotropic virus type 1 by dendritic cells. Journal of virology. 2009;83(21):10908–21. doi: 10.1128/JVI.01054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kampani K, Quann K, Ahuja J, Wigdahl B, Khan ZK, Jain P. A novel high throughput quantum dot-based fluorescence assay for quantitation of virus binding and attachment. Journal of virological methods. 2007;141(2):125–32. doi: 10.1016/j.jviromet.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geijtenbeek TB, van Kooyk Y. Pathogens target DC-SIGN to influence their fate DC-SIGN functions as a pathogen receptor with broad specificity. Apmis. 2003;111:698–714. doi: 10.1034/j.1600-0463.2003.11107803.x. [DOI] [PubMed] [Google Scholar]
- 13.Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–60. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- 14.East L, Isacke CM. The mannose receptor family. Biochim Biophys Acta. 2002;1572:364–86. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Pomares L. The mannose receptor. Journal of Leukocyte Biology. 2012;92(6):1177–86. doi: 10.1189/jlb.0512231. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XS, Brondyk W, Lydon JT, Thurberg BL, Piepenhagen PA. Biotherapeutic target or sink: analysis of the macrophage mannose receptor tissue distribution in murine models of lysosomal storage diseases. Journal of Inherited Metabolic Disease. 2011;34(3):795–809. doi: 10.1007/s10545-011-9285-9. [DOI] [PubMed] [Google Scholar]
- 17.Le Cabec V, Emorine LJ, Toesca I, Cougoule C, Maridonneau-Parini I. The human macrophage mannose receptor is not a professional phagocytic receptor. J Leukoc Biol. 2005;77:934–43. doi: 10.1189/jlb.1204705. [DOI] [PubMed] [Google Scholar]
- 18.Leitinger B, Hohenester E. Mammalian collagen receptors. Matrix Biol. 2007;26:146–55. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheong C, Matos I, Choi J-H, Dandamudi DB, Shrestha E, Longhi MP, et al. Microbial Stimulation Fully Differentiates Monocytes to DC-SIGN/CD209+ Dendritic Cells for Immune T Cell Areas. Cell. 2010;143(3):416–29. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turville SG, Cameron PU, Handley A, Lin G, Pohlmann S, Doms RW, et al. Diversity of receptors binding HIV on dendritic cell subsets. Nature Immunology. 2002;3(10):975–83. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- 22.Jameson B, Baribaud F, Pohlmann S, Ghavimi D, Mortari F, Doms RW, et al. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. Journal of virology. 2002;76(4):1866–75. doi: 10.1128/JVI.76.4.1866-1875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rappocciolo G, Piazza P, Fuller CL, Reinhart TA, Watkins SC, Rowe DT, et al. DC-SIGN on B lymphocytes is required for transmission of HIV-1 to T lymphocytes. PLoS pathogens. 2006;2(7):e70. doi: 10.1371/journal.ppat.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dominguez-Soto A, Sierra-Filardi E, Puig-Kroger A, Perez-Maceda B, Gomez-Aguado F, Corcuera MT, et al. Dendritic Cell-Specific ICAM-3-Grabbing Nonintegrin Expression on M2-Polarized and Tumor-Associated Macrophages Is Macrophage-CSF Dependent and Enhanced by Tumor-Derived IL-6 and IL-10. The Journal of Immunology. 2011;186(4):2192–200. doi: 10.4049/jimmunol.1000475. [DOI] [PubMed] [Google Scholar]
- 25.Soilleux EJ, Morris LS, Leslie G, Chehimi J, Luo Q, Levroney E, et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. Journal of Leukocyte Biology. 2002;71(3):445–57. [PubMed] [Google Scholar]
- 26.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–85. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 27.Geijtenbeek TBH, Krooshoop DlJEB, Bleijs DA, van Vliet SJ, van Duijnhoven GCF, Grabovsky V, et al. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nature immunology. 2000;1(4):353–7. doi: 10.1038/79815. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Vallejo JJ, van Liempt E, da Costa Martins P, Beckers C, van het Hof B, Gringhuis SI, et al. DC-SIGN mediates adhesion and rolling of dendritic cells on primary human umbilical vein endothelial cells through Lewis Y antigen expressed on ICAM-2. Molecular immunology. 2008;45(8):2359–69. doi: 10.1016/j.molimm.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, et al. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. The Journal of Immunology. 2006;177(6):3677–85. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 30.Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26:605–16. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Dominguez-Soto A, Aragoneses-Fenoll L, Martin-Gayo E, Martinez-Prats L, Colmenares M, Naranjo-Gomez M, et al. The DC-SIGN-related lectin LSECtin mediates antigen capture and pathogen binding by human myeloid cells. Blood. 2007;109(12):5337–45. doi: 10.1182/blood-2006-09-048058. [DOI] [PubMed] [Google Scholar]
- 32.Powlesland AS, Ward EM, Sadhu SK, Guo Y, Taylor ME, Drickamer K. Widely divergent biochemical properties of the complete set of mouse DC-SIGN-related proteins. J Biol Chem. 2006;281:20440–9. doi: 10.1074/jbc.M601925200. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Nieto S, Johal RK, Shakesheff KM, Emara M, Royer PJ, Chau DY, et al. Laminin and fibronectin treatment leads to generation of dendritic cells with superior endocytic capacity. PLoS ONE. 2010:5. doi: 10.1371/journal.pone.0010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viebig NK, Andrews KT, Kooyk Yv, Lanzer M, Knolle PA. Evaluation of the role of the endocytic receptor L-SIGN for cytoadhesion of Plasmodium falciparum-infected erythrocytes. Parasitology Research. 2005;96(4):247–52. doi: 10.1007/s00436-005-1360-4. [DOI] [PubMed] [Google Scholar]
- 35.Geijtenbeek TB, Groot PC, Nolte MA, van Vliet SJ, Gangaram-Panday ST, van Duijnhoven GC, et al. Marginal zone macrophages express a murine homologue of DC-SIGN that captures blood-borne antigens in vivo. Blood. 2002;100:2908–16. doi: 10.1182/blood-2002-04-1044. [DOI] [PubMed] [Google Scholar]
- 36.Takahara K, Yashima Y, Omatsu Y, Yoshida H, Kimura Y, Kang YS, et al. Functional comparison of the mouse DC-SIGN, SIGNR1, SIGNR3 and Langerin, C-type lectins. Int Immunol. 2004;16:819–29. doi: 10.1093/intimm/dxh084. [DOI] [PubMed] [Google Scholar]
- 37.Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, et al. Langerin, a Novel C-Type Lectin Specific to Langerhans Cells, Is an Endocytic Receptor that Induces the Formation of Birbeck Granules. Immunity. 2000;12(1):71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 38.Pipirou Z, Powlesland AS, Steffen I, Pohlmann S, Taylor ME, Drickamer K. Mouse LSECtin as a model for a human Ebola virus receptor. Glycobiology. 2011;21(6):806–12. doi: 10.1093/glycob/cwr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mowry RW, Millican RC. A histochemical study of the distribution and fate of dextran in tissues of the mouse. Am J Pathol. 1953;29:523–45. [PMC free article] [PubMed] [Google Scholar]
- 40.Knolle PA, Germann T, Treichel U, Uhrig A, Schmitt E, Hegenbarth S, et al. Endotoxin Down-Regulates T Cell Activation by Antigen-Presenting Liver Sinusoidal Endothelial Cells. The Journal of Immunology. 1999;162(3):1401–7. [PubMed] [Google Scholar]
- 41.Shu SA, Lian ZX, Chuang YH, Yang GX, Moritoki Y, Comstock SS, et al. The role of CD11c+ hepatic dendritic cells in the induction of innate immune responses. Clinical and Experimental Immunology. 2007;149(2):335–43. doi: 10.1111/j.1365-2249.2007.03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grayson MH, Chaplin DD, Karl IE, Hotchkiss RS. Confocal fluorescent intravital microscopy of the murine spleen. Journal of Immunological Methods. 2001;256(1–2):55–63. doi: 10.1016/s0022-1759(01)00437-9. [DOI] [PubMed] [Google Scholar]
- 43.Mwandumba HC, Russell DG, Nyirenda MH, Anderson J, White SA, Molyneux ME, et al. Mycobacterium tuberculosis Resides in Nonacidified Vacuoles in Endocytically Competent Alveolar Macrophages from Patients with Tuberculosis and HIV Infection. The Journal of Immunology. 2004;172(7):4592–8. doi: 10.4049/jimmunol.172.7.4592. [DOI] [PubMed] [Google Scholar]
- 44.Caulfield JP, Farquhar MG. The Permeability of Glomerular Capillaries to Graded Dextrans. Identification of the Basement Membrane as the Primary Filtration Barrier. The Journal of Cell Biology. 1974;63(3):883–903. doi: 10.1083/jcb.63.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Broderick C, Duncan L, Taylor N, Dick AD. IFN-γ and LPS-Mediated IL-10-Dependent Suppression of Retinal Microglial Activation. Investigative Ophthalmology & Visual Science. 2000;41(9):2613–22. [PubMed] [Google Scholar]
- 46.Groger M, Holnthoner W, Maurer D, Lechleitner S, Wolff K, Mayr BB, et al. Dermal microvascular endothelial cells express the 180-kDa macrophage mannose receptor in situ and in vitro. The Journal of Immunology. 2000:165. doi: 10.4049/jimmunol.165.10.5428. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi K, Donovan MJ, Rogers RA, Ezekowitz RA. Distribution of murine mannose receptor expression from early embryogenesis through to adulthood. Cell Tissue Res. 1998;292:311–23. doi: 10.1007/s004410051062. [DOI] [PubMed] [Google Scholar]
- 48.Bashirova AA, Geijtenbeek TB, van Duijnhoven GC, van Vliet SJ, Eilering JB, Martin MP, et al. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J Exp Med. 2001;193:671–8. doi: 10.1084/jem.193.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henze E, Schelbert HR, Collins JD, Najafi A, Barrio JR, Bennett LR. Lymphoscintigraphy with Tc-99m-labeled dextran. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1982;23(10):923–9. [PubMed] [Google Scholar]
- 50.Padera TP, Stoll BR, So PT, Jain RK. Conventional and high-speed intravital multiphoton laser scanning microscopy of microvasculature, lymphatics, and leukocyte-endothelial interactions. Mol Imaging. 2002;1(1):9–15. doi: 10.1162/15353500200200004. [DOI] [PubMed] [Google Scholar]
- 51.Ribera J, Pauta M, Melgar-Lesmes P, Tugues S, Fernandez-Varo G, Held KF, et al. Increased nitric oxide production in lymphatic endothelial cells causes impairment of lymphatic drainage in cirrhotic rats. Gut. 2013;62(1):138–45. doi: 10.1136/gutjnl-2011-300703. [DOI] [PubMed] [Google Scholar]
- 52.Linehan S. The mannose receptor is expressed by subsets of APC in non-lymphoid organs. BMC immunology. 2005:6. doi: 10.1186/1471-2172-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kato M, Neil TK, Fearnley DB, McLellan AD, Vuckovic S, Hart DN. Expression of multilectin receptors and comparative FITC-dextran uptake by human dendritic cells. Int Immunol. 2000;12:1511–9. doi: 10.1093/intimm/12.11.1511. [DOI] [PubMed] [Google Scholar]
- 54.Sadaka C, Marloie-Provost M-A, Soumelis V, Benaroch P. Developmental regulation of MHC II expression and transport in human plasmacytoid-derived dendritic cells. Blood. 2009;113(10):2127–35. doi: 10.1182/blood-2008-10-178152. [DOI] [PubMed] [Google Scholar]
- 55.Xu H, Liew LN, Kuo IC, Huang CH, Goh DL-M, Chua KY. The modulatory effects of lipopolysaccharide-stimulated B cells on differential T-cell polarization. Immunology. 2008;125(2):218–28. doi: 10.1111/j.1365-2567.2008.02832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cambi A, Beeren I, Joosten B, Fransen JA, Figdor CG. The C type lectin DC SIGN internalizes soluble antigens and HIV 1 virions via a clathrin dependent mechanism. European journal of immunology. 2009;39:1923–8. doi: 10.1002/eji.200939351. [DOI] [PubMed] [Google Scholar]
- 57.Navarrete A-M, Delignat S, Teillaud J-L, Kaveri SV, Lacroix-Desmazes SBb, Bayry J. CD4+ CD25+ regulatory T cell-mediated changes in the expression of endocytic receptors and endocytosis process of human dendritic cells. Vaccine. 2011;29:2649–52. doi: 10.1016/j.vaccine.2011.01.095. [DOI] [PubMed] [Google Scholar]
- 58.Piemonti L, Monti P, Allavena P, Leone BE, Caputo A, Di Carlo V. Glucocorticoids increase the endocytic activity of human dendritic cells. Int Immunol. 1999;11:1519–26. doi: 10.1093/intimm/11.9.1519. [DOI] [PubMed] [Google Scholar]
- 59.Wollenberg A, Mommaas M, Oppel T, Schottdorf E-M, Gunther S, Moderer M. Expression and Function of the Mannose Receptor CD206 on Epidermal Dendritic Cells in Inflammatory Skin Diseases. Journal of Investigative Dermatology. 2002;118(2):327–34. doi: 10.1046/j.0022-202x.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nature Cell Biology. 1999;1(6):362–8. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- 61.Knolle PA, Uhrig A, Hegenbarth S, Loser E, Schmitt E, Gerken G, et al. IL-10 down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells through decreased antigen uptake via the mannose receptor and lowered surface expression of accessory molecules. Clin Exp Immunol. 1998;114:427–33. doi: 10.1046/j.1365-2249.1998.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kang YS, Yamazaki S, Iyoda T, Pack M, Bruening SA, Kim JY, et al. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int Immunol. 2003;15:177–86. doi: 10.1093/intimm/dxg019. [DOI] [PubMed] [Google Scholar]
- 63.Lanoue A, Clatworthy MR, Smith P, Green S, Townsend MJ, Jolin HE, et al. SIGN-R1 contributes to protection against lethal pneumococcal infection in mice. J Exp Med. 2004;200:1383–93. doi: 10.1084/jem.20040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koivusalo M, Welch C, Hayashi H, Scott CC, Kim M, Alexander T, et al. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. The Journal of Cell Biology. 2010;188(4):547–63. doi: 10.1083/jcb.200908086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gu Z, Noss EH, Hsu VW, Brenner MB. Integrins traffic rapidly via circular dorsal ruffles and macropinocytosis during stimulated cell migration. The Journal of cell biology. 2011;193(1):61–70. doi: 10.1083/jcb.201007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schnatwinkel C, Christoforidis S, Lindsay MR, Uttenweiler-Joseph S, Wilm M, Parton RG, et al. The Rab5 Effector Rabankyrin-5 Regulates and Coordinates Different Endocytic Mechanisms. PLoS Biol. 2004;2(9) doi: 10.1371/journal.pbio.0020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Araki N, Hatae T, Yamada T, Hirohashi S. Actinin-4 is preferentially involved in circular ruffling and macropinocytosis in mouse macrophages: analysis by fluorescence ratio imaging. Journal of Cell Science. 2000;113(18):3329–40. doi: 10.1242/jcs.113.18.3329. [DOI] [PubMed] [Google Scholar]
- 68.Garrett WS, Chen L-M, Kroschewski R, Ebersold M, Turley S, Trombetta S, et al. Developmental Control of Endocytosis in Dendritic Cells by Cdc42. Cell. 2000;102(3):325–34. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 69.Racoosin EL, Swanson JA. Macropinosome maturation and fusion with tubular lysosomes in macrophages. The Journal of Cell Biology. 1993;121(5):1011–20. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3(6):783–91. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 71.Dowrick P, Kenworthy P, McCann B, Warn R. Circular ruffle formation and closure lead to macropinocytosis in hepatocyte growth factor/scatter factor-treated cells. European journal of cell biology. 1993;61(1):44–53. [PubMed] [Google Scholar]
- 72.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochemical Journal. 2004;377(Pt 1):159–69. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schroder U, Arfors KE, Tangen O. Stability of fluorescein labeled dextrans< i> in vivo and< i> in vitro. Microvascular Research. 1976;11:33–9. [PubMed] [Google Scholar]
- 74.Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proceedings of the National Academy of Sciences. 1978;75(7):3327–31. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nature immunology. 14(8):812–20. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gaudin Rl, Berre S, Cunha de Alencar B, Decalf Jrm, Schindler M, Gobert Fo-X, et al. Dynamics of HIV-Containing Compartments in Macrophages Reveal Sequestration of Virions and Transient Surface Connections. PLoS ONE. 8(7) doi: 10.1371/journal.pone.0069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fiegl D, Kagebein D, Liebler-Tenorio EM, Weisser T, Sens M, Gutjahr M, et al. Amphisomal Route of MHC Class I Cross-Presentation in Bacteria-Infected Dendritic Cells. The Journal of Immunology. 2013;190(6):2791–806. doi: 10.4049/jimmunol.1202741. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Z, Meng Y, Guo Y, He X, Li’u Q, Wan’g X, et al. Rehmannia glutinosa polysaccharide induces maturation of murine bone marrow derived Dendritic cells (BMDCs) International Journal of Biological Macromolecules. 2013;54:136–43. doi: 10.1016/j.ijbiomac.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 79.Bauer R, Mezger M, Blockhaus C, Schmitt A-L, Kurzai O, Einsele H, et al. 40-O-[2-Hydroxyethyl] rapamycin modulates human dendritic cell function during exposure to Aspergillus fumigatus. Journal of Basic Microbiology. 2011;52(3):269–76. doi: 10.1002/jobm.201100071. [DOI] [PubMed] [Google Scholar]
- 80.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. doi: 10.1038/nature12138. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hope JC, Guzman E, Cubillos-Zapata C, Stephens SA, Gilbert SC, Prentice H, et al. Migratory sub-populations of afferent lymphatic dendritic cells differ in their interactions with Mycobacterium bovis Bacille Calmette Guerin. Vaccine. 2011;30(13):2357–67. doi: 10.1016/j.vaccine.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 82.Gronwall A, Ingelman B. Dextran as a substitute for plasma. Nature. 1945;155:45. [Google Scholar]
- 83.Vickery AL. The Fate of Dextran in Tissues of the Acutely Wounded: A Study of the Histologic Localization of Dextran in Tissues of Korean Battle Casualties*. The American Journal of Pathology. 1956:32. [PMC free article] [PubMed] [Google Scholar]
- 84.Kulakov VN, Pimenova GN, Matveev VA, Sedov VV, Vasil’ev AE. Pharmacokinetic study of medicinal polymers: Models based on dextrans. Pharmaceutical Chemistry Journal. 1985;19:240–4. [Google Scholar]
- 85.Yamaoka T, Tabata Y, Ikada Y. Body distribution profile of polysaccharides after intravenous administration. Drug Delivery. 1993;1:75–82. [Google Scholar]
- 86.Mehvar R, Robinson MA, Reynolds JM. Molecular weight dependent tissue accumulation of dextrans: in vivo studies in rats. J Pharm Sci. 1994;83:1495–9. doi: 10.1002/jps.2600831024. [DOI] [PubMed] [Google Scholar]
- 87.Kaneo Y, Uemura T, Tanaka T, Kanoh S. Polysaccharides as drug carriers: biodisposition of fluorescein-labeled dextrans in mice. Biol Pharm Bull. 1997;20:181–7. doi: 10.1248/bpb.20.181. [DOI] [PubMed] [Google Scholar]
- 88.Howard JM, Teng CT, Loeffler RK, Johnsen A. Studies of dextrans of various molecular sizes. Annals of Surgery. 1956:143. doi: 10.1097/00000658-195603000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosenfeld EL, Lukomskaya IS. The splitting of dextran and isomaltose by animal tissues. Clinica chimica acta. 1957;2:105–14. doi: 10.1016/0009-8981(57)90090-6. [DOI] [PubMed] [Google Scholar]
- 90.MacGregor E, Janecek S, Svensson B. Relationship of sequence and structure to specificity in the alfa-amylase family of enzymes. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology. 2001;1546:1–20. doi: 10.1016/s0167-4838(00)00302-2. [DOI] [PubMed] [Google Scholar]
- 91.Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, de Castro E, et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Research. 2012;40(W1):W597–W603. doi: 10.1093/nar/gks400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schomburg I, Chang A, Placzek S, Söhngen C, Rother M, Lang M, et al. BRENDA in 2013: integrated reactions, kinetic data, enzyme function data, improved disease classification: new options and contents in BRENDA. Nucleic Acids Research. 2013;41(Database issue):D764–72. doi: 10.1093/nar/gks1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cargill WH, Bruner HD. Metabolism of C-14 labeled dextran in the mouse. J Pharmacol. 1951:103. [Google Scholar]
- 94.Gray I. Metabolism of plasma expanders studied with carbon-14-labeled dextran. American Journal of Physiology--Legacy Content. 1953:174. doi: 10.1152/ajplegacy.1953.174.3.462. [DOI] [PubMed] [Google Scholar]
- 95.Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–30. [PubMed] [Google Scholar]
- 96.Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, et al. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–99. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Astarie-Dequeker C, N’Diaye EN, Le Cabec V, Rittig MG, Prandi J, Maridonneau-Parini I. The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infection and immunity. 1999:67. doi: 10.1128/iai.67.2.469-477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nguyen DG, Hildreth JEK. Involvement of macrophage mannose receptor in the binding and transmission of HIV by macrophages. European journal of immunology. 2003;33:483–93. doi: 10.1002/immu.200310024. [DOI] [PubMed] [Google Scholar]
- 99.Milone MC, Fitzgerald-Bocarsly P. The Mannose Receptor Mediates Induction of IFN-α in Peripheral Blood Dendritic Cells by Enveloped RNA and DNA Viruses. The Journal of Immunology. 1998;161(5):2391–9. [PubMed] [Google Scholar]
- 100.Bandivdekar AH. P1.031 CD4 Independent Binding of HIV to Human Mannose Receptor on Vaginal Epithelial Cells and Sperm. Sexually Transmitted Infections. 89(Suppl 1):A83–A. [Google Scholar]
- 101.Crespo H, Reina R, Glaria I, Ramírez H, de Andrés X, Jáuregui P, et al. Identification of the ovine mannose receptor and its possible role in Visna/Maedi virus infection. Vet Res. 2011:42. doi: 10.1186/1297-9716-42-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Crespo H, Jauregui P, Glaria I, Sanjosé L, Polledo L, García-Marín JF, et al. Mannose receptor may be involved in small ruminant lentivirus pathogenesis. Veterinary research. 2012;43(1):1–6. doi: 10.1186/1297-9716-43-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marodi L, Schreiber S, Anderson DC, MacDermott RP, Korchak HM, Johnston RB. Enhancement of macrophage candidacidal activity by interferon-gamma. Increased phagocytosis, killing, and calcium signal mediated by a decreased number of mannose receptors. Journal of Clinical Investigation. 1993;91(6):2596–601. doi: 10.1172/JCI116498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Newman SL, Holly A. Candida albicans Is Phagocytosed, Killed, and Processed for Antigen Presentation by Human Dendritic Cells. Infection and immunity. 2001;69(11):6813–22. doi: 10.1128/IAI.69.11.6813-6822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reading PC, Miller JL, Anders EM. Involvement of the Mannose Receptor in Infection of Macrophages by Influenza Virus. Journal of virology. 2000;74(11):5190–7. doi: 10.1128/jvi.74.11.5190-5197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miller JL, de Wet BJM, Martinez-Pomares L, Radcliffe CM, Dwek RA, Rudd PM, et al. The Mannose Receptor Mediates Dengue Virus Infection of Macrophages. PLoS Pathog. 2008;4(2) doi: 10.1371/journal.ppat.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Op den Brouw ML, Binda RS, Geijtenbeek TBH, Janssen HLA, Woltman AM. The mannose receptor acts as hepatitis B virus surface antigen receptor mediating interaction with intrahepatic dendritic cells. Virology. 2009;393(1):84–90. doi: 10.1016/j.virol.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 108.Everts B, Hussaarts L, Driessen NN, Meevissen MHJ, Schramm G, Ham AJvd, et al. Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. The Journal of experimental medicine. 2012;209(10):1753–67. doi: 10.1084/jem.20111381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hofmann H, Pohlmann S. DC-SIGN: Access Portal for Sweet Viral Killers. Cell Host & Microbe. 2011;10(1):5–7. doi: 10.1016/j.chom.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 110.de Witte L, de Vries RD, van der Vlist M, Yüksel S, Litjens M, de Swart RL, et al. DC-SIGN and CD150 have distinct roles in transmission of measles virus from dendritic cells to T-lymphocytes. PLoS pathogens. 2008;4(4) doi: 10.1371/journal.ppat.1000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schulert GS, Allen L-AH. Differential infection of mononuclear phagocytes by Francisella tularensis: role of the macrophage mannose receptor. Journal of Leukocyte Biology. 2006;80(3):563–71. doi: 10.1189/jlb.0306219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang P, Skurnik M, Zhang S-S, Schwartz O, Kalyanasundaram R, Bulgheresi S, et al. Human dendritic cell-specific intercellular adhesion molecule-grabbing nonintegrin (CD209) is a receptor for Yersinia pestis that promotes phagocytosis by dendritic cells. Infection and immunity. 2008;76(5):2070–9. doi: 10.1128/IAI.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wilson ME, Pearson RD. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infection and immunity. 1988;56(2):363–9. doi: 10.1128/iai.56.2.363-369.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ueno N, Wilson ME. Receptor-mediated phagocytosis of Leishmania: implications for intracellular survival. Trends in parasitology. 2012;28(8):335–44. doi: 10.1016/j.pt.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tailleux L, Schwartz O, Herrmann JL, Pivert E, Jackson M, Amara A, et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med. 2003;197:121–7. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sarkar R, Mitra D, Chakrabarti S. HIV-1 Gp120 Protein Downregulates Nef Induced IL-6 Release in Immature Dentritic Cells through Interplay of DC-SIGN. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0059073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cambi A, Gijzen K, de Vries IJM, Torensma R, Joosten B, Adema GJ, et al. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. European journal of immunology. 2003;33(2):532–8. doi: 10.1002/immu.200310029. [DOI] [PubMed] [Google Scholar]
- 118.Londrigan SL, Tate MD, Brooks AG, Reading PC. Cell-surface receptors on macrophages and dendritic cells for attachment and entry of influenza virus. Journal of Leukocyte Biology. 2012;92:97–106. doi: 10.1189/jlb.1011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hillaire MLB, Nieuwkoop NJ, Boon ACM, de Mutsert G, Vogelzang-van Trierum SE, Fouchier RAM, et al. Binding of DC-SIGN to the Hemagglutinin of Influenza A Viruses Supports Virus Replication in DC-SIGN Expressing Cells. PLoS ONE. 8(2) doi: 10.1371/journal.pone.0056164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marzi A, Gramberg T, Simmons G, Möller P, Rennekamp AJ, Krumbiegel M, et al. DC-SIGN and DC-SIGNR Interact with the Glycoprotein of Marburg Virus and the S Protein of Severe Acute Respiratory Syndrome Coronavirus. Journal of virology. 2004;78(21):12090–5. doi: 10.1128/JVI.78.21.12090-12095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Y, Buckles E, Whittaker GR. Expression of the C-type lectins DC-SIGN or L-SIGN alters host cell susceptibility for the avian coronavirus, infectious bronchitis virus. Veterinary Microbiology. 2012;157(3–4):285–93. doi: 10.1016/j.vetmic.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goncalves A-R, Moraz M-L, Pasquato A, Helenius A, Lozach P-Y, Kunz S. Role of DC-SIGN in Lassa Virus Entry into Human Dendritic Cells. Journal of virology. 87(21):11504–15. doi: 10.1128/JVI.01893-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martinez MG, Bialecki MA, Belouzard S, Cordo SM, Candurra NlA, Whittaker GR. Utilization of human DC-SIGN and L-SIGN for entry and infection of host cells by the New World arenavirus, Junín virus. Biochemical and Biophysical Research Communications. 2013;441(3):612–7. doi: 10.1016/j.bbrc.2013.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lozach P-Y, Lortat-Jacob H, Lavalette ADLD, Staropoli I, Foung S, Amara A, et al. DC-SIGN and L-SIGN Are High Affinity Binding Receptors for Hepatitis C Virus Glycoprotein E2. Journal of Biological Chemistry. 2003;278(22):20358–66. doi: 10.1074/jbc.M301284200. [DOI] [PubMed] [Google Scholar]
- 125.Tassaneetrithep B, Burgess TH, Granelli-Piperno A, Trumpfheller C, Finke J, Sun W, et al. DC-SIGN (CD209) Mediates Dengue Virus Infection of Human Dendritic Cells. The Journal of experimental medicine. 2003;197(7):823–9. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hottz ED, Oliveira MF, Nunes PCG, Nogueira RMR, Valls-de-Souza R, Da Poian AT, et al. Dengue induces platelet activation, mitochondrial dysfunction and cell death through mechanisms that involve DC-SIGN and caspases. Journal of Thrombosis and Haemostasis. 11(5):951–62. doi: 10.1111/jth.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Davis CW, Nguyen H-Y, Hanna SL, Sánchez MD, Doms RW, Pierson TC. West Nile Virus Discriminates between DC-SIGN and DC-SIGNR for Cellular Attachment and Infection. Journal of virology. 2006;80(3):1290–301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Barkhash AV, Perelygin AA, Babenko VN, Brinton MA, Voevoda MI. Single nucleotide polymorphism in the promoter region of the CD209 gene is associated with human predisposition to severe forms of tick-borne encephalitis. Antiviral research. 2012;93(1):64–8. doi: 10.1016/j.antiviral.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 129.Johnson TR, McLellan JS, Graham BS. Respiratory Syncytial Virus Glycoprotein G Interacts with DC-SIGN and L-SIGN To Activate ERK1 and ERK2. Journal of virology. 2012;86(3):1339–47. doi: 10.1128/JVI.06096-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Halary F, Amara A, Lortat-Jacob H, Messerle M, Delaunay T, Houles C, et al. Human Cytomegalovirus Binding to DC-SIGN Is Required for Dendritic Cell Infection and Target Cell trans-Infection. Immunity. 2002;17(5):653–64. doi: 10.1016/s1074-7613(02)00447-8. [DOI] [PubMed] [Google Scholar]
- 131.Rappocciolo G, Jenkins FJ, Hensler HR, Piazza P, Jais M, Borowski L, et al. DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. The Journal of Immunology. 2006;176(3):1741–9. doi: 10.4049/jimmunol.176.3.1741. [DOI] [PubMed] [Google Scholar]
- 132.Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, et al. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. Journal of virology. 2008;82(10):4793–806. doi: 10.1128/JVI.01587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alvarez CP, Lasala F, Carrillo J, Muniz O, Corbi AL, Delgado R. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J Virol. 2002;76:6841–4. doi: 10.1128/JVI.76.13.6841-6844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Appelmelk BJ, van Die I, van Vliet SJ, Vandenbroucke-Grauls CM, Geijtenbee’k TB, van Kooyk Y. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J Immunol. 2003;170:1635–9. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- 135.Caparros E, Serrano D, Puig-Kroger A, Riol L, Lasala F, Martinez I, et al. Role of the C-type lectins DC-SIGN and L-SIGN in Leishmania interaction with host phagocytes. Immunobiology. 2005;210(2–4):185–93. doi: 10.1016/j.imbio.2005.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lefevre L, Lugo-Villarino G, Meunier E, Valentin A, Olagnier D, Authier Hl, et al. The C-type Lectin Receptors Dectin-1, MR, and SIGNR3 Contribute Both Positively and Negatively to the Macrophage Response to Leishmania infantum. Immunity. 2013;38(5):1038–49. doi: 10.1016/j.immuni.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 137.van Die I, van Vliet SJ, Nyame AK, Cummings RD, Bank CMC, Appelmelk B, et al. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology. 2003;13(6):471–8. doi: 10.1093/glycob/cwg052. [DOI] [PubMed] [Google Scholar]
- 138.Klimstra WB, Nangle EM, Smith MS, Yurochko AD, Ryman KD. DC-SIGN and L-SIGN Can Act as Attachment Receptors for Alphaviruses and Distinguish between Mosquito Cell- and Mammalian Cell-Derived Viruses. Journal of virology. 2003;77(22):12022–32. doi: 10.1128/JVI.77.22.12022-12032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Iyori M, Ohtani M, Hasebe A, Totsuka Y, Shibata K-i. A role of the Ca2+ binding site of DC-SIGN in the phagocytosis of E. coli. Biochemical and Biophysical Research Communications. 2008;377(2):367–72. doi: 10.1016/j.bbrc.2008.09.142. [DOI] [PubMed] [Google Scholar]
- 140.Bloem K, Garcia-Vallejo JJ, Vuist IM, Cobb BA, van Vliet SJ, van Kooyk Y. Interaction of the Capsular Polysaccharide A from Bacteroides fragilis with DC-SIGN on Human Dendritic Cells is Necessary for Its Processing and Presentation to T Cells. Frontiers in Immunology. 2013:4. doi: 10.3389/fimmu.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koppel EA, Ludwig IS, Hernandez MS, Lowary TL, Gadikota RR, Tuzikov AB, et al. Identification of the mycobacterial carbohydrate structure that binds the C-type lectins DC-SIGN, L-SIGN and SIGNR1. Immunobiology. 2004;209:117–27. doi: 10.1016/j.imbio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 142.Taylor PR, Brown GD, Herre J, Williams DL, Willment JA, Gordon S. The Role of SIGNR1 and the β-Glucan Receptor (Dectin-1) in the Nonopsonic Recognition of Yeast by Specific Macrophages. The Journal of Immunology. 2004;172(2):1157–62. doi: 10.4049/jimmunol.172.2.1157. [DOI] [PubMed] [Google Scholar]
- 143.Koppel EA, Wieland CW, van den Berg VCM, Litjens M, Florquin S, van Kooyk Y, et al. Specific ICAM-3 grabbing nonintegrin-related 1 (SIGNR1) expressed by marginal zone macrophages is essential for defense against pulmonary Streptococcuspneumoniae infection. European journal of immunology. 2005;35(10):2962–9. doi: 10.1002/eji.200526216. [DOI] [PubMed] [Google Scholar]
- 144.Koppel EA, Litjens M, van den Berg VC, van Kooyk Y, Geijtenbeek TBH. Interaction of SIGNR1 expressed by marginal zone macrophages with marginal zone B cells is essential to early IgM responses against Streptococcus pneumoniae. Molecular immunology. 2008;45(10):2881–7. doi: 10.1016/j.molimm.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 145.Tanne A, Ma B, Boudou F, Tailleux L, Botella H, Badell E, et al. A murine DC-SIGN homologue contributes to early host defense against Mycobacterium tuberculosis. J Exp Med. 2009;206:2205–20. doi: 10.1084/jem.20090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pohlmann S, Soilleux EJ, Baribaud F, Leslie GJ, Morris LS, Trowsdale J, et al. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc Natl Acad Sci U S A. 2001;98:2670–5. doi: 10.1073/pnas.051631398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gardner JP, Durso RJ, Arrigale RR, Donovan GP, Maddon PJ, Dragic T, et al. L-SIGN (CD 209L) is a liver-specific capture receptor for hepatitis C virus. Proceedings of the National Academy of Sciences. 2003;100(8):4498–503. doi: 10.1073/pnas.0831128100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Van Liempt E, Imberty A, Bank CM, Van Vliet SJ, Van Kooyk Y, Geijtenbeek TB, et al. Molecular basis of the differences in binding properties of the highly related C-type lectins DC-SIGN and L-SIGN to Lewis X trisaccharide and Schistosoma mansoni egg antigens. J Biol Chem. 2004;279:33161–7. doi: 10.1074/jbc.M404988200. [DOI] [PubMed] [Google Scholar]
- 149.Simmons G, Reeves JD, Grogan CC, Vandenberghe LH, Baribaud Fdr, Whitbeck JC, et al. DC-SIGN and DC-SIGNR Bind Ebola Glycoproteins and Enhance Infection of Macrophages and Endothelial Cells. Virology. 2003;305(1):115–23. doi: 10.1006/viro.2002.1730. [DOI] [PubMed] [Google Scholar]
- 150.Hunger RE, Sieling PA, Ochoa MT, Sugaya M, Burdick AE, Rea TH, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. Journal of Clinical Investigation. 2004;113(5):701–8. doi: 10.1172/JCI19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MAWP, de Gruijl T, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nature Medicine. 2007;13(3):367–71. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 152.de Jong MAWP, Vriend LEM, Theelen B, Taylor ME, Fluitsma D, Boekhout T, et al. C-type lectin Langerin is a β-glucan receptor on human Langerhans cells that recognizes opportunistic and pathogenic fungi. Molecular immunology. 2010;47(6):1216–25. doi: 10.1016/j.molimm.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.van der Vlist M, Geijtenbeek TBH. Langerin functions as an antiviral receptor on Langerhans cells. Immunology and Cell Biology. 2011;88(4):410–5. doi: 10.1038/icb.2010.32. [DOI] [PubMed] [Google Scholar]
- 154.Liu B, Wan’g M, Wan’g X, Zhao D, Liu D, Liu J, et al. Liver Sinusoidal Endothelial Cell Lectin Inhibits CTL-Dependent Virus Clearance in Mouse Models of Viral Hepatitis. The Journal of Immunology. 190(8):4185–95. doi: 10.4049/jimmunol.1203091. [DOI] [PubMed] [Google Scholar]
- 155.Gramberg T, Hofmann H, Möller P, Lalor PF, Marzi A, Geier M, et al. LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus. Virology. 2005;340(2):224–36. doi: 10.1016/j.virol.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Powlesland AS, Fisch T, Taylor ME, Smith DA, Tissot B, Dell A, et al. A Novel Mechanism for LSECtin Binding to Ebola Virus Surface Glycoprotein through Truncated Glycans. Journal of Biological Chemistry. 2008;283(1):593–602. doi: 10.1074/jbc.M706292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.L’i Y, Hao B, Kuai X, Xing G, Yang J, Chen J, et al. C-type lectin LSECtin interacts with DC-SIGNR and is involved in hepatitis C virus binding. Molecular and Cellular Biochemistry. 2009;327(1–2):183–90. doi: 10.1007/s11010-009-0056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shimojima M, Ströher U, Ebihara H, Feldmann H, Kawaoka Y. Identification of Cell Surface Molecules Involved in Dystroglycan-Independent Lassa Virus Cell Entry. Journal of virology. 2012;86(4):2067–78. doi: 10.1128/JVI.06451-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shkurupy VA. Tuberculosis-induced granulomatosis. Cytophysiology and targeted therapy [in Russian] 2007 [Google Scholar]
- 160.Elkington PT, D’Armiento JM, Friedland JS. Tuberculosis immunopathology: the neglected role of extracellular matrix destruction. Science translational medicine. 2011;3(71):71ps6–ps6. doi: 10.1126/scitranslmed.3001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dartois V. The path of anti-tuberculosis drugs: from blood to lesions to mycobacterial cells. Nature Reviews Microbiology. 2014;12(3):159–67. doi: 10.1038/nrmicro3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shkurupy VA, Kurunov JN, Arkhipov SA. [Antibacterial efficiency of prolonged isoniazid formulation in experiment] [In Russian] Problemy tuberkuleza. 1997;(2):54–6. [PubMed] [Google Scholar]
- 163.Mantegazza AR, Savina A, Vermeulen M, Pérez L, Geffner J, Hermine O, et al. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood. 2008;112(12):4712–22. doi: 10.1182/blood-2008-01-134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shkurupy VA, Selyatitskaya VG, Tsyrendorzhiev DD, Pal’chikova NA, Kurilin VV, Travin MA, et al. Effects of modified amphotericin in experimental systemic candidiasis. Bull Exp Biol Med. 2007;143:392–4. doi: 10.1007/s10517-007-0138-3. [DOI] [PubMed] [Google Scholar]
- 165.Polyakova IN, Ginak AI, Moskvichev BV, Shitikova GS. Chemical modification of polysacharides with rimantadine and the properties of derived product [in Russian] Isvestia VolgGTU. 2011;2((75)(8)):99–102. [Google Scholar]
- 166.Potapova OV, Shkurupiy VA. Effect of dialdehyde dextran on structural changes in the liver and lungs during chronic BCG granulomatosis. Bull Exp Biol Med. 2008;146:861–3. doi: 10.1007/s10517-009-0399-0. [DOI] [PubMed] [Google Scholar]
- 167.Potapova OV, Shkurupiy VA, Sharkova TV, Troitskiy AV, Lusgina NG, Shestopalov AM. Preventive Efficacy of Oxidized Dextran and Pathomorphological Processes in Mouse Lungs in Avian Influenza A/H5N1. Bulletin of Experimental Biology and Medicine. 2011;150:707–10. doi: 10.1007/s10517-011-1229-8. [DOI] [PubMed] [Google Scholar]
- 168.Shkurupy V, Guseva E, Potapov’a O, Nadeev A. Morphological Changes in the Brain of Mice with Systemic Candidiasis Treated with Composition of Amphotericin B and Oxidized Dextran. Bulletin of Experimental Biology and Medicine. 2011:1–4. doi: 10.1007/s10517-011-1267-2. [DOI] [PubMed] [Google Scholar]
- 169.Shkurupy VA, Kozyaev MA, Potapov’a OV. Morphological Study of the Efficiency of Isoniazid and Dialdehyde Dextran Composition in the Treatment of Mice with BCG Granulomatosis. Bulletin of experimental biology and medicine. 2008;146:853–6. doi: 10.1007/s10517-009-0401-x. [DOI] [PubMed] [Google Scholar]
- 170.Ryan AA, Wozniak TM, Shklovskaya E, O’Donnell MA, Groth BFdS, Britton WJ, et al. Improved Protection against Disseminated Tuberculosis by Mycobacterium bovis Bacillus Calmette-Guerin Secreting Murine GM-CSF Is Associated with Expansion and Activation of APCs. The Journal of Immunology. 2007;179(12):8418–24. doi: 10.4049/jimmunol.179.12.8418. [DOI] [PubMed] [Google Scholar]
- 171.Tkachev VO, Kolesnikova OP, Troitskii AV, Shkurupy VA. Effect of oxidized dextrans on NO synthase and arginase activities of mouse macrophages. Bull Exp Biol Med. 2008;146:83–5. doi: 10.1007/s10517-008-0214-3. [DOI] [PubMed] [Google Scholar]
- 172.Tkachev VO, Zaikovskaya MV, Troitsky AV, Luzgina NG, Shkurupy VA. The effect of oxidized dextrans on generation of reactive oxygen species by murine peritoneal exudate phagocytes. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry. 2012;6(2):144–8. [Google Scholar]
- 173.Pustylnikov S. Dextrans as Ligands of Mannose Receptor and DC-SIGN Family Receptors – A Novel Means of Modulating Th1/Th2 Immune Responses (Abstract Book) Annals of Allergy, Asthma & Immunology. 2012;109:A108–P248. [Google Scholar]
- 174.Pustylnikov S, DuBuske L. Inhibiting the binding of allergens having glycosylated structures by dextrans as a potential immunomodulatory strategy. European Academy of Allergy and Clinical Immunology and World Allergy Organization Congress, 2013; Milan, Italy. June 22–26, 2013; 2013. p. 885. [Google Scholar]
- 175.Royer P-J, Emara M, Yang C, Al-Ghouleh A, Tighe P, Jones N, et al. The Mannose Receptor Mediates the Uptake of Diverse Native Allergens by Dendritic Cells and Determines Allergen-Induced T Cell Polarization through Modulation of IDO Activity. The Journal of Immunology. 2010;185(3):1522–31. doi: 10.4049/jimmunol.1000774. [DOI] [PubMed] [Google Scholar]
- 176.Emara M, Royer P-J, Mahdavi J, Shakib F, Ghaemmaghami AM. Retagging Identifies Dendritic Cell-specific Intercellular Adhesion Molecule-3 (ICAM3)-grabbing Non-integrin (DC-SIGN) Protein as a Novel Receptor for a Major Allergen from House Dust Mite. Journal of Biological Chemistry. 2011;287(8):5756–63. doi: 10.1074/jbc.M111.312520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Salazar F, Sewell HF, Shakib F, Ghaemmaghami AM. The role of lectins in allergic sensitization and allergic disease. Journal of Allergy and Clinical Immunology. 2013;132(1):27–36. doi: 10.1016/j.jaci.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 178.Pustylnikov S, Zavjalov E, Litvinova E. Dextran affects the immune response of BALB/c mice to intranasal challenge with inactivated mycobacterium H37Rv - Abstract in Russian. Russian Laboratory Animal Science Association Conference, 2013; Novosibirsk, Russia. September 25–28; 2013; 2013. p. 39. [Google Scholar]
- 179.Pustylnikov SVMAG, Durymanov AG. Dextran Extends an Average Length of Life of Mice after Avian Influenza H5N1 Infection. General Meeting of the American Society of Microbiology 2014; 2014 May 17–20, 2014; Boston. 2014. [Google Scholar]
- 180.Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. New England Journal of Medicine. 1997;336(15):1072–8. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 181.Wu L. Biology of HIV mucosal transmission. Current Opinion in HIV and AIDS. 2008;3(5):534. doi: 10.1097/COH.0b013e32830634c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Arrighi J-Fo, Pion M, Garcia E, van Kooyk Y, Geijtenbeek TB, Piguet V. DC-SIGN-mediated Infectious Synapse Formation Enhances X4 HIV-1 Transmission from Dendritic Cells to T Cells. The Journal of experimental medicine. 2004;200(10):1279–88. doi: 10.1084/jem.20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hodges A, Sharrocks K, Edelmann M, Baban D, Moris A, Schwartz O, et al. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nature Immunology. 2007;8(6):569–77. doi: 10.1038/ni1470. [DOI] [PubMed] [Google Scholar]
- 184.Avota E, Gulbins E, Schneider-Schaulies S. DC-SIGN mediated sphingomyelinase-activation and ceramide generation is essential for enhancement of viral uptake in dendritic cells. PLoS pathogens. 2011;7(2):e1001290. doi: 10.1371/journal.ppat.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gringhuis SI, van der Vlist M, van den Berg LM, den Dunnen J, Litjens M, Geijtenbeek TBH. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nature Immunology. 2010;11(5):419–26. doi: 10.1038/ni.1858. [DOI] [PubMed] [Google Scholar]
- 186.Este JA, Telenti A. HIV entry inhibitors. The Lancet. 2007;370(9581):81–8. doi: 10.1016/S0140-6736(07)61052-6. [DOI] [PubMed] [Google Scholar]
- 187.Feinberg H, Mitchell DA, Drickamer K, Weis WI. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science. 2001;294(5549):2163–6. doi: 10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- 188.Wen J, Pang L, Estes D. The roles of combination of dextran with gp120 on human macrophage immune response in HIV infection. The Journal of Immunology. 2012;188:168.16. [Google Scholar]
- 189.Chehimi J, Luo Q, Azzoni L, Shawver L, Ngoubilly N, June R, et al. HIV-1 transmission and cytokine-induced expression of DC-SIGN in human monocyte-derived macrophages. Journal of Leukocyte Biology. 2003;74(5):757–63. doi: 10.1189/jlb.0503231. [DOI] [PubMed] [Google Scholar]
- 190.Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science (New York, NY) 2010;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Neff CP, Kurisu T, Ndolo T, Fox K, Akkina R. A topical microbicide gel formulation of CCR5 antagonist maraviroc prevents HIV-1 vaginal transmission in humanized RAG-hu mice. PLoS ONE. 2011;6(6):e20209. doi: 10.1371/journal.pone.0020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ingelman B. Dextran and its use as a plasma substitute. Acta chem scand. 1947;1:731–8. [Google Scholar]
- 193.Virnik AD, Khomyakov KP, Skokova IF. Dextran and its derivatives. Russian Chemical Reviews. 1975:44. [Google Scholar]
- 194.Larsen C. Dextran prodrugs - structure and stability in relation to therapeutic activity. Advanced Drug Delivery Reviews. 1989;3:103–54. [Google Scholar]
- 195.Mehvar R. Dextrans for targeted and sustained delivery of therapeutic and imaging agents. J Control Release. 2000;69:1–25. doi: 10.1016/s0168-3659(00)00302-3. [DOI] [PubMed] [Google Scholar]
- 196.Dhaneshwar SS, Kandpal M, Gairola N, Kadam SS. Dextran: A promising macromolecular drug carrier. Indian journal of pharmaceutical sciences. 2006:68. [Google Scholar]
- 197.Shrivastava PK, Shrivastava SK. Dextran Polysaccharides: Successful Macromolecular Carrier for Drug Delivery. Int J Ph Sci. 2009;1:353–68. [Google Scholar]
- 198.Varshosaz J. Dextran conjugates in drug delivery. Expert Opinion on Drug Delivery. 2012;9:509–23. doi: 10.1517/17425247.2012.673580. [DOI] [PubMed] [Google Scholar]
- 199.Tang Q, Cao B, Lei X, Sun B, Zhang Y, Cheng G. Dextran-Peptide Hybrid for Efficient Gene Delivery. Langmuir. 2014 doi: 10.1021/la500905z. [DOI] [PubMed] [Google Scholar]
- 200.Pearce OMT, Fisher KD, Humphries J, Seymour LW, Smith A, Davis BG. Glycoviruses: chemical glycosylation retargets adenoviral gene transfer. Angewandte Chemie International Edition. 2005;44(7):1057–61. doi: 10.1002/anie.200461832. [DOI] [PubMed] [Google Scholar]
- 201.Keler T, Ramakrishna V, Fanger MW. Mannose receptor-targeted vaccines. Expert Opinion on Biological Therapy. 2004;4(12):1953–62. doi: 10.1517/14712598.4.12.1953. [DOI] [PubMed] [Google Scholar]
- 202.Gao J, Chen P, Singh Y, ZhX, Szekely Z, Stein S, et al. Novel Monodisperse PEGtide Dendrons: Design, Fabrication, and Evaluation of Mannose Receptor-Mediated Macrophage Targeting. Bioconjugate Chemistry. 24(8):1332–44. doi: 10.1021/bc400011v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tacken PJ, ter Huurne M, Torensma R, Figdor CG. Antibodies and carbohydrate ligands binding to DC-SIGN differentially modulate receptor trafficking. European journal of immunology. 2012;42(8):1989–98. doi: 10.1002/eji.201142258. [DOI] [PubMed] [Google Scholar]
- 204.Hesse C, Ginter W, Förg T, Mayer CT, Baru AM, Arnold-Schrauf C, et al. In vivo targeting of human DC-SIGN drastically enhances CD8+T-cell-mediated protective immunity. European journal of immunology. 2013 doi: 10.1002/eji.201343429. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 205.Thepaut M, Guzzi C, Sutkeviciute I, Sattin S, Ribeiro-Viana R, Varga N, et al. Structure of a Glycomimetic Ligand in the Carbohydrate Recognition Domain of C-type Lectin DC-SIGN. Structural Requirements for Selectivity and Ligand Design. Journal of the American Chemical Society. 2013;135(7):2518–29. doi: 10.1021/ja3053305. [DOI] [PubMed] [Google Scholar]
- 206.Varga N, Sutkeviciute I, Guzzi C, McGeagh J, Petit-Haertlein I, Gugliotta S, et al. Selective targeting of dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) with mannose-based glycomimetics: synthesis and interaction studies of bis(benzylamide) derivatives of a pseudomannobioside. Chemistry (Weinheim an der Bergstrasse, Germany) 2013;19(15):4786–97. doi: 10.1002/chem.201202764. [DOI] [PubMed] [Google Scholar]
- 207.Anderluh M, Chang C-F. Carbohydrates - Comprehensive Studies on Glycobiology and Glycotechnology. InTech; 2012. DC-SIGN Antagonists - A Paradigm of C-Type Lectin Binding Inhibition. [Google Scholar]
- 208.Webster NL, Crowe SM. Matrix metalloproteinases, their production by monocytes and macrophages and their potential role in HIV-related diseases. Journal of Leukocyte Biology. 2006;80(5):1052–66. doi: 10.1189/jlb.0306152. [DOI] [PubMed] [Google Scholar]
- 209.Stahl P, Schlesinger PH, Sigardson E, Rodman JS, Lee YC. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980;19(1):207–15. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- 210.Guo Y, Feinberg H, Conroy E, Mitchell DA, Alvarez R, Blixt O, et al. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nature structural & molecular biology. 2004;11(7):591–8. doi: 10.1038/nsmb784. [DOI] [PubMed] [Google Scholar]