Abstract
Insulin resistance with adipose tissue dysfunction and dysregulation in the production and secretion of adipokines is one of the hallmarks of metabolic syndrome. We have previously reported that increased levels of the heme oxygenase (HO) system, HO-1/HO-2 results in increased levels of adiponectin. Despite documentation of the existence of the anti-inflammatory axis HO-adiponectin, a possible protein–protein interaction between HO and adiponectin has not been examined. Here, we investigated the existence of protein interactions between HO-2 and adiponectin in the maintenance of adipocyte function during metabolic syndrome by integrating phenotypic and in silico studies. Compared to WT animals, HO-2 null mice displayed an increase in both visceral and subcutaneous fat content and reduced circulating adiponectin levels. The decrease in adiponectin was reversed by upregulation of HO-1. HO-2 depletion was associated with increased adipogenesis in cultured mesenchymal stem cells (MSCs) and decreased adiponectin levels in the culture media. In addition, HO-1 siRNA decreased adiponectin release. HO-2 was found to bind to the monomeric form of adiponectin, according to poses and calculated energies. HO-2-adiponectin interactions were validated by the two-hybrid system assay. In conclusion, protein–protein interactions between HO-2 and adiponectin highlight the role of HO-2 as a molecular chaperone for adiponectin assembly, while HO-1 increases adiponectin levels. Thus, crosstalk between HO-2 and HO-1 could be manipulated in a therapeutic approach to ameliorate the deleterious effects of obesity and the metabolic syndrome.
Keywords: Metabolic syndrome, Adiponectin, Heme oxygenase, Protein interactions
1. Introduction
The heme oxygenase (HO) isoforms HO-1 and HO-2 are endoplasmic reticulum (ER) proteins responsible for the degradation of heme from denatured heme proteins, resulting in the formation of equimolar amounts of carbon monoxide (CO), iron and biliverdin, the latter a powerful antioxidant [1,2]. HO-2 (constitutive isoform) contributes to basal physiological functions while HO-1 (inducible isoform) represents the major cytoprotective moiety of the HO system, by scavenging reactive oxygen species (ROS) and preventing apoptosis [1,3]. Several lines of evidence suggest that the HO system plays a central physiological role in diabetes and obesity. Hyperglycemia suppresses HO-1 expression [4] and increases the levels of cellular heme and as the result of a decrease in HO activity. Adipose tissue dysfunction together with insulin resistance, is one of the hallmarks of metabolic syndrome as the result of a decrease in HO-1 expression. An increase in HO-1 levels provides protection for adiponectin [5], presumably by decreasing production, increasing glutathione and EC-SOD levels and protecting adiponectin thiol groups against ROS [6]. Furthermore, adipose tissue plays an important role in insulin resistance through the production and secretion of a variety of cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, leptin and adiponectin [7,8]. Adiponectin is a relatively abundant protein secreted exclusively from adipocytes [7,8] that plays an anti-inflammatory role in the pathophysiology of metabolic disorders [9–11]. In addition to anti-inflammatory properties, adiponectin is involved in cell proliferation and differentiation [12]. Reduced adiponectin levels have been implicated in the development of obesity, diabetes and cardiovascular disease, all characterized by an increase in ROS [13–15]. Adiponectin does not circulate as a monomer, but rather associates into multimeric higher-order structures via disulphide bonds linking collagenous domains [16]. Secretion of the adiponectin oligomers is controlled, in the endoplasmic reticulum, by molecular chaperones [17]. Heat Shock Proteins (HSP) or chaperones favor the correct folding of newly synthesized proteins, and they are actively involved in both repairing denatured proteins and promoting their degradation. However, the molecular mechanisms by which these chaperones promote high molecular weight (HMW) adiponectin formation remain unclear because a basic understanding of adiponectin oligomer assembly is lacking. The aim of the present study was to elucidate possible interactions between HO-2 and adiponectin in the maintenance of adipocyte function during metabolic syndrome by integrating phenotypic and in silico studies. We show that HO-2 is intimately involved as a molecular chaperone in adiponectin assembly.
2. Materials and methods
2.1. Animal experimentation
All animal experiments followed an institutionally approved protocol (New York Medical College, IACUC) in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The HO-2-null mice are direct descendants of the HO-2 mutants. The original HO-2 knockout was developed by Poss and Tonegawa [18]. These well characterized HO-2-null mice have a C57BL/6 ×129/Sv genetic background, that was used on age- and gender-matched controls [19]. Cobalt protoporphyrin (CoPP), an inducer of HO-1, was administered intraperitoneally (i.p.) once a week (3 mg/kg) for six weeks.
2.2. Cell culture and treatment
Cells were isolated from bone marrow and cultured as previously described [11]. The adipogenic media comprised complete DMEM-high glucose supplemented with 10% (v/v) FBS, 10 mg/ml insulin, 0.5 mM dexamethasone and 0.1 mM indomethacin. Cells were treated with three different predesigned siRNAs of the HO-1 gene (Sigma–Aldrich, St. Louis, MO). According to the manufacture’s protocol, adipogenic media containing 10 nM siRNA using NTER was replaced every 48 h.
2.3. Oil Red O staining and lipid droplet size
For Oil Red O staining, 0.21% Oil Red O in 100% isopropanol was used. MSC-derived adipocytes, after 14 days, were fixed in 10% formaldehyde, washed in Oil-red O for 10 min, rinsed with 60% isopropanol, and the Oil Red O eluted by adding 100% isopropanol for 10 min and OD measured at 490 nm.
2.4. Adiponectin measurement
Adiponectin (HMW) was determined in mice serum and in cell culture condition media using an ELISA assay (Pierce Biotechnology Inc. Woburn, MA).
2.5. Docking studies
X-ray structures of Adiponectin (PDB_ENTRY = 1C28) and HO-2 (PDB_ENTRY = 2Q32) were retrieved from the Brookhaven Protein Databank (PDB) (www.rcsb.org). Monomeric form of adiponectin was obtained by removing chains b and c from the trimeric structure. The software Firedock [20] was used to dock of candidates generated by PatchDock [21]. Docking solutions were scored and ranked according to the binding energies. Binding regions were selected using both proximity and structure criteria: among regions within 5 Å of interacting surfaces the ones showing a secondary structure motif (α-helix or β-sheet) were selected. Binding regions were visualized using the RasMol software, version 2.7.5.1 (http://www.rasmol.org).
2.6. Reverse translation and codon adaptation
In order to avoid infrequent E. coli codons, which may cause poor synthesis and/or expression of chimeric proteins in bacterial hosts, the amino acid sequences of interest of both adiponectin and HO-2 domains were conceptually reverse translated using the software available on line at URL http://genomes.urv.es/OPTIMIZER/, which takes into account the relative differences in the codon adaptation indexes for mouse and E.coli. Oligonucleotides were designed with tagged adaptors in order to create protruding ends compatible with BamHI-cut vectors (Table 1). Appropriate oligonucleotide pairs were annealed at the concentration of 50 pmol/μl by incubation at 98 °C for 1 min, followed by slow cooling to room temperature, then quickly frozen at −20 °C before use.
Table 1.
Amino acid sequences of domains putatively involved in binding, along with corresponding reverse-translated DNA sequences. Characters in italics in DNA sequence refers to protruding nucleotides making BamHI cohesive ends, which results in the addition of a GS dipeptide at both sides of inserts.
| Domain | Forward DNA sequence (coding) | Reverse DNA sequence |
|---|---|---|
| TGFLLYHDT | 5′- GA TCC ACC GGC TTC CTG CTG TAT CAC GAC ACC G -3′ | 5′- GA TCC GGT GTC GTG ATA GAC CAG GAA GCC GGT G -3′ |
| NELDQAGSTLARE | 5′- GA TCC AAC GAA CTG GAC CAG GCG GGT TCT ACC CTG GCG CGT GAA G -3′ | 5′- GA TCC TTC ACG CGC CAG GGT AGA ACC CGC CTG GTC CAG TTC GTT G -3′ |
2.7. Cloning vectors
The Bacteriomatch system (Agilent) was used as a two hybrid system [22]. Plasmid vectors pBT and pTRG were cut with BamHI and ligated with annealed oligonucleotides coding for HO-2 and adiponectin fragments, respectively corresponding to domains putatively involved in binding (Table 1). Ligated constructs were used to transform XL1-Blue MRF’ Kan Escherichia coli cells, in the presence of either Chloramphenicol (for pBT vector) or Tetracycline (for pTRG vector), respectively. Clones were randomly selected and subjected to PCR analysis with primers adipo_2215U1 and HO2_2388L15 for recombinant pBT-HO2, and adipo_849U1 and adipo_1018L for recombinant pTRG-AD (Table 2). Three positive clones from each transformation were sequenced, in order to demonstrate the correct insertion of the oligonucleotide insert.
Table 2.
Sequence of primers used for PCR.
| Primers | Sequence |
|---|---|
| adipo_2215U1 | 5′-GATCAGGGATAGCGGTCAG-3′ |
| HO2_2388L15 | 5′-CGCGCCAGGGTAGAAC-3′ |
| adipo_849U1 | 5′-ATCGAAATGGAAACCAACG-3′ |
| adipo_1018L | 5′-GGATCCGGTGTCGTGA-3′ |
2.8. Two hybrid assay
Recombinant clones of choice, namely pBT-HO2 and pTRG-AD, were co-transformed in BacterioMatch II Validation Reporter Competent Cells hosts (Agilent) and selected on LB plates containing both Chloramphenicol and Tetracycline, as recommended [23]. Bacterial suspensions were then serially diluted 1:10 in saline and 5 μl of each dilution was spotted on M9 plates containing either Choramphenicol and Tetracycline (M9 + C + T), Choramphenicol, Tetracycline and 3-aminotriazole (M9 + C + T + 3AT), or Choramphenicol, Tetracycline, 3-aminotriazole and Streptomycin (M9 + C + T + 3AT + S). IPTG was normally added to M9 plates at a final concentration of 0.05 mM. In some experiments, the concentration was raised to 0.1 mM in order to ameliorate the expression of HIS3 and aadA reporter genes. Colony growth was monitored for up to 5 days of incubation at 37 °C. Negative controls (E. coli host co-transformed with both vectors) were subjected to the same assay in parallel.
2.9. Statistical analyses
Statistical significance between experimental groups was determined by the Fisher method of analysis of multiple comparisons. For comparison between treatment groups, the null hypothesis was tested by either a single factor ANOVA for multiple groups or the unpaired t-test for two groups. p < 0.05 was regarded as significant.
3. Results
3.1. In vivo effects of HO-2 deletion on body weight, fat content and adiponectin
Key indicators of metabolic syndrome in the HO-2(−/−) mice were as previously described [19]. The increase in HO-2 KO mice body weight when compared to WT (p < 0.01) was associated with increases of 27 and 42% (p < 0.01) in subcutaneous and visceral fat, respectively (Fig. 1A and B and Table 3). Plasma glucose levels in HO-2 null mice were higher (p < 0.01) than those in WT mice (Table 3). PCR quantification demonstrated that HO-2 deletion is associated with a significant decrease (p < 0.05) in mRNA levels of adiponectin in visceral fat tissue (Fig. 1C). Levels of plasma adiponectin in HO-2(−/−) mice were significantly lower (p < 0.01) than those in age-matched WT mice (Fig. 1D). This effect was reversed when HO-2 KO mice were treated with CoPP.
Fig. 1.
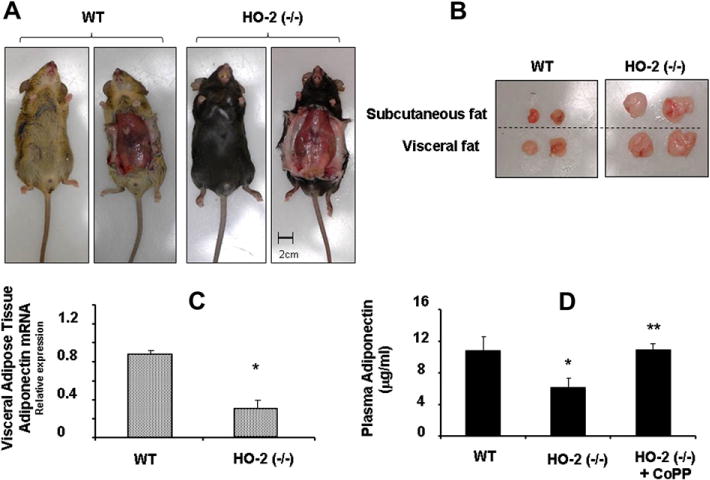
(A) Representative pictures showing WT and HO-2(−/−) mice. Top, body appearance; (B) bottom, dissected subcutaneous and visceral fat of WT and HO-2(−/−). (C) mRNA expression of adiponectin in visceral fat tissue from WT and HO-2(−/−) mice. Values are means ± SE; n = 4, ⁄p < 0.05 versus WT mice. (D) Serum adiponectin levels from WT and HO-2(−/−) mice ± CoPP. Values are means ± SE; n = 6, *p < 0.05 versus WT mice; **p < 0.01 versus HO-2(−/−).
Table 3.
In vivo effects of HO-2 depletion on body weight, adiposity and blood glucose.
| Wild type | HO-2 −/− | |
|---|---|---|
| Body weight (g) | 22.5 ± 1.3 | 30.4 ± 1.6* |
| Subcutaneous fat (g) | 1.16 ± 0.11 | 1.48 ± 0.09* |
| Visceral fat (g) | 1.32 ± 0.13 | 1.87 ± 0.07* |
| Visceral/subcutaneous fat | 1.07 ± 0.10 | 1.4 ± 0.12* |
| Blood glucose (mg/dl) | 106.2 ± 2.6 | 153.9 ± 6.7* |
Values are means ± SE, n = 4.
p < 0.01 versus WT mice.
3.2. In vitro effects of HO-2 and HO-1 deletion on adipocytes
As seen in Fig. 2, bone marrow derived MSCs from HO-2(−/−) mice demonstrated an increase in adipogenesis and accumulation of lipid droplets compared to the adipocytes derived from WT mice. The results showed a significant decrease (p < 0.01) of adiponectin in the culture media of HO-2(−/−)-derived adipocytes when compared to WT. Inhibition of HO activity, by specific HO-1 siRNAs reduced the levels of released adiponectin from MSC-derived adipocytes (Fig. 2D) indicating that inhibition of both HO-1 and HO-2 decreased adiponectin formation.
Fig. 2.
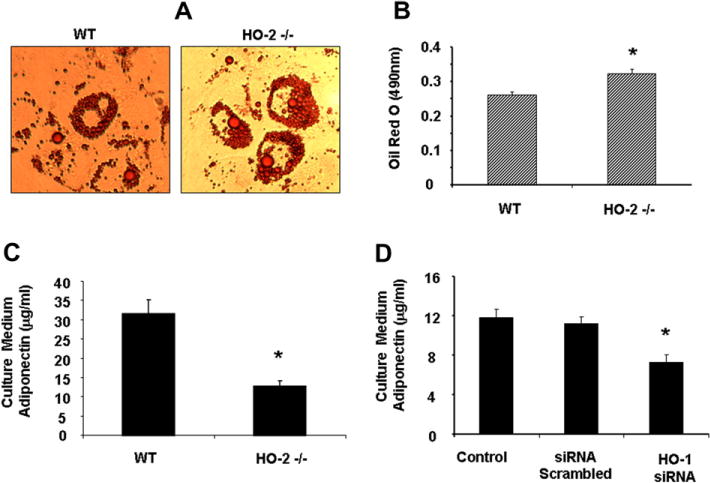
(A) Representative pictures showing WT and HO-2 −/− MSCs during adipocyte differentiation. (B) Adipogenesis was measured as the relative absorbance of Oil Red O at day 15. Data are expressed as mean ± SE (*p < 0.05 compared to WT). (C) Expression of adiponectin levels from culture media samples in WT and HO-2(−/−) MSC-derived adipocytes. (D) Effect of HO-1 siRNA on adiponectin levels from culture media samples in MSC-derived adipocytes. Results are calculated as μg/ml for adiponectin (*p < 0.05 versus control).
3.3. Protein interaction between HO-2/adiponectin and experimental validation
According to the calculated energies of the complexes, HO-2 can bind to the monomeric form of adiponectin while complexes between the multimeric form of adiponectin and HO-2 show unfavorable energies, thus preventing binding. Two hypothetical symmetrical binding regions making contact with two different regions of adiponectin are shown (Fig. 3A). Multiple structural motifs appear to be involved in both the recognition and binding process between HO-2 and adiponectin. In particular, an extended and structured area of 13 amino acids (a.a. 235–247) in HO-2 seems to interact with a specific sequence of adiponectin (a.a. 238–246). Interestingly one single Arginine (R) residue on the HO was found in the two hypothetical contact regions. It is known that the regulation motif of the HO is a short a.a. sequence containing this residue. The in silico adiponectin-HO-2 domains interaction was experimentally validated using the Bacteriomatch two-hybrid system. A Sequence alignment is substantial in order to select which sequence should be tested through the two hybrid system. Homologous sequences were selected then a multiple sequence alignment was carried out using the software ClustalW (http://www.ebi.ac.uk/Tools/msa/clustalw2). Specie-different HO-2 isoforms were compared. The compared regions were: sequences 235–247 and 119–129. The most conservative sequences were cloned and tested. In the bacterial system used, the in vivo interaction between domains allows histidine autotrophy in a his− strain when grown in a M9 minimal medium. Growth in minimal medium persisted even in the presence of 3-AT, an inhibitor of histidine biosynthesis. Finally, resistance to Streptomycin was detected in most clones, even if an uneven phenotypic effect was observed (Fig. 3B). Since Streptomycin resistance was due to transcription of an aadD cistron, this represents the final demonstration of an effective interaction between domains. It should be noted that protein interaction in mouse involves duplicated domains, whereas in bacterial systems interaction only occurs among single domains.
Fig. 3.
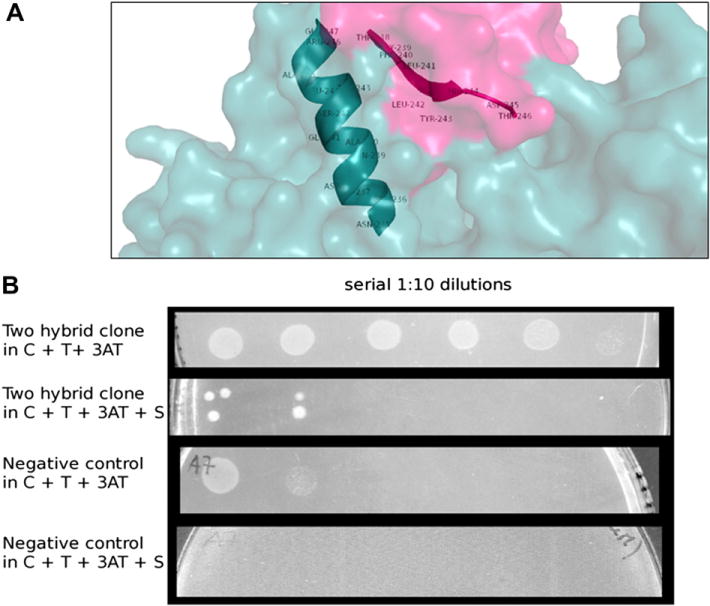
(A) Human heme oxygenase 2-Human adiponectin complex. Residues 238–246 (TGFLLYHDT) from adiponectin and 235–247 (NELDQAGSTLARE) from HO-2 are involved in the interaction. (B) Growth properties of a representative two-hybrid clone (rows 1,2) and a negative control clone (rows 3,4). Suspensions of overnight cultures, were serially (1:10) diluted in saline and spotted onto M9-based selective agar plates. C: Chloramphenicol; T: Tetracycline; 3AT: 3-Amino-1,2,4-triazole; S: Streptomycin.
4. Discussion
Although it was considered to be an inert energy-storage depot, adipose tissue is now considered a major endocrine organ, secreting a plethora of bioactive molecules including adiponectin [24]. Unlike most other adipokines, serum levels of adiponectin are decreased in obesity and major obesity-related pathologies [25–27]. This 30-kDa glycoprotein is synthesized as a single polypeptide in the ER and associates intracellularly, into trimeric, hexameric and other HMW complexes [28]. Despite the fact that different adiponectin oligomers possess distinct biological functions, these oligomeric forms, once released from adipocytes, are not interchangeable, suggesting that adiponectin oligomeric complex distribution in the circulation is primarily controlled at the level of secretion from adipocytes [29,30]. The data presented here shows that HO-2 null mice are characterized by a disruption of metabolic homeostasis manifested by increases in body weight, adiposity, insulin resistance and oxidative stress. Hence, diminished activity of the HO-1/HO-2 system contributes to increased oxidative stress and consequently to derangements in the regulation of adipogenesis. Both HO-1 and HO-2 play a vital role in the regulation of adipogenesis in vitro [31]. Our data (unpublished results) demonstrated that HO-1 siRNA mediated up-regulation of peroxisome proliferator activator receptor (PPARγ), fatty acid binding protein (FABP4) and down regulation/inhibition of Wnt pathway (activated by the major downstream target of adiponectin, AMPK), preventing HO-1 from reducing adipocyte hypertrophy in MSCs. These data supports the hypothesis that adipocytes of smaller size are considered to be healthy, insulin sensitive and capable of producing adiponectin. We show here, an increase in adipogenesis and accumulation of lipid droplets of MSC-derived adipocyte from HO-2 null mice compared to MSC-derived adipocytes from WT mice.
In addition, the levels of adiponectin reported in our in vitro studies mirror those found in vivo. Indeed, HO-2 −/− animals exhibited, when compared to age-matched WT animals, a significant (p < 0.05) decrease in plasma adiponectin and in mRNA adiponectin levels. Interestingly, an increase in HO-1 expression in HO-2 KO mice upregulated plasma adiponectin levels thus corroborating the biological protective effects of both HO-1 and HO-2 in obesity and inflammation and clearly defining the existence of an HO-adiponectin regulatory axis, the HO component of which involves both HO-1 and HO-2. HMW adiponectin levels in circulation change depending upon the metabolic conditions [32]. HMW adiponectin levels are reduced in diabetes and cardiovascular disease states and this decrease could be a consequence of a defect in adiponectin oligomerization. The formation of inter- and intra-molecular disulfide bonds is essential for the maturation of cysteine-containing secretory proteins. However, the formation of sulfide bonds, within the lumen of the ER, is regulated and dependent on a number of oxidoreductases that catalyze disulfide bond exchange. Protein disulfide isomerase (PDI) plays a critical role in this process because of its two distinct properties, one as a molecular chaperone and secondly as a protein-folding catalyst. The role of chaperone proteins is to surround a protein during the folding process, sequestering the protein until folding is complete. PDI contains 2 CXXC (C, cysteine; X, any amino acid) active sites necessary for disulfide bond formation and isomerization [6]. However, these sites are not essential for the molecular chaperone activity of PDI during the refolding of client proteins. HO-2 does not contain CXXC sites, suggesting that HO-2 cannot function specifically as a protein-folding catalyst. Our docking results provide insights in deconvoluting and rationalizing the phenotypic interactions. The interaction between HO-2 and adiponectin could induce a conformational change in adiponectin and thus favor a disulfide bond exchange reaction with PDI, emphasizing the role of HO-2 as a molecular chaperone in the assembly of adiponectin. The specific binding of HO-2 to the monomeric form of adiponectin and when coupled the unfavorable thermodynamics with the multimeric form, suggest that HO-2 is directly involved in the oligomerization of monomeric adiponectin and not in the secretion of the oligomeric forms. The in silico interaction between adiponectin and HO-2 was validated by the two-hybrid system assay. To the best of our knowledge, this is the first report demonstrating an interaction between HO-2/adiponectin and the essential role of HO-2 in stimulating the maturation and the correct folding of adiponectin.
We demonstrate that HO-2 provides a novel function in metabolic syndrome by inducing oligomerization of adiponectin and in addition, may act directly on MSC-derived adipocytes and their progenitors to regulate adipogenesis and adipocytes hypertrophy via interaction with adiponectin. The inability of HO-2 null mice to sustain normal levels of adiponectin may be the result of a decrease in HO-1 expression and an increase in ROS, leading to a miss-folding of adiponectin during protein synthesis which is increased by CoPP-treatment of HO-2 KO mice. It is therefore reasonable to suggest that HO-1 and HO-2 crosstalk exists to regulate adiposity by sustaining the oligomerization of active adiponectin. Finally, our data suggest that HO may possess other important biological actions besides the turn-over regulation of heme proteins. Indeed, HO-1 may migrate to the nuclei under various pathological conditions to control the transcription of early response genes and to regulate heme content [33,34]. In addition, previous reports have indicated the existence of protein–protein interactions involving both HO-1 and HO-2 [31,35,36]. Taken together, our data provide a portal on the role of HO-2 in the development of adiposity and the basis for a new pharmacological strategy for the treatment of metabolic syndrome and its associated vascular dysfunction. We endeavored to understand the principles that determine specificity of molecular recognition at protein–protein interfaces. Thus elucidating the dynamics of protein–protein complexes could lead to the designing of new drugs with the potential of restoring impaired level of adiponectin.
Acknowledgments
This work was supported by grants from the NIH (HL55601 to N.G.A. and HL34300 to M.L.S.). All authors had full access to the data and take responsibility for its integrity. All authors have read and agree with the manuscript as written. We thank Jennifer Brown for her outstanding editorial assistance in the preparation of the manuscript.
Abbreviations
- HO
heme oxygenase
- MSCs
mesenchymal stem cells
- CoPP
cobalt-protoporphyrin IX
- ROS
reactive oxygen species
References
- 1.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 2.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med. 2005;39:1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, et al. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension. 2009;53:508–515. doi: 10.1161/HYPERTENSIONAHA.108.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quan S, Kaminski PM, Yang L, Morita T, Inaba M, Ikehara S, et al. Heme oxygenase-1 prevents superoxide anion-associated endothelial cell sloughing in diabetic rats. Biochem Biophys Res Commun. 2004;315:509–516. doi: 10.1016/j.bbrc.2004.01.086. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF, et al. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57:1526–1535. doi: 10.2337/db07-1764. [DOI] [PubMed] [Google Scholar]
- 6.Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, et al. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol. 2007;27:3716–3731. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bluher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes. 2009;117:241–250. doi: 10.1055/s-0029-1192044. [DOI] [PubMed] [Google Scholar]
- 8.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 9.Abraham NG, Li M, Vanella L, Peterson SJ, Ikehara S, Asprinio D. Bone marrow stem cell transplant into intra-bone cavity prevents type 2 diabetes: role of heme oxygenase-adiponectin. J Autoimmun. 2008;30:128–135. doi: 10.1016/j.jaut.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Burgess A, Li M, Vanella L, Kim DH, Rezzani R, Rodella L, et al. Adipocyte heme oxygenase-1 induction attenuates metabolic syndrome in both male and female obese mice. Hypertension. 2010;56:1124–1130. doi: 10.1161/HYPERTENSIONAHA.110.151423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanella L, Kim DH, Sodhi K, Barbagallo I, Burgess AP, Falck JR, et al. Crosstalk between EET and HO-1 downregulates Bach1 and adipogenic marker expression in mesenchymal stem cell derived adipocytes. Prostaglandins Other Lipid Mediat. 2011;96:54–62. doi: 10.1016/j.prostaglandins.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahal OM, Simmen RC. Paracrine-acting adiponectin promotes mammary epithelial differentiation and synergizes with genistein to enhance transcriptional response to estrogen receptor beta signaling. Endocrinology. 2011;152:3409–3421. doi: 10.1210/en.2011-1085. [DOI] [PubMed] [Google Scholar]
- 13.Sambuceti G, Morbelli S, Vanella L, Kusmic C, Marini C, Massollo M, et al. Diabetes impairs the vascular recruitment of normal stem cells by oxidant damage; reversed by increases in pampk, heme oxygenase-1 and adiponectin. Stem Cells. 2009;27:399–407. doi: 10.1634/stemcells.2008-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang J, Li Y, Huang Y, Lam KS, Hoo RL, Wong WT, et al. Adiponectin prevents diabetic premature senescence of endothelial progenitor cells and promotes endothelial repair by suppressing the p38 MAP kinase/p16INK4A signaling pathway. Diabetes. 2010;59:2949–2959. doi: 10.2337/db10-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 16.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–259. [PubMed] [Google Scholar]
- 17.Briggs DB, Jones CM, Mashalidis EH, Nunez M, Hausrath AC, Wysocki VH, et al. Disulfide-dependent self-assembly of adiponectin octadecamers from trimers and presence of stable octadecameric adiponectin lacking disulfide bonds in vitro. Biochemistry (Moscow) 2009;48:12345–12357. doi: 10.1021/bi9015555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poss KD, Thomas MJ, Ebralidze AK, O’Dell TJ, Tonegawa S. Hippocampal long-term potentiation is normal in heme oxygenase-2 mutant mice. Neuron. 1995;15:867–873. doi: 10.1016/0896-6273(95)90177-9. [DOI] [PubMed] [Google Scholar]
- 19.Sodhi K, Inoue K, Gotlinger K, Canestraro M, Vanella L, Kim DH, et al. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther. 2009;331:906–916. doi: 10.1124/jpet.109.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrusier N, Nussinov R, Wolfson HJ. FireDock: fast interaction refinement in molecular docking. Proteins. 2007;69:139–159. doi: 10.1002/prot.21495. [DOI] [PubMed] [Google Scholar]
- 21.Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res. 2005;33:W363–W367. doi: 10.1093/nar/gki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joung JK, Ramm EI, Pabo CO. A bacterial two-hybrid selection system for studying protein–DNA and protein–protein interactions. Proc Natl Acad Sci USA. 2000;97:7382–7387. doi: 10.1073/pnas.110149297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serebriiskii IG, Milech N, Golemis EA. A bacterial/yeast merged two-hybrid system: protocol for bacterial screening. Methods Mol Biol. 2007;408:291–315. doi: 10.1007/978-1-59745-547-3_16. [DOI] [PubMed] [Google Scholar]
- 24.Cinti S. The adipose organ, Prostaglandins Leukot. Essent Fatty Acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 26.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 27.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 28.Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. Biochem J. 2010;425:41–52. doi: 10.1042/BJ20091045. [DOI] [PubMed] [Google Scholar]
- 29.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 30.Tsao TS, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, et al. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 31.Burgess AP, Vanella L, Bellner L, Gotlinger K, Falck JR, Abraham NG, et al. Heme oxygenase (HO-1) rescue of adipocyte dysfunction in HO-2 deficient mice via recruitment of epoxyeicosatrienoic acids (EETs) and adiponectin. Cell Physiol Biochem. 2012;29:99–110. doi: 10.1159/000337591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 33.Li Volti G, Ientile R, Abraham NG, Vanella A, Cannavo G, Mazza F, et al. Immunocytochemical localization and expression of heme oxygenase-1 in primary astroglial cell cultures during differentiation: effect of glutamate. Biochem Biophys Res Commun. 2004;315:517–524. doi: 10.1016/j.bbrc.2004.01.090. [DOI] [PubMed] [Google Scholar]
- 34.Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- 35.Li Volti G, Galvano F, Frigiola A, Guccione S, Di GC, Forte S, et al. Potential immunoregulatory role of heme oxygenase-1 in human milk: a combined biochemical and molecular modeling approach. J Nutr Biochem. 2010;21:865–871. doi: 10.1016/j.jnutbio.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Weng YH, Yang G, Weiss S, Dennery PA. Interaction between heme oxygenase-1 and -2 proteins. J Biol Chem. 2003;278:50999–51005. doi: 10.1074/jbc.M307644200. [DOI] [PubMed] [Google Scholar]


