Summary
Mucosal sites such as the intestine, oral cavity, nasopharynx, and vagina all have associated commensal flora. The surface of the eye is also a mucosal site, but proof of a living, resident ocular microbiome remains elusive. Here, we used a mouse model of ocular surface disease to reveal that commensals were present in the ocular mucosa and had functional immunological consequences. We isolated one such candidate commensal, Corynebacterium mastitidis and showed that this organism elicited a commensal-specific interleukin 17 response from γδ T cells in the ocular mucosa that was central to local immunity. The commensal-specfic response drove neutrophil recruitment and the release of antimicrobials into the tears, and protected the eye from pathogenic Candida albicans or Pseudomonas aeruginosa infection. Our findings provide direct evidence that a resident commensal microbiome exists on the ocular surface and identify the cellular mechanisms underlying its effects on ocular immune homeostasis and host defense.
Graphical abstract
Although the eye is a mucosal site, there has been a long-standing controversy regarding whether a resident microbiome exists on the ocular surface. St. Leger et al. show that a microorganism that lives on the conjunctiva tunes local mucosal immunity and protects the eye from pathogenic infection.
Introduction
The ocular surface is a mucosal tissue that lies at the interface between the environment and the host immune system and is continually exposed to microbes such as bacteria, fungi, and viruses. It has long been a question whether the ocular mucosa, similar to other mucosal sites, harbors a regular consortium of microbes representing a microbiome. This is contentious because proof of a living microbiome associated with ocular tissue is lacking. The ability of the ocular surface environment to support a resident microbiome is in question due to constant tear washing and the profoundly antimicrobial nature of ocular secretions that contain lysozyme, antimicrobial peptides, immunoglobulin A, complement and other substances (McDermott, 2013). Genetic analyses as well as conjunctival swabs reveal limited amounts (in number and variety) of non-pathogenic and pathogenic bacteria at the ocular surface, but a regular microbial “signature” remains elusive (Dong et al., 2011; Graham et al., 2007; Lee et al., 2012; Shin et al., 2016; Willcox, 2013; Zhou et al., 2014). Current experimental limitations are unable to resolve whether these bacteria are resident (long-term colonizers as opposed to transient, originating from the environment), metabolically active, or even alive. Although there is evidence that bacteria at the ocular surface may affect disease (de Paiva et al., 2016; Kugadas et al., 2016; Zaidi et al., 2014), this does not speak to their status as ocular surface commensals, or how they interact with the immune system to be functionally relevant to local immunity. Rather, these studies conclude that gut microbes might play a major role in disease outcome.
Immune function within the conjunctiva is provided by the eye-associated lymphoid tissue, which is the mucosal tissue of the ocular surface, and includes the lacrimal gland, conjunctiva, and tear ducts. While the lacrimal glands and tear ducts are primarily responsible for the production and drainage of tears, the conjunctiva is thought to help mediate immune responses to promote the health and integrity of the cornea (Knop and Knop, 2005). Within the conjunctiva are immune follicles (Agnifili et al., 2014; Knop and Knop, 2000; Siebelmann et al., 2013), similar to the isolated lymphoid follicles of the gut (ILFs) (Kanamori et al., 1996). These develop after birth and contain all the necessary cells for the generation of immune responses, including antigen-presenting cells (APCs) of myeloid origin, B cells and T cells, up to 50% of which are γδ T cells (Zhang et al., 2012). In experimental mouse models, cells comprising the conjunctival follicles can respond to antigenic stimulation, innate receptor stimulation, and topical application of bacteria (Siebelmann et al., 2013). Thus, microbes can trigger local immune responses at the ocular surface.
Data from clinical studies indicate that ocular use of topical antibiotics correlates positively with fungal infection, suggesting that disruption of the interactions between the immune system and ocular surface microbes renders the eye susceptible to disease (Tanure et al., 2000). In the present study we identified Corynebacterium mastitidis (C. mast) as a candidate resident commensal and dissected the immunological interactions that led to an enhancement of host defense at the ocular surface. Using a mouse model, we demonstrated that C. mast, a commensal organism found in both humans and mice (Bernard et al., 2016; Eguchi et al., 2008; Kobayashi et al., 2015), was able to uniquely colonize the ocular surface and caused γδ T cells within the ocular mucosa to produce interleukin 17 (IL-17). This interaction resulted in the production and release of antimicrobial molecules into the tears and confered protection from invasive Candida albicans and Pseudomonas aeruginosa infection of the ocular surface. We used Koch's postulates (originally coined to link a microbe to a disease phenotype), as criteria to support that the protective phenotype we observed is directly attributable to the presence of C. mast. Our findings indicate that true commensalism with benefit to the host can exist at the ocular surface and uncover the importance of local γδ T cell response in this process. We suggest that tuning of the local immune response by commensals may be necessary to maintain immune homeostasis in the ocular mucosa and may play a broad role in diseases of the ocular surface.
Results
Locally produced IL -17 recruits neutrophils to the conjunctiva in the steady state
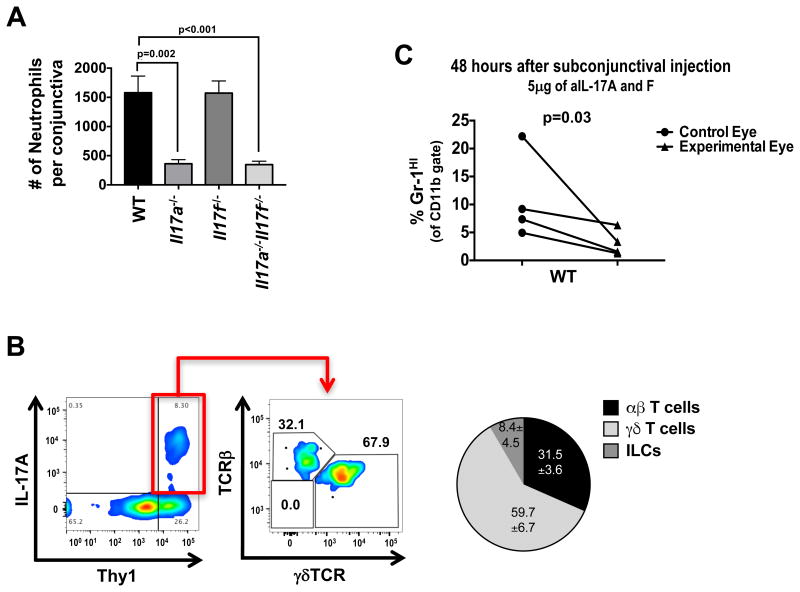
Interleukin (IL)-17 plays a pathogenic role in ocular surface diseases such as Dry Eye Disease (DED) and various forms of infectious keratitis (Chauhan et al., 2009; Suryawanshi et al., 2011; Zaidi et al., 2012). However, in our facility, mice deficient in IL-17A alone or in both IL-17A and IL-17F harbored significantly fewer neutrophils in the conjunctiva at steady state compared to WT controls and single Il17f-/- (Figure 1A). About 50% of Il17a-/-Il17f-/- (but not Il17a-/- or Il17f-/-) mice, developed ocular surface inflammation, which was prevented by including antibiotics in their drinking water. Il17a-/-Il17f-/- were previously reported to be prone to spontaneous Staphylococcus aureus infection (Ishigame et al., 2009). This suggested that, while IL-17A is dominant among the two cytokines in recruiting neutrophils, both contribute to control the outgrowth of inflammation-causing bacteria.
Figure 1. Locally produced IL-17 recruits neutrophils to the conjunctiva.
(A) Neutrophils were quantified by flow cytometry from the conjunctiva of WT, Il17a-/-, Il17f-/-, or Il17a-/-Il17f-/- mice under steady state conditions. (B) Flow plots represent IL-17A production in cells from the conjunctiva after 4 hour PMA and ionomycin stimulation. TCR staining in the right plot represents the gated IL17A+ population in the left plot. Pie chart shows the contribution of IL-17A from each cell subset. (C) WT mice were given single subconjunctival injection of anti-IL-17A & F in one eye and PBS in the contralateral eye. After 48 hours, conjunctiva was harvested and assessed for neutrophils by flow cytometry. Data are representative of ≥5 (A) or 2 experiments (B & C). Symbols represent individual mice. Statistical significance was determined using an (A) unpaired two-way students t test and (C) paired one-way students t test.
Experiments aimed at identifying the IL-17-producing cell population in the conjunctiva revealed that IL-17 was produced by Thy1+ cells (Figure 1B). Consistently, γδ T cells accounted for over 50% of the IL-17-producing cells in the steady state, with lower amounts being produced by αβ T cells and with a modest contribution by TCR-negative cells, consistent with the recently described group 3 innate lymphoid cells (ILC3) (Figure 1B). Neutralization of IL-17 by a subconjunctival injection of anti-IL-17 antibodies was followed after only 48 hours by a significant reduction in neutrophils in the injected eye, compared to the contralateral eye that received an injection of PBS (Figure 1C). These data suggested that IL-17 is continually produced by γδ T cell locally, and that this production was required to maintain neutrophil presence in the conjunctiva during steady state.
An ocular surface bacterium elicits production of IL-17 by conjunctival γδ T cells
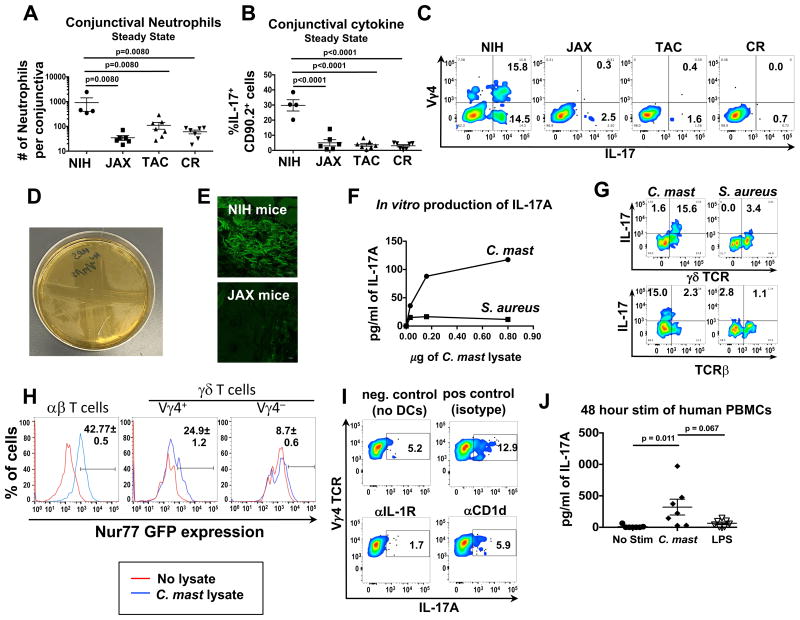
In the course of these studies, we noted that wild-type (WT) C57BL/6 mice bred in our facility consistently had a significantly higher neutrophil presence in the conjunctiva during steady state conditions compared to mice from major commercial vendors (Figure 2A). This correlated with significantly more IL-17+ cells in the conjunctiva of our mice (Figure 2 B). As shown in Fig 1, most of the IL-17 producing cells in the conjunctiva in steady state were γδ T cells; of these about half were Vγ4+ subset (by positive staining with the anti-Vγ4 antibody UC3-10A6) (Dent et al., 1990) (Figure 2C). Of the non-Vγ4 IL-17 producers, less than 5% were γδ T cells while the remaining were αβ T cells and ILCs. In parallel, a higher percentage of γδ T cells in the cervical lymph nodes of mice from our facility demonstrated an activated phenotype (CD44hiCD62Llow) compared to parallel γδ T cells from vendor mice (Figure S1).
Figure 2. Microbial stimulation of conjunctival T cells results in production of IL-17A from γδ T cells.
(A-C) Single-cell suspensions from the conjunctiva of mice from NIH, Jackson Laboratories (“JAX”), Taconic Biosciences (“TAC”), or Charles River (“CR”) were either assessed for (A) neutrophil numbers by flow cytometry or (B & C) IL-17A production after a 4 hours stimulation with PMA and ionomycin in the presence of brefeldin A for 4 hours. (D) C. mastitidis cultured on TSA plates. The streaks are ophthalmic gentamicin gel (Gentak™) applied to the agar showing bacterial sensitivity. (E) Frozen sections of whole eyes (with eyelids) were stained with fluorescent probes against Corynebacterium spp. Pictures are representative of 3 mice. (F & G) Lysates from C. mastitidis or S. aureus were incubated with FACS isolated CD11b+CD11c+and Thy1+ cells from conjunctival tissue. After 72 hours, (F) supernatants were collected and IL-17A was measured by ELISA. (G) brefeldin A was added the last 6 hours of culture, and γδ TCR and IL-17A expression was assessed by flow cytometry. (H) 1 × 104 αβ, Vγ4+ γδ , and Vγ4- γδ Nr4a1GFP reporter mice were incubated with 1 × 105 splenic DCs pulsed with 3 μg for 48 hours. Numbers represent GFP expression ± SEM in cell populations as assessed by flow cytometry. Data are from triplicate wells in an individual experiment. (I) 1×104 FACS isolated γδ T cells from the cervical LN and 1×105 MACS isolated splenic CD11c+ cells were incubated with 3 μg of C. mast lysate and either αIL-1R or αCD1d for 72 hours. Brefeldin A was added the last 6 hours of culture and IL-17A was assessed by flow cytometry. (J) Lysates from C. mastitidis or LPS were incubated with PBMCs from healthy donors for 48 hours at 37°C. Supernatants were collected and assessed for IL-17A by ELISA. Data is pooled from 7 healthy donors from 3 independent experiments (Statistical significance was determined by ANOVA). Bars in A, B, D and F represent the mean ± SEM. (A & C) Symbols represent individual mice from two experiments. (H & I) Data are representative of 2 or 3 independent experiments, respectively. See also Figures S1 & S2.
We hypothesized that IL-17-inducing ocular surface bacteria might be present in our mouse colony, but absent in vendor colonies, similarly to the case of segmented filamentous bacteria (SFB) that are found in Taconic but not in Jackson mice (Ivanov et al., 2009). We therefore spread homogenized conjunctivae of mice from our facility on various agar plates. Similar to previous reports (Graham et al., 2007; Kugadas et al., 2016), various Staphylococci spp. could be cultured from murine conjunctival tissue (limited in numbers of CFU - data not shown). In addition, on trypticase soy agar (TSA) plates that had been incubated for 7 days we observed numerous small, translucent colonies representing a Gram+, gentamicin-sensitive bacterium (Figure 2D), which we subsequently identified by 16S sequencing as Corynebacterium mastitidis (C. mast - Figure S2). Corynebacterium spp. are frequently found in the ocular tissue in human individuals and C. mast is a known skin commensal (Kobayashi et al., 2015; Shin et al., 2016). This organism was consistently cultured from conjunctiva of C57BL/6 mice from our colony as well as from mice sent to us by a collaborator at Washington University, but could not be cultured from conjunctiva of vendor mice. The bacteria could also be detected by Corynebacterium-specific fluorescence in situ hybridization (FISH) (Mark Welch et al., 2016) in NIH, but not in vendor mice (Fig 2E). To assess whether the C. mast we identified was stimulatory to immune cells, we co-incubated C. mast lysate with fluorescence activated cell sorting (FACS) isolated CD11b+CD11c+ dendritic cells and Thy1+ cells from ocular tissue of C57BL/6 mice from our colony. C. mast lysate induced IL-17 production from γδ T cells in a dose dependent manner, which was not the case when the γδ T cells were incubated with Staphylococcus aureus lysate (Figure 2F & 2G).
We asked whether the interaction of C. mast with γδ T cells leading to IL-17 production was purely innate, or involved an interaction with the γδ TCR. To examine this, we used Nr4a1GFP reporter mice, in which intensity of GFP fluorescence reflects the Nur77 gene expression, and hence the strength of antigen receptor signaling (Moran et al., 2011). Three populations of T cells, (αβ T cells, Vγ4+ γδ T cells, and Vγ4- γδ T cells) were isolated by FACS from the cervical lymph nodes of Nr4a1GFP reporter mice bred in our colony. These cells were stimulated with C. mast lysate-pulsed splenic CD11c+ DCs for 48 hours. Although C. mast can stimulate αβ T cells (Kobayashi et al., 2015), we noted that Vγ4+ γδ T cells also expressed GFP, suggesting that γδ T cells can also respond to C. mast, and that the responding population is the Vγ4+ subset (Figure 2H). IL-17 production by the Vγ4 population was dependent on presence of the dendritic cells, and no IL-17 was induced by stimulation of γδ T cells alone (data not shown). Antibody blockade of IL-1 signaling or of the non-classical MHC molecule CD1d inhibited IL-17 production from Vγ4 T cells, supporting involvement of a TCR dependent interaction (Figure 2I).
The C. mast lysate was able to elicit a rapid (48h) IL-17 production from PBMCs of healthy human donors (Figure 2J) suggesting presence of immunity to this organism. Overall, these data illustrated that C. mast, which was isolated from murine conjunctivae, stimulated IL-17 production from mouse γδ T cells and human PBMCs.
Disruption of ocular surface bacteria reduces the immune signature in conjunctival tissue and compromises local host defense against Candida albicans infection
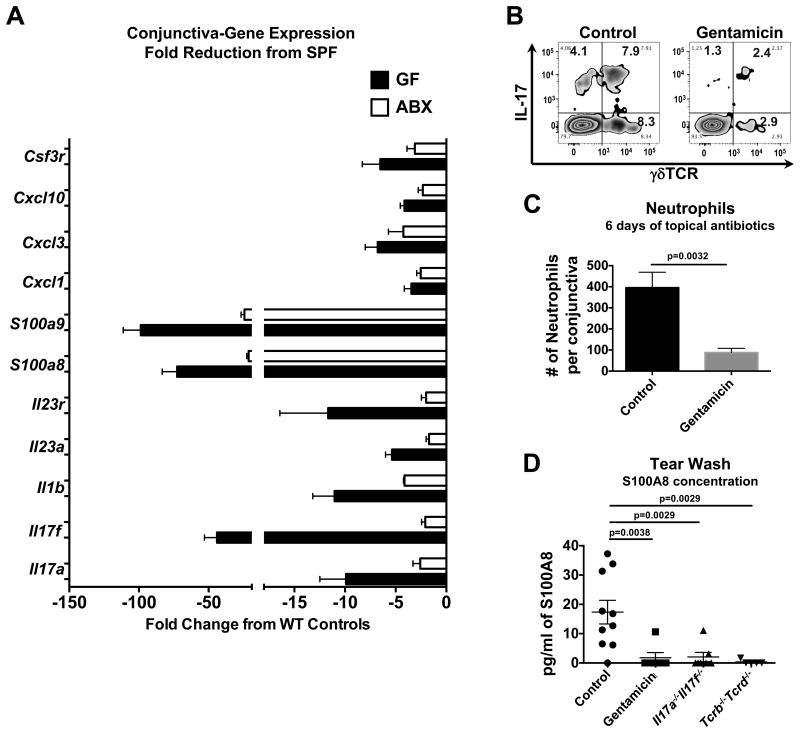
If bacterial presence is responsible for the immune signature in the conjunctiva, it should be reduced if ocular surface bacteria are eliminated. We examined conjunctival tissue of germ-free (GF) mice as well as of mice from our specific pathogen free (SPF) facility that were treated topically for 6 days with gentamicin ophthalmic gel (0.3%, Gentak™), a topical antibiotic commonly prescribed for human ocular infection (Dias et al., 2013) to which C. mast is susceptible in vitro (Figure 2D). The antibiotic-treated as well as the GF mice had reductions in gene expression compared to WT controls including reductions in Il17a and Il17f as well as downstream effectors of IL-17: Cxcl1, Cxcl3, Cxcl10, S100a8, S100a9 (Figure 3A & Figure S3A). Consistent with the gene expression data, we saw concurrent reductions in IL-17-producing γδ T cells (Figure 3B) and neutrophil recruitment to the ocular mucosa (Figure 3C) in gentamicin-treated mice. Additionally, S100A8, a major anti-microbial peptide of the ocular surface when dimerized with S100A9 (Tong et al., 2014), was routinely found in the tears of WT control mice but was not detected in mice treated with gentamicin gel or in Il17a-/-Il17f-/- or Tcrb-/-Tcrd-/- mice (Figure 3D). We concluded from these data that local bacteria at the ocular surface maintained ocular immunity, and transient disruption of bacteria via antibiotics resulted in a reduction in immune-related mechanisms. In addition, T cell-generated IL-17 appears to be critical for the production and release of the anti-microbial, S100A8, into the tears, suggesting that bacteria-T cell interaction was essential for antimicrobial immunity at the ocular surface. Similarly, in conjunctival tissue of germ free (GF) mice, neutrophils and IL-17 were reduced (Figure S3B & S3C), as is soluble immunoglobulin (sIgA) in the tears and lacrimal gland (Figure S3D) and these mice had a poorly developed lacrimal gland cytokine response (Figure S3E & S3F).
Figure 3. Disruption of ocular bacteria reduces the immune signature in conjunctival tissue.
(A-C) WT mice were treated topically with PBS or gentamicin ophthalmic gel daily for 6 days. (A) RNA from conjunctival tissue control WT, antibiotic WT, Germ Free (GF) mice was assessed using Nanostring Technologies' Immunology Gene panel. Bars represent the mean fold change ± SEM in gene expression compared to WT controls. (B) Single-cell suspensions from conjunctival tissue were stimulated with PMA/Ionomycin in the presence of brefeldin A for 4 hours. After stimulation intracellular IL-17A and the γδ TCR was assessed by flow cytometry. (C) Conjunctival neutrophils were quantified by flow cytometry. Bars represent the mean number ± SEM of neutrophils from each eye (n≥10 from 4 independent experiments, statistical significance was determined using Students T test). (D) The ocular surface was washed with 10μl of PBS and diluted in 190 μl of PBS. Dilutions were then assessed for S100A8 concentration using ELISA. Symbols represent the concentration from an individual mouse. Statistical significance was determined using ANOVA. See also Figure S3.
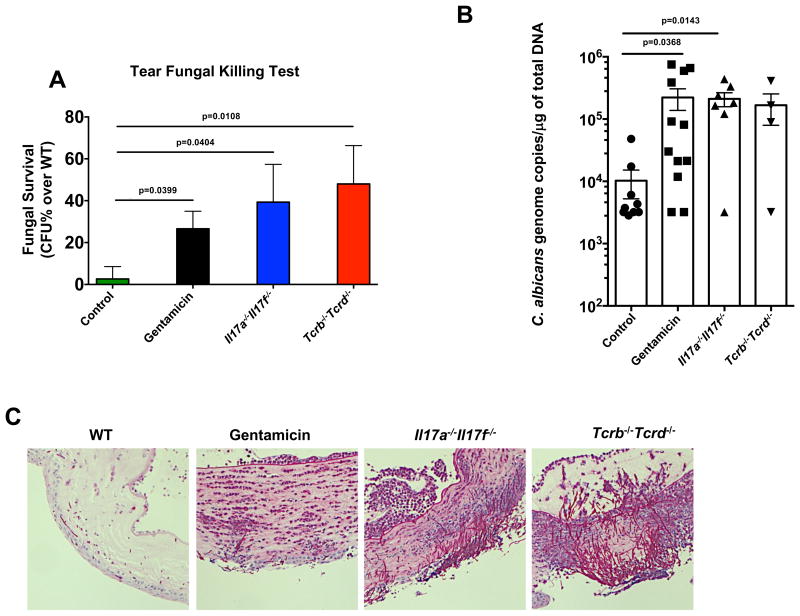
Based on the data depicted in Figure 3, combined with reports in the literature implicating antibiotic use in the rise of ocular fungal infection (Tanure et al., 2000), we wished to examine whether disruption of ocular bacteria would lead to a higher susceptibility to fungal infections of the ocular surface. First, we tested in vitro the ability of tear fluid from unmanipulated or gentamicin-treated WT mice to kill C. albicans, the most common human fungal pathogen, compared to tears of immunodeficient Il17a-/-Il17f-/- and Tcrb-/-Tcrd-/- mice, both of which were colonized with C. mast as determined by culture (data not shown) and FISH data (Figure S4). Significantly more viable C. albicans remained alive and culturable after a 1 hour incubation with tear fluid from antibiotic-treated than from untreated WT mice, resembling the immunodeficient situation (Figure 4A). We next used a model of ocular Candida albicans infection to assess host defense in vivo. C. albicans was applied to the ocular surface of anesthetized mice after gently dabbing the conjunctiva with a cotton swab and corneas were collected for analysis after 15h. Antibiotic-treated mice had 10-fold more C. albicans DNA in corneal homogenates as detected by qPCR (Figure 4B) as well as pronounced fungal penetration into the cornea and tissue destruction (Figures 4C & S4), resembling the situation in immunodeficient mice. In the aggregate, our results thus far suggested that bacteria at the ocular surface, which can be eliminated by antibiotic treatment, were necessary to tune local host defense for protection against fungal infection.
Figure 4. Local antibiotic treatment compromises host resistance to Candida albicans at the ocular surface.
WT mice were topically treated with PBS or gentamicin ophthalmic gel daily for 6 days. After 6 days, (A) the ocular surface of both eyes from a single mouse was washed with 10ul of PBS and fungicidal activity was tested in vitro. Bars represent the mean percentage ± SEM increase in fungal survival after 60 minutes compared to WT controls. (B & C) Then the WT groups, Il17a-/-Il17f-/-, and Tcrb Tcrd-/- mice were ocularly infected with 5 × 105 CFU of Candida albicans. Briefly, mice were anesthetized and the ocular surface was gently dabbed with gauze. C. albicans (strain SC5314) was then applied in 5μl of PBS and remained on the surface for 30 minutes until mice awoke. Fifteen hours after infection, mice were sacrificed. (B) qPCR was used to measure the concentration of fungal DNA in corneal homogenates. Symbols represent individual mice pooled from 2 independent experiments. Statistical significance was determined using Kruskal-Wallis Test. (C) Representative PAS stains of corneas at the end point. See also Figure S4.
The putative ocular commensal C. mastitidis stably colonizes the ocular surface and induces a local immune signature
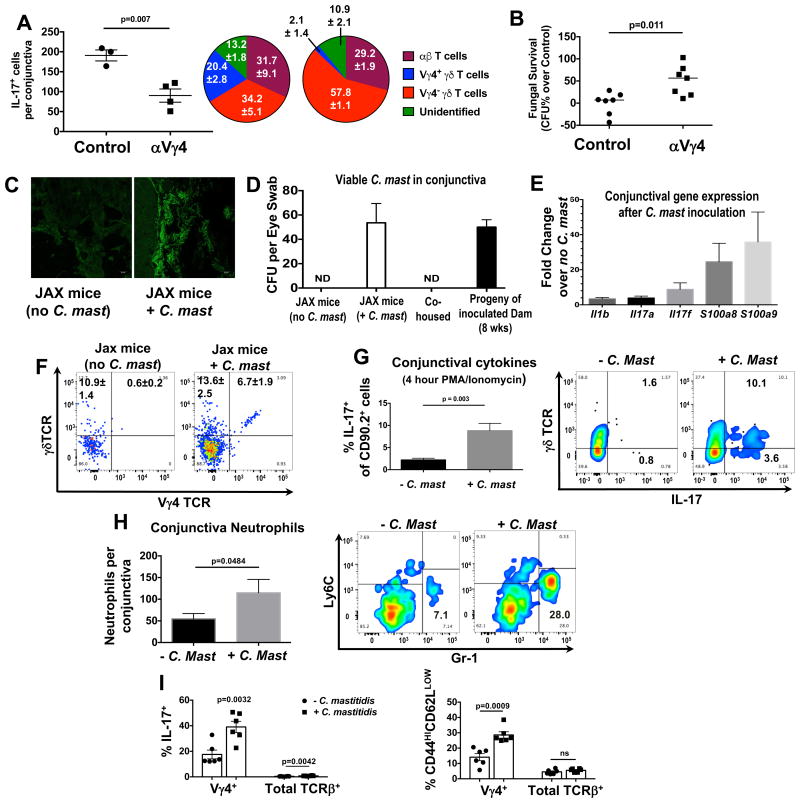
To link the C. mast dependent protective effects to Vγ4+ γδ T cells, we eliminated them from NIH-bred mice using antibody depletion. This resulted in a significant reduction in conjunctival IL-17 production as well as in fungal killing ability of the tear fluid (Figure 5A & B). These data suggested that Vγ4+ T cells, which recognized C. mast, produced sufficient levels of IL-17 to directly enhance the production of anti-microbial components of tears. Therefore, colonization with C. mast should confer a protective γδ T cell-IL-17 phenotype.
Figure 5. Ocular colonization with C. mastitidis induces immunity at the ocular surface and the eye draining lymph nodes.
(A) NIH bred mice were depleted of Vy4+ T cells 4 days before sacrifice, as described in Methods. Conjunctival single-cell suspensions were stimulated with PMA and ionomycin + brefeldin A and IL-17 production was assessed by flow. Bars represent the mean ± SEM of IL-17+ cells after stimulation. Symbols represent individual mice from a single experiment that is representative of two experiments. Pie Charts represent the percent contribution ± SEM of IL-17 production by the various cell populations. (B) Tear wash from these mice was tested for fungicidal activity in vitro, as in Fig 4A. (C-I) Mice from JAX Laboratories were given PBS or were inoculated with 1 × 108 CFU of C. mast. once every three days, totaling three inoculations. (C) After three weeks, frozen sections of whole eyes (with eyelids) were stained with fluorescent probes against Corynebacterium spp. Pictures are representative of 3 mice. (D) After 5 weeks, swabs of conjunctiva were assessed for C. mast. CFU. Swabs were also taken from co-housed mice or 8 wk-old mice born from ocular inoculated dams. (E-I) Three weeks after the final inoculation, mice were sacrificed and the conjunctiva was assessed for (E) immunology-related gene expression, (F) T cells, (G) IL-17A+ cells after 4 hour PMA and ionomycin stimulation, and (H) neutrophil numbers. (C) Bars represent the mean fold change in gene expression ± SEM over uninoculated mice (5 mice from 2 independent experiments) as determined by the Nanostring Mouse Immunology Panel. (F) Representative flow plots displaying the frequency of Vγ4+ γδ T cells of Thy1+ cells in the conjunctiva ± SEM (6 mice from 2 independent experiments. (G & H) Bars represent the mean (G) frequency of IL-17+ cells or (H) number of neutrophils ± SEM in the conjunctiva after inoculation with C. mast (n≥6 from at least 2 experiments). (I) Cervical lymph nodes were harvested and either assessed by flow cytometry for IL-17A production after 4 hour PMA and ionomycin stimulation or for the expression of T cell activation markers, CD44 and CD62L. Symbols represent individual mice and bars represent the mean frequency of IL-17A+ cells or CD44HICD62LLOW cells ± SEM. Statistical significance was determined using students T test (G & H) or ANOVA (I). See also Figure S5.
The ability of bacteria at the ocular surface to affect local immunity did not prove that they are a true resident commensal, similar to the commensals populating other mucosal tissues. To demonstrate this, it was essential to establish stable commensalism in a mouse strain that was not colonized with C. mast. SPF mice at major vendors such as Jackson Laboratories appeared to lack detectable colonization with C. mast and the associated C. mast-dependent immune responses (Figure 2 & Figure 5). Using a modified colonization protocol (Naik et al., 2015; Scharschmidt et al., 2015), we were able to establish commensalism in ocular tissue by lightly dabbing the conjunctiva with a cotton swab and applying 1 × 108 CFU of C. mast once every 3 days for a total of 3 inoculations. The inoculation procedure induced a stable colonization that could be detected by FISH or swabbing as long as 5 weeks after the final inoculation. Of note, co-housing of non-colonized Jax mice with C. mast+ mice from our facility for up to 8 weeks did not result in ocular C mast transmission in terms of culturable bacteria or associated immune response, supporting the notion that this was true commensalism rather than self-re-inoculation from skin or feces. However, all offspring of an inoculated dam had detectable C. mast at the ocular surface when tested 8 weeks after birth, confirming vertical transmission of C. mast to the ocular mucosa within the colony (Figure 5C & D).
Jax mice ocularly colonized with C. mast displayed no ocular surface pathology and acquired an enhanced immune response within the ocular tissue, which included increases in expression of multiple immune-related genes (Figure 5E), expansion of Vγ4+ IL-17 producing γδ T cells (Figure 5F & G) and enhanced neutrophil recruitment (Figure 5H). In addition, IL-17-producing γδ T cells were expanded within the eye-draining cervical lymph nodes and displayed an activated phenotype (Figure 5I). In contrast, there was little change in the IL-17 production and activation phenotype of γδ T cells in peripheral lymphoid compartments, supporting the local nature of the γδ T cell response to ocular C. mast colonization (Figure S5).
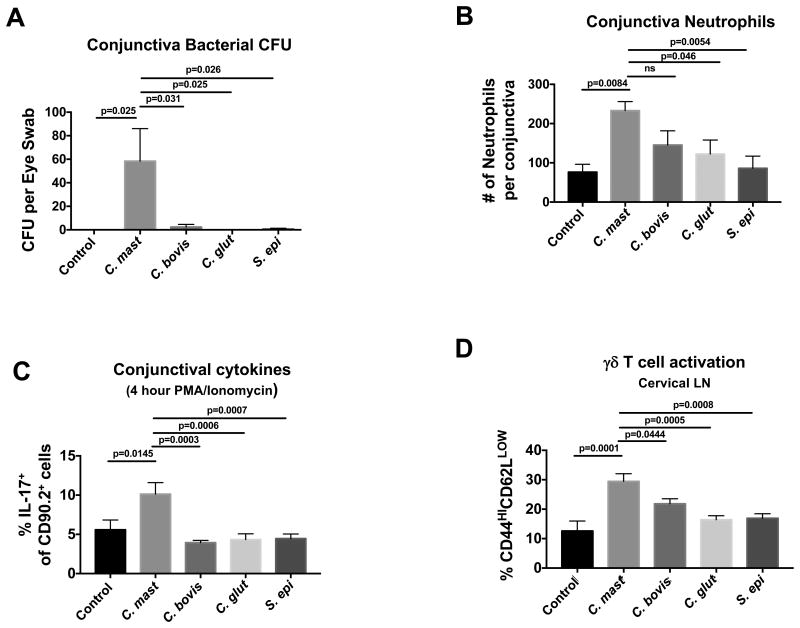
The ability to stably colonize the ocular surface and induce a local immune signature was not a general property possessed by all bacteria, and may not apply to other Corynebacterium spp. We inoculated the conjunctiva of Jax mice with equal numbers of C. mast, C. bovis, C. glutamicum, or Staphylococcus epidermidis, a skin commensal that is frequently identified on the eye. After 3 weeks, only C. mast was detectable on the ocular surface, and only C. mast-inoculated mice exhibited enhanced numbers of neutrophils, IL-17, and γδ T cell activation in the conjunctiva (Figure 6). These findings appear to reveal a unique ability of C. mast to colonize and induce an immune response in ocular tissue.
Figure 6. C. mastitidis, but not other bacteria, persists at the ocular surface and induces immunity.
Mice from JAX Laboratories were given PBS or were inoculated with 1 × 108 CFU of the noted bacteria once every three days totaling three inoculations. After 3 weeks, mice were sacrificed and the conjunctiva was assessed for (A) live bacteria (CFU), (B) neutrophils, and (C) IL-17A producing cells. (D) Cervical lymph nodes were harvested and either assessed by flow cytometry for the expression of T cell activation markers, CD44 and CD62L. Symbols represent individual mice from 2 independent experiments. Bars represent the mean (A) CFU of bacteria (B) number of neutrophils or (C) frequency of IL-17A+ cells or (D) frequency of CD44HICD62LLOW cells ± SEM. Statistical significance was determined using One-Way ANOVA.
C. mast colonization of the ocular surface protects from pathogenic fungal and bacterial infection
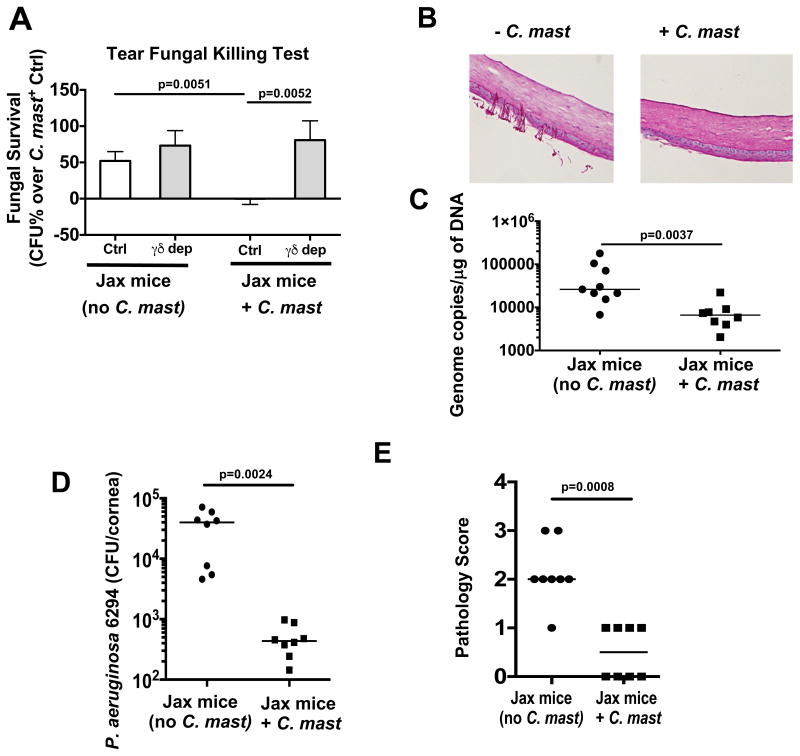
As association of C. mast with the ocular surface of Jax mice appeared to induce an immune signature and expansion of γδ T cells in previously C. mast-negative Jax mice, we asked whether this gain of function would translate to enhanced host resistance to infection with pathogenic organisms. Three weeks after C. mast-association of Jax mice, their tear fluid became more effective at killing C. albicans in vitro compared to the tears of non-associated Jax mice. This was dependent on γδ T cells, as their depletion reduced IL-17 within the eye-draining lymph nodes (Figure S6) and abrogated the enhanced fungal killing ability (Figure 7A). Next, the ocular surface was inoculated with 5 × 105 CFU of C. albicans and the ability of C. albicans to establish infection was examined. At 8 hours post inoculation, mice without C. mast already had fungal filaments embedded in ocular tissue, whereas mice with C. mast had pristine corneas (Figure 7B). In line with this, there was significantly more C. albicans DNA found in corneas of C. mast-negative mice (Figure 7C). To expand our observations from fungal infection to bacterial infection as well, we inoculated C. mast-negative and C. mast-positive Jax mice with Pseudomonas aeruginosa, a common bacterial pathogen of the human ocular surface, and measured bacterial burden and corneal pathology 48h after inoculation. As with C. albicans, mice colonized with C. mast had a significantly lower bacterial burden in their eyes and a lower pathology score (Figure 7 D & E). These data demonstrate that C. mast as an ocular commensal modulated ocular immunity and maintained a γδ T cell-dependent IL-17 response that protected the ocular surface from infection by invasive C. albicans and P. aeruginosa.
Figure 7. Immunity induced by local C. mast colonization protects the ocular surface from pathogenic fungal or bacterial infection.
Mice from JAX Laboratories were given PBS or were inoculated as described previously. Three weeks after the final inoculation, (A) the ocular surface of both eyes from a single mouse was washed with 10ul of PBS and fungicidal activity was tested in vitro. (B & C) The ocular surface was then ocularly infected with 5 × 105 CFU of C. albicans (strain SC5314) and corneas were harvested after 8 hours. (B) Representative PAS stains of cornel sections. (C) qPCR was used to measure the concentration of fungal DNA in corneal homogenates. (D & E) After inoculation with C. mast, mice were ocularly infected with 1×106 CFU of Pseudomonas aeruginosa (strain 6294) After 48 hours, (D) bacterial burden and (E) pathology scores were assessed. Symbols represent samples from individual mice from 2 independent experiments. Statistical significance was determined using Mann-Whitney Test (A & E) or students T test (C & D). See also Figure S6.
Discussion
In the current study, we present evidence that may help resolve the long-standing controversy of whether a resident microbiome exists at the ocular surface. While many published reports now freely use the terminology “resident microbiota” in relation to bacteria detected on the ocular surface, evidence that these organisms have not arrived minutes ago from the surroundings and will not be promptly eliminated by the ocular antimicrobial environment, has not been provided previously. As proof of concept, we demonstrated that a specific commensal, Corynebacterium mastitidis, stably colonized the ocular surface and enhanced the host's ability to resist pathogenic fungal and bacterial infections. Lack of horizontal transmission upheld the notion that C. mast was a bona fide ocular commensal, and that its persistence was not a result of continuous self-re-inoculation from skin or feces. We further dissected its interaction with the local conjunctival immune system and demonstrated that its presence had functional consequences for host defense at the ocular surface. As an ocular commensal conferring a beneficial (rather than a disease) phenotype, C. mast satisfied all four of Koch's postulates for a causative agent, namely: (1) C. mast was present in mice displaying a distinct immune signature in the conjunctiva, but was absent in mice without this immune phenotype; (2) C. mast could be isolated from ocular tissue of mice displaying the phenotype and grown in pure culture; (3) inoculation of C. mast into mice free of C. mast colonization conferred the protective immune phenotype; (4) C. mast could be re-isolated from the inoculated hosts which display the protective immune phenotype.
Our data also uncovered a nonredundant function of γδ T cells, particularly the Vγ4 subset, in IL-17 production in response to C. mast and in host defense at the ocular surface. High numbers of γδ T cells are typically found at barrier surfaces (gut, lung, skin, reproductive tract) and they appear to be somewhat specialized in terms of their TCR usage and diversity. For example, Vγ5 is found in mouse skin, Vγ7 in gut, Vγ6 in the uterus, and Vγ4 in human gut (Goodman and Lefrancois, 1989; Vantourout and Hayday, 2013). As innate T cells, γδ T cells can produce cytokines in response to innate receptor stimulation and previous work has also shown potential recognition of antigens through the γδ TCR (Ramirez-Valle et al., 2015; Sheridan et al., 2013), but little is known about the antigens or MHC restrictions involved in these interactions. High numbers of γδ T cells are also observed in the conjunctiva (Zhang et al., 2012), but no study has thus far investigated what selects and activates them. Our data suggested that C. mast potentially possessesd antigen(s) that triggered Vγ4 T cells at least in part through a TCR driven interaction. The C. mast-derived antigen appears to be presented to the Vγ4 TCR by CD11c+CD11b+ DCs on the non-classical MHC class 1 molecule, CD1d. Our data also supported a role for innate stimulation, as evidenced by the requirement for IL-1 in order to elicit IL-17 production in the γδ T-DC cocultures. An interesting next step would involve identifying the putative antigenic epitope(s) and second signals involved in regulating the γδ T cell response to C. mast in the conjunctiva. Nevertheless, the current data demonstrated that in response to these stimuli Vγ4 T cells accumulated in the conjunctiva and eye-draining LN, where they expressed surface activation markers (CD44HI, CD62LLOW) and produced IL-17, conferring resistance to colonization with pathogenic organisms. A similar protective relationship is found in the gut between SFB and CD4+ T cells, which prevent C. rodentium infection, and between S. epidermidis and CD8+ T cells in the skin, which prevent C. albicans infection (Ivanov et al., 2009; Naik et al., 2015). Thus, in this regard as well, C. mast appeared to behave similarly to other commensals.
In humans, fungi have been linked to posterior ocular diseases like endophthalmitis and chorioretinitis as well as anterior diseases like keratitis. Filamentous fungal keratitis caused by Fusarium or Aspergillus is more commonly associated with tropical climates and ocular trauma; however, keratitis caused by yeast-like fungi, Candida spp., are more closely linked to temperate climates and pre-existing conditions like insufficient tear quality, defective eyelid function, diabetes mellitus, or immunosuppression (Lakhundi et al., 2017; Nivenius and Montan, 2015; Oude Lashof et al., 2011; Romano et al., 1976; Thomas and Kaliamurthy, 2013).
Widespread use of antibiotics is a known risk factor associated with vaginal candidiasis (Ferrer, 2000); however, a causal connection to elimination of bacterial flora has not yet been demonstrated. Antibiotic-induced dysbiosis of the intestinal microbes, specifically in Bacteroides spp., is linked to a reduction in the cathelicidin antimicrobial peptide, CRAMP (LL-37 in humans), which results in the outgrowth of intestinal Candida spp. leading to other systemic pathologies (Fan et al., 2015). In this study, we showed that C. mast acted in a similar fashion by eliciting the production of IL-17 from γδ T cells and its downstream effectors like the antimicrobial, S100A8-S100A9 dimer, which was released into the tears. This process enhanced the killing ability of tear fluid, which directly contributed to the defense of the ocular surface from not only C. albicans but also of P. aeruginosa, a leading cause of bacteria-induced corneal infection and potential blindness.
C. mast is a known skin commensal; however, its presence in the conjunctiva has also been described (Bernard et al., 2016). Our findings showed that C. mast can actively colonize ocular tissue and rather than maintaining its better known rod-like morphology, it appeared to take on a filamentous morphology similar to Corynebacterium spp. reported in the oral cavity. Since some strains of bacteria filament due to stress, the filamentous nature of C. mast supported the notion that despite the ability to persist on the ocular surface, C. mast may perceive this environment as hostile. Indeed, other related strains of Corynebacteria, C. bovis and C. glutamicum, did not appear to be able to colonize the ocular surface. It will be of interest in future studies to dissect what properties make a microorganism into a successful ocular commensal and to examine the presence of C. mast in the human conjunctiva in health vs. disease.
It is conceivable that C. mast might, under some conditions, act also as a pathobiont in the eye. Under conditions of dysbiosis in the skin, this and other Corynebacterium spp. are linked to increases in IL-17 and atopic dermatitis (Kobayashi et al., 2015). While immunosufficient WT mice harboring, or colonized de novo with C. mast, demonstrated no ocular surface pathology as a result, we did detect Corynebacterium by FISH in the conjunctiva of immunodeficient Tcrb-/-Tcrd-/- and Il17a-/-Il17f-/- mice, which developed spontaneous ocular surface inflammation. This suggested, but did not prove, that under conditions of immune deficiency C. mast might act as a pathobiont. Although the load of Corynebacterium in eyes of immunodeficient mice by fluorescence quantitation was lower than in WT, this by itself neither supported nor excluded C. mast as a potential pathobiont. These mice also harbor other bacteria, including S. aureus, and the immune status of the host can disrupt the community compostion of resident bacteria (dysbiosis). We propose that deficient production of IL-17 and its downstream antibacterial mediators would diminish the ability of the host to control C. mast itself as well as other microbes, permitting development of pathology. An in-depth analysis of the complex host-bacterial interrelationships on the ocular surface of an immunodeficient host may provide insights into the factors that affect the balance between a mutualistic and a pathobiont situation.
Many other questions arise, which should be addressed in future investigations. It is currently unknown what makes C. mast able to persist and successfully colonize the ocular surface, whereas a number of related bacteria fail to do so. In addition, why is this bacterium not eliminated from the ocular surface by the IL-17 response that it itself induces, bears further investigation. Further biological and structural investigation into this issue might help to better understand how microbes can evade the anti-microbial mechanisms to colonize ocular mucosa. Finally, it will be of interest to address why horizontal transmission is ineffective and how is C. mast transmitted from mother to offspring.
The ocular surface is an easily accessible site and the possibility of altering microbial communities at the ocular surface has exciting implications on ocular disease. Our findings provided proof of concept that resident ocular commensals exist and have consequences for the establishment and maintenance of ocular immune homeostasis; they furthermore identified a major mechanism whereby this is regulated through interaction of the commensal with local γδ T cells, which afforded protection from pathogenic microorganisms. These findings may have clinical implications not only on the use of antibiotics for ocular surface disease but also for the development of potential probiotic and prebiotic therapies for ocular disease.
Star Methods
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Rachel Caspi (caspir@mail.nih.gov).
Experimental Model and Subject Details
Mice
NIH WT C57BL/6 mice were bred in our facility under SPF conditions. Where noted, WT C57BL/6 mice were purchased from the Jackson Laboratory (“JAX”), Taconic Biosciences (“TAC”), or Charles River Laboratories (“CR”). Nr4a1GFP mice were purchased from Jackson Laboratory and bred in our animal facility. Il17a-/-Il17f-/- mice were generated at The Institute of Medical Science, University of Tokyo, as previously described (Ishigame et al., 2009) and were bred in our facility. Tcrb-/-Tcrd-/- mice (JAX stock # 002122) were generously provided by Dr. Marco Colonna (Washington University). Germ free (GF) mice were a gift from Dr. Yasmine Belkaid (NIAID, NIH) or were purchased from Taconic Farms. For most in vivo animal studies, male and female mice aged 6-10 weeks were used. Only female mice aged 6-10 weeks were used for germ free and inoculation experiments (Figures 5-7, Supplemental Figure S3 and Supplemental Figures S5-S6). Littermates were randomized and used in all experiments but were separated prior to antibiotic treatment or inoculation with bacteria. All mice except germ free were housed under NIH SPF conditions. In cases with germ free mice, mice were shipped directly to the laboratory and sacrificed immediately. All mouse studies were performed in full compliance with IACUC approved protocols (NEI-581 and NIAID/LCID 14E) and institutional guidelines.
Human studies
Research was in compliance with guidelines of the National Institutes of Health Institutional Review Board (Protocol #6145) and all procedures conformed to the tenets of the Declaration of Helsinki. Healthy human PBMCs were received by the NIH blood bank. Red bloods cells (RBCs) were separated using centrifugation (2000g × 15 min. at 25°C) and the ACCUSPIN™ System-Histopaque®-1077. Remaining RBCs were lysed after a 1 min incubation at room temperature (RT) with ACK lysing buffer (Sigma). 5 × 105 Cells were plated in 96 well round bottom plate in 200 μl of DMEM + 10% FBS. In addition cells were stimulated with 10μg of C. mast lysate for 72 hours.
Bacteria
C. mast was originally isolated from conjunctival homogenates that were plated on TSA + 5% blood agar plates kept at 37°C in anaerobic conditions for 7 days. After original isolation, C. mast was propagated using TSA with 5% blood agar plates at 37oC in aerobic conditions. Further, C. mast can be grown in blood heart infusion broth with 1% Tween 80 aerobically with shaking at 37°C. P. aeruginosa was grown on blood agar plates overnight at 37°C, and bacterial concentration was adjusted according to spectrophotometry. C. glutamicum, C. bovis, and S. epidermidis were grown according to ATCC instructions.
Fungi
A single colony was chosen from C. albicans (strain SC5314—used for all experiments) grown for 48 hours at 37°C on YPD (yeast extract peptone dextrose) agar plates. That colony was used to inoculate YPD broth containing penicillin and streptomycin (Mediatech Inc, VA). The yeast was grown at 30oC, with shaking, and serially passaged three times. Growth periods ranged from 18-24 hours at each passage. After the final passage, the yeast cells were harvested va centrifugation at 1400 rpm for 7 minutes, washed twice with PBS, counted using a hemocytometer and adjusted to 5 × 105 CFU/5μl for infection.
Method Details
γδ T cell depletion
Vγ4 γδ T cells in Figure 5 (Clone: UC3-10A6) and total γδ T cells in Figures S6 & 7 (UC7-13D5) were depleted using antibodies from Bio X Cell. Antibodies were administered intraperitoneally in a dose of 500 μg per 200 μl of PBS four days before tear fluid assessment.
Antibiotic treatment
Antibiotic treatment regimen consisted applying an ophthalmic formulation of 0.3% gentamicin gel (Gentak™) to the ocular surface once daily for 6 days.
Bacterial inoculation
Mice were first anesthetized using isoflurane. The conjunctiva was gently dabbed with a KimWipe (Kimberly-Ckar, Radnor PA) or cotton swab to disrupt the tear film. 1 × 108 colony forming units (CFU) of bacteria in 5ml of PBS was applied as a drop to the ocular surface. This procedure was performed once every 3 days for a total of 3 inoculations.
Fungal challenge
Mice were anesthetized with a ketamine/xylazine mixture. The tear film was disrupted using a KimWipe, as above, and 5 × 105 CFU of C. albicans (Strain SC5314) in 5ml of PBS were topically applied to the ocular surface. Mice were then euthanized at 8, 15, or 48 hours post infection and eyes were collected for qPCR analysis for fungal DNA (described below) or were fixed in 10% neutral buffered formalin, Periodic Acid-Schiff (PAS) stained, and sectioned (Histoserv Inc.) for imaging analysis and the highlighting og fungal organisms.
Bacterial challenge
Pseudomonas aeruginosa (strain: 6294) culture and infections were carried out as described previously (Gadjeva et al., 2010; Kugadas et al., 2016; Preston et al., 1995). For evaluation of corneal pathology, daily scores are recorded by a blinded observer according to a graded scale of 0 to 4 as follows: 0, eye macroscopically identical to the uninfected contra-lateral control eye; 1, faint opacity partially covering the pupil; 2, dense opacity covering the pupil; 3, dense opacity covering the entire anterior segment; and 4, perforation of the cornea, phthisis bulbi (shrinkage of the eye), or both.
Tissue harvest and processing
Mice were euthanized by extensive cardiac perfusion to minimize tissue contamination by blood-borne immune cells remaining in the vasculature. Conjunctiva was harvested by excising the eyelid and bulbar conjunctiva, combined, minced and exposed to collagenase for 1 hour at 37° C while shaking. Cervical and submandibular lymph nodes were isolated, minced and exposed to collagenase for 30 minutes at 37° C while shaking. After collagenase treatment, conjunctiva and draining lymph node cells were separately filtered through a 40μm filter using a 3 cc syringe plunger. After this, conjunctival cells were filtered a final time using a Corning Falcon Test Tube with Cel Strainer Snap Cap (Corning 352235).
Conjunctival C. mast quantification
Conjunctivae were swabbed immediately prior to sacrifice and were plated on TSA + 5% blood agar plates. After 72 hours, CFUper swab was calculated by counting individual colonies.
Corneal P. aeruginosa quantification
Corneas were harvested 24 or 48 h after infection, homogenized in 0.05% Triton X100 in 5% FBS-F12, diluted in 5% FBS-F12, and plated for bacterial counts.
Corneal C. albicans quantification (qPCR)
Isolated corneas were homogenized in 300 μl of sterile TENTS (10mM Tris-HCl, pH 8.0 containing 1mM EDTA, 100mM NaCl, 2% Triton X-100 and 1% SDS), mixed with 200 μl phenol:chloroform:isoamyl alcohol (25:24:1, Invitrogen), 300 μl zirconia/silica beads (0.5 mm, BioSpec Products) and homogenized further on FastPrep®-24 (MP Biomedical). After addition of 200 μl TE, pH 8.0, the tubes were briefly vortexed and centrifuged, and the DNA was precipitated from the aqueous phase using 100% ethanol. The DNA was pelleted by centrifugation, washed with 70% ethanol, air-dried, and resuspended in TE, pH 8.0. For qPCR, 20 ng of total DNA, PerfecTa Fast mix (Quanta Biosciences), 400 nM C. albicans specific primers (Willger et al., 2014) and 400 nM hydrolysis probe (AGC ATC TTC CCG CACTTC CCA AA, with 6-FAM at 5′- and Iowa Black quencher at 3′-end) were run in duplicate on a 7500 Real Time PCR machine (Applied Biosystems). The cycling protocol consisted of an initial denaturation step at 95°C (10 min), followed by 40 cycles of 95°C (5 s) and 60°C (30 s). The number of C. albicans genome copies was quantitated by normalizing against a series of standards, prepared by “spiking” uninfected corneas with known CFU quantities (genome copies) of C. albicans.
Tear harvest
For killing assays or ELISAs of tear fluid, after mice were anesthetized with a ketamine/xylazine mixture, 10 μl of PBS was pipetted up and down ten times on the ocular surface of one eye. The same 10 μl was then pipetted up and down ten times on the other eye. This volume was then diluted in 190 μl of PBS. 50 μl was used for each ELISA replicate and 50 μl was used for the fungal killing assay (described below).
Candida killing by tear fluid
104 CFUs of C. albicans strain SC5314, washed and resuspended in 10mM sodium phosphate buffer (pH 7.4), were mixed 1:1 with the tear samples that were diluted 1:20 in PBS. Immediately after mixing, 10 μl volumes were withdrawn, serially diluted and plated on YPD-agar for CFU enumerations (t0). The tear-Candida suspensions were incubated for 1h in a 37°C water-bath, and the tears' killing capacity was assessed by enumerating Candida CFU at t60 relative to CFUs at t0. Fungal survival (Figures 4A, 5B, 7A) was determined by first dividing CFU numbers at t60 by t0 to get “% living” of each sample. The average of the “% living” of the reference group (Figure 4A-“Control”, Figure 5B-Control, Figure 7A-Jax mice + C. mast Ctrl) was calculated. Final values were then calculated by taking experimental “% living” values and dividing by the reference values.
Gene expression analysis
Conjunctival tissue was harvested and immediately homogenized for 30 seconds in RLT buffer using a hand-held tissue homogenizer. RNA was isolated using the RNeasy Mini kit from Qiagen. For analysis, 100 ng of RNA was assessed for the expression of immunology related genes using the Mouse Immunology Kit from Nanostring Technologies (Seattle, WA). Samples were processed at the National Cancer Institute, NIH, gene sequencing core. Copies of mRNA were normalized according to the internal control housekeeping genes. Finally, fold change in gene expression was calculated using the nSolver analysis software (Nanostring Technologies v. 2.5).
Flow cytometry and cell sorting
Conjunctival or lymph node single-cell suspensions were acquired as described above. Single-cell suspensions were stained with fluorescently-conjungated antibodies described in Table 1 for 20 minutes at 4°C. For cytokine assessment, cells were fixed and permeabilized according to instructions for the BD Cytofix/Cytoperm kit (554714) and stained with fluorescently-conjugated antibodies against IL-17A and IFNγ. All flow samples were acquired on a MACS Quant Analyzer 10 (Miltenyi Biotec) and analyzed using Flowjo v. 10. For cell sorting, conjunctiva or draining lymph node samples were stained normally and sorted using a BD FACSAria II or BD FACSAria Fusion (BD Biosciences).
In vitro cell culture
Lymphoid cells were cultured in HL-1 medium supplemented with essential amino acids, β2-mercaptoethanol, and 4% heat-inactivated fetal bovine calf serum (Hyclone, UT) (Cortes et al., 2008). Where noted, immune cells from the conjunctiva or cervical/submandibular lymph nodes were dispersed into single-cell suspensions and stained with fluorescent antibodies. Cell populations of interest were then isolated using Fluorescence Activated Cell Sorting (FACS) using the FACSAria II (BD Biosciences, San Jose, CA). For conjunctival cell cultures, at least 1 × 103 counted events of each Thy1+ and DC+ populations we co-incubated in triplicate in 96-well plate; 2 × 105 bulk LN cells were used for LN cultures. The indicated amount of bacterial lysate was added to each culture, where noted. Cultures were then stimulated for 72 hours. Brefeldin A (Golgi-plug) was added in the last 6 hours of culture. Supernatants were assessed for IL-17 content by ELISA (R&D Systems) and cells were assessed for intracellular IL-17 by flow cytometry. Where noted, CD1d and IL-1R were inhibited by antibodies (5 μg per reaction) purchased from BD Biosciences (clones: 1B1 and 35F5, respectively). In some experiments, bulk single-cell suspensions of conjunctival tissue or LN tissue were stimulated with PMA/Ionomycin in presence of brefeldin A for 4 hours.
Fluorescence in situ hybridization (FISH)
Conjunctiva with eyelids were isolated and immediately frozen in Cryomolds with OCT compound (Tissue-Tek). 10μm sections were prepared on X-tra pre cleaned micro slides using Leica Biosystems cryostat (Leica Biosystems Inc.) and were fixed in 4% paraformaldehyde for 20 minutes at room temperature. AlexaFluor 488-labeled oligonucleotide probe (5′ CCG GAA TTT CAC AGA CGA CG 3′) for Corynebacterium was purchased from Thermo Fisher and FISH was carried out using standard protocol (Mark Welch et al., 2016). Probe was diluted in hybridization solution [900 mM NaCl, 20 mM Tris, pH 7.5, 0.05% SDS, 20% (vol/vol) formamide] and used at 1.5ng/μl concentration. Samples were incubated with probe at 46 °C for 4 hours in a chamber humidified with 20% (vol/vol) form- amide. Slides were then washed three times in wash buffer [900 mM NaCl, 20 mM Tris, pH 7.5, 0.05% SDS, 5 mM EDTA] at 46 °C for 15 minutes. Slides were then washed with cold water and mounted in Vectashield (Vectorlabs). Sections were imaged using a Zeiss LSM 880 Confocal Microscope with a 63× 1.4 N.A. Plan-Apochromat objective at 1 airy unit using excitation with 488 nm laser line. Images were processed and analyzed using Fiji.
Imaging analysis and quantification of C. mast by FISH (for Supplemental Figure S4)
To calculate the fluorescence intensities in conjunctival samples, regions of interest (ROI) were determined and analyzed by FIJI software. Images were processed as follows:
z-proj-max-save image
process-math-subtract = 15000
process – noise- despeckle
process-binary-options-check black background
process-binary-make binary-save binary
edit-selection-create selection
Edit – selection – make inverse (this created ROI)-add as ROI to ROI manager
Use ROI created from binary on the max z proj image and measure
Quantitation and Statistical Analysis
Data were analyzed on GraphPad Prism software v 7.0. Statistical tests, n values, replicate experiments, and p values are all located in the figures and/or legends.
Supplementary Material
Highlights.
Corynebacterium mastitidis colonizes the mouse conjunctiva.
C. mastitidis induces interleukin-17 production from mucosal γδ T cells.
Introduction of the commensal to mice that lack it protects the eye from infection
Topical antibiotics cause the ocular surface to be more susceptible to infection.
Acknowledgments
The authors wish to thank The NEI Flow Cytometry Core for assistance in cell sorting and analysis, and Dr. Marco Colonna for sharing Tcrb-/-Tcrd-/- mice maintained in his colony. This work was supported by the Division of Intramural Research (DIR), National Eye Institute (NEI), and the National Institute of Allergy & Infectious Diseases (NIAID), NIH. This work was also supported by grants from NIH: K99EY025761 (AJS) R01EY022054 (MG) as well as grants from the Prevention of Blindness Society of Metropolitan Washington (AJS) and the American Association of Immunology Fellowship Award (MG). The authors declare no conflicts of interest.
Footnotes
AUTHOR CONTRIBUTIONS: AJS, RRC, MSL and MG designed the experiments. AJS, JVD, RAD, AK, KK, FA and PS performed and analyzed the experiments. YI, MSL and MG contributed unique biological materials and expertise and edited the manuscript. AJS and RRC wrote the manuscript. RRC conceptualized and supervised the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnifili L, Mastropasqua R, Fasanella V, Di Staso S, Mastropasqua A, Brescia L, Mastropasqua L. In vivo confocal microscopy of conjunctiva-associated lymphoid tissue in healthy humans. Invest Ophthalmol Vis Sci. 2014;55:5254–5262. doi: 10.1167/iovs.14-14365. [DOI] [PubMed] [Google Scholar]
- Bernard KA, Pacheco AL, Loomer C, Burdz T, Wiebe D, Huyhn C, Kaplen B, Olson AB, Cnockaert M, Eguchi H, et al. Corynebacterium lowii sp. nov. and Corynebacterium oculi sp. nov., derived from Human Clinical Disease and an Emended Description of Corynebacterium mastitidis. Int J Syst Evol Microbiol. 2016 doi: 10.1099/ijsem.0.001059. [DOI] [PubMed] [Google Scholar]
- Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR, Dana R. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182:1247–1252. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes LM, Mattapallil MJ, Silver PB, Donoso LA, Liou GI, Zhu W, Chan CC, Caspi RR. Repertoire analysis and new pathogenic epitopes of IRBP in C57BL/6 (H-2b) and B10.RIII (H-2r) mice. Invest Ophthalmol Vis Sci. 2008;49:1946–1956. doi: 10.1167/iovs.07-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paiva CS, Jones DB, Stern ME, Bian F, Moore QL, Corbiere S, Streckfus CF, Hutchinson DS, Ajami NJ, Petrosino JF, Pflugfelder SC. Altered Mucosal Microbiome Diversity and Disease Severity in Sjogren Syndrome. Sci Rep. 2016;6:23561. doi: 10.1038/srep23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent AL, Matis LA, Hooshmand F, Widacki SM, Bluestone JA, Hedrick SM. Self-reactive gamma delta T cells are eliminated in the thymus. Nature. 1990;343:714–719. doi: 10.1038/343714a0. [DOI] [PubMed] [Google Scholar]
- Dias C, Goncalves M, Joao A. Epidemiological study of hospital-acquired bacterial conjunctivitis in a level III neonatal unit. ScientificWorldJournal. 2013;2013:163582. doi: 10.1155/2013/163582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q, Brulc JM, Iovieno A, Bates B, Garoutte A, Miller D, Revanna KV, Gao X, Antonopoulos DA, Slepak VZ, Shestopalov VI. Diversity of bacteria at healthy human conjunctiva. Invest Ophthalmol Vis Sci. 2011;52:5408–5413. doi: 10.1167/iovs.10-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi H, Kuwahara T, Miyamoto T, Nakayama-Imaohji H, Ichimura M, Hayashi T, Shiota H. High-level fluoroquinolone resistance in ophthalmic clinical isolates belonging to the species Corynebacterium macginleyi. J Clin Microbiol. 2008;46:527–532. doi: 10.1128/JCM.01741-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D, Coughlin LA, Neubauer MM, Kim J, Kim MS, Zhan X, Simms-Waldrip TR, Xie Y, Hooper LV, Koh AY. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nature medicine. 2015;21:808–814. doi: 10.1038/nm.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer J. Vaginal candidosis: epidemiological and etiological factors. Int J Gynaecol Obstet. 2000;71(Suppl 1):S21–27. doi: 10.1016/s0020-7292(00)00350-7. [DOI] [PubMed] [Google Scholar]
- Gadjeva M, Nagashima J, Zaidi T, Mitchell RA, Pier GB. Inhibition of macrophage migration inhibitory factor ameliorates ocular Pseudomonas aeruginosa-induced keratitis. PLoS Pathog. 2010;6:e1000826. doi: 10.1371/journal.ppat.1000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T, Lefrancois L. Intraepithelial lymphocytes. Anatomical site, not T cell receptor form, dictates phenotype and function. J Exp Med. 1989;170:1569–1581. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JE, Moore JE, Jiru X, Moore JE, Goodall EA, Dooley JS, Hayes VE, Dartt DA, Downes CS, Moore TC. Ocular pathogen or commensal: a PCR-based study of surface bacterial flora in normal and dry eyes. Invest Ophthalmol Vis Sci. 2007;48:5616–5623. doi: 10.1167/iovs.07-0588. [DOI] [PubMed] [Google Scholar]
- Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop E, Knop N. The role of eye-associated lymphoid tissue in corneal immune protection. J Anat. 2005;206:271–285. doi: 10.1111/j.1469-7580.2005.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop N, Knop E. Conjunctiva-associated lymphoid tissue in the human eye. Invest Ophthalmol Vis Sci. 2000;41:1270–1279. [PubMed] [Google Scholar]
- Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, Kong HH, Amagai M, Nagao K. Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity. 2015;42:756–766. doi: 10.1016/j.immuni.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugadas A, Christiansen SH, Sankaranarayanan S, Surana NK, Gauguet S, Kunz R, Fichorova R, Vorup-Jensen T, Gadjeva M. Impact of Microbiota on Resistance to Ocular Pseudomonas aeruginosa-Induced Keratitis. PLoS Pathog. 2016;12:e1005855. doi: 10.1371/journal.ppat.1005855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhundi S, Siddiqui R, Khan NA. Pathogenesis of microbial keratitis. Microbial pathogenesis. 2017;104:97–109. doi: 10.1016/j.micpath.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Lee SH, Oh DH, Jung JY, Kim JC, Jeon CO. Comparative ocular microbial communities in humans with and without blepharitis. Invest Ophthalmol Vis Sci. 2012;53:5585–5593. doi: 10.1167/iovs.12-9922. [DOI] [PubMed] [Google Scholar]
- Mark Welch JL, Rossetti BJ, Rieken CW, Dewhirst FE, Borisy GG. Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci U S A. 2016;113:E791–800. doi: 10.1073/pnas.1522149113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AM. Antimicrobial compounds in tears. Exp Eye Res. 2013;117:53–61. doi: 10.1016/j.exer.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015;520:104–108. doi: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivenius E, Montan P. Candida albicans should be considered when managing keratitis in Atopic keratoconjunctivitis. Acta ophthalmologica. 2015;93:579–580. doi: 10.1111/aos.12708. [DOI] [PubMed] [Google Scholar]
- Oude Lashof AM, Rothova A, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, Schlamm HT, Oborska IT, Rex JH, Kullberg BJ. Ocular manifestations of candidemia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53:262–268. doi: 10.1093/cid/cir355. [DOI] [PubMed] [Google Scholar]
- Preston MJ, Fleiszig SM, Zaidi TS, Goldberg JB, Shortridge VD, Vasil ML, Pier GB. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect Immun. 1995;63:3497–3501. doi: 10.1128/iai.63.9.3497-3501.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Valle F, Gray EE, Cyster JG. Inflammation induces dermal Vgamma4+ gammadeltaT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proc Natl Acad Sci U S A. 2015;112:8046–8051. doi: 10.1073/pnas.1508990112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A, Segal E, Eylan E, Stein R. Treatment of external ocular Candida infections with 5-fluorocytosine. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1976;172:282–286. doi: 10.1159/000307726. [DOI] [PubMed] [Google Scholar]
- Scharschmidt TC, Vasquez KS, Truong HA, Gearty SV, Pauli ML, Nosbaum A, Gratz IK, Otto M, Moon JJ, Liese J, et al. A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity. 2015;43:1011–1021. doi: 10.1016/j.immuni.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan BS, Romagnoli PA, Pham QM, Fu HH, Alonzo F, 3rd, Schubert WD, Freitag NE, Lefrancois L. gammadelta T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity. 2013;39:184–195. doi: 10.1016/j.immuni.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Price K, Albert L, Dodick J, Park L, Dominguez-Bello MG. Changes in the Eye Microbiota Associated with Contact Lens Wearing. MBio. 2016;7 doi: 10.1128/mBio.00198-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebelmann S, Gehlsen U, Huttmann G, Koop N, Bolke T, Gebert A, Stern ME, Niederkorn JY, Steven P. Development, alteration and real time dynamics of conjunctiva-associated lymphoid tissue. PLoS One. 2013;8:e82355. doi: 10.1371/journal.pone.0082355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawanshi A, Veiga-Parga T, Rajasagi NK, Reddy PB, Sehrawat S, Sharma S, Rouse BT. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol. 2011;187:1919–1930. doi: 10.4049/jimmunol.1100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanure MA, Cohen EJ, Sudesh S, Rapuano CJ, Laibson PR. Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea. 2000;19:307–312. doi: 10.1097/00003226-200005000-00010. [DOI] [PubMed] [Google Scholar]
- Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2013;19:210–220. doi: 10.1111/1469-0691.12126. [DOI] [PubMed] [Google Scholar]
- Tong L, Lan W, Lim RR, Chaurasia SS. S100A proteins as molecular targets in the ocular surface inflammatory diseases. Ocul Surf. 2014;12:23–31. doi: 10.1016/j.jtos.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox MD. Characterization of the normal microbiota of the ocular surface. Exp Eye Res. 2013;117:99–105. doi: 10.1016/j.exer.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Willger SD, Grim SL, Dolben EL, Shipunova A, Hampton TH, Morrison HG, Filkins LM, O'Toole GA, Moulton LA, Ashare A, et al. Characterization and quantification of the fungal microbiome in serial samples from individuals with cystic fibrosis. Microbiome. 2014;2:40. doi: 10.1186/2049-2618-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi T, Zaidi T, Cywes-Bentley C, Lu R, Priebe GP, Pier GB. Microbiota-driven immune cellular maturation is essential for antibody-mediated adaptive immunity to Staphylococcus aureus infection in the eye. Infect Immun. 2014;82:3483–3491. doi: 10.1128/IAI.01951-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi TS, Zaidi T, Pier GB, Priebe GP. Topical neutralization of interleukin-17 during experimental Pseudomonas aeruginosa corneal infection promotes bacterial clearance and reduces pathology. Infect Immun. 2012;80:3706–3712. doi: 10.1128/IAI.00249-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Volpe EA, Gandhi NB, Schaumburg CS, Siemasko KF, Pangelinan SB, Kelly SD, Hayday AC, Li DQ, Stern ME, et al. NK cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoS One. 2012;7:e36822. doi: 10.1371/journal.pone.0036822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Holland MJ, Makalo P, Joof H, Roberts CH, Mabey DC, Bailey RL, Burton MJ, Weinstock GM, Burr SE. The conjunctival microbiome in health and trachomatous disease: a case control study. Genome Med. 2014;6:99. doi: 10.1186/s13073-014-0099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.