Abstract
Neurocardiology is an emerging specialty that addresses the interaction between the brain and the heart, i.e. the effects of cardiac injury on the brain, and the effects of brain injury on the heart. This review article focuses on cardiac dysfunction in the setting of stroke such as ischemic stroke, brain hemorrhage and subarachnoid hemorrhage (SAH). The majority of post stroke deaths are attributed to neurological damage, and cardiovascular complications are the second leading cause of post stroke mortality. Accumulating clinical and experimental evidence suggests a causal relationship between brain damage and heart dysfunction. Thus, it is important to determine whether cardiac dysfunction is triggered by stroke, is an unrelated complication, or is the underlying cause of stroke. Stroke induced cardiac damage may lead to fatality or potentially lifelong cardiac problems (such as heart failure), or to mild and recoverable damage such as neurogenic stress cardiomyopathy (NSC) and Takotsubo cardiomyopathy. The role of location and lateralization of brain lesions after stroke in brain-heart interaction, clinical biomarkers and manifestations of cardiac complications, and underlying mechanisms of brain-heart interaction following stroke, such as: the hypothalamic pituitary adrenal axis (HPA); catecholamine surge; sympathetic and parasympathetic regulation; microvesicles (MV’s); microRNAs; gut microbiome, immunoresponse and systemic inflammation are discussed.
Keywords: Stroke, Cardiac-dysfunction, Brain-Heart axis, Inflammation, Gut-microbiome
Subject Terms: Ischemia, Mechanisms
1. Introduction
Cardiac injury is common in patients with cerebrovascular disease1–3. In 1947, Byer and colleagues first reported that cerebral vascular disease can cause myocardial damage and arrhythmia4. The specialty that deals with the brain-heart connection is now referred to as neurocardiology5. Typically, stroke (ischemic stroke, brain hemorrhage and subarachnoid hemorrhage (SAH)) induces neurovascular uncoupling and disrupts cerebral auto-regulation, which then renders cerebral blood flow directly dependent upon cardiac function6. Multiple interactions occur among the various forms of cardiovascular and cerebrovascular diseases. Myocardial injury, ischemia like electrocardiographic (ECG) changes and arrhythmias are frequently encountered in acute stroke patients, even in the absence of primary heart disease, which support a central nervous system (CNS) origin of these ECG abnormalities1–3, 7. Using a meta analysis, including 25 studies with a total of 2,690 patients, Bilt et al. found that cardiac dysfunction is associated with an increased risk of death, delayed cerebral ischemia and poor outcome after SAH8. On the basis of accumulating clinical evidence, it is likely that there exists a causal relationship between brain damage and heart dysfunction. It is important to determine whether cardiac dysfunction in patients is triggered by stroke, is an unrelated complication, or is the underlying cause of stroke. Therefore, investigating the brain-heart interaction after stroke is highly clinically significant.
Scope of this review
A multitude of brain injuries such as stroke (ischemic stroke, brain hemorrhage or SAH), traumatic brain injury (TBI), brain tumor and various causes of intracranial hypertension can lead to cardiac dysfunction, arrhythmias and heart failure. Brain-heart syndrome broadly refers to heart damage caused by various brain disorders. The ability to accurately diagnose the occurrence and development of brain-heart syndrome is highly valued in the clinic. This review article focuses on brain-heart interaction after stroke of CNS origin. The role of location and lateralization of brain lesions in brain-heart interaction, clinical manifestations, pathophysiology and mechanisms of brain-heart interaction after stroke are discussed.
Cardiac damage and stroke in the light of risk factors and pre-existing heart disease
Cardiac complications leading to morbidity and even mortality in the days immediately following an acute stroke include heart attack, congestive heart failure, cardiac arrest and abnormal heart rhythms like atrial fibrillation5. Converging risk factors for cerebrovascular and cardiovascular diseases such as hypertension, diabetes, high cholesterol, and age, exacerbate cardiac injury irrespective of stroke cause or subtype9. Therefore, severe heart problems are more likely due to systemic dysfunction induced vascular damage, inflammation and immune responses such as in hypertension and diabetes, rather than a direct neural causation, although brain damage may act to worsen cardiac dysfunction10, 11. The Framingham study reported that stroke incidence more than doubled in the presence of coronary heart disease, more than tripled with hypertension, increased four fold with cardiac failure, and increased five fold with atrial fibrillation9. Approximately 20% of ischemic strokes are caused by cardiac disease, the major risk factor being atrial fibrillation9. In addition to being the most common tachyarrhythmia in acute stroke, atrial fibrillation is also highly associated with an increased risk of systemic thromboembolism9, 12. A history of cardiovascular diseases and hypertension increases incidence of ECG abnormalities in comparison to ischemic stroke patients with no primary heart disease13. The aging population is largely affected by heart failure due to structural or functional damage to the heart affecting ventricular blood filling and/or ejection fraction14. In this review, to specifically elucidate the brain-heart interaction of CNS origin in the setting of stroke, we discuss the mechanisms of brain-heart interaction after stroke without pre-existing heart disease. How diabetes, hypertension, age, sex, atrial fibrillation, and pre-existing heart disease influence brain-heart interaction are beyond the scope of this review and are not discussed.
2. Cardiac damage as a consequence of stroke
The second leading cause of post stroke death is cardiovascular complications15. Broadly, stroke induced cardiac damage may lead to fatality, potentially lifelong cardiac problems (such as heart failure), or mild and recoverable damage such as neurogenic stress cardiomyopathy (NSC) and Takotsubo cardiomyopathy. Cardiac dysfunction manifested during the acute phase after brain injury usually resolves over the following several weeks alongside improvement of neurological function15. Stabilization of the patient in relation to the type of stroke takes precedence over treatment of the heart dysfunction. NSC is diagnosed by reduced left ventricular ejection fraction (LVEF), ventricular wall motion abnormalities, and elevated serum cardiac enzymes. While Takotsubo cardiomyopathy shares the symptoms and transient nature of NSC, it is caused by psychological stress in the absence of physical damage to the brain. The telltale sign of Takotsubo cardiomyopathy is apical ballooning, caused by a weakening of the heart’s muscular cells. Still, Takotsubo cardiomyopathy and NSC may induce ventricular wall motion abnormalities in other regions of the heart16, 17. Many studies focus on the impaired contraction of the ventricle which results in low ejection fraction, but it appears that the relaxation of the ventricle is also disrupted in NSC. Clinical symptoms of cardiac dysfunction after brain hemorrhagic stroke and ischemic stroke are summarized in Table 1.
Table 1.
Summary of clinical manifestation of cardiac dysfunction following stroke in patients
| Subarachnoid hemorrhage (SAH) | Ischemic stroke (IS) | Comments | |
|---|---|---|---|
| Takotsubo cardiomyopathy (TTC) |
|
|
|
| Stress-induced cardiomyopathy |
|
|
|
|
|||
| Abnormal cardiac function and ECG abnormalities |
|
|
|
|
|||
| Secondary Complications |
|
||
| Recovery of Cardiac function | |||
Abbreviations: cTn: Cardiac Troponin; ECG: Electrocardiogram; EF: Ejection fraction; IS: Ischemic stroke; LV: Left ventricular; LVDD: Left ventricular diastolic dysfunction; LVEF: Left ventricular ejection fraction; LVH: Left ventricular hypertrophy; RWMA: Regional wall motion abnormalities; SAH: Subarachnoid hemorrhage; TTC: Takotsubo cardiomyopathy; WMA: Wall motion abnormalities.
2.1 Hemorrhagic stroke induced cardiac damage
Experimental animal studies as well as clinical data indicate a gradient of NSC, where ECG abnormalities are found in 40–100% of SAH patients and 5% SAH patients have serious cardiac arrhythmias18. Cardiac arrhythmias are associated with an increased risk of cardiovascular comorbidity, prolonged hospital stay and poor outcome or death after SAH18. The life-threatening ventricular arrhythmia, is also associated with elevated creatine phosphokinase-myocardial fraction (CKMB) mass and increased Troponin T level in left side of intracerebral hemorrhagic stroke patients19. 80% of patients exhibit ischemic-like ECG changes within 1 year after intracerebral hemorrhage or SAH20. In SAH patients, the Hunt Hess grading score of severity shows that ECG changes are observed consistently at the lower grades, but echocardiography changes are found predominately in patients with higher grades21. It is likely that less severe ECG changes precede structural alterations, as QTc (the interval between peak contraction and repolarization) prolongation does not predict ventricular wall motion abnormalities, but more severe ECG changes like inverted T waves and elevated Troponin levels, secreted by damaged sarcomeres, can predict ventricular wall motion abnormalities16. Lee et al. found that in spontaneous and traumatic brain hemorrhage patients22, 7.2% (no previous history of cardiac disease patients) show acute cardiac dysfunction and 43% have LV hypertrophy22. Acute cardiac dysfunction is independently associated with in-hospital death22, 23, and associated with a diminished 1-year functional outcome20. Left-ventricular diastolic dysfunction (LVDD) reflects abnormalities in atrial filling by venous return, atrial contraction, and ventricular relaxation capabilities. While LVDD is a cause of cardio-embolic stroke (present in 15%-25% of the population, increasing with age and hypertension24), more than half of SAH patients develop LVDD, despite suffering a stroke of non-cardioembolic origin.
2.2 Ischemic stroke induced cardiac damage
The risk of cardiac complications increases proportionally to the severity of ischemic stroke and neurological deficits15. Likewise, impaired cardiac function as a consequence of severe acute ischemic stroke is a predictor of worse functional outcome and secondary complications25. Clinically relevant secondary complications such as vasospasm, delayed cerebral ischemia and pulmonary edema require active management. Recent evidence suggests that ischemic stroke can cause cardiac dysfunction even in the absence of risk factors and pre existing heart disease26. During the first 3 months after acute ischemic stroke, 19.0% of patients suffer from at least one serious cardiac adverse event; 28.5% have LVEF less than 50%; and 13–29% have systolic dysfunction27, 28. Approximately 67% of acute ischemic stroke patients have ECG abnormalities of ischemic and/or arrhythmic in the first 24 hours after stroke29. Cardiac arrhythmias are common reasons for death after acute ischemic stroke26. About 88% of stroke patients who sustain damage to the insular cortex in the right cerebral hemisphere develop myocardial injury in the weeks following ischemic stroke1.
Stroke (lacunar subtype) also induces heart problems in up to 70% of patients, with clinical manifestations such as ECG changes, reduced LVEF, ventricular wall motion abnormalities, and increases in serum cardiac enzymes28, 30. However, there is a paucity of studies investigating the mechanisms of small or lacunar stroke induced cardiac dysfunction in both clinical and experimental research.
2.3 Myocardial enzymes
Cardiac Troponin I (cTnI) is considered a more specific and sensitive biomarker for the detection of cardiac damage and LV dysfunction than CK-MB31. CK-MB is not completely cardiac specific and may increase in skeletal muscle injury, kidney failure, intramuscular injection, strenuous exercise, and after exposure to several toxins and drugs31. CK-MB elevations are most likely of non-cardiac origin in patients with large hemispheric stroke31. Circulating cTnI is associated with clinical outcomes and cardiac function ST-segment elevation myocardial infarction patients32 as well as with advanced myocardial hypertrophy, fibrosis and increased cardiovascular death33, 34.
Serum cTnI or cTnT (Cardiac Troponin T) levels are indicators of cardiac damage after ischemic stroke, intracerebral hemorrhage stroke and SAH. Elevation of serum myocardial enzymes is reported in 11–21% of SAH patients and in 1–17% of ischemic stroke patients35. SAH patients have higher levels of circulating high-sensitivity Troponin T (hsTnT) and N-terminal fragment of B-type natriuretic peptide (NTproBNP)36, 37. 22% of brain hemorrhage patients have Troponin I elevation and 59% have NT-proBNP elevation38. In acute ischemic stroke patients, 5–8% patients have high level of circulating cTnI and cTnT39, 40, and 65% patients have increased levels of circulating NT-proBNP40. A recent study reported that patients who suffered acute ischemic stroke in the dorsal anterior insular cortical region of the right hemisphere had elevated relative changes of high sensitivity cTnT levels, which can induce autonomic dysbalance, sympathetic activation and myocardial injury41.
The level of hsTnT and NTproBNP are strongly related with poor long-term outcome in SAH patients, and are associated with increased stroke severity, mortality, and worse neurological functional outcome after ischemic stroke36, 42, 43. However, using multivariate regression analysis, Etgen et al. reported that neither cardiac Troponins nor NT-proBNP influence morbidity and mortality if other risk factors are considered44. Nigro et al found that BNP, but not hsTnT level, predicts short and long term prognosis after cerebrovascular events42. Among the reported independent predictors of elevated cTnI are female sex, larger body surface area and higher heart rate45. The American Stroke Association recommends a baseline ECG and baseline Troponin assessment in patients with acute stroke to identify concurrent myocardial ischemia or cardiac arrhythmias46. Myocardial enzymes and biomarkers of cardiac dysfunction after brain hemorrhage and ischemic stroke are summarized in Table 2.
Table 2.
Summary of biomarkers of cardiac dysfunction following stroke in patients
| Subarachnoid hemorrhage (SAH) | Ischemic stroke (IS) | Comments | |
|---|---|---|---|
| Creatine kinase-MB (CK-MB) |
|
|
|
| Cardiac Troponins (cTn) (cTn T and cTn I) |
|
|
|
| C-Reactive Protein (CRP) |
|
||
| N-terminal pro-brain natriuretic peptide (NT-proBNP) |
|
|
|
Abbreviations: CK-MB: Creatine kinase-MB; CRP: C-Reactive Protein; cTn: Cardiac Troponin; IS: Ischemic stroke; LV: Left ventricular; LVDD: Left ventricular diastolic dysfunction; NT-proBNP: N-terminal pro-brain natriuretic peptide; SAH: Subarachnoid hemorrhage; TTC: Takotsubo cardiomyopathy.
2.4 Heart rate variability (HRV)
Heart rate variability serves as an assessment tool for autonomic cardiac control and is a measurement of the variation in the heart beat interval (interval between successive R peaks in the QRS complex of ECG) reflecting the instantaneous fluctuation in heart rate. Heart rate fluctuation may result from the neurohumoral regulation of the body in order to adapt to different physiological conditions, or in response to certain pathological conditions. Heart rhythm oscillations may be categorized into 4 primary frequency bands: high-frequency (HF), low-frequency (LF), very-low-frequency (VLF), and ultra-low-frequency (ULF). Respiration modulates vagal (parasympathetic) activity which contributes to the HF component of heart rate variability47. LF is thought to be the combined sympathetic and vagal response to arterial baroreceptors48. Although not an accurate measure, it is commonly interpreted that a low LF to HF ratio reflects greater parasympathetic activity relative to sympathetic activity, while a high LF to HF ratio indicates higher sympathetic activity relative to parasympathetic activity47. Dysfunction in the CNS can have downstream effects on spinal cord pre-ganglionic autonomic nerves and result in HRV changes. Changes in HRV may represent an inadequate response of the autonomic nervous system to the stress associated with a more severe condition49. Studies have indicated a neural source of HRV changes in ischemic stroke patients50. In SAH patients, lowered LF/HF ratio is decreased in patients with pulmonary edema and indicates poor quality recovery and high mortality51. Left hemisphere lesions in ischemic stroke patients induce increased HF (parasympathetic) rhythms, while right hemisphere lesions increase the LF/HF ratio52. HRV decrease is associated with acute stroke severity, post stroke depression, poor-quality recovery, and mortality49, 53.
3. Mechanisms of brain-heart interaction after stroke
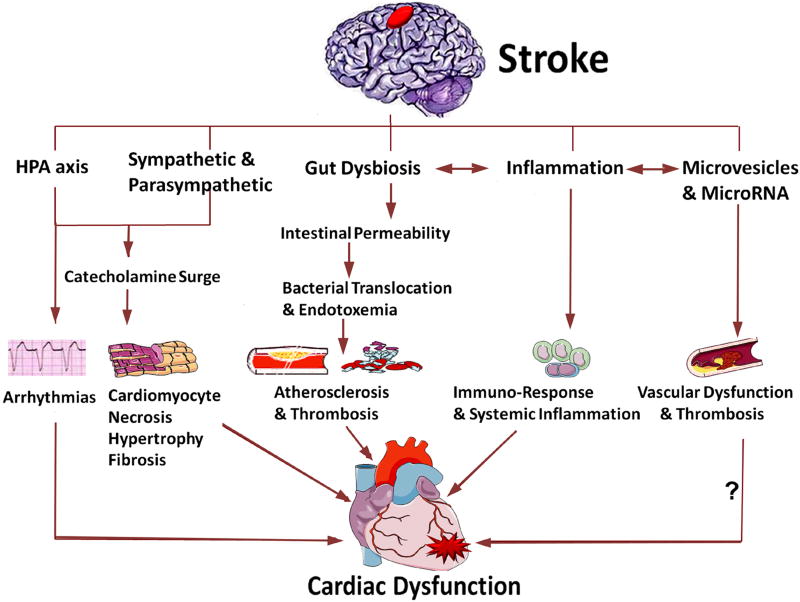
The mechanisms of brain heart interaction resulting in cardiac dysfunction after injury to the brain are discussed below and summarized in Figure 1.
Figure 1. Summary of mechanisms for brain heart interaction after stroke.
Cardiac dysfunction after stroke may be caused by several mechanisms, including activation of the HPA axis, sympathetic and parasympathetic regulation, catecholamine surge, gut microbiome dysbiosis, immune responses and inflammation as well as the release of microvesicles and microRNA (? indicates that future studies are warranted).
3.1 Hypothalamic-pituitary-adrenal (HPA) axis, catecholamine surge and sympathetic and parasympathetic regulation after stroke
Many patients develop early-onset depression related to the degree of their functional deficits, or late-onset depression54, 55. Post stroke depression including anxiety, fear, and stress is psychological as well as biochemical in nature, and is largely dependent on the extent of neurological functional deficits54, 55. Ischemic stress can cause Takotsubo cardiomyopathy15. The HPA axis is a master regulator of body hormones that integrates emotion, stress, physical activity state, and metabolism56. The HPA axis consisting of the complex interactions between the 3 endocrine glands: hypothalamus, pituitary and adrenal glands, is an important part of the neuroendocrine system. The hypothalamic paraventricular nucleus is the main control center of the HPA, and secretes corticotropin-releasing factor such as corticotrophin-releasing-hormone and vasopressin, that stimulate the pituitary gland to release adreno-cortico-tropic-hormone (ACTH), especially under stressful conditions57. ACTH stimulates the adrenal gland to release the steroid hormone cortisol. Serum cortisol levels have been correlated with stroke severity and extent of insular damage58. Prolonged elevation of cortisol may be neurotoxic and has been associated with increased post stroke mortality59. The paraventricular nucleus also projects directly to the rostral ventrolateral medulla, which integrates cardiac afferents, baroreceptor activity, and inputs from higher brain regions into sympathetic outflow to the heart. Stimulation of the hypothalamus activates sympathetic output and induces ECG abnormalities, arrhythmias, and myocardial necrosis60. Activation of the paraventricular nucleus of the hypothalamus in rats subjected to ischemic stroke causes arrhythmias mediated by glutamate via activation of N-methyl-D-aspartic acid receptors61. Reduction of paraventricular nucleus activity promotes improved recovery of cardiac function after myocardial infarction62. Activation of the HPA axis after ischemic stroke causes a significant increase in catecholamines63. The catecholamine surge hypothesis is the most widely accepted mechanism of brain-heart interaction.
The catecholamine surge theory has been closely linked to heart damage after physical and emotional stressors. Catecholamine surge can cause cardiac hypertrophy or myocardial ischemia64, 65. The autonomic nervous system regulates the release of catecholamines from the adrenal glands. Brain injury can cause elevated sympathetic tone with an additional increase in catecholamine secretion66. Neurologic injury causes excessive circulating catecholamines and massive catecholamine release from myocardial nerve endings66. The myocardium adjacent to the nerve is damaged67. The sympathetic nerve can directly release catecholamines and thereby induce cardiomyocyte toxicity. Long-term elevation of serum catecholamine produces cardio toxicity68 and may trigger edema in regions of hypokinesia, transient fibrosis, inflammation and contraction band necrosis69, 70. Experimental animal studies have demonstrated an increase in plasma catecholamine levels following ischemic stroke that was directly proportional to the incidence of myocardial lesions and cardiac damage71. The increased catecholamine levels is correlated with QT-interval prolongation and myocardial damage after SAH, while hypothalamic stimulation induces ECG changes without associated myocardial damage72, 73.
Catecholamines act on the heart via β receptors to increase the strength and rate of contraction74. The β receptors couple to the stimulatory G protein and activate adenylyl cyclase, and increase cytosolic cAMP. cAMP binds to the regulatory subunits of protein kinase A, which phosphorylates sarcolemmal L-type Ca2+ channels and sarcoplasmic phospholamban, which over loads mitochondria75. Mitochondrial Ca2+ overload triggers oxidative stress, and the subsequent opening of their inner membrane permeability transition pore along with osmotic swelling and loss of ATP synthesis, leads to myocardial cell death75. While pre-treatment with beta blockers has been reported to decrease the incidence of myocardial lesion76 and β blockers are commonly prescribed after Takotsubo cardiomyopathy, some studies have reported a lack of significant effect of β blockers on recurrence of Takotsubo syndrome rate77.
Sympathetic and parasympathetic physiology; as well as regulation of brain heart interaction has been described in detail elsewhere78. The forebrain plays an important role in the regulation of the autonomic nervous system in patients with hemorrhagic and ischemic stroke. Stimulating the orbital surface of the frontal lobe and the anterio reingulate gyrus affects blood pressure and heart rate79. The insular cortex is regarded as a vital part of the central autonomic network80. Ischemic lesions in the insular cortex increase the risk of cardiac complication and can cause blood pressure variations, cardiac arrhythmias and myocytolysis81, 82. The location of an ischemic lesion also affects cardiac function after stroke and studies have reported cardiac dysfunction after ischemic damage to both left and right insular cortex52, 80, 83. It appears that the right hemisphere primarily controls sympathetic activity, while parasympathetic activity is mainly controlled by the left hemisphere71, 72. Therefore, a right insular lesion decreases sympathetic tone and results in parasympathetic over activity80. Right insular lesions are associated with higher mortality at an early-stage compared with other sites84. Brain infarctions in the left hemisphere are associated with fewer incidences of arrhythmias, an increased risk of adverse cardiac outcome, increased long term mortality and decreased cardiac wall motion compared to stroke in other locations85, 86. Elevated activity of the sympathetic nervous system observed in the acute phase of SAH induces myocardial damage and contributes to the development of cardiac dysfunction87. Therefore, intensive heart monitoring will be particularly essential for patients with insular cortical damage. Figure 2 summarizes the role of the HPA axis, catecholamine surge and sympathetic regulation in mediating cardiac dysfunction after stroke.
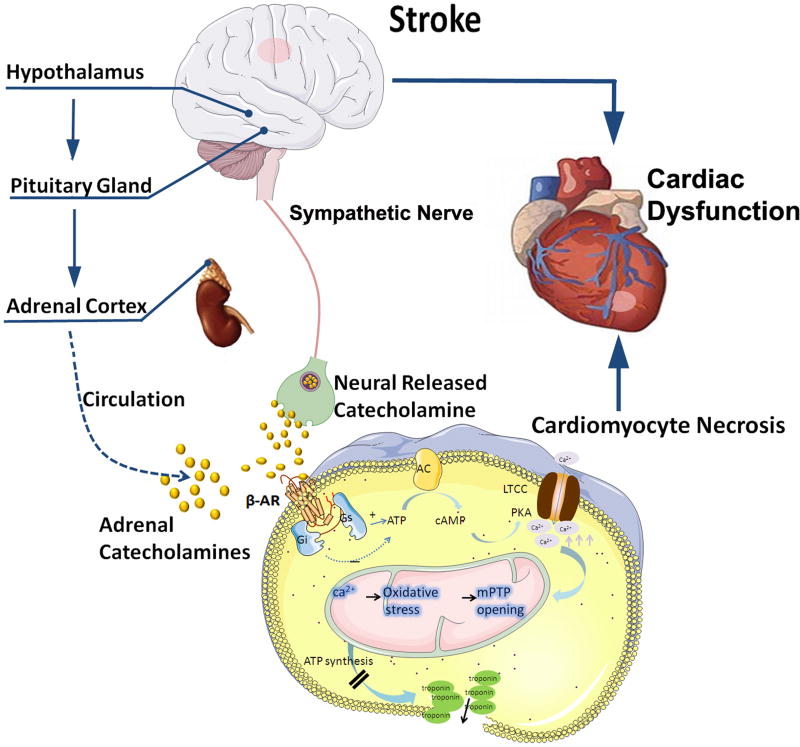
Figure 2. The HPA axis, catecholamine surge and sympathetic and parasympathetic regulation mediate cardiac dysfunction after stroke.
Catecholamines act on β receptors, β receptors couple to the stimulatory G protein (Gs) and activate adenylyl cyclase (AC), and increase cytosolic cAMP. cAMP binds to the regulatory subunits of protein kinase A (PKA), which phosphorylates sarcolemmal L-type Ca2+ channels (LTCC) and sarcoplasmic phospholamban, which over loads mitochondria. Mitochondrial Ca2+ overload triggers oxidative stress, and the subsequent opening of their inner membrane permeability transition pore with ensuing osmotic swelling and loss of ATP synthesis, leads to myocardial cell death.
3.2 Blood brain barrier disruption after stroke
The neurovascular unit (NVU) is a functional unit encompassing the anatomical and metabolic interactions between the neuronal, glial and vascular components of the brain. The blood brain barrier (BBB) composed of endothelial cells, astrocytic end-feet, pericytes, and a thick basement membrane is at the core of the NVU88, 89. The BBB serves as a dynamic interface between the brain and the circulatory system limiting the entry of potential neurotoxins and microscopic and hydrophilic molecules88, 89. The BBB also protects the brain from variations in blood composition and helps maintain constant and optimal cerebral metabolism and neuronal activity. Disruption of the NVU and BBB is a major step in the pathophysiological cascade after stroke and contributes to secondary tissue damage. Increased BBB permeability disturbs cerebral auto regulation, leads to neuronal damage, and facilitates the invasion of inflammatory factors into the injured brain90. In a vicious cycle, BBB disruption promotes entry of inflammatory factors into the ischemic brain, and inflammation increases BBB permeability. Increased BBB permeability is found in almost half the patients at 24 hours post cardiac surgery even in the absence of stroke91. Additionally, cardiac dysfunction and cardiac surgery can lead to cerebral ischemia and stroke via hypoperfusion and cardiac embolization, respectively, as well as trigger systemic inflammatory responses that may either induce or aggravate BBB disruption91. BBB disruption facilitates the entry of brain derived antigens and extracellular vesicles derived from injured brain cells to enter the blood stream and encounter peripheral immune cells, as described in detail in the following section. Therefore, there exists a close relationship between cardiac function and BBB integrity, but the effects are most likely indirect.
3.3 Immunoresponse and systemic inflammation after stroke
Immune system activation leading to inflammation after stroke is an important factor in stroke progression. The complex interplay of the local and systemic inflammatory responses after stroke involves many immune cell types, circulating signals, and is important in understanding both infection and other secondary complications. The complex role of inflammation in stroke pathogenesis has been reviewed elsewhere92, 93. In the following section, we discuss inflammatory and immune responses that may mediate brain-heart interaction after stroke, which is summarized in Figure 3.
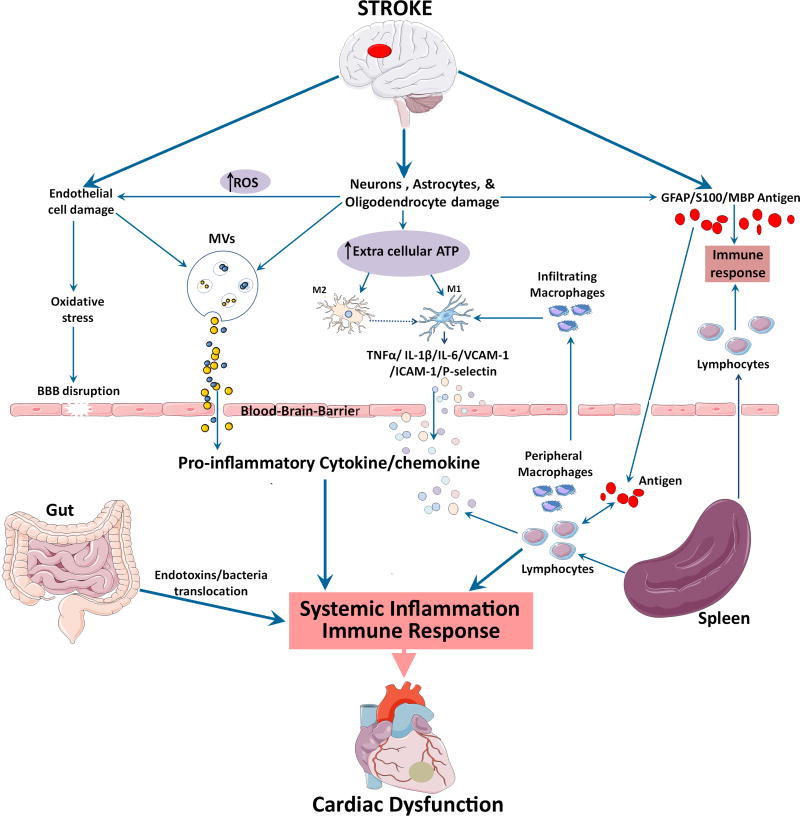
Figure 3. Role of immune response and inflammation in mediating cardiac dysfunction after stroke.
Local inflammatory responses in the brain, endothelial cell damage and increased oxidative stress lead to BBB disruption after stroke. Release of proinflammatory cytokine and chemokines can be stimulated by damaged endothelial cells and astrocytes, activated resident microglia and infiltrating macrophages that assume M1 phenotype, as well as the spleen. Systemic inflammatory responses are mainly driven by a number of cytokines, chemokines, stress hormones, as well as parasympathetic and sympathetic regulation. Gut microbiome dysbiosis after stroke, can also lead to bacterial and endotoxin translocation to the blood, leading to systemic inflammation. Damage associated molecular patterns (DAMPs) released by dying brain cells after ischemic stroke release brain derived antigens which can pass the ruptured BBB and enter the systemic circulation. In the presence of systemic inflammation, the antigen specific autoimmune response leads to the secretion of proinflammatory cytokines.
In the acute phase of brain injury, local inflammatory responses in the brain parenchyma include microgliosis, astrogliosis, and cytokine/chemokine secretion which occur concomitantly with endothelial cell activation94. Ischemia can lead to endothelial cell damage directly or indirectly via the release of reactive oxygen species95. Damaged endothelial cells can increase oxidative stress and rupture the BBB95. In addition, damaged endothelial cells and astrocytes at the site of inflammation can shed extracellular vesicles that rapidly cross the BBB and enter the bloodstream96. Upon reaching peripheral organs such as the liver, the protein and microRNA (miR) cargo of these endothelial cell and astrocyte derived extracellular vesicles regulate acute cytokine response and mediate trafficking of peripheral immune cells to injured brain tissue96. Extracellular vesicles are emerging as intrinsic communication mediators between the brain and immune system96. The role of microvesicles (MVs) and miR as mediators of brain damage induced heart dysfunction is discussed in detail later in this review.
BBB disruption facilitates the infiltration of peripheral macrophages and neutrophils into brain after stroke onset97, 98. Subsequent to ischemia onset, there is an increase in extracellular ATP as a result of neuronal and glial depolarization and damaged plasma membrane of injured brain cells93. High extracellular ATP levels can activate resident microglia and stimulate inflammatory cytokine production93. Microglia and macrophages can assume a neurodegenerative M1 phenotype and increase inflammation by releasing proinflammatory cytokines. In the initial few minutes to hours after stroke onset, there is an increased production of proinflammatory cytokines (Interleukins (IL-6, IL-1β), TNF-α, etc), integrins, adhesion molecules (VCAM-1, ICAM-1, P-selectin, etc), chemokines and their receptor molecules93, 99, 100. These proinflammatory molecules can further damage the BBB, initiate the recruitment of peripheral immune cells into the cerebral microcirculation and ischemic brain, as well as pass through the BBB into the circulation inducing systemic inflammation100. On the other hand, the microglia and macrophages can also assume an anti-inflammatory M2 phenotype and clear cellular debris from the injured brain93.
Systemic inflammatory responses are mainly driven by a number of cytokines, chemokines, stress hormones, as well as parasympathetic and sympathetic regulation101. In acute ischemic stroke, lymphocyte influx begins about 48 hours after stroke, and the invading T lymphocytes promote detrimental inflammatory cascades and induce delayed brain damage97, 99. While an optimal level leukocyte infiltration is beneficial to the ischemic brain, in excess it leads to increased intracranial pressure resulting in poor outcome or mortality93, 99, 100. The spleen plays a central role in mediating the peripheral immune response to ischemic stroke by increasing circulating lymphocytes and proinflammatory cytokines and chemokines such as TNF-α, monocyte chemoattractant protein-1 (MCP-1), IFN-γ, IL-6, and IL-2, exacerbating inflammation in the acute phase after stroke102. Increased TNF-α level induces degradation of Troponin I which has been associated with impaired cardiac contractility103. Splenectomy 8 weeks after chronic heart failure has been reported to significantly improve left ventricular systolic function and reduce cardiomyocyte hypertrophy, suggesting a role of spleen in mediating cardiac function104. At a later time point, splenic atrophy and decrease in T cell proliferation and inflammatory cytokines secretion lead to a state of immunosuppression105.
Damage associated molecular patterns (DAMPs) including various heat-shock proteins are released by dying brain cells after ischemic stroke106. Injured astrocytes, neurons and oligodendrocytes release brain derived antigens such as glial fibrillary acidic protein, S100, and myelin basic protein (MBP)107. DAMPs and brain derived antigens can also pass the ruptured BBB and enter the systemic circulation. DAMPS can activate Toll-like receptors (TLRs) and lead to cytokine and chemokine production108. In experimental stroke, lymphocyte response to the brain derived antigens such as MBP, leads to antigen specific secretion of transforming growth factor (TGF-β), consistent with a regulatory T cells (TREG) response109. However, in the presence of systemic inflammation, the antigen specific autoimmune response is characterized by T helper cells 1 (Th1) and TLR activation and secretion of proinflammatory interferon (IFN-γ), IL-2, etc110, 111. TLR-4 is expressed on the cell surface of cardiomyocytes. TLR-4 activation and chronic inflammation with increased release of proinflammatory cytokines such as TNF-α IL-6, IL-1β have been implicated in developing heart failure and poor prognosis of heart disease112. TGF-β induces IL-6 increase while decreasing MCP-1 and VCAM1 in vascular pericytes, and is also instrumental in the transition from fibroblasts to myofibroblasts that may play a role in cardiac fibrosis observed in NSC113, 114.
Systemic inflammatory responses are activated after spontaneous intracerebral hemorrhage, and ischemic stroke as well as SAH115. Systemic inflammatory responses carry an increased risk of subsequent intracranial complications such as vasospasm and normal pressure hydrocephalus, as well as systemic secondary complications116. The immune system is involved in this process in the early phases after stroke, and correlates with ischemic stroke and SAH progression116. Systemic inflammatory response syndrome (SIRS) was found in approximately 50% of SAH patients on admission and 85% of patients developed SIRS within the first four days after admission117. SIRS is indicated by abnormal leukocyte counts, high respiratory and heart rate (>90 bmp), and abnormal body temp, and can increase the risk of developing vasospasm and secondary complications117. In ischemic stroke patients, the number of CD74+ cells (the MHC class II invariant chain present on all class II expressing cells, including monocytes, macrophages and dendritic cells) were significantly increased in peripheral blood mononuclear cells compared to healthy controls118. The increased CD74 positive cells were mainly on CD4+ T cells, monocytes and dendritic cells118. In heart diseases such as myocardial infarction, elevation of circulating blood monocytes is associated with poor cardiac function and predicts poor recovery of left ventricular systolic function at 3 months post injury119.
There is also some evidence of cross-talk between inflammatory responses and sympathetic activation in ischemic and hemorrhagic stroke120, 121. The proinflammatory cytokines released by damaged neuronal and glial cells stimulate the posterior hypothalamus to increase sympathetic output and circulating catecholamine level122–124. Bilt et al. found that catecholaminergic stress after brain hemorrhage coincided with influx of inflammatory cells into the heart and induced cardiac damage after SAH121. Neutrophils were found embedded in the inflamed myocardium along with thrombi121. The coupling of catecholamine release with parasympathetic dysfunction regulates inflammation that induces myocardial dysfunction, thrombi formation, and cardiomyocyte cell death121. Therefore, inflammation may play an important role and as a link between brain injury and cardiac damage after ischemic stroke and SAH. The role of immune response and inflammation after stroke in mediating cardiac dysfunction is summarized in Figure 3.
Gut microbiome dysbiosis after stroke, can lead to intestinal paralysis and bacterial and endotoxin translocation to blood leading to systemic inflammation125–127, as discussed in detail in the following section.
3.4 Gut microbiome dysbiosis after stroke
Our knowledge and understanding of the human microbiome and its involvement in various diseases are increasing. In particular, there is an emerging body of evidence based from experimental studies that suggests an interaction between the gut microbiome and heart as well as the gut microbiota and the CNS; referred to as gut-heart axis and brain-gut axis, respectively. The gut blood barrier (GBB) mainly regulates the absorption of nutrients and water while preventing toxins and pathogenic microorganisms from entering the blood stream. In gastrointestinal, metabolic, cardiovascular and cerebrovascular diseases, disruption of the GBB integrity resulting in increased intestinal permeability enables microbiota derived molecules to enter the blood stream125.
Brain-Gut axis
The brain-gut axis can affect normal brain functioning as well as influence the pathological cascade of events in neurological diseases including stroke and TBI128, 129. The brain-gut axis comprising neural and humoral pathways with signaling molecules such as cytokines, hormones, and neuropeptides can regulate immune response and lymphocyte population129, 130. Alterations to the normal gut microbiota or gut dysbiosis caused by acute brain lesions can affect neuroinflammatory and immune responses in the brain and worsen neurological function131. In patients with large-artery atherosclerotic ischemic stroke or transient ischemic attack, significant gut dysbiosis is indicated by increased opportunistic bacteria and decreased beneficial bacteria132. It has also been suggested that commensal gut microbiota exert protection against ischemic damage, and their depletion or dysbiosis increases post stroke mortality in mice and influences stroke outcome133. In addition, the extent of increase of proteobacteria in the gut of stroke patients is proportional to stroke severity132. Dietary choline and l-carnitine are transformed by gut microbiota to trimethylamine, and its oxidation yields the pro-atherogenic metabolite which is largely considered a disease marker134.
In ischemic stroke, gut dysbiosis via stress-mediated intestinal paralysis can alter T cell homeostasis, promote migration of T cells from the intestine to the ischemic brain, and increase pro-inflammatory responses resulting in poor stroke outcome130. In mice subjected to transient focal cerebral ischemia, trafficking of intestinal T cells from the gut to the meninges of the brain increases neuroinflammation and the secretion of pro-inflammatory cytokine IL-17; which can stimulate the production of several other cytokines and chemokines and facilitate the infiltration of cytotoxic immune cells and neutrophils into the injured brain130. In mice subjected to transient focal cerebral ischemia, stroke increased gut permeability proportional to stroke severity, promoted bacterial translocation from the gut to mesenteric lymph nodes and peripheral organs such as spleen, liver and lungs, as well as triggered adaptive and innate immune responses135. In ischemic stroke patients, gut dysbiosis and increased bacterial counts of Lactobacillus ruminis subgroup in the fecal gut microbiota was associated with elevated systemic inflammation and altered metabolism136. Bacterial metabolites have also been implicated to mediate communication between the commensal gut microbiota and the immune system tipping the balance in favor of pro-inflammatory mechanism137.
Gut-heart axis
Increased gut permeability can promote inflammatory responses while systemic inflammation can increase gut permeability125. Bacterial and endotoxin translocation to the blood stream, increased pro-inflammatory cytokines, and systemic inflammation can induce or exacerbate cardiac dysfunction126, 127. In an independent large clinical cohort, 3 gut microbiota dependent metabolites including choline, trimethylamine-N-oxide (TMAO) and betaine were found to be predictors of cardiovascular disease134. Gut microbes can directly contribute to platelet hyper reactivity and increase the risk of thrombosis via TMAO generation138. Altered TMAO levels has been associated with impaired cardiac function, heart attack and heart failure126, 138. Elevated TMAO levels is the plasma of patients presenting with chest pain was found to be a predictor of short term and long term risk of myocardial infarction, stroke, need for revascularization, or even death139. Figure 4 summarizes the role of gut dysbiosis in mediating cardiac damage after stroke. Future studies are warranted to investigate the role of the gut microbiome in cardiac damage after stroke and investigate whether stroke induced guy dysbiosis promotes a pro-inflammatory cascade which then leads to dysfunction of other organs including the heart.
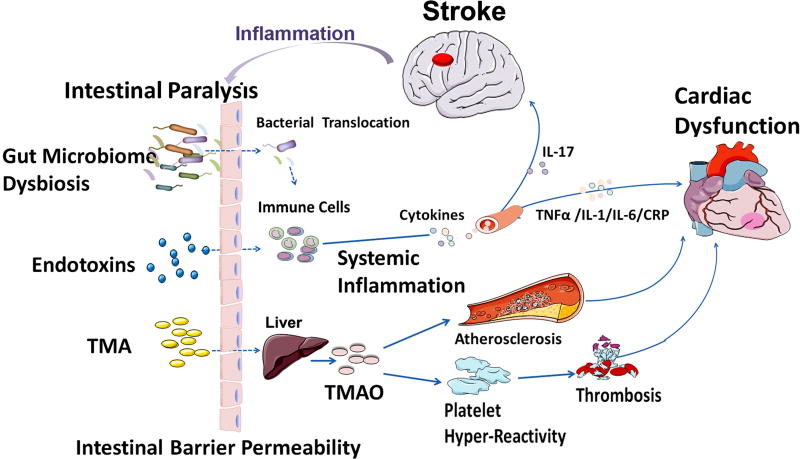
Figure 4. The brain-gut axis and gut-heart axis may mediate cardiac damage after stroke.
Stroke increases intestinal barrier permeability, enabling bacterial and endotoxin translocation to bloodstream inducing inflammatory responses and proinflammatory cytokine production. Cytokine production can trigger inflammation, fibrosis and microvascular and myocardial dysfunction. TMAO is the hepatic oxidation product of the microbial metabolite TMA. TMAO promotes the development of hyper responsive platelet phenotype and enhances elevating the risk for heart failure and/or heart attack.
4. Future perspectives
While there exists knowledge gaps in our understanding of the mechanisms of brain-heart interaction in the setting of stroke, emerging evidence suggests that MVs and their cargo miR may function as mediators of inter-organ and intercellular communication. Important unanswered questions relating to the role of MVs/miR in brain-heart interaction include: How do changes in miRs in the brain affect cardiac function and vice versa? Do changes in miR expression correspond to stages of disease and recovery? Are MVs containing miR’s transported by the circulation from the brain to heart (as well as other organs), thereby inducing damage to target tissue? Since miRs can affect immune response, it is also possible that injured brain cells and resident and infiltrating inflammatory cells release a variety of MVs carrying miRs which upon reaching cardiac tissue have adverse effects on cardiac function? The following section explores this perspective, as summarized in Figure 5. Further studies are warranted to elucidate the role of these intercellular communication mediators in facilitating interaction between the brain and distant organs such as the heart in neurological diseases.
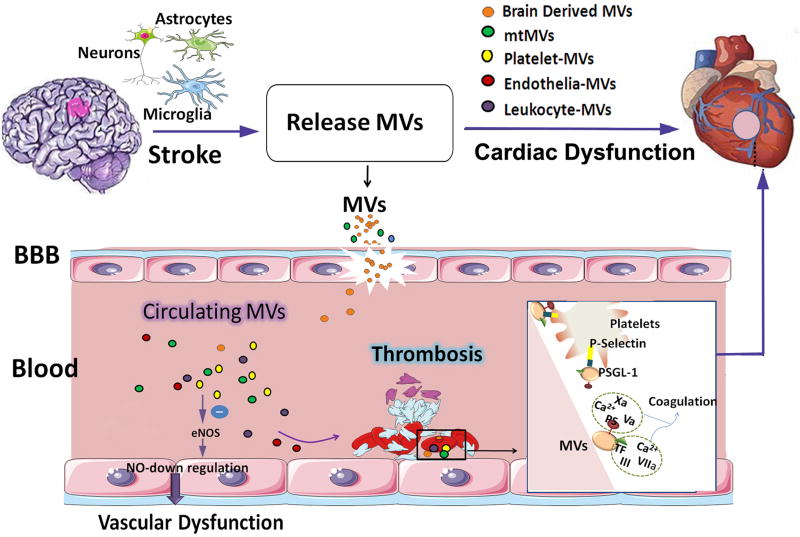
Figure 5. The role of microvesicles released by damaged astrocytes, neurons and microglia after stroke in mediating cardiac damage warrants future investigation.
Stroke induced elevation of circulating platelets and microvesicles (MVs) are associated with endothelial dysfunction and coagulation leading to thrombosis. MVs induce endothelial dysfunction by decreasing nitric oxide (NO) synthesis as the result of endothelial nitric oxide synthase (eNOS) function inhibition and an increase in caveolin-1. The outer leaflet of the MVs membrane contains two pro-coagulants: phosphatidylserine and tissue factor. MVs can bind to coagulation factors and promote their activation. In addition, MVs harbor tissue factor, which can initiate extrinsic coagulation pathways and promote the assembly of clotting enzymes leading to thrombin generation. At sites of vascular injury, P-selectin exposure by activated endothelial cells or platelets leads to the rapid recruitment of MVs bearing the P-selectin glycoprotein ligand-1 (PSGL-1). The interactions between PSGL-1 and platelet P-selectin are necessary to concentrate tissue factor activity at the thrombus.
4.1 Microvesicles
MVs are lipid bilayer-covered cell fragments that range in diameter from 100 nm- 1 µm and are released into the extracellular environment by several cell types typically during cell activation or cell death via necrosis or apoptosis. MVs are produced by the outward budding and fission of plasma membrane. In addition to intercellular communication via cell contact, MVs constitute an important mode of communication by serving as vehicles for intercellular transfer of membrane and cytosolic proteins, lipids, and RNA either locally or over long distances140. Since MVs retain the signature membrane protein composition of the parent cell, they can be classified by flow cytometry into different cellular MVs, such as endothelial-MVs, leukocyte-MVs and platelet-MVs or brain derived MV. MV shedding can also be triggered by pathological activation of inflammatory processes and activation of coagulation or complement systems, or even by shear stress in the circulation. MVs have a bilayered phospholipid structure exposing coagulant-active phosphatidylserine and expressing various membrane receptors, and they serve as cell to cell shuttles for bioactive molecules such as lipids, growth factors, miR, and mitochondria140. MV’s have been implicated in endothelial dysfunction, inflammation, and thrombosis141. In addition, MVs may also be targeted as a therapeutic delivery tool by either inhibiting their release from diseased cells or by manipulating their cargo to enable shuttling of secretory molecules such as cytokines, chemokines, and growth factors142.
4.1.1 Circulating MVs
Many circulating MVs in the blood derive from cells that are in contact with the bloodstream such as platelets and endothelial cells. Circulating MV level is elevated in patients with ischemic stroke, intracerebral hemorrhage stroke as well as SAH; and is associated with poor clinical outcome143, 144. A major source of MV’s in stroke patients’ is vascular endothelial cells145. Blood derived MV’s stimulate release of endothelial cell cytokine IL-6145. Correlation between IL-6 expression and incidence of vasospasm has been demonstrated previously146. Therefore, in peripheral blood vessels, including cardiac vasculature, IL-6 can induce vascular spasm and lead to coronary syndrome. In addition, endothelial cells secrete MV’s which can inhibit endothelial nitric oxide synthase (eNOS)147. Anti-eNOS activity leads to a decline in NO production, causing endothelial dysfunction and impaired vascular relaxation148, 149. Endothelial MV was significantly increased in SAH patients, and the increased endothelial cell MV is related with symptomatic cerebral vasospasm143, and predicts infarction post-SAH150. MVs may play an important role for secondary complications in patients with ischemic and hemorrhagic stroke151.
Prior studies have demonstrated that platelet MV’s have 50–100 fold higher specific pro-coagulant activity than activated platelets152, and are hence associated with thrombosis. Platelets play an important role in maintaining hemostasis such as thrombosis, and immune response. Platelets are activated by inflammation, infection, or injury, and after their activation MVs are released from platelets. In intracerebral hemorrhage, cerebrospinal fluid and plasma pro-coagulant MV levels are significantly increased, and are related with stroke pathogenesis144. Platelet MVs may also serve as a link between vascular coagulation and inflammation in cardiovascular disease153. Altered platelet activity due to MVs derived from platelets and/or injured brain can lead to thromboembolic events and associated cardiac complications after brain injury such as stroke or TBI153. Circulating MVs derived from brain, endothelial cells and blood cells may promote procoagulant activity and thrombin generation153. However, MVs can contribute to the onset and progression of some neurodegenerative and neuroinflammatory diseases, as well as to the development and regeneration of the nervous system. Activated platelets shed MVs, which contain a variety of growth factors augmenting endogenous neural progenitor and stem cells angiogenesis and neurogenesis, which may be utilized for therapy for ischemic stroke154.
4.1.2 Neural source of MVs
Neurons, astrocytes, microglia, as well as neural stem cells release MVs under normal and pathological conditions. Neural-MVs can contribute to the onset and progression of some neurodegenerative and neuroinflammatory diseases, as well as to the development and regeneration of the nervous system after stroke155. Microglial-MVs and astrocyte-MVs store and release the inflammatory cytokine IL-1β156, 157. In addition, MVs contribute to IL-1β release from glial cells157. In the acute phase of injury after TBI in mice, circulating platelet numbers did not change, however, platelet contribution to clot formation and the number of total circulating MV’s decreased significantly158. When these TBI mice were treated with MV’s derived from the brain tissue of sham mice, platelet contribution to clot formation normalized158. This indicates that brain tissue secreted MVs generated after TBI are likely to mediate post traumatic hypercoagulable state after TBI158. Tan et al. found that MVs were produced from injured hippocampal cells, transmigrated through the disrupted endothelial barrier in a platelet-dependent manner, and activated platelets, and thereby induce coagulopathy in TBI model158. Zhao et al found that brain-derived mitochondrial microparticles (mtMPs) significantly increased in the circulating after TBI159. The mtMPs synergized with platelets to facilitate vascular leakage by disrupting the endothelial barrier. The disrupted endothelial barrier allowed the release of mtMPs into the systemic circulation to promote coagulation and enhanced fibrinolysis, vascular fibrin deposition, and thrombosis159. The underlying mechanisms and role played by MV’s in mediating cardiac complications after cerebral injury are emerging and warrant further study. Increased coagulation and thrombosis may induce heart damage after brain injury.
4.2 MicroRNA
MiRs are short sequences of non-coding RNA (ca. 22 nucleotides) which regulate gene expression both transcriptionally and post-transcriptionally160. MiRs can regulate several genes, pathways and biological networks, either acting alone or in concert with other miR’s. MiRs regulate many biological processes regulating tissue repair including angiogenesis, inflammation and hypoxia–response160. About 50 circulating miRs are believed to be associated with cardiovascular diseases including miR-1, miR-16, miR-27b, miR-30d, miR-126, miR-133, miR-143, miR-145, miR-208 and the let-7 family161. Several miRs with key cardiac and vascular function have been reported to be affected after stroke including: miR-23, miR-24, miR-29, miR-30, miR-103, miR-222 were up regulated, while miR-126 was down regulated162. MVs also contain abundant miRNAs.
Endothelial cell specific miR-126 has a key role in maintaining vascular integrity and regulating angiogenesis163. Circulating miR-126 level is significantly decreased in patients with ischemic stroke until at least 24 weeks164. Endothelial cell dysfunction is a principal step in atherosclerosis. MiR-126 deficiency has been highly associated with heart failure, atrial fibrillation and coronary artery disease and may be associated with stroke induced severe cardiac complications163, 165. Coronary artery disease or ischemic heart disease includes stable and unstable angina, myocardial infarction, and sudden cardiac death. In myocardial infarction, circulating miR-1 increases, and miR-126 decreases in proportion with cTnI plasma concentration levels166. Vascular miR-126 has been demonstrated to be “consumed” or taken up by the heart from the bloodstream according to a transcoronary gradient167. It has been demonstrated that ischemic stroke decreases serum and heart miR-126 expression, as well induces post stroke cardiac dysfunction168. Conditional specific endothelial cell miR-126 knockout mice exhibited enhanced cardiac dysfunction and increased cardiomyocyte hypertrophy, fibrosis, and inflammatory factor expression after ischemic stroke compared to miR-126 knockout control stroke mice168. The study demonstrated that decreased miR-126 may play a role in brain-heart interaction after ischemic stroke168.
In addition, circulating miR-145 also significantly increases within 24 hours of cerebral ischemia, and circulating miR-145 level is positively correlated with elevated serum inflammatory factor IL-6169. MiR-145 modulates endothelial cell function in angiogenesis and vessel stabilization170. Circulating miR-145 changes has also been reported in patients with coronary artery disease and acute myocardial infarction171. Therefore, other miRs may also regulate brain-heart interaction.
5. Conclusions
In summary, brain-heart syndrome is very commonly encountered in the clinic, and affects the prognosis, morbidity and mortality of patients. The secondary brain damage caused by cardiac dysfunction as well as impaired systemic and brain homeostasis is a serious problem that requires attention. Although there is a necessity for specific interventions for the prevention and treatment of post stroke cardiac complications, there is a dearth of data to guide the management of these complications. Investigation of clinical indicators to diagnose the adverse brain-heart disease at early stage will enable improved clinical management and reduce mortality. Research of novel therapies for the protection of heart after stroke warrants investigation with the potential to be applied to a large proportion of stroke patients over a wide time window.
Acknowledgments
None
Sources of Funding: This work was supported by National Institute of Neurological Disorders and Stroke R01 NS083078-01A1 (JC), R01 NS099030-01 (JC) and R01NS097747 (JC), American heart association 17POST33410580 (PV) and by the National Natural Science Foundation of China grant 81300993 and 81571145 (TY).
Non-standard Abbreviations and Acronyms
- ACTH
Adreno-cortico-tropic-hormone
- ATP
Adenosine triphosphate
- BBB
Blood brain barrier
- CKMB
Creatine phosphokinase-myocardial fraction
- CNS
Central nervous system
- cTnI
Cardiac Troponin I
- cTnT
Cardiac Troponin T
- DAMPs
Damage associated molecular patterns
- ECG
Electrocardiographic
- eNOS
Endothelial nitric oxide synthase
- GBB
Gut blood barrier
- HF
High-frequency
- HPA
Hypothalamic-pituitary-adrenal
- HRV
Heart rate variability
- hsTnT
High-sensitivity Troponin T
- IL
Interleukin
- LF
Low-frequency
- LVDD
Left-ventricular diastolic dysfunction
- LVEF
Left ventricular ejection fraction
- MBP
Myelin basic protein
- MCP-1
Monocyte chemoattractant protein-1
- MiR
MicroRNA
- mtMPs
Mitochondrial microparticles
- MVs
Microvesicles
- NSC
Neurogenic stress cardiomyopathy
- NTproBNP
N-terminal fragment of B-type natriuretic peptide
- NVU
Neurovascular unit
- SAH
Subarachnoid hemorrhage
- SIRS
Systemic inflammatory response syndrome
- TBI
Traumatic brain injury
- TGF-β
Transforming growth factor
- Th1
T helper cells 1
- TLRs
Toll-like receptors
- TMAO
Trimethylamine-N-oxide
- TREG
Regulatory T cells
- ULF
Ultra-low-frequency
- VLF
Very-low-frequency
Footnotes
Disclosures: None
References
- 1.Ay H, Koroshetz WJ, Benner T, Vangel MG, Melinosky C, Arsava EM, Ayata C, Zhu M, Schwamm LH, Sorensen AG. Neuroanatomic correlates of stroke-related myocardial injury. Neurology. 2006;66:1325–1329. doi: 10.1212/01.wnl.0000206077.13705.6d. [DOI] [PubMed] [Google Scholar]
- 2.Oppenheimer SM. Neurogenic cardiac effects of cerebrovascular disease. Current opinion in neurology. 1994;7:20–24. doi: 10.1097/00019052-199402000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Tokgozoglu SL, Batur MK, Topcuoglu MA, Saribas O, Kes S, Oto A. Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke; a journal of cerebral circulation. 1999;30:1307–1311. doi: 10.1161/01.str.30.7.1307. [DOI] [PubMed] [Google Scholar]
- 4.Byer E, Ashman R, Toth LA. Electrocardiograms with large, upright t waves and long q-t intervals. American heart journal. 1947;33:796–806. doi: 10.1016/0002-8703(47)90025-2. [DOI] [PubMed] [Google Scholar]
- 5.Samuels MA. The brain-heart connection. Circulation. 2007;116:77–84. doi: 10.1161/CIRCULATIONAHA.106.678995. [DOI] [PubMed] [Google Scholar]
- 6.Tranmer Bruce I, Keller Ted S, Kindt Glenn W, Archer David. Loss of cerebral regulation during cardiac output variations in focal cerebral ischemia. Journal of Neurosurgery. 1992;77:253–259. doi: 10.3171/jns.1992.77.2.0253. [DOI] [PubMed] [Google Scholar]
- 7.Cheshire WP, Jr, Saper CB. The insular cortex and cardiac response to stroke. Neurology. 2006;66:1296–1297. doi: 10.1212/01.wnl.0000219563.87204.7d. [DOI] [PubMed] [Google Scholar]
- 8.van der Bilt IA, Hasan D, Vandertop WP, Wilde AA, Algra A, Visser FC, Rinkel GJ. Impact of cardiac complications on outcome after aneurysmal subarachnoid hemorrhage: A meta-analysis. Neurology. 2009;72:635–642. doi: 10.1212/01.wnl.0000342471.07290.07. [DOI] [PubMed] [Google Scholar]
- 9.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 10.Verdecchia P, Porcellati C, Reboldi G, Gattobigio R, Borgioni C, Pearson TA, Ambrosio G. Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation. 2001;104:2039–2044. doi: 10.1161/hc4201.097944. [DOI] [PubMed] [Google Scholar]
- 11.Selvetella G, Notte A, Maffei A, Calistri V, Scamardella V, Frati G, Trimarco B, Colonnese C, Lembo G. Left ventricular hypertrophy is associated with asymptomatic cerebral damage in hypertensive patients. Stroke; a journal of cerebral circulation. 2003;34:1766–1770. doi: 10.1161/01.STR.0000078310.98444.1D. [DOI] [PubMed] [Google Scholar]
- 12.Petersen P. Thromboembolic complications in atrial fibrillation. Stroke. 1990;21:4–13. doi: 10.1161/01.str.21.1.4. [DOI] [PubMed] [Google Scholar]
- 13.Khechinashvili G, Asplund K. Electrocardiographic changes in patients with acute stroke: A systematic review. Cerebrovasc Dis. 2002;14:67–76. doi: 10.1159/000064733. [DOI] [PubMed] [Google Scholar]
- 14.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshimura S, Toyoda K, Ohara T, Nagasawa H, Ohtani N, Kuwashiro T, Naritomi H, Minematsu K. Takotsubo cardiomyopathy in acute ischemic stroke. Ann Neurol. 2008;64:547–554. doi: 10.1002/ana.21459. [DOI] [PubMed] [Google Scholar]
- 16.van der Bilt IA, Hasan D, van den Brink RB, Cramer MJ, van der Jagt M, van Kooten F, Regtien JG, van den Berg MP, Groen RJ, Cate FJ, Kamp O, Gotte MJ, Horn J, Girbes AR, Vandertop WP, Algra A, Rinkel GJ, Wilde AA, Investigators S. Time course and risk factors for myocardial dysfunction after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2015;76:700–705. doi: 10.1227/NEU.0000000000000699. discussion 705–706. [DOI] [PubMed] [Google Scholar]
- 17.Ghadri JR, Cammann VL, Napp LC, Jurisic S, Diekmann J, Bataiosu DR, Seifert B, Jaguszewski M, Sarcon A, Neumann CA, Geyer V, Prasad A, Bax JJ, Ruschitzka F, Luscher TF, Templin C, International Takotsubo R. Differences in the clinical profile and outcomes of typical and atypical takotsubo syndrome: Data from the international takotsubo registry. JAMA cardiology. 2016;1:335–340. doi: 10.1001/jamacardio.2016.0225. [DOI] [PubMed] [Google Scholar]
- 18.Frontera JA, Parra A, Shimbo D, Fernandez A, Schmidt JM, Peter P, Claassen J, Wartenberg KE, Rincon F, Badjatia N, Naidech A, Connolly ES, Mayer SA. Cardiac arrhythmias after subarachnoid hemorrhage: Risk factors and impact on outcome. Cerebrovascular diseases. 2008;26:71–78. doi: 10.1159/000135711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estanol BV, Marin OS. Cardiac arrhythmias and sudden death in subarachnoid hemorrhage. Stroke. 1975;6:382–386. doi: 10.1161/01.str.6.4.382. [DOI] [PubMed] [Google Scholar]
- 20.Junttila E, Vaara M, Koskenkari J, Ohtonen P, Karttunen A, Raatikainen P, Ala-Kokko T. Repolarization abnormalities in patients with subarachnoid and intracerebral hemorrhage: Predisposing factors and association with outcome. Anesthesia and analgesia. 2013;116:190–197. doi: 10.1213/ANE.0b013e318270034a. [DOI] [PubMed] [Google Scholar]
- 21.Jangra K, Grover VK, Bhagat H, Bhardwaj A, Tewari MK, Kumar B, Panda NB, Sahu S. Evaluation of the effect of aneurysmal clipping on electrocardiography and echocardiographic changes in patients with subarachnoid hemorrhage: A prospective observational study. Journal of neurosurgical anesthesiology. 2016 doi: 10.1097/ANA.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 22.Lee M, Oh JH, Lee KB, Kang GH, Park YH, Jang WJ, Chun WJ, Lee SH, Lee IC. Clinical and echocardiographic characteristics of acute cardiac dysfunction associated with acute brain hemorrhage- difference from takotsubo cardiomyopathy. Circulation journal : official journal of the Japanese Circulation Society. 2016;80:2026–2032. doi: 10.1253/circj.CJ-16-0395. [DOI] [PubMed] [Google Scholar]
- 23.Hays A, Diringer MN. Elevated troponin levels are associated with higher mortality following intracerebral hemorrhage. Neurology. 2006;66:1330–1334. doi: 10.1212/01.wnl.0000210523.22944.9b. [DOI] [PubMed] [Google Scholar]
- 24.Park HK, Kim BJ, Yoon CH, Yang MH, Han MK, Bae HJ. Left ventricular diastolic dysfunction in ischemic stroke: Functional and vascular outcomes. Journal of stroke. 2016;18:195–202. doi: 10.5853/jos.2015.01697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milionis H, Faouzi M, Cordier M, D’Ambrogio-Remillard S, Eskandari A, Michel P. Characteristics and early and long-term outcome in patients with acute ischemic stroke and low ejection fraction. International journal of cardiology. 2013;168:1082–1087. doi: 10.1016/j.ijcard.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 26.Kolin A, Norris JW. Myocardial damage from acute cerebral lesions. Stroke. 1984;15:990–993. doi: 10.1161/01.str.15.6.990. [DOI] [PubMed] [Google Scholar]
- 27.Prosser J, MacGregor L, Lees KR, Diener HC, Hacke W, Davis S, Investigators V. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke. 2007;38:2295–2302. doi: 10.1161/STROKEAHA.106.471813. [DOI] [PubMed] [Google Scholar]
- 28.Rauh R, Fischereder M, Spengel FA. Transesophageal echocardiography in patients with focal cerebral ischemia of unknown cause. Stroke; a journal of cerebral circulation. 1996;27:691–694. doi: 10.1161/01.str.27.4.691. [DOI] [PubMed] [Google Scholar]
- 29.LAVY S, YAAR I, MELAMED E, STERN S. The effect of acute stroke on cardiac functions as observed in an intensive stroke care unit. Stroke. 1974;5:775–780. doi: 10.1161/01.str.5.6.775. [DOI] [PubMed] [Google Scholar]
- 30.McDermott MM, Lefevre F, Arron M, Martin GJ, Biller J. St segment depression detected by continuous electrocardiography in patients with acute ischemic stroke or transient ischemic attack. Stroke; a journal of cerebral circulation. 1994;25:1820–1824. doi: 10.1161/01.str.25.9.1820. [DOI] [PubMed] [Google Scholar]
- 31.Ay H, Arsava EM, Saribas O. Creatine kinase-mb elevation after stroke is not cardiac in origin: Comparison with troponin t levels. Stroke; a journal of cerebral circulation. 2002;33:286–289. doi: 10.1161/hs0102.101544. [DOI] [PubMed] [Google Scholar]
- 32.Hall TS, Hallen J, Krucoff MW, Roe MT, Brennan DM, Agewall S, Atar D, Lincoff AM. Cardiac troponin i for prediction of clinical outcomes and cardiac function through 3-month follow-up after primary percutaneous coronary intervention for st-segment elevation myocardial infarction. American heart journal. 2015;169:257–265. e251. doi: 10.1016/j.ahj.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Apple FS, Steffen LM, Pearce LA, Murakami MM, Luepker RV. Increased cardiac troponin i as measured by a high-sensitivity assay is associated with high odds of cardiovascular death: The minnesota heart survey. Clinical chemistry. 2012;58:930–935. doi: 10.1373/clinchem.2011.179176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubo T, Kitaoka H, Okawa M, Yamanaka S, Hirota T, Baba Y, Hayato K, Yamasaki N, Matsumura Y, Yasuda N, Sugiura T, Doi YL. Combined measurements of cardiac troponin i and brain natriuretic peptide are useful for predicting adverse outcomes in hypertrophic cardiomyopathy. Circulation journal : official journal of the Japanese Circulation Society. 2011;75:919–926. doi: 10.1253/circj.cj-10-0782. [DOI] [PubMed] [Google Scholar]
- 35.Bugnicourt JM, Rogez V, Guillaumont MP, Rogez JC, Canaple S, Godefroy O. Troponin levels help predict new-onset atrial fibrillation in ischaemic stroke patients: A retrospective study. European neurology. 2010;63:24–28. doi: 10.1159/000258679. [DOI] [PubMed] [Google Scholar]
- 36.Oras J, Grivans C, Bartley A, Rydenhag B, Ricksten SE, Seeman-Lodding H. Elevated high-sensitive troponin t on admission is an indicator of poor long-term outcome in patients with subarachnoid haemorrhage: A prospective observational study. Critical care. 2016;20:11. doi: 10.1186/s13054-015-1181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruder N, Rabinstein A. Cardiovascular and pulmonary complications of aneurysmal subarachnoid hemorrhage. Neurocritical care. 2011;15:257–269. doi: 10.1007/s12028-011-9598-4. [DOI] [PubMed] [Google Scholar]
- 38.Garrett MC, Komotar RJ, Starke RM, Doshi D, Otten ML, Connolly ES. Elevated troponin levels are predictive of mortality in surgical intracerebral hemorrhage patients. Neurocrit Care. 2010;12:199–203. doi: 10.1007/s12028-009-9245-5. [DOI] [PubMed] [Google Scholar]
- 39.Di Angelantonio E, Fiorelli M, Toni D, Sacchetti ML, Lorenzano S, Falcou A, Ciarla MV, Suppa M, Bonanni L, Bertazzoni G, Aguglia F, Argentino C. Prognostic significance of admission levels of troponin i in patients with acute ischaemic stroke. Journal of neurology, neurosurgery, and psychiatry. 2005;76:76–81. doi: 10.1136/jnnp.2004.041491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cushman M, Judd SE, Howard VJ, Kissela B, Gutierrez OM, Jenny NS, Ahmed A, Thacker EL, Zakai NA. N-terminal pro-b-type natriuretic peptide and stroke risk: The reasons for geographic and racial differences in stroke cohort. Stroke; a journal of cerebral circulation. 2014;45:1646–1650. doi: 10.1161/STROKEAHA.114.004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krause T, Werner K, Fiebach JB, Villringer K, Piper SK, Haeusler KG, Endres M, Scheitz JF, Nolte CH. Stroke in right dorsal anterior insular cortex is related to myocardial injury. Ann Neurol. 2017;81:502–511. doi: 10.1002/ana.24906. [DOI] [PubMed] [Google Scholar]
- 42.Nigro N, Wildi K, Mueller C, Schuetz P, Mueller B, Fluri F, Christ-Crain M, Katan M. Bnp but not s-ctnln is associated with cardioembolic aetiology and predicts short and long term prognosis after cerebrovascular events. PloS one. 2014;9:e102704. doi: 10.1371/journal.pone.0102704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yip HK, Sun CK, Chang LT, Chen MC, Liou CW. Time course and prognostic value of plasma levels of n-terminal pro-brain natriuretic peptide in patients after ischemic stroke. Circulation journal : official journal of the Japanese Circulation Society. 2006;70:447–452. doi: 10.1253/circj.70.447. [DOI] [PubMed] [Google Scholar]
- 44.Etgen T, Baum H, Sander K, Sander D. Cardiac troponins and n-terminal pro-brain natriuretic peptide in acute ischemic stroke do not relate to clinical prognosis. Stroke; a journal of cerebral circulation. 2005;36:270–275. doi: 10.1161/01.STR.0000151364.19066.a1. [DOI] [PubMed] [Google Scholar]
- 45.Tung P, Kopelnik A, Banki N, Ong K, Ko N, Lawton MT, Gress D, Drew B, Foster E, Parmley W, Zaroff J. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke. 2004;35:548–551. doi: 10.1161/01.STR.0000114874.96688.54. [DOI] [PubMed] [Google Scholar]
- 46.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 47.Billman GE. The lf/hf ratio does not accurately measure cardiac sympatho-vagal balance. Frontiers in Physiology. 2013;4:26. doi: 10.3389/fphys.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papaioannou V, Pneumatikos I, Maglaveras N. Association of heart rate variability and inflammatory response in patients with cardiovascular diseases: Current strengths and limitations. Frontiers in Physiology. 2013;4:174. doi: 10.3389/fphys.2013.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korpelainen JT, Sotaniemi KA, Huikuri HV, Myllya VV. Abnormal heart rate variability as a manifestation of autonomic dysfunction in hemispheric brain infarction. Stroke. 1996;27:2059–2063. doi: 10.1161/01.str.27.11.2059. [DOI] [PubMed] [Google Scholar]
- 50.Korpelainen JT, Sotaniemi KA, Mäkikallio A, Huikuri HV, Myllylä VV. Dynamic behavior of heart rate in ischemic stroke. Stroke. 1999;30:1008–1013. doi: 10.1161/01.str.30.5.1008. [DOI] [PubMed] [Google Scholar]
- 51.Chen WL, Huang CH, Chen JH, Tai HC, Chang SH, Wang YC. Ecg abnormalities predict neurogenic pulmonary edema in patients with subarachnoid hemorrhage. Am J Emerg Med. 2016;34:79–82. doi: 10.1016/j.ajem.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 52.Colivicchi F, Bassi A, Santini M, Caltagirone C. Cardiac autonomic derangement and arrhythmias in right-sided stroke with insular involvement. Stroke. 2004;35:2094–2098. doi: 10.1161/01.STR.0000138452.81003.4c. [DOI] [PubMed] [Google Scholar]
- 53.Naver HK, Blomstrand C, Wallin BG. Reduced heart rate variability after right-sided stroke. Stroke. 1996;27:247–251. doi: 10.1161/01.str.27.2.247. [DOI] [PubMed] [Google Scholar]
- 54.Loubinoux I, Kronenberg G, Endres M, Schumann-Bard P, Freret T, Filipkowski RK, Kaczmarek L, Popa-Wagner A. Post-stroke depression: Mechanisms, translation and therapy. Journal of Cellular and Molecular Medicine. 2012;16:1961–1969. doi: 10.1111/j.1582-4934.2012.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hachinski V. Post-stroke depression, not to be underestimated. Lancet. 1999;353:1728. doi: 10.1016/S0140-6736(99)00139-7. [DOI] [PubMed] [Google Scholar]
- 56.Rosmond R, Bjorntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. Journal of internal medicine. 2000;247:188–197. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- 57.Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40:573–629. doi: 10.1016/0301-0082(93)90035-q. [DOI] [PubMed] [Google Scholar]
- 58.Christensen H, Boysen G, Johannesen HH. Serum-cortisol reflects severity and mortality in acute stroke. J Neurol Sci. 2004;217:175–180. doi: 10.1016/j.jns.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 59.Barugh AJ, Gray P, Shenkin SD, MacLullich AM, Mead GE. Cortisol levels and the severity and outcomes of acute stroke: A systematic review. J Neurol. 2014;261:533–545. doi: 10.1007/s00415-013-7231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melville KI, Blum B, Shister HE, Silver MD. Cardiac ischemic changes and arrhythmias induced by hypothalamic stimulation. The American journal of cardiology. 1963;12:781–791. doi: 10.1016/0002-9149(63)90281-9. [DOI] [PubMed] [Google Scholar]
- 61.Jia S, Xia Q, Zhang B, Wang L. Involvement of the paraventricular nucleus in the occurrence of arrhythmias in middle cerebral artery occlusion rats. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2015;24:844–851. doi: 10.1016/j.jstrokecerebrovasdis.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 62.Infanger DW, Cao X, Butler SD, Burmeister MA, Zhou Y, Stupinski JA, Sharma RV, Davisson RL. Silencing nox4 in the paraventricular nucleus improves myocardial infarction-induced cardiac dysfunction by attenuating sympathoexcitation and periinfarct apoptosis. Circ Res. 2010;106:1763–1774. doi: 10.1161/CIRCRESAHA.109.213025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fassbender K, Schmidt R, Mossner R, Daffertshofer M, Hennerici M. Pattern of activation of the hypothalamic-pituitary-adrenal axis in acute stroke Relation to acute confusional state, extent of brain damage, and clinical outcome. Stroke; a journal of cerebral circulation. 1994;25:1105–1108. doi: 10.1161/01.str.25.6.1105. [DOI] [PubMed] [Google Scholar]
- 64.Samuels MA. Neurogenic heart disease: A unifying hypothesis. Am J Cardiol. 1987;60:15j–19j. doi: 10.1016/0002-9149(87)90678-3. [DOI] [PubMed] [Google Scholar]
- 65.Schomig A. Catecholamines in myocardial ischemia Systemic and cardiac release. Circulation. 1990;82:Ii13–22. [PubMed] [Google Scholar]
- 66.Mertes PM, Carteaux JP, Jaboin Y, Pinelli G, el Abassi K, Dopff C, Atkinson J, Villemot JP, Burlet C, Boulange M. Estimation of myocardial interstitial norepinephrine release after brain death using cardiac microdialysis. Transplantation. 1994;57:371–377. doi: 10.1097/00007890-199402150-00010. [DOI] [PubMed] [Google Scholar]
- 67.Jacob WA, Van Bogaert A, De Groodt-Lasseel MH. Myocardial ultrastructure and haemodynamic reactions during experimental subarachnoid haemorrhage. Journal of molecular and cellular cardiology. 1972;4:287–298. doi: 10.1016/0022-2828(72)90076-4. [DOI] [PubMed] [Google Scholar]
- 68.Costa VM, Carvalho F, Bastos ML, Carvalho RA, Carvalho M, Remiao F. Contribution of catecholamine reactive intermediates and oxidative stress to the pathologic features of heart diseases. Current medicinal chemistry. 2011;18:2272–2314. doi: 10.2174/092986711795656081. [DOI] [PubMed] [Google Scholar]
- 69.Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277–286. doi: 10.1001/jama.2011.992. [DOI] [PubMed] [Google Scholar]
- 70.Nef HM, Mollmann H, Hilpert P, Troidl C, Voss S, Rolf A, Behrens CB, Weber M, Hamm CW, Elsasser A. Activated cell survival cascade protects cardiomyocytes from cell death in tako-tsubo cardiomyopathy. Eur J Heart Fail. 2009;11:758–764. doi: 10.1093/eurjhf/hfp076. [DOI] [PubMed] [Google Scholar]
- 71.Hachinski VC, Smith KE, Silver MD, Gibson CJ, Ciriello J. Acute myocardial and plasma catecholamine changes in experimental stroke. Stroke. 1986;17:387–390. doi: 10.1161/01.str.17.3.387. [DOI] [PubMed] [Google Scholar]
- 72.Weinberg SJ, Fuster JM. Electrocardiographic changes produced by localized hypothalamic stimulations*†. Annals of Internal Medicine. 1960;53:332–341. doi: 10.7326/0003-4819-53-2-332. [DOI] [PubMed] [Google Scholar]
- 73.Cruickshank JM, Neil-Dwyer G, Stott AW. Possible role of catecholamines, corticosteroids, and potassium in production of electrocardiographic abnormalities associated with subarachnoid haemorrhage. British Heart Journal. 1974;36:697–706. doi: 10.1136/hrt.36.7.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moss RL, Fitzsimons DP, Ralphe JC. Cardiac mybp-c regulates the rate and force of contraction in mammalian myocardium. Circ Res. 2015;116:183–192. doi: 10.1161/CIRCRESAHA.116.300561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saini HK, Tripathi ON, Zhang S, Elimban V, Dhalla NS. Involvement of na+/ca2+ exchanger in catecholamine-induced increase in intracellular calcium in cardiomyocytes. American journal of physiology. Heart and circulatory physiology. 2006;290:H373–380. doi: 10.1152/ajpheart.00613.2005. [DOI] [PubMed] [Google Scholar]
- 76.Hunt D, Gore I. Myocardial lesions following experimental intracranial hemorrhage: Prevention with propranolol. American heart journal. 1972;83:232–236. doi: 10.1016/0002-8703(72)90142-1. [DOI] [PubMed] [Google Scholar]
- 77.Madias JE. What is the recurrence rate of takotsubo syndrome in patients treated with beta-blockers and angiotensin converting enzyme inhibitors/angiotensin receptor blockers? International journal of cardiology. 2016;219:394–395. doi: 10.1016/j.ijcard.2016.06.119. [DOI] [PubMed] [Google Scholar]
- 78.Tahsili-Fahadan P, Geocadin RG. Heart-brain axis: Effects of neurologic injury on cardiovascular function. Circ Res. 2017;120:559–572. doi: 10.1161/CIRCRESAHA.116.308446. [DOI] [PubMed] [Google Scholar]
- 79.Hall RE, Livingston RB, Bloor CM. Orbital cortical influences on cardiovascular dynamics and myocardial structure in conscious monkeys. J Neurosurg. 1977;46:648–653. [PubMed] [Google Scholar]
- 80.Soros P, Hachinski V. Cardiovascular and neurological causes of sudden death after ischaemic stroke. The Lancet. Neurology. 2012;11:179–188. doi: 10.1016/S1474-4422(11)70291-5. [DOI] [PubMed] [Google Scholar]
- 81.Sander D, Winbeck K, Klingelhofer J, Etgen T, Conrad B. Prognostic relevance of pathological sympathetic activation after acute thromboembolic stroke. Neurology. 2001;57:833–838. doi: 10.1212/wnl.57.5.833. [DOI] [PubMed] [Google Scholar]
- 82.Ozdemir O, Hachinski V. Brain lateralization and sudden death: Its role in the neurogenic heart syndrome. Journal of the neurological sciences. 2008;268:6–11. doi: 10.1016/j.jns.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 83.Min J, Farooq MU, Greenberg E, Aloka F, Bhatt A, Kassab M, Morgan JP, Majid A. Cardiac dysfunction after left permanent cerebral focal ischemia: The brain and heart connection. Stroke. 2009;40:2560–2563. doi: 10.1161/STROKEAHA.108.536086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Christensen H, Boysen G, Christensen AF, Johannesen HH. Insular lesions, ecg abnormalities, and outcome in acute stroke. Journal of neurology, neurosurgery, and psychiatry. 2005;76:269–271. doi: 10.1136/jnnp.2004.037531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laowattana S, Zeger SL, Lima JA, Goodman SN, Wittstein IS, Oppenheimer SM. Left insular stroke is associated with adverse cardiac outcome. Neurology. 2006;66:477–483. doi: 10.1212/01.wnl.0000202684.29640.60. discussion 463. [DOI] [PubMed] [Google Scholar]
- 86.Algra A, Gates PC, Fox AJ, Hachinski V, Barnett HJ. Side of brain infarction and long-term risk of sudden death in patients with symptomatic carotid disease. Stroke; a journal of cerebral circulation. 2003;34:2871–2875. doi: 10.1161/01.STR.0000099964.34430.2D. [DOI] [PubMed] [Google Scholar]
- 87.Masuda T, Sato K, Yamamoto S, Matsuyama N, Shimohama T, Matsunaga A, Obuchi S, Shiba Y, Shimizu S, Izumi T. Sympathetic nervous activity and myocardial damage immediately after subarachnoid hemorrhage in a unique animal model. Stroke; a journal of cerebral circulation. 2002;33:1671–1676. doi: 10.1161/01.str.0000016327.74392.02. [DOI] [PubMed] [Google Scholar]
- 88.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 89.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiology of Disease. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 90.Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain, Behavior, and Immunity. 2017;60:1–12. doi: 10.1016/j.bbi.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 91.Merino JG, Latour LL, Tso A, Lee KY, Kang DW, Davis LA, Lazar RM, Horvath KA, Corso PJ, Warach S. Blood-brain barrier disruption after cardiac surgery. AJNR. American journal of neuroradiology. 2013;34:518–523. doi: 10.3174/ajnr.A3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iadecola C, Anrather J. The immunology of stroke: From mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gauberti M, Montagne A, Quenault A, Vivien D. Molecular magnetic resonance imaging of brain-immune interactions. Front Cell Neurosci. 2014;8:389. doi: 10.3389/fncel.2014.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Doll DN, Barr TL, Simpkins JW. Cytokines: Their role in stroke and potential use as biomarkers and therapeutic targets. Aging and Disease. 2014;5:294–306. doi: 10.14336/AD.2014.0500294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dickens AM, Tovar-y-Romo LB, Yoo S-W, Trout AL, Bae M, Kanmogne M, Megra B, Williams DW, Witwer KW, Gacias M, Tabatadze N, Cole RN, Casaccia P, Berman JW, Anthony DC, Haughey NJ. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Science Signaling. 2017:10. doi: 10.1126/scisignal.aai7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Emsley HC, Smith CJ, Gavin CM, Georgiou RF, Vail A, Barberan EM, Hallenbeck JM, del Zoppo GJ, Rothwell NJ, Tyrrell PJ, Hopkins SJ. An early and sustained peripheral inflammatory response in acute ischaemic stroke: Relationships with infection and atherosclerosis. J Neuroimmunol. 2003;139:93–101. doi: 10.1016/s0165-5728(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 98.Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- 99.Liesz A, Zhou W, Mracsko E, Karcher S, Bauer H, Schwarting S, Sun L, Bruder D, Stegemann S, Cerwenka A, Sommer C, Dalpke AH, Veltkamp R. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011;134:704–720. doi: 10.1093/brain/awr008. [DOI] [PubMed] [Google Scholar]
- 100.Yilmaz G, Granger DN. Leukocyte recruitment and ischemic brain injury. Neuromolecular Med. 2010;12:193–204. doi: 10.1007/s12017-009-8074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.An C, Shi Y, Li P, Hu X, Gan Y, Stetler RA, Leak RK, Gao Y, Sun BL, Zheng P, Chen J. Molecular dialogs between the ischemic brain and the peripheral immune system: Dualistic roles in injury and repair. Prog Neurobiol. 2014;115:6–24. doi: 10.1016/j.pneurobio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 103.Adams V, Linke A, Wisloff U, Doring C, Erbs S, Krankel N, Witt CC, Labeit S, Muller-Werdan U, Schuler G, Hambrecht R. Myocardial expression of murf-1 and mafbx after induction of chronic heart failure: Effect on myocardial contractility. Cardiovasc Res. 2007;73:120–129. doi: 10.1016/j.cardiores.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 104.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: Critical importance of the cardiosplenic axis. Circ Res. 2014;114:266–282. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory t cells and circulating macrophages. Journal of immunology (Baltimore, Md. : 1950) 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 106.Shichita T, Hasegawa E, Kimura A, Morita R, Sakaguchi R, Takada I, Sekiya T, Ooboshi H, Kitazono T, Yanagawa T, Ishii T, Takahashi H, Mori S, Nishibori M, Kuroda K, Akira S, Miyake K, Yoshimura A. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med. 2012;18:911–917. doi: 10.1038/nm.2749. [DOI] [PubMed] [Google Scholar]
- 107.Becker K. Autoimmune responses to brain following stroke. Transl. Stroke Res. 2012;3:310–317. doi: 10.1007/s12975-012-0154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marsh BJ, Williams-Karnesky RL, Stenzel-Poore MP. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience. 2009;158:1007–1020. doi: 10.1016/j.neuroscience.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–1644. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dabbagh K, Lewis DB. Toll-like receptors and t-helper-1/t-helper-2 responses. Current opinion in infectious diseases. 2003;16:199–204. doi: 10.1097/00001432-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 111.Becker KJ, Kalil AJ, Tanzi P, Zierath DK, Savos AV, Gee JM, Hadwin J, Carter KT, Shibata D, Cain KC. Autoimmune responses to brain following stroke are associated with worse outcome. Stroke; a journal of cerebral circulation. 2011;42:2763–2769. doi: 10.1161/STROKEAHA.111.619593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mann DL. Innate immunity and the failing heart: The cytokine hypothesis revisited. Circulation research. 2015;116:1254–1268. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rustenhoven J, Aalderink M, Scotter EL, Oldfield RL, Bergin PS, Mee EW, Graham ES, Faull RL, Curtis MA, Park TI, Dragunow M. Tgf-beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J Neuroinflammation. 2016;13:37. doi: 10.1186/s12974-016-0503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Viereck J, Bang C, Foinquinos A, Thum T. Regulatory rnas and paracrine networks in the heart. Cardiovasc Res. 2014;102:290–301. doi: 10.1093/cvr/cvu039. [DOI] [PubMed] [Google Scholar]
- 115.Vahidy FS, Parsha KN, Rahbar MH, Lee M, Bui TT, Nguyen C, Barreto AD, Bambhroliya AB, Sahota P, Yang B, Aronowski J, Savitz SI. Acute splenic responses in patients with ischemic stroke and intracerebral hemorrhage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016;36:1012–1021. doi: 10.1177/0271678X15607880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yoshimoto Y, Tanaka Y, Hoya K. Acute systemic inflammatory response syndrome in subarachnoid hemorrhage. Stroke; a journal of cerebral circulation. 2001;32:1989–1993. doi: 10.1161/hs0901.095646. [DOI] [PubMed] [Google Scholar]
- 117.Dhar R, Diringer MN. The burden of the systemic inflammatory response predicts vasospasm and outcome after subarachnoid hemorrhage. Neurocritical care. 2008;8:404–412. doi: 10.1007/s12028-008-9054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang L, Kong Y, Ren H, Li M, Wei CJ, Shi E, Jin WN, Hao J, Vandenbark AA, Offner H. Upregulation of cd74 and its potential association with disease severity in subjects with ischemic stroke. Neurochem Int. 2016 doi: 10.1016/j.neuint.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsujioka H, Imanishi T, Ikejima H, Kuroi A, Takarada S, Tanimoto T, Kitabata H, Okochi K, Arita Y, Ishibashi K, Komukai K, Kataiwa H, Nakamura N, Hirata K, Tanaka A, Akasaka T. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54:130–138. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 120.Winklewski PJ, Radkowski M, Demkow U. Cross-talk between the inflammatory response, sympathetic activation and pulmonary infection in the ischemic stroke. J Neuroinflammation. 2014;11:213. doi: 10.1186/s12974-014-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van der Bilt IA, Vendeville JP, van de Hoef TP, Begieneman MP, Lagrand WK, Kros JM, Wilde AA, Rinkel GJ, Niessen HW. Myocarditis in patients with subarachnoid hemorrhage: A histopathologic study. Journal of critical care. 2016;32:196–200. doi: 10.1016/j.jcrc.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 122.Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- 123.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 124.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 125.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Frontiers in Cellular Neuroscience. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nagatomo Y, Wilson Tang WH. Intersections between microbiome and heart failure: Revisiting the gut hypothesis. Journal of cardiac failure. 2015;21:973–980. doi: 10.1016/j.cardfail.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Adrie C, Parlato M, Salmi L, Adib-Conquy M, Bical O, Deleuze P, Fitting C, Cavaillon JM, Monchi M. Bacterial translocation and plasma cytokines during transcatheter and open-heart aortic valve implantation. Shock (Augusta, Ga.) 2015;43:62–67. doi: 10.1097/SHK.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 128.Sun J, Wang F, Ling Z, Yu X, Chen W, Li H, Jin J, Pang M, Zhang H, Yu J, Liu J. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Research. 2016;1642:180–188. doi: 10.1016/j.brainres.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 129.Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterology & Motility. 2012;24:405–413. doi: 10.1111/j.1365-2982.2012.01906.x. [DOI] [PubMed] [Google Scholar]
- 130.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδt cells. Nature medicine. 2016;22:516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, Dichgans M, Liesz A. Microbiota dysbiosis controls the neuroinflammatory response after stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yin J, Liao SX, He Y, Wang S, Xia GH, Liu FT, Zhu JJ, You C, Chen Q, Zhou L, Pan SY, Zhou HW. Dysbiosis of gut microbiota with reduced trimethylamine-oxide level in patients with large artery atherosclerotic stroke or transient ischemic attack. Journal of the American Heart Association. 2015;4:1–12. doi: 10.1161/JAHA.115.002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Winek K, Engel O, Koduah P, Heimesaat MM, Fischer A, Bereswill S, Dames C, Kershaw O, Gruber AD, Curato C, Oyama N, Meisel C, Meisel A, Dirnagl U. Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke; a Journal of Cerebral Circulation. 2016;47:1354–1363. doi: 10.1161/STROKEAHA.115.011800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, Feldstein AE, Britt EB, Fu X, Chung Y-M, Wu Y, Schauer P, Smith JD, Allayee H, Tang WHW, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Crapser J, Ritzel R, Verma R, Venna VR, Liu F, Chauhan A, Koellhoffer E, Patel A, Ricker A, Maas K, Graf J, McCullough LD. Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging. 2016;8:1049–1063. doi: 10.18632/aging.100952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yamashiro K, Tanaka R, Urabe T, Ueno Y, Yamashiro Y, Nomoto K, Takahashi T, Tsuji H, Asahara T, Hattori N. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PloS one. 2017;12:e0171521. doi: 10.1371/journal.pone.0171521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory t-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WH, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite tmao enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Raber L, Windecker S, Rodondi N, Nanchen D, Muller O, Miranda MX, Matter CM, Wu Y, Li L, Wang Z, Alamri HS, Gogonea V, Chung YM, Tang WH, Hazen SL, Luscher TF. Gut microbiota-dependent trimethylamine n-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38:814–824. doi: 10.1093/eurheartj/ehw582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Blann A, Shantsila E, Shantsila A. Microparticles and arterial disease. Seminars in thrombosis and hemostasis. 2009;35:488–496. doi: 10.1055/s-0029-1234144. [DOI] [PubMed] [Google Scholar]
- 142.Martinez MC, Tual-Chalot S, Leonetti D, Andriantsitohaina R. Microparticles: Targets and tools in cardiovascular disease. Trends in pharmacological sciences. 2011;32:659–665. doi: 10.1016/j.tips.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 143.Lackner P, Dietmann A, Beer R, Fischer M, Broessner G, Helbok R, Marxgut J, Pfausler B, Schmutzhard E. Cellular microparticles as a marker for cerebral vasospasm in spontaneous subarachnoid hemorrhage. Stroke; a journal of cerebral circulation. 2010;41:2353–2357. doi: 10.1161/STROKEAHA.110.584995. [DOI] [PubMed] [Google Scholar]
- 144.Huang M, Hu YY, Dong XQ. High concentrations of procoagulant microparticles in the cerebrospinal fluid and peripheral blood of patients with acute basal ganglia hemorrhage are associated with poor outcome. Surgical neurology. 2009;72:481–489. doi: 10.1016/j.surneu.2008.12.016. discussion 489. [DOI] [PubMed] [Google Scholar]
- 145.Mesri M, Altieri DC. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a jnk1 signaling pathway. The Journal of biological chemistry. 1999;274:23111–23118. doi: 10.1074/jbc.274.33.23111. [DOI] [PubMed] [Google Scholar]
- 146.Schoch B, Regel JP, Wichert M, Gasser T, Volbracht L, Stolke D. Analysis of intrathecal interleukin-6 as a potential predictive factor for vasospasm in subarachnoid hemorrhage. Neurosurgery. 2007;60:828–836. doi: 10.1227/01.NEU.0000255440.21495.80. discussion 828–836. [DOI] [PubMed] [Google Scholar]
- 147.Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, Boulanger CM. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. Journal of the American Society of Nephrology : JASN. 2005;16:3381–3388. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 148.Agouni A, Lagrue-Lak-Hal AH, Ducluzeau PH, Mostefai HA, Draunet-Busson C, Leftheriotis G, Heymes C, Martinez MC, Andriantsitohaina R. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. The American journal of pathology. 2008;173:1210–1219. doi: 10.2353/ajpath.2008.080228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Boulanger CM, Scoazec A, Ebrahimian T, Henry P, Mathieu E, Tedgui A, Mallat Z. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation. 2001;104:2649–2652. doi: 10.1161/hc4701.100516. [DOI] [PubMed] [Google Scholar]
- 150.Sanborn MR, Thom SR, Bohman LE, Stein SC, Levine JM, Milovanova T, Maloney-Wilensky E, Frangos S, Kumar MA. Temporal dynamics of microparticle elevation following subarachnoid hemorrhage. Journal of neurosurgery. 2012;117:579–586. doi: 10.3171/2012.6.JNS111163. [DOI] [PubMed] [Google Scholar]
- 151.Boettinger S, Lackner P. Cellular microparticles in subarachnoid hemorrhage. Translational stroke research. 2015;6:342–344. doi: 10.1007/s12975-015-0413-y. [DOI] [PubMed] [Google Scholar]
- 152.Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, Ataullakhanov FI. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thrombosis and haemostasis. 2007;97:425–434. [PubMed] [Google Scholar]
- 153.Horn P, Erkilet G, Veulemans V, Kropil P, Schurgers L, Zeus T, Heiss C, Kelm M, Westenfeld R. Microparticle-induced coagulation relates to coronary artery atherosclerosis in severe aortic valve stenosis. PloS one. 2016;11:e0151499. doi: 10.1371/journal.pone.0151499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Varon D, Haion Y, Brill A, Leker R. Cell-driven angiogenesis and neurogenesis after stroke is regulated by platelet’s microparticles. Blood. 2008;112:1899–1899. [Google Scholar]
- 155.Porro C, Trotta T, Panaro MA. Microvesicles in the brain: Biomarker, messenger or mediator? Journal of neuroimmunology. 2015;288:70–78. doi: 10.1016/j.jneuroim.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 156.Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C. Astrocyte-derived atp induces vesicle shedding and il-1 beta release from microglia. Journal of immunology. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 157.Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E, Matteoli M, Verderio C. Acid sphingomyelinase activity triggers microparticle release from glial cells. The EMBO journal. 2009;28:1043–1054. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tian Y, Salsbery B, Wang M, Yuan H, Yang J, Zhao Z, Wu X, Zhang Y, Konkle BA, Thiagarajan P, Li M, Zhang J, Dong JF. Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood. 2015;125:2151–2159. doi: 10.1182/blood-2014-09-598805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao Z, Wang M, Tian Y, Hilton T, Salsbery B, Zhou EZ, Wu X, Thiagarajan P, Boilard E, Li M, Zhang J, Dong JF. Cardiolipin-mediated procoagulant activity of mitochondria contributes to traumatic brain injury-associated coagulopathy in mice. Blood. 2016;127:2763–2772. doi: 10.1182/blood-2015-12-688838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen J, Venkat P, Zacharek A, Chopp M. Neurorestorative therapy for stroke. Front Hum Neurosci. 2014;8:382. doi: 10.3389/fnhum.2014.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, Socci ND, Hermida L, Fulci V, Chiaretti S, Foa R, Schliwka J, Fuchs U, Novosel A, Muller RU, Schermer B, Bissels U, Inman J, Phan Q, Chien M, Weir DB, Choksi R, De Vita G, Frezzetti D, Trompeter HI, Hornung V, Teng G, Hartmann G, Palkovits M, Di Lauro R, Wernet P, Macino G, Rogler CE, Nagle JW, Ju J, Papavasiliou FN, Benzing T, Lichter P, Tam W, Brownstein MJ, Bosio A, Borkhardt A, Russo JJ, Sander C, Zavolan M, Tuschl T. A mammalian microrna expression atlas based on small rna library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, Jeyaseelan K. Expression profile of micrornas in young stroke patients. PloS one. 2009;4:e7689. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microrna mir-126 governs vascular integrity and angiogenesis. Developmental cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Long G, Wang F, Li H, Yin Z, Sandip C, Lou Y, Wang Y, Chen C, Wang DW. Circulating mir-30a, mir-126 and let-7b as biomarker for ischemic stroke in humans. BMC Neurology. 2013;13:1–10. doi: 10.1186/1471-2377-13-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Qiang L, Hong L, Ningfu W, Huaihong C, Jing W. Expression of mir-126 and mir-508-5p in endothelial progenitor cells is associated with the prognosis of chronic heart failure patients. International journal of cardiology. 2013;168:2082–2088. doi: 10.1016/j.ijcard.2013.01.160. [DOI] [PubMed] [Google Scholar]
- 166.Long G, Wang F, Duan Q, Chen F, Yang S, Gong W, Wang Y, Chen C, Wang DW. Human circulating microrna-1 and microrna-126 as potential novel indicators for acute myocardial infarction. Int J Biol Sci. 2012;8:811–818. doi: 10.7150/ijbs.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.De Rosa S, Fichtlscherer S, Lehmann R, Assmus B, Dimmeler S, Zeiher AM. Transcoronary concentration gradients of circulating micrornas. Circulation. 2011;124:1936–1944. doi: 10.1161/CIRCULATIONAHA.111.037572. [DOI] [PubMed] [Google Scholar]
- 168.Chen J, Cui C, Yang X, Xu J, Venkat P, Zacharek A, Yu P, Chopp M. Mir-126 affects brain-heart interaction after cerebral ischemic stroke. Transl. Stroke Res. 2017:1–12. doi: 10.1007/s12975-017-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral micrornaome. J Cereb Blood Flow Metab. 2009;29:675–687. doi: 10.1038/jcbfm.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Climent M, Quintavalle M, Miragoli M, Chen J, Condorelli G, Elia L. Tgfbeta triggers mir-143/145 transfer from smooth muscle cells to endothelial cells, thereby modulating vessel stabilization. Circulation research. 2015;116:1753–1764. doi: 10.1161/CIRCRESAHA.116.305178. [DOI] [PubMed] [Google Scholar]
- 171.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Roxe T, Muller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating micrornas in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 172.van der Bilt I, Hasan D, van denBrink R, Cramer MJ, van der Jagt M, van Kooten F, Meertens J, van den Berg M, Groen R, Ten Cate F, Kamp O, Gotte M, Horn J, Groeneveld J, Vandertop P, Algra A, Visser F, Wilde A, Rinkel G, Investigators S. Cardiac dysfunction after aneurysmal subarachnoid hemorrhage: Relationship with outcome. Neurology. 2014;82:351–358. doi: 10.1212/WNL.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 173.Tanabe M, Crago EA, Suffoletto MS, Hravnak M, Frangiskakis JM, Kassam AB, Horowitz MB, Gorcsan J., 3rd Relation of elevation in cardiac troponin i to clinical severity, cardiac dysfunction, and pulmonary congestion in patients with subarachnoid hemorrhage. The American journal of cardiology. 2008;102:1545–1550. doi: 10.1016/j.amjcard.2008.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wybraniec MTM-SK, Kryzch L. Neurocardiogenic injury in subarachnoid hemorrhage: A wide spectrum of catecholamin-mediated brain-heart interactions. Cardiol J. 2014;21:220–228. doi: 10.5603/CJ.a2014.0019. [DOI] [PubMed] [Google Scholar]
- 175.Seo JY, Lee KB, Lee JG, Kim JS, Roh H, Ahn MY, Park BW, Hyon MS. Implication of left ventricular diastolic dysfunction in cryptogenic ischemic stroke. Stroke; a journal of cerebral circulation. 2014;45:2757–2761. doi: 10.1161/STROKEAHA.114.006108. [DOI] [PubMed] [Google Scholar]
- 176.Christensen H, Fogh Christensen A, Boysen G. Abnormalities on ecg and telemetry predict stroke outcome at 3 months. Journal of the neurological sciences. 2005;234:99–103. doi: 10.1016/j.jns.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 177.Lane RD, Wallace JD, Petrosky PP, Schwartz GE, Gradman AH. Supraventricular tachycardia in patients with right hemisphere strokes. Stroke; a journal of cerebral circulation. 1992;23:362–366. doi: 10.1161/01.str.23.3.362. [DOI] [PubMed] [Google Scholar]
- 178.Naidech AM, Kreiter KT, Janjua N, Ostapkovich ND, Parra A, Commichau C, Fitzsimmons B-FM, Connolly ES, Mayer SA. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation. 2005;112:2851–2856. doi: 10.1161/CIRCULATIONAHA.105.533620. [DOI] [PubMed] [Google Scholar]
- 179.Banki N, Kopelnik A, Tung P, Lawton MT, Gress D, Drew B, Dae M, Foster E, Parmley W, Zaroff J. Prospective analysis of prevalence, distribution, and rate of recovery of left ventricular systolic dysfunction in patients with subarachnoid hemorrhage. Journal of neurosurgery. 2006;105:15–20. doi: 10.3171/jns.2006.105.1.15. [DOI] [PubMed] [Google Scholar]
- 180.Ahtarovski KA, Iversen KK, Christensen TE, Andersson H, Grande P, Holmvang L, Bang L, Hasbak P, Lonborg JT, Madsen PL, Engstrom T, Vejlstrup NG. Takotsubo cardiomyopathy, a two-stage recovery of left ventricular systolic and diastolic function as determined by cardiac magnetic resonance imaging. European heart journal cardiovascular Imaging. 2014;15:855–862. doi: 10.1093/ehjci/jeu004. [DOI] [PubMed] [Google Scholar]
- 181.Mayer SA, Lin J, Homma S, Solomon RA, Lennihan L, Sherman D, Fink ME, Beckford A, Klebanoff LM. Myocardial injury and left ventricular performance after subarachnoid hemorrhage. Stroke. 1999;30:780–786. doi: 10.1161/01.str.30.4.780. [DOI] [PubMed] [Google Scholar]
- 182.Oras J, Grivans C, Dalla K, Omerovic E, Rydenhag B, Ricksten SE, Seeman-Lodding H. High-sensitive troponin t and n-terminal pro b-type natriuretic peptide for early detection of stress-induced cardiomyopathy in patients with subarachnoid hemorrhage. Neurocritical care. 2015;23:233–242. doi: 10.1007/s12028-015-0108-y. [DOI] [PubMed] [Google Scholar]
- 183.Sandhu R, Aronow WS, Rajdev A, Sukhija R, Amin H, D’Aquila K, Sangha A. Relation of cardiac troponin i levels with in-hospital mortality in patients with ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage. The American journal of cardiology. 2008;102:632–634. doi: 10.1016/j.amjcard.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 184.Rost NS, Wolf PA, Kase CS, Kelly-Hayes M, Silbershatz H, Massaro JM, D’Agostino RB, Franzblau C, Wilson PW. Plasma concentration of c-reactive protein and risk of ischemic stroke and transient ischemic attack: The framingham study. Stroke; a journal of cerebral circulation. 2001;32:2575–2579. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- 185.Di Napoli M, Papa F, Bocola V. C-reactive protein in ischemic stroke: An independent prognostic factor. Stroke; a journal of cerebral circulation. 2001;32:917–924. doi: 10.1161/01.str.32.4.917. [DOI] [PubMed] [Google Scholar]
- 186.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and hdl cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–2011. doi: 10.1161/01.cir.97.20.2007. [DOI] [PubMed] [Google Scholar]