Abstract
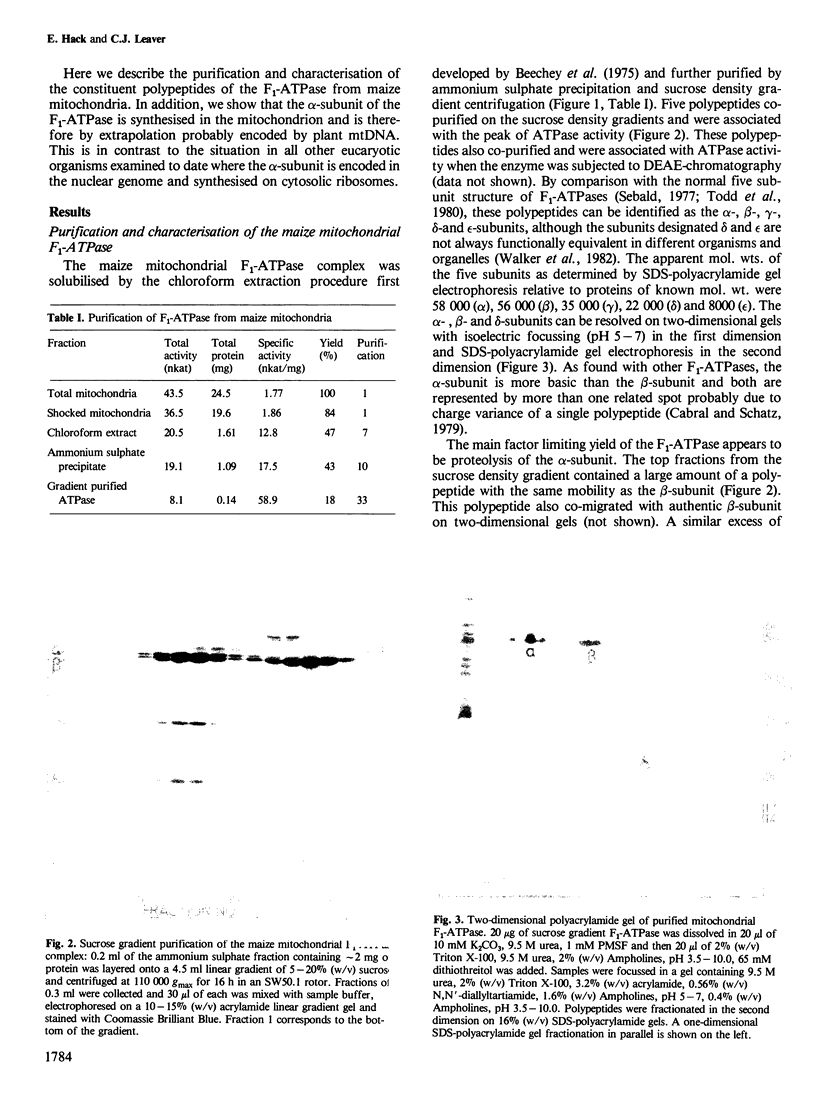
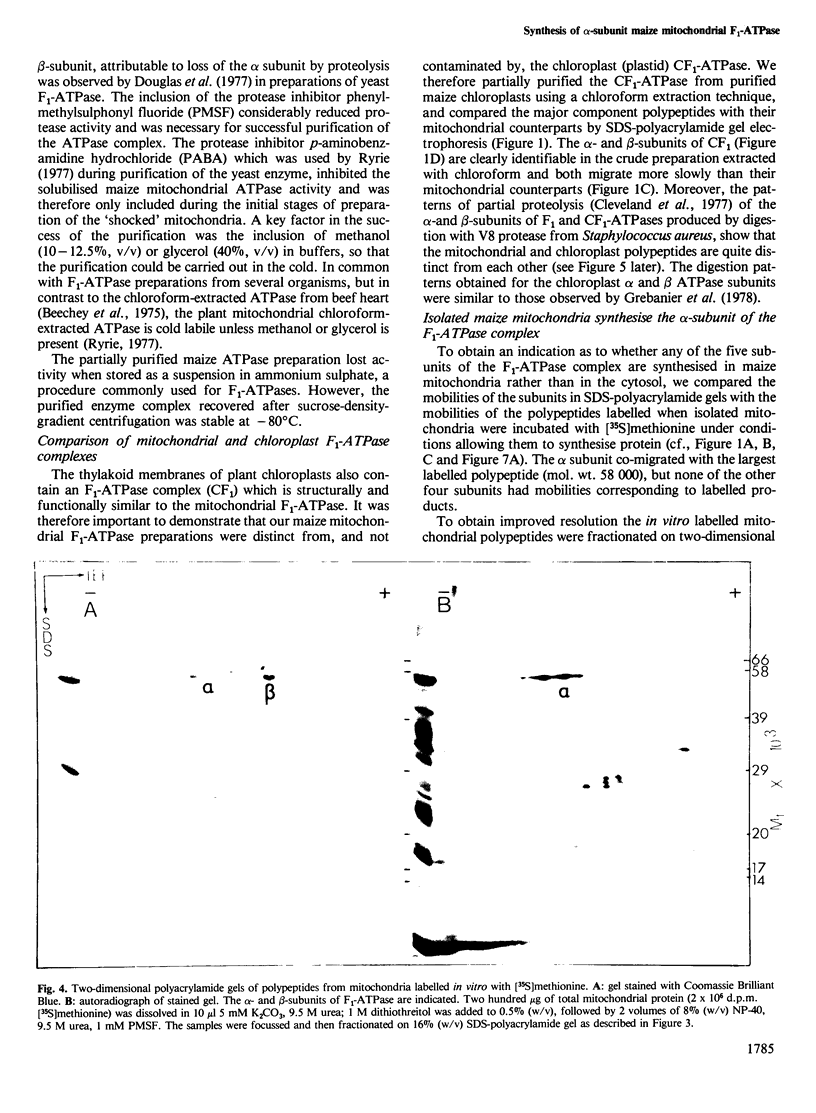
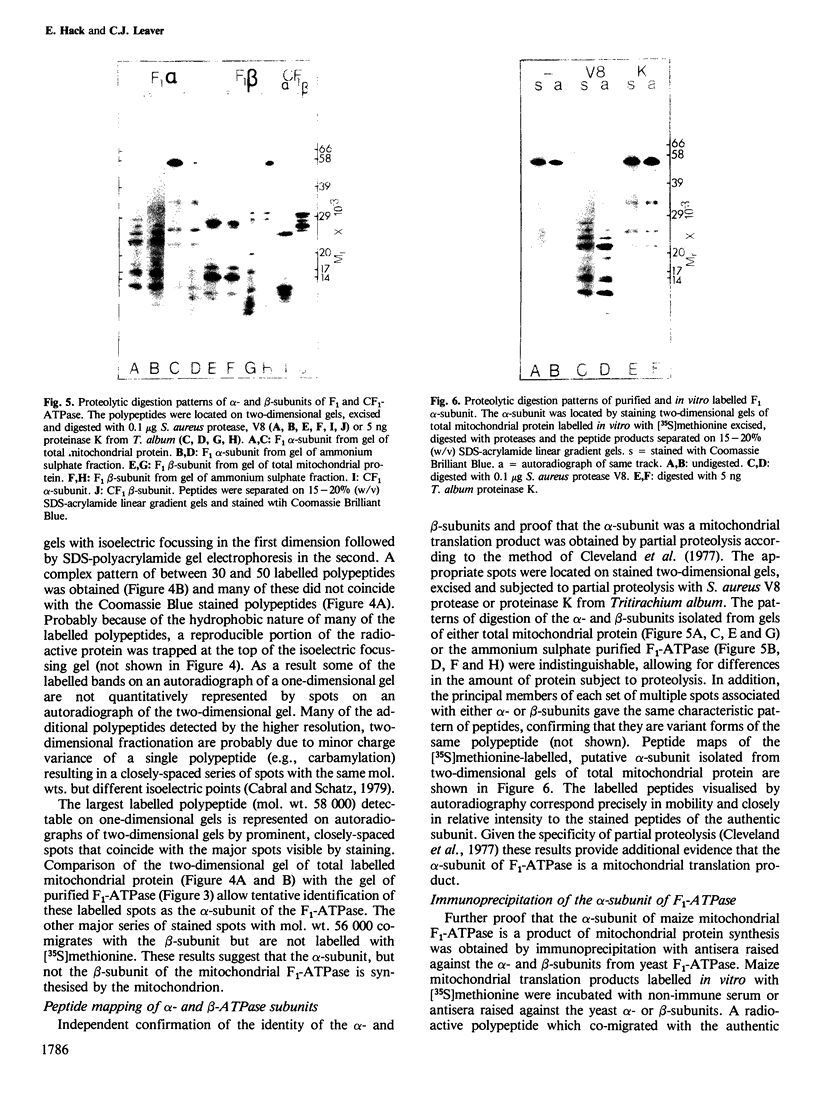
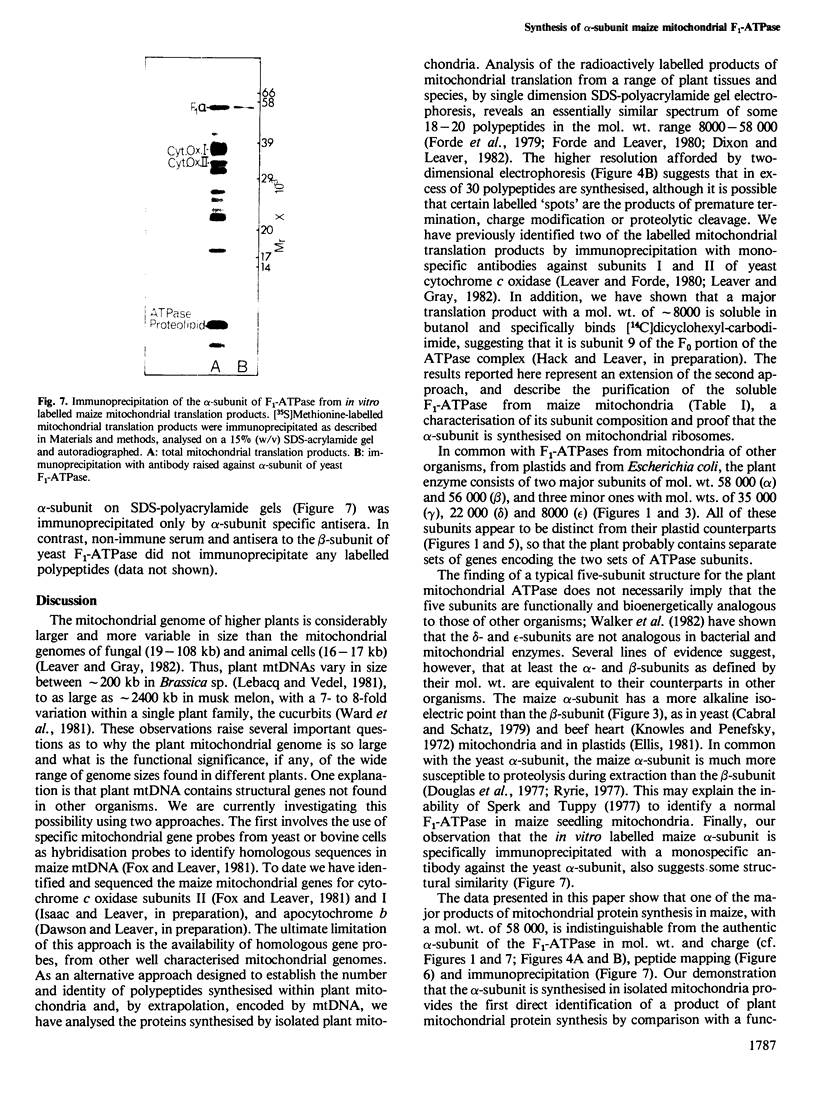
The F1-ATPase complex has been purified from maize (Zea mays L.) mitochondria and shown to consist of five subunits with mol. wts. of 58 000 (α), 56 000 (β), 35 000 (γ), 22 000 (δ) and 8000 (ε). The α-subunit co-migrates on one- and two- dimensional isoelectric focussing-SDS polyacrylamide gels with the major polypeptide synthesised by isolated mitochondria. One-dimensional proteolytic peptide mapping and immunoprecipitation confirms that the α-subunit is a mitochondrial translation product and therefore presumably encoded in mitochondrial DNA. This contrasts with the situation in animal and fungal cells where all five subunits of the F1-ATPase are encoded by the nuclear genome and synthesised on cytosolic ribosomes.
Keywords: F1-ATPase, maize, mitochondria, plant
Full text
PDF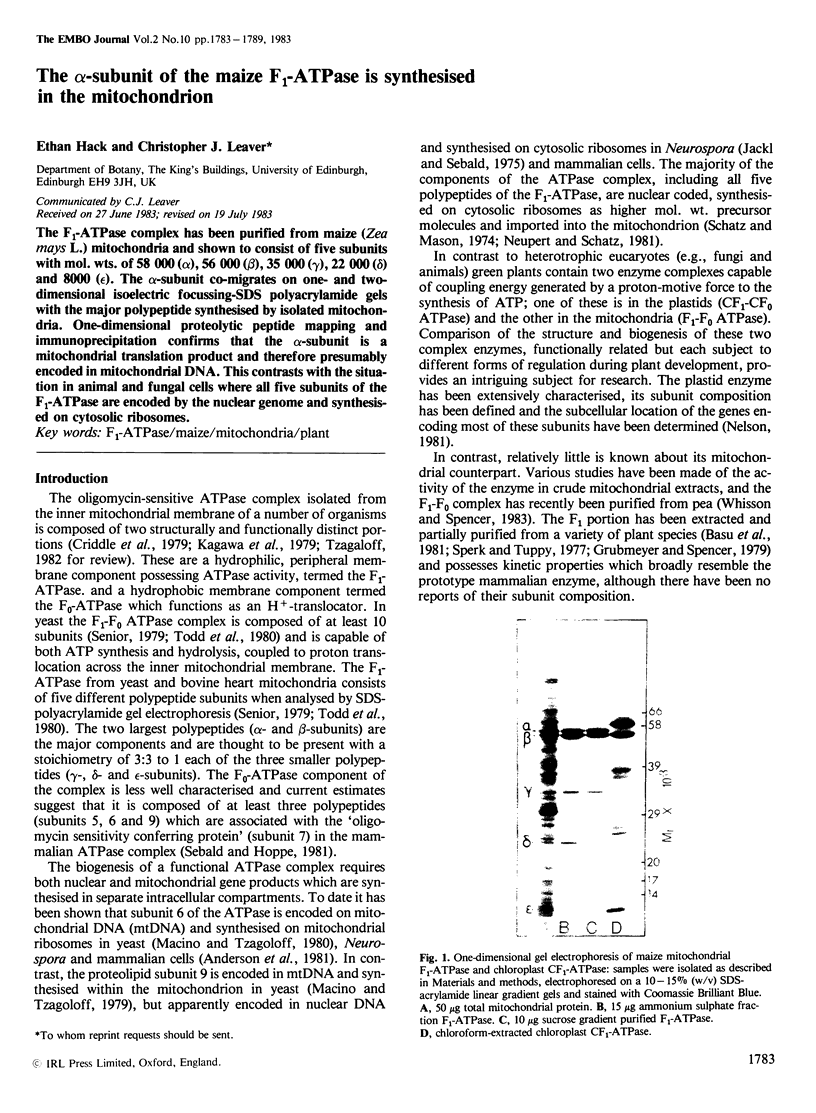


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Beechey R. B., Hubbard S. A., Linnett P. E., Mitchell A. D., Munn E. A. A simple and rapid method for the preparation of adenosine triphosphatase from submitochondrial particles. Biochem J. 1975 Jun;148(3):533–537. doi: 10.1042/bj1480533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral F., Schatz G. High resolution one- and two- dimensional electrophoretic analysis of mitochondrial membrane polypeptides. Methods Enzymol. 1979;56:602–613. doi: 10.1016/0076-6879(79)56057-1. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J., Weston P. D. Cathepsin D. Characteristics of immunoinhibition and the confirmation of a role in cartilage breakdown. Biochem J. 1971 Jun;123(1):1–13. doi: 10.1042/bj1230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas M. G., Koh Y., Dockter M. E., Schatz G. Aurovertin binds to the beta subunit of yeast mitochondrial ATPase. J Biol Chem. 1977 Dec 10;252(23):8333–8335. [PubMed] [Google Scholar]
- Forde B. G., Leaver C. J. Nuclear and cytoplasmic genes controlling synthesis of variant mitochondrial polypeptides in male-sterile maize. Proc Natl Acad Sci U S A. 1980 Jan;77(1):418–422. doi: 10.1073/pnas.77.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B. G., Oliver R. J., Leaver C. J. In Vitro Study of Mitochondrial Protein Synthesis during Mitochondrial Biogenesis in Excised Plant Storage Tissue. Plant Physiol. 1979 Jan;63(1):67–73. doi: 10.1104/pp.63.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., Leaver C. J. The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell. 1981 Nov;26(3 Pt 1):315–323. doi: 10.1016/0092-8674(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Grebanier A. E., Coen D. M., Rich A., Bogorad L. Membrane proteins synthesized but not processed by isolated maize chloroplasts. J Cell Biol. 1978 Sep;78(3):734–746. doi: 10.1083/jcb.78.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst M. N., Basha S. M., Baumbach G. A., Mansfield E. H., Roberts R. M. Alkaline urea solubilization, two-dimensional electrophoresis and lectin staining of mammalian cell plasma membrane and plant seed proteins. Anal Biochem. 1980 Mar 1;102(2):399–408. doi: 10.1016/0003-2697(80)90174-8. [DOI] [PubMed] [Google Scholar]
- Jackl G., Sebald W. Identification of two products of mitochondrial protein synthesis associated with mitochondrial adenosine triphosphatase from Neurospora crassa. Eur J Biochem. 1975 May;54(1):97–106. doi: 10.1111/j.1432-1033.1975.tb04118.x. [DOI] [PubMed] [Google Scholar]
- Kagawa Y., Sone N., Hirata H., Yoshida M. Structure and function of H+-ATPase. J Bioenerg Biomembr. 1979 Aug;11(3-4):39–78. doi: 10.1007/BF00743196. [DOI] [PubMed] [Google Scholar]
- Knowles A. F., Penefsky H. S. The subunit structure of beef heart mitochondrial adenosine triphosphatase. Isolation procedures. J Biol Chem. 1972 Oct 25;247(20):6617–6623. [PubMed] [Google Scholar]
- LaPolla R. J., Lambowitz A. M. Mitochondrial ribosome assembly in Neurospora crassa. Purification of the mitochondrially synthesized ribosomal protein, S-5. J Biol Chem. 1981 Jul 10;256(13):7064–7067. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leaver C. J., Hack E., Forde B. G. Protein synthesis by isolated plant mitochondria. Methods Enzymol. 1983;97:476–484. doi: 10.1016/0076-6879(83)97156-2. [DOI] [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: partial sequence of a mitochondrial ATPase gene in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Jan;76(1):131–135. doi: 10.1073/pnas.76.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: sequence analysis of a yeast mitochondrial ATPase gene containing the oli-2 and oli-4 loci. Cell. 1980 Jun;20(2):507–517. doi: 10.1016/0092-8674(80)90637-6. [DOI] [PubMed] [Google Scholar]
- Mariottini P., Chomyn A., Attardi G., Trovato D., Strong D. D., Doolittle R. F. Antibodies against synthetic peptides reveal that the unidentified reading frame A6L, overlapping the ATPase 6 gene, is expressed in human mitochondria. Cell. 1983 Apr;32(4):1269–1277. doi: 10.1016/0092-8674(83)90308-2. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ryrie I. J. The yeast mitochondrial adenosine triphosphatase complex. Purification, subunit composition, and some effects of protease inhibitors. Arch Biochem Biophys. 1977 Dec;184(2):464–475. doi: 10.1016/0003-9861(77)90456-8. [DOI] [PubMed] [Google Scholar]
- Sebald W. Biogenesis of mitochondrial ATPase. Biochim Biophys Acta. 1977 Jun 21;463(1):1–27. doi: 10.1016/0304-4173(77)90002-7. [DOI] [PubMed] [Google Scholar]
- Sperk G., Tuppy H. Differences between Adenosine Triphosphatases from Monocotylous and Dicotylous Plants. Plant Physiol. 1977 Feb;59(2):155–157. doi: 10.1104/pp.59.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Terpstra P., Zanders E., Butow R. A. The association of var1 with the 38 S mitochondrial ribosomal subunit in yeast. J Biol Chem. 1979 Dec 25;254(24):12653–12661. [PubMed] [Google Scholar]
- Todd R. D., Griesenbeck T. A., Douglas M. G. The yeast mitochondrial adenosine triphosphatase complex. Subunit stoichiometry and physical characterization. J Biol Chem. 1980 Jun 10;255(11):5461–5467. [PubMed] [Google Scholar]
- Walker J. E., Runswick M. J., Saraste M. Subunit equivalence in Escherichia coli and bovine heart mitochondrial F1F0 ATPases. FEBS Lett. 1982 Sep 20;146(2):393–396. doi: 10.1016/0014-5793(82)80960-5. [DOI] [PubMed] [Google Scholar]
- Ward B. L., Anderson R. S., Bendich A. J. The mitochondrial genome is large and variable in a family of plants (cucurbitaceae). Cell. 1981 Sep;25(3):793–803. doi: 10.1016/0092-8674(81)90187-2. [DOI] [PubMed] [Google Scholar]
- Whisson M. B., Spencer M. S. Solubilization and partial purification of n,n'-dicyclohexylcarbodiimide-sensitive ATPase from pea cotyledon mitochondria. Plant Physiol. 1983 Apr;71(4):707–711. doi: 10.1104/pp.71.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis H. M., Winget G. D., Racker E. Requirement of the delta subunit of chloroplast coupling factor 1 for photophosphorylation. J Biol Chem. 1977 Mar 10;252(5):1814–1818. [PubMed] [Google Scholar]










