Abstract
Traditional Chinese medication (TCM) has analgesic and anti-inflammatory effects in patients with knee osteoarthritis (OA). We conducted the first systematic review of the best quantitative and qualitative evidence currently available in order to evaluate the effectiveness of TCM in relieving pain in knee OA. A comprehensive literature search was conducted using three English and four Chinese biomedical databases from their inception through March 1, 2015. We included randomized controlled trials of TCM for knee OA with intervention durations of at least two weeks. The effects of TCM on pain and other clinical symptoms were measured with the visual analog scale (VAS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). The total effectiveness rate, which was used to assess overall pain, physical performance and wellness, was also measured. Two researchers independently extracted data on study design, population characteristics, duration, intervention, outcomes, risk of bias, and primary results. We performed a random-effects meta-analysis when appropriate. We also explored factors that could explain the heterogeneity by conducting subgroup and meta-regression analyses. Twenty-three studies, totaling 2362 subjects, met the eligibility criteria. Treatments were formulated with an average of 8 Chinese herbs and were prescribed based on the traditional Chinese diagnostic method of syndrome differentiation. The mean treatment duration was seven weeks, with oral administration occurring one to three times a day. Compared with non-steroidal anti-inflammatory drugs and intra-articular hyaluronate injections, 18 of the studies showed significantly improved VAS pain scores (Mean Difference [MD] = 0.56; 95% confidence interval [CI], 0.18 to 0.94; p = 0.004), six of the studies showed significantly improved WOMAC pain subscale scores (MD = 2.23; 95% CI, 0.56 to 3.91; p = 0.009), and 16 of the trials showed significantly improved total effectiveness rates (risk ratio = 1.12; 95% CI, 1.05 to 1.19; p = 0.0003). In addition, TCM showed a lower risk of adverse events than standard western treatments. This evidence suggests that TCM is safe and effective for improving pain, function, and wellness in treatments of knee OA. However, there is inherent clinical heterogeneity (diverse TCM formulations, controls, and treatment regimens) among the included trials. Despite these limitations, the potential analgesic effects of TCM warrant further methodologically rigorous research to determine the clinical implications of TCM on pain management in knee OA.
Keywords: Traditional Chinese Medication, Knee Osteoarthritis, Pain, Treatment, Review
Introduction
Knee osteoarthritis (OA) is a progressive, multi-factorial disease and is the most frequent cause of dependency in lower limb tasks in aging populations (Abou-Raya et al., 2014; Felson, 2006). There are currently no effective disease-modifying remedies available to treat knee OA (Jotanovic et al., 2014).
Several recent guidelines have questioned the safety and efficacy of the standard western medical treatment for OA. For example, the American Academy of Orthopaedic Surgeons’ 2013 guideline provided “inconclusive” recommendations for both acetaminophen and intra-articular corticosteroids (Brown, 2013). Oral nonsteroidal anti-inflammatory drugs (NSAIDs) were only conditionally recommended by both the OA Research Society International (McAlindon et al., 2014) and the American College of Rheumatology (ACR) (Hochberg et al., 2012). However, the long-term utilization of nonselective NSAIDs have been reported to increase the risk of side effects (Nagi et al., 2014). Thus, complementary and integrative therapies are heavily favored in treatments of OA. In fact, a growing number of patients with chronic musculoskeletal pain report utilizing such therapy, specifically traditional Chinese medication (TCM) (Barnes et al., 2008).
An ancient, traditional treatment, the use of TCM in treatments of arthritis has been empirically tested and refined over thousands of years in Asian countries (Cheung, 2011; Eisenberg et al., 1998; Fan et al., 2005; Jiang et al., 2014; Zhong et al., 2014). In the earliest published Chinese medical works, such as “Inner Classic of the Yellow Emperor” (475 B.C.–221 B.C.) and “Treatise on Febrile and Miscellaneous Diseases” (200 A.D.–210 A.D.), the etiology, pathogenesis, and TCM treatment for arthritis is well documented, especially regarding the beneficial outcomes (Zhang, 2010). To date, the popularity of TCM continues to grow in Asian countries and across the world for the treatment of pain disorders, including knee OA (Maroon et al., 2010; Teekachunhatean et al., 2004; Im et al., 2014; Yang et al., 2014). Compared to other herbal medicine, TCM consists of specific medicinal ingredients which target the biological processes underlying diseases, which are based on specific symptom differentiation (Zhang et al., 2014; Ling et al., 2015). Recent evidence suggests that TCM may actively ameliorate pain by exerting analgesic, anti-inflammatory, and blood circulation effects (Zhang et al., 2008; Zhou et al., 2011, 2013).
Although TCM has long been regarded historically as a key component in the treatment of arthritis in China, and this mode of treatment continues to disseminate around the world, quantitative evidence to assess its effects is still lacking. The biological actions and potential interactions of TCM with other prescription medications remain unclear (Pan et al., 2014; Wang et al., 2014a). We conducted the first meta-analysis of all available data to determine the effects of TCM on pain relief in patients with knee OA in order to better inform future research and clinical practice.
Methods
Search Strategy
We conducted a comprehensive literature search on 3 English and 4 Chinese biomedical databases from inception through March 1, 2015. These databases included PubMed, the Cochrane Library, Springer, Chinese National Knowledge Infrastructure, Chongqing VIP, Wanfang, and Chinese Biomedical Databases. In addition, we manually searched publication records from the Shanghai University of traditional Chinese medicine Library. The search terms included Chinese medication, Chinese herbs, electuary, pill, granule, decoction, powder, pellet, knee osteoarthritis, pain, randomized controlled trial, and clinical trial.
Eligibility Criteria
We defined TCM as practitioner-prescribed herbal medicines that treated knee OA based on syndrome differentiation, according to traditional medical theory. We included randomized controlled trials (RCTs) that compared TCM with standard western treatments in adults with knee osteoarthritis. To be eligible for this study, each trial was required to have at least 2 weeks of TCM intervention, more than 10 subjects in each group, and had to publish original data. There was no language restriction on the literature search. We excluded review articles and case reports (Fig. 1).
Figure 1.
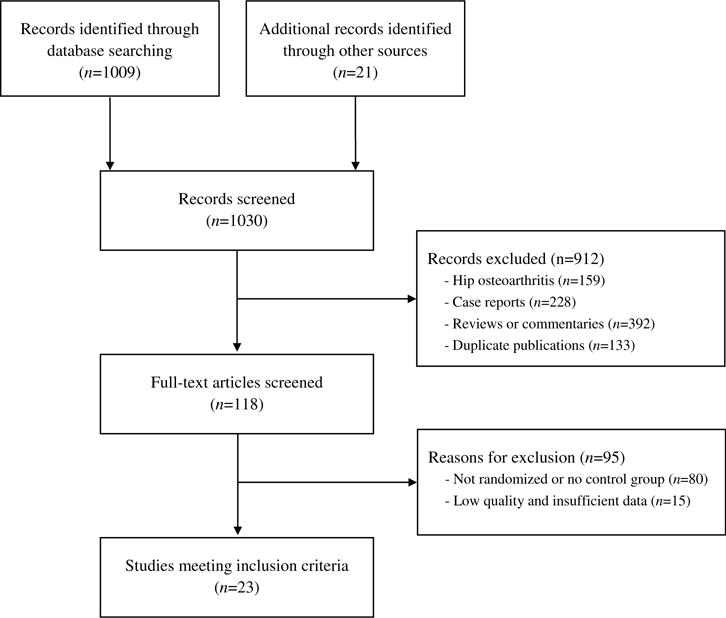
Study selection flow chart.
Study Selection
Two authors independently screened all potential eligible studies. Titles and abstracts were first screened to exclude irrelevant citations. The full texts of all potentially relevant abstracts were retrieved and screened according to the study eligibility criteria. Disagreements were resolved by consensus or discussion with a third author. The diagnostic criterion derived from the ACR (Altman et al., 1986), and the Chinese Orthopedic Association criteria (Chinese Orthopaedic Association, 2010) were both accepted (Table 1).
Table 1.
Characteristics of 23 Randomized Controlled Trials of Traditional Chinese Medication for Knee OA.
| Source | Diagnostic Criteria | N (Female %) | Age (yr) | Disease Duration (Months) | Traditional Chinese Medication | Controls | Outcomes Assessments | Results (TCM vs. Control) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Cao et al. (2005) | ACR knee OA criteria (1986) | 60 (68%) | 63 | 132 | Blood-nourishing and hard-softening capsule (Ingredients: White peony root, Largeleaf Gentian Root and Liquoric Root), 1.05 g, three times a day. 4 wks/1 course. | Glucosamine, 750 mg, three times a day. 4 wks/1 course. | VAS Pain WOMAC Pain | 1.8 vs. 2.0 11 vs. 14 |
> 0.05 > 0.05 |
| Deng (2008) | ACR knee OA criteria (1986) | 100 (58%) | 59 | 114 | Gancao Fuzi Decoction (Ingredients: Liquoric Root 6 g, Prepared Common Monkshood Daughter Root 9 g, Largehead Atractylodes Rhizome 12 g, and Cassia Twig 9 g), once a day. 2 wks/1 course. | Diclofenac sodium, 75 mg, once a day. 2 wks/1 course. | VAS Pain Total effectiveness rate | 1.55 vs. 1.50 92% vs. 88% |
> 0.05 > 0.05 |
| Ji et al. (2008) | ACR knee OA criteria (1986) | 50 (60%) | 59 | 132 | Tong Bi Granule (Ingredients: Doubleteeth pubescent angilica root, Chinese Angelica, Twotooth achyranthes root, Giant Knotweed Rhizome, Chinese Taxillus Twig, Epimedium Herb, etc.), three times a day. 6 wks/1 course. | Glucosamine, 250 mg three times a day. 6 wks/1 course. | VAS Pain Total effectiveness rate | 2.6 vs. 3.4 90% vs.75% |
< 0.05 < 0.05 |
| Deng and Ding (2009) | ACR knee OA criteria (1986) | 160 (64%) | 60 | 12 | Prepared Common Monkshood Daughter Root Decoction (Ingredients: Pilose Asiabell Root 9 g, Prepared Common Monkshood Daughter Root 6 g, Largehead Atractylodes Rhizome 12 g, Indian Buead 9 g, White peony root 9 g), once a day. 2 wks/1 course. | Meloxicam, 75 mg, once a day, an hour after the meal. 2 wks/1 course. | VAS Pain Total effectiveness rate | 2.86 vs. 3.29 91% vs. 94% |
> 0.05 > 0.05 |
| Ma et al. (2009) | ACR knee OA criteria (1986) | 118 (90%) | 52 | 84 | Self-made Decoction (Ingredients: Peach Seed 10 g, Safflower 12 g, Szechuan Lovage Rhizome 10 g, Chinese Angelica 12 g, Red Paeony Root 10 g, Twotooth Achyranthes Root 12 g, Chinese Taxillus Twig 15 g, Eucommia Bark 12 g, Clematis Root 12 g), three times a day. 12 wks/1 course. | Celecoxib, 200 mg, once a day. 12wks/1 course. | VAS Pain Total effectiveness rate | 2.41 vs. 3.18 80% vs. 59% |
< 0.05 < 0.05 |
| Qi et al. (2009) | ACR knee OA criteria (1986) | 337 (56%) | 55 | 60 | Yang Yuan Rou Gan Decoction (Ingredients: Astragalus root 30 g, Rehmannia Root 20 g, Malaytea Scurfpea Fruit 15 g, White peony root 30 g, Ginseng 15 g, Tuber Fleeceflower Root 12 g, Chinese Angelical 2 g, Desertliving Cistanche 10 g, Fortune’s Drynaria Rhizome 10 g, Chinese Thorowax Root 10 g, Dendrobium 30 g, Common Floweringquince Fruit 10 g, Twotooth Achyranthes Root 10 g, Liquoric Root 10 g), twice a day. 1 month/1 course. | Glucosamine, 750 mg, three times a day. 1 month/1 course. | VAS Pain Total effectiveness rate | 1.22 vs. 2.46 92% vs. 79% |
< 0.05 < 0.05 |
| Zhang et al. (2009) | ACR knee OA criteria (1986) | 60 (58%) | 53 | 76 | Decoction of suppling qi and activating blood circulation (Ingredients: Astragalus root 30 g, Pilose Asiabell Root 30 g, Largehead Atractylodes Rhizome 20 g, Suberect Spatholobus Stem 30 g, Donkey-hide Glue 15 g, Szechuan Lovage Rhizome 15 g, White peony root 20 g, Chinese Angelica 15 g, Costustoot 6 g, Chinese Thorowax Root 12 g, Chinese Starjasmine Stem 20 g, Clematis Root 30 g), twice a day. 2 courses, 3 months/1 course. | Celecoxib, 100 mg, twice a day. 2 courses, 3 months/1 course. | WOMAC Pain | 6.03 vs. 7.14 | < 0.01 |
| Xie et al. (2010) | ACR knee OA criteria (1986) | 176 (81%) | 60 | 96 | Self-made Decoction (Ingredients: Amur Corktree Bark 0.5 g, Coix Seed 1.4 g, Swordlike Atractylodes Rhizome 2.2g, Largehead Atractylodes Rhizome 3.0 g, Radix Stephaniae Tehrandrae 0.6 g, Salviae miltiorrhizae 1.8 g, Indian Buead 0.5 g, Doubleteeth Pubescent Angilica Root 2.5 g, Chinese Taxillus Twig 0.9 g, Twotooth Achyranthes Root 2.5 g), twice a day. 4 wks/1 course. | Ibuprofen, 0.6g, twice a day. 4 wks/1 course. | VAS Pain Total effectiveness rate | 2.6 vs. 4.2 93% vs. 86% |
< 0.01 < 0.05 |
| Kang et al (2011) | ACR knee OA criteria (1986) | 114 (79%) | 53 | 102 | Wangbi Tablet (Ingredients: Rehmannia Root, Common Anemarrhena Rhizome, Epimedium Herb, Himalayan Teasel Root, Malaytea Scurfpea Fruit, East Asian Tree Fem Rhizome, Common Clubmoss Herb, Safflower, White peony root, Cassia Twig, Doubleteeth Pubescent Angilica Root, Divaricate Saposhnikovia Root, Clematis Root), 2.0 g, three times a day. 8 wks/1 course. | Diclofenac sodium, 25 mg once or twice a day. 8 wks/1 course. | VAS Pain Total effectiveness rate | 1.93 vs. 1.93 97% vs. 71% |
> 0.05 < 0.05 |
| Liu and Xue (2011) | ACR knee OA criteria (1986) | 80 (55%) | 58 | 24 | Bi Qi capsule (Ingredients: Pilose Asiabell Root, Salviae miltiorrhizae, Twotooth Achyranthes Root, Largehead Atractylodes Rhizome, Sanchi, Szechuan Lovage Rhizome, strychnine, Liquoric Root), 1.2 g, three times a day. 4 wks/1 course. | Diclofenac sodium, 75 mg twice a day. 4 wks/1 course. | VAS Pain Total effectiveness rate | 3.89 vs. 4.79 93% vs. 63% |
< 0.05 < 0.05 |
| Fan et al (2012) | ACR knee OA criteria (1986) | 152 (58%) | 50 | 50 | Danggui Sini Decoction (Ingredients: Chinese Angelica 15 g, Cassia Twig 10 g, White peony root 10 g, Manchurian Wildginger 3 g, Rice-paperplant Pith 10 g, Twotooth Achyranthes Root 10 g, Slenderstyle Acanthopanax Bark 5 g, Malaytea Scurfpea Fruit 10 g, Fortune’s Drynaria Rhizome 10 g, Coix Seed 5 g, Common Floweringquince Fruit 10 g, Safflower 5 g, Liquoric Root 10 g), twice a day. 4 courses, 2 wks/1 course. | Celecoxib, 200 mg once a day. 4 courses, 2 wks/1 course. | VAS Pain Total effectiveness rate | 2.8 vs. 2.7 93% vs. 87% |
> 0.05 < 0.05 |
| Gao et al., (2012) | ACR knee OA criteria (1986) | 96 (71%) | 58 | 6 | Bushen Huoxue Qubi Decoction (Ingredients: Epimedium Herb 15 g, Fortune’s Drynaria Rhizome 15 g, Astragalus root 50 g, Chinese Angelica 10 g, Salviae miltiorrhizae 15 g, Slender style Acanthopanax Bark 10 g, Oriental Bittersweet Root 30 g, Bitter Orange 10 g, Liquoric Root 15 g), twice a day 4 wks/1 course. | Celecoxib, 200 mg once a day. 4 wks/1 course. | VAS Pain | 1.56 vs. 1.94 | < 0.05 |
| Li et al. (2012) | ACR knee OA criteria (1986) | 89 (63%) | 61 | 18 | Small Meridian-Activating Pill (Ingredients: Common Monkshood Mother Root, Kusnezoff Monkshood Root, Chinese Angelica, Szechuan Lovage Rhizome, White peony root, Earthworm, Frankincense, Myrrh, Nutgrass Galingale Rhizome, Rhizoma arisaematis), twice a day. 2 months/1 course. | Glucosamine, 250 mg, twice a day. 2 months/1 course. | VAS Pain | 4.38 vs. 6.94 | < 0.05 |
| Liu et al. (2012) | Chinese Orthopedic Association criteria (2007)** | 57 (61%) | 61 | 28 | Compound Xuanju Capsule (Ingredients: Black Ant, Epimedium Herb, Barbary Wolfberry Fruit, Common Cnidium Fruit), 1.68 g, three times a day. 6 wks/1 course. | Celecoxib, 200 mg once a day. 6 wks/1 course. | VAS Pain Total effectiveness rate | 1.34 vs. 1.12 86% vs. 89% |
> 0.05 > 0.05 |
| Sun et al. (2012) | Chinese Orthopedic Association criteria (2007)** | 60 (73%) | 57 | 54 | Jintiange Capsule (Artificial Tiger Bone Extract), 1.2 g, three times a day. 4 wks/1 course. | Diclofenac sodium, 75 mg once a day. 4 wks/1 course. | WOMAC Pain | 13.78 vs. 14.01 | > 0.05 |
| Tao et al. (2012) | ACR knee OA criteria | 120 (56%) | 60 | 50 | Gubitong Decoction (Ingredients: Fortune’s Drynaria Rhizome 18 g, Eucommia Bark 20 g, East Asian Tree Fem Rhizome 25 g, Malaytea Scurfpea Fruit 10 g, Fritillary 15 g, Ovientvine 20 g, Suberect Spatholobus Stem 20 g, Epimedium Herb 10 g), twice a day. 8 wks/1 course. | Glucosamine, 480 mg twice a day. 8 wks/1 course. | VAS Pain | 3.09 vs. 3.16 | > 0.05 |
| Yang (2012) | ACR knee OA criteria (1986) | 40 (58%) | 62 | No Data | Gancao Fuzi Decoction (Ingredients: Prepared Common Monkshood Daughter Root 15 g, Liquoric Root 10 g, Largehead Atractylodes Rhizome 15 g, Cassia Twig 20 g), twice a day. 5 wks/1 course. | Hyaluronate injection 1 × 2ml/wk. 5 wks/1 course. | VAS Pain Total effectiveness rate | 3.1 vs. 3.0 70% vs. 80% |
> 0.05 > 0.05 |
| Lin et al. (2013) | Chinese Orthopedic Association criteria (2007)** | 120 (65%) | 57 | 75 | Jiawei Simiao San Decoction (Ingredients: Eucommia Bark 15 g, Chinese Taxillus Twig 30 g, Swordlike Atractylodes Rhizome 12 g, Amur Corktree Bark 12 g, Twotooth Achyranthes Root 12 g, Coix Seed 15 g, Beautiful Sweetgum Fruit 12 g, Sanchi 10 g, Pangolin Scales 6 g), twice a day. 8 wks/1 course. | Alendronate sodium, 70 mg, once a week. 8 wks/1 course. | VAS Pain Total effectiveness rate | 3.51 vs. 3.43 68% vs. 60% |
> 0.05 < 0.05 |
| Zhu et al. (2013) | ACR knee OA criteria (1986) | 86 (71%) | 65 | 16 | Zhengqing Fengtongning Pill (Ovientvine Extract), 60 mg, twice a day. 12 wks/1 course. | Glucosamine, 240 mg twice a day. 12 wks/1 course. | WOMAC Pain Total effectiveness rate | 3.6 vs. 4.5 95% vs. 91% |
< 0.05 < 0.05 |
| Fu and Teng (2014) | ACR knee OA criteria (1986) | 86 (60%) | 61 | 30 | Xitong Decoction (Ingredients: Eucommia Bark 15 g, White peony root 15 g, Twotooth Achyranthes Root 15 g, Rehmannia Root 15 g, Indian Buead 12 g, Chinese Angelica 12 g, Safflower 12 g, Salviae miltiorrhizae 12 g, Suberect Spatholobus Stem 12 g, Ceratostigma willmottianum 12 g, Liquoric Root 6 g), twice a day. 8 wks/1 course. | Glucosamine, 628 mg three times a day. 8 wks/1 course. | WOMAC Pain Total effectiveness rate | 3.19 vs. 8.51 86% vs. 70% |
< 0.05 < 0.05 |
| Han (2014) | ACR knee OA criteria (1986) | 60 (62%) | 57 | No Data | Yiqi Jianpi Huoxue Tongluo Decoction (Ingredients: Astragalus root 45 g, Largehead Atractylodes Rhizome 10 g, Indian Buead 10 g, Coix Seed 15 g, Dendrobium 15 g, White peony root 15 g, Twotooth Achyranthes Root 10 g, Red Paeony Root 10 g, Chinese Angelica 10 g, Common Floweringquince Fruit 15 g, etc.), twice a day. 4 wks/1 course. | Meloxicam, 7.5 mg once a day. 4 wks/1 course. | VAS Pain Total effectiveness | 2.43 vs. 3.48 90% vs. rate 77 % |
< 0.01 < 0.05 |
| Lin and Wang (2014) | Chinese Orthopedic Association criteria (2007)** | 78 (62%) | 55 | 52 | Jintiange Capsule (Artificial Tiger Bone Extract), 1.0 g, three times a day. 12 wks/1 course. | Alendronate sodium, 70 mg, once a week. 12 wks/1 course. | VAS Pain Total effectiveness | 2.42 vs. 4.17 77% vs. rate 59% |
< 0.05 < 0.05 |
| Tang et al. (2014) | Chinese Orthopedic Association criteria (2007)** | 63 (44%) | 55 | 22 | Yanghe Decoction (Ingredients: Rehmannia Root 30 g, Cassia Bark 15 g, Ephedra Herb 5 g, Pickled Ginger 6 g, White Mustard Seed 15 g, Deerhorn Glue 15 g, Liquoric Root 15 g), twice a day. 4 wks/1 course. | Glucosamine, 750 mg, three times a day. 4 wks/1 course. | WOMAC Pain | 6.31 vs. 9.33 | < 0.05 |
ACR = American College of Rheumatology; N = number of patients included; yr = year; g = gram; VAS = visual analog scale; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Total effectiveness rate assessing the overall pain, physical function and wellness.
Chinese Orthopaedic Association diagnostic criteria: 1. Recurrent knee joint pain in the last month; 2. Narrowed joint space, subchondral cyst formation and bone sclerosis, or osteophytosis around joint margin on the radiographs in standing or load position; 3. Evidence of clear, transparent, and viscous joint effusion at least twice; white cell count < 2000 ml; 4. Middle-aged and aged patients (40 years old or older); 5. Morning stiffness ≤ 30 min; 6. Palpable bone crepitation (fremitus) on movement of joint. Diagnosis of knee OA can be made if the following conditions are satisfied: 1+2, 1+3+5+6 or 1+4+5+6.
Pain intensity was measured using the visual analogue scale (VAS) or the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). The pain subscale was the pre-specified primary outcome in this study. The total effectiveness rate, which assessed overall pain, physical performance, and wellness, was the secondary outcome. The total effectiveness rate (Zheng, 2002) was assessed based on the number of patients in each of the following categories: “Clinically cured,” (the pain and swelling of joints had disappeared and active function had returned to normal); “Significant improvement,” (the pain and swelling of joints was alleviated and active function had improved significantly); “Improvement,” (the pain and swelling of joints was partially alleviated and active function had improved); and “Not cured,” (the pain and swelling of joints remained unchanged and there was no improvement of active function). The total effectiveness rate (%) was determined as the quotient of the number of cured and improved patients divided by the total number of the patients.
Data Collection and Quality Assessment
We extracted data from the selected studies using a pre-designed data extraction table, including publication information, the origin of the study, the study setting, the time frame of the study, age, gender, the author’s definition of knee OA, detailed information of interventions and controls, outcome measures, summary of results, main conclusion and adverse reactions. The accuracy of the data extraction was verified by another author (Table 1).
We assessed the risk of bias for each study using the items in Cochrane Collaboration’s tool for assessing risk of bias in randomized trials with modifications (Higgins et al., 2011), which included the following items: adequacy of randomization; allocation concealment; similarity of study groups at baseline; blinding; equal treatment of groups throughout the study; completeness of follow-up; and intention to treat (participants were analyzed in the groups to which they were randomly assigned) (Table 3).
Table 3.
Quality Assessment of the Included Studies.
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Outcome Reporting | Other Bias |
|---|---|---|---|---|---|---|---|
| Cao et al. (2005) | L | L | L | U | L | U | U |
| Deng (2008) | U | U | H | U | L | U | U |
| Ji et al. (2008) | U | U | H | U | L | U | U |
| Deng and Ding (2009) | L | U | H | U | L | U | U |
| Ma et al. (2009) | L | U | H | U | L | U | U |
| Qi et al. (2009) | U | U | H | U | L | U | U |
| Zhang et al. (2009) | U | U | H | U | L | U | U |
| Xie et al. (2010) | U | U | H | U | L | U | U |
| Kang et al. (2011) | U | U | H | U | L | U | U |
| Liu and Xue (2011) | U | U | H | U | L | U | U |
| Fan et al. (2012) | L | U | H | U | L | U | U |
| Gao et al. (2012) | U | U | H | U | L | U | U |
| Li et al. (2012) | U | U | H | U | L | U | U |
| Liu et al. (2012) | U | U | H | U | L | U | U |
| Sun et al. (2012) | U | U | H | U | L | U | U |
| Tao et al. (2012) | U | U | H | U | L | U | U |
| Yang (2012) | U | U | H | U | L | U | U |
| Lin et al. (2013) | U | U | H | U | L | U | U |
| Zhu et al. (2013) | U | U | H | U | L | U | U |
| Fu and Teng (2014) | L | U | H | U | L | U | U |
| Han (2014) | L | U | H | U | L | U | U |
| Lin and Wang (2014) | L | L | H | U | L | U | U |
| Tang et al. (2014) | L | U | H | U | L | U | U |
L = low risk of bias; H = high risk of bias; U = unclear risk of bias.
Data Synthesis and Statistical Analysis
We qualitatively synthesized all included studies (see Table 1). Included studies on pain were synthesized based on the VAS scale and the WOMAC pain subscale separately. The VAS score ranged from 0-points (no pain) to 10-points (worst possible pain). The WOMAC pain subscale was assessed with the following five items: pain during walking, stair climbing, resting, weight bearing, and pain at night. Each subscale used the following descriptors: none (0 points), mild (1 point), moderate (2 points), severe (3 points), and extreme (4 points).
To meta-analyze pain intensity, we combined the studies using the group mean difference (MD) in the VAS scale or WOMAC pain subscale scores after intervention. The MD was calculated by subtracting the post-intervention mean VAS or WOMAC pain score of the control group from that of the TCM group. A positive MD indicates an effect favorable for TCM compared with the controls.
To meta-analyze the total effectiveness rate, we combined studies using the risk ratio and compared TCM with the controls. A risk ratio of total effectiveness rate greater than 1 indicated that TCM is more effective than controls.
Regarding the significant clinical heterogeneity, the DerSimonian-Laird random-effects model was used to perform pooling (DerSimonian and Laird, 1986). The statistical heterogeneity across the included studies was estimated using the Cochran Q statistic (which was considered significant when the p value was less than 0.10) and was quantified with the I2 index (Higgins et al., 2003). Subgroup and meta-regression analyses were performed to explore whether the type of controls, duration of treatment, baseline mean pain scores, and mean change in pain scores in the control group could explain the extent of the heterogeneity across the included studies. Analyses were conducted using RevMan V5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration) and the metareg command in Stata (Harbord and Higgins, 2008). All reported p values were two sided and a p value < 0.05 was considered to be statistically significant.
Results
The detailed study selection process is summarized in Fig. 1. After initially screening 1030 abstracts, we excluded 912 abstracts, which did not meet the inclusion criteria. We retrieved and reviewed 118 full articles. Ninety-five articles were excluded due to lack of randomization or the absence of a control group, and data insufficient for meta-analysis. Finally, 23 RCTs, which included 2362 patients and were published between 2005 and 2014, met our inclusion criteria. Only one study was in English (Cao et al., 2005).
The characteristics of the 23 trials are summarized in Table 1. The mean age was 58 years, with a range of 50 to 65. 62% of the participants were women. The disease duration ranged from 6 to 132 months, and the treatment duration ranged from 2 to 24 weeks with 1 to 4 courses of treatment in both groups. Study participants were diagnosed with knee OA by the American College of Rheumatology criteria (18 studies) or the Chinese Orthopedic Association criteria (five studies).
TCM prescriptions in the intervention groups included an average of 8 Chinese herbs, ranging from 1 to 20 herbs. The TCM was administered orally one to three times a day. The herbs most commonly prescribed, and their known pharmacological actions, are summarized in Table 2. The control groups received oral doses of NSAIDs, glucosamine, alendronate sodium, or intra-articular hyaluronate injections as treatment. NSAIDs included celecoxib (100 mg or 200 mg), diclofenac sodium (25 mg or 75 mg), meloxicam (7.5 mg or 75 mg), or ibuprofen (0.6 g), one to three times a day. Glucosamine (240 mg to 750 mg) was prescribed two to three times a day. Intra-articular injections of sodium hyaluronate (20 mg/2 mL) and alendronate sodium (70 mg) were administered once a week.
Table 2.
Nomenclature and Pharmacological Actions of Common Herbs in Traditional Chinese Medication.
| Common Name | Pinyin Name | Latin Name | Pharmacological Actions | Included Formulas |
|---|---|---|---|---|
| White peony root | Bai Shao | Radix paeoniae alba | Decreases the expression of substance P, inhibits platelet aggregation and thrombosis (Teekachunhatean et al, 2004; Yang et al, 2012). | 9 formulas |
| Rhizoma arisaematis | Nan Xing | Rhizoma pinelliae seu arisaematis | Analgesic effects (Vickers et al., 1998). | 2 formulas |
| Monkshood root | Chuan Wu | Radix aconiti | Analgesic and anti-inflammatory effects (Tang et al, 2014; Tao et al., 2012). | 2 formulas |
| Clematis root | Wei Ling Xian | Radix clematidis | Inhibits metalloproteinase expression and protects against cytokine-induced (β-cell damage (Zheng et al., 2002; Zhou et al., 2013). | 4 formulas |
| Chinese taxillus twig | Sang Ji Sheng | Herba taxilli | Inhibits vascular endothelial growth factor and hypoxia-inducible factor-1α expression (Zhang et al., 2009). | 4 formulas |
| Doubleteeth pubescent angilica root | Du Huo | Radix angelicae pubescentis | Inhibits articular cartilage damage, synovium inflammation, and chondrocyte apoptosis; inhibits release of Interleukin-1β and tumor necrosis factor-α (Zhang et al., 2008). | 3 formulas |
| Twotooth achyranthes root | Niu Xi | Radix achyranthis bidentatae | Promotes chondrocyte proliferation by activating the Wnt/β-catenin signaling pathway (Wang et al, 2013; Wei et al., 1999). | 9 formulas |
| Chinese angelica | Dang Gui | Radix angelicae sinensis | Prevents cartilage destruction in osteoarthritis and promotes cartilage repair (Weng et al, 2014; Xie et al., 2001). | 9 formulas |
| Salviae miltiorrhizae | Dan Shen | Radix salviae miltiorrhizae | Vasodilates, increases platelet production to promote hemostasis, anti-inflammatory (Xie et al, 2010; Zhou et al., 2011). | 4 formulas |
| Safflower | Hong Hua | Flos carthami | Prevents clot formation, vasodilates, attenuates apoptosis (Xu et al., 2015). | 4 formulas |
Meta-Analysis
In the 23 eligible RCTs, we assessed the quantitative treatment effects using the measured pain levels of 18 trials that used the VAS scale (Fig. 2A) and six trials that used the WOMAC pain subscale (Fig. 2B). One trial (Cao et al., 2005) measured pain both on the VAS scale and WOMAC pain subscale simultaneously. Sixteen trials evaluated overall pain, physical performance and wellness using total effectiveness rate (Fig. 3).
Figure 2.
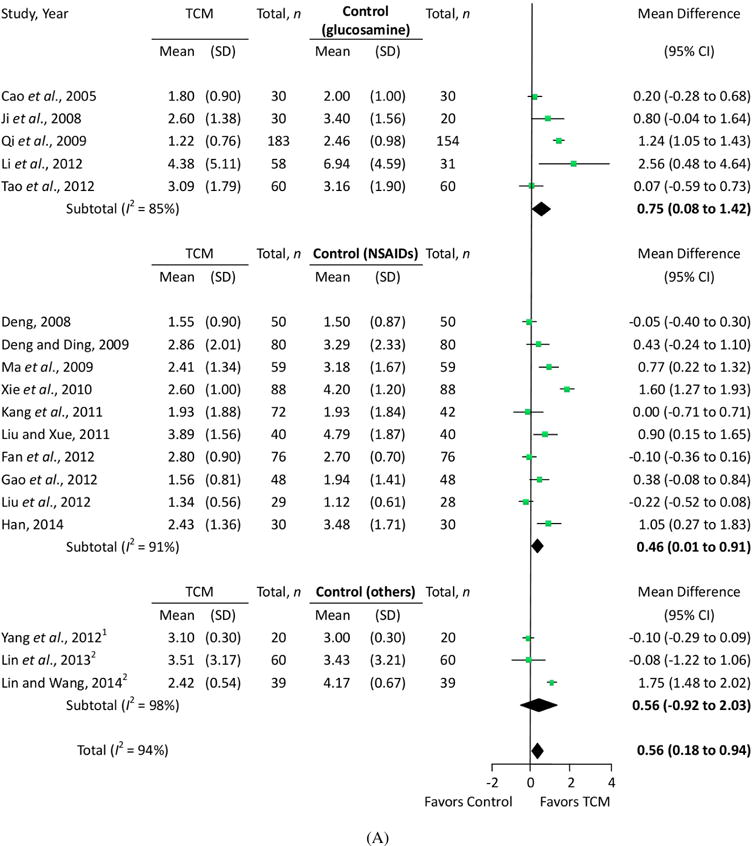
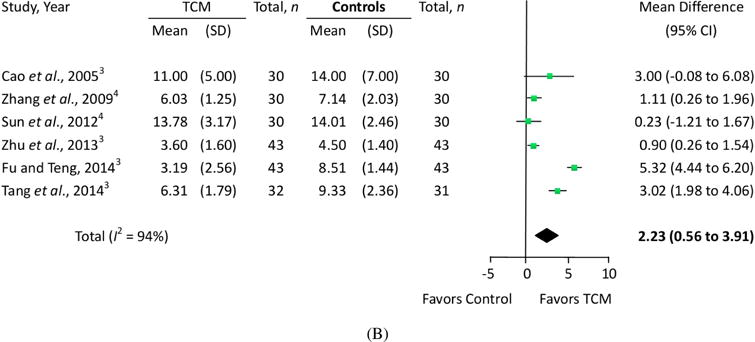
Effects of traditional Chinese medication on pain. (A) VAS pain and (B) WOMAC pain. Other controls: 1The control group was given the dose via an intra-articular hyaluronate injection. 2The control group received oral alendronate sodium. 3The control group received oral glucosamine. 4The control group received oral NSAIDs.
Figure 3.
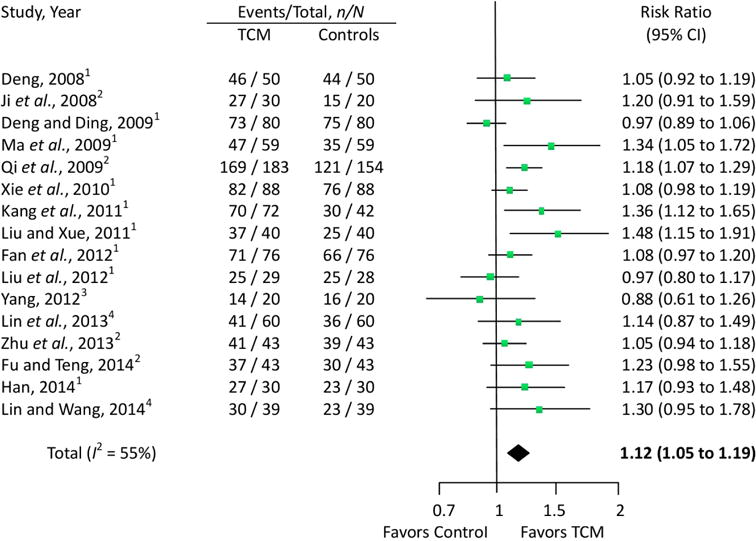
Effects of TCM on overall effectiveness. Controls: 1The control group received oral NSAIDs; 2The control group received oral glucosamine; 3The control group was given the dose via an intra-articular hyaluronate injection; 4The control group received oral alendronate sodium.
Knee Pain
Eighteen trials involving 2007 patients were used to perform a meta-analysis of the pain outcomes using the VAS scale. The results of the random-effects meta-analysis indicated that patients in the TCM groups had significantly lower pain scores than those in the glucosamine, NSAIDs, alendronate sodium and intra-articular hyaluronate injection control groups (MD = 0.56; 95% confidence interval [CI], 0.18 to 0.94; p = 0.004) after 2–12 weeks of treatment. The heterogeneity (I2) score of VAS was 94% (Fig. 2A).
A subgroup analysis exploring the variations in the treatment effects by different controls showed that trials comparing TCM with glucosamine had a higher effect size (MD = 0.75; 95% CI, 0.08 to 1.42; p = 0.03) than those comparing TCM with NSAIDs (MD = 0.46; 95% CI, 0.01 to 0.91; p = 0.04). The heterogeneity, however, was only slightly reduced in each subgroup meta-analysis (Fig. 2A). Meta-regression was performed to further explore whether the treatment durations (weeks), the baseline VAS score, or the degree of pain reduction in the control group (change in VAS score in the control group from pretreatment) could explain the variations in the effect sizes. The analyses showed that the larger reduction in VAS pain in the control group was significantly associated with a smaller effect size (p = 0.002), and this factor can explain almost half of the heterogeneity (adjusted R2 = 48.17%). Neither the treatment duration nor the baseline VAS score significantly explained the heterogeneity (Fig. 4A).
Figure 4.
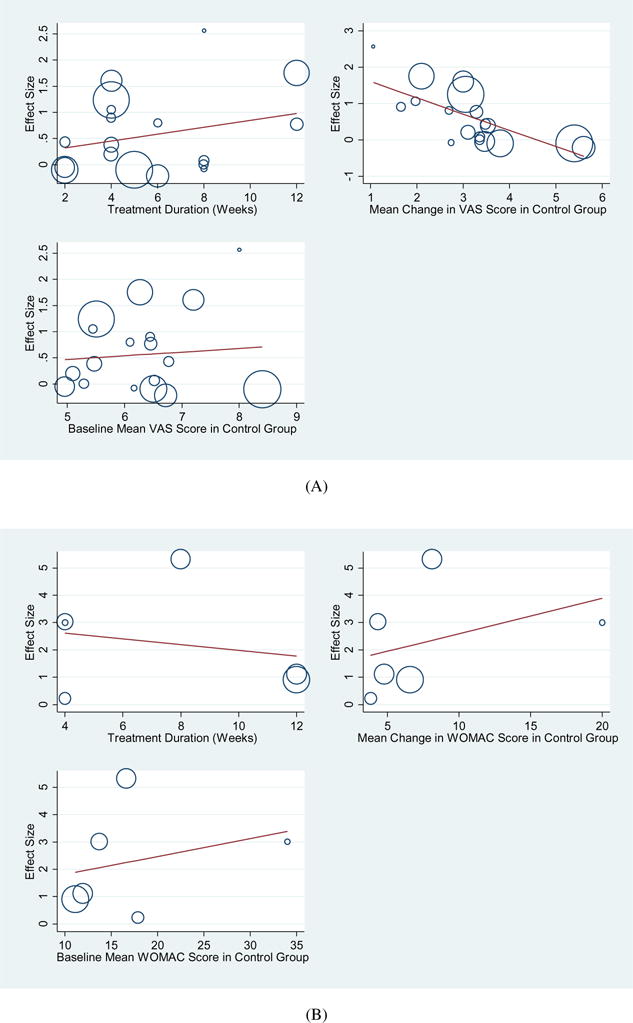
(A) Meta-regression to explore the associations between treatment durations (weeks), baseline VAS score, or degree of pain reduction in the control group and effect sizes on VAS outcomes across 18 trials. (B) Meta-regression to explore the associations between treatment durations (weeks), baseline WOMAC scores, or the degree of pain reduction in the control group and effect sizes on WOMAC outcome across 6 trials. VAS = visual analog scale; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
We also performed a meta-analysis of WOMAC pain subscale scores, combining six trials and involving 415 patients. The results showed that patients in the TCM groups had significantly improved WOMAC pain subscale scores after 4–24 weeks of treatment, compared with scores of the glucosamine and NSAIDs control groups (MD = 2.23; 95% CI, 0.56 to 3.91; p = 0.009). The heterogeneity (I2) score of VAS was 94% (Fig. 2B). Similarly, the subgroup analysis showed that trials comparing TCM with glucosamine had higher effect size (MD = 3.05; 95% CI, 0.65 to 5.46; p = 0.013) than those comparing TCM with NSAIDs (MD = 0.87; 95% CI, 0.10 to 1.64; p = 0.027), but there was still significant heterogeneity in the glucosamine control subgroup (I2 = 94%). Meta-regression analyses showed that none of the three factors (treatment durations, baseline VAS scores, or degrees of pain reduction in the control group) could significantly explain the heterogeneity (Fig. 4B).
Overall, these studies suggest that TCM was associated with significant pain reduction in patients with knee OA, compared with knee OA patients under standard western control interventions.
Total Effectiveness Rate
Sixteen trials involving 1814 patients were used in the meta-analysis of the total effectiveness rate of TCM compared to NSAIDs, glucosamine, alendronate sodium and intra-articular hyaluronate injection controls. The results from our random-effects model meta-analysis showed that TCM improved the clinical effectiveness rates by 12% (Risk Ratio = 1.12; 95% CI, 1.05 to 1.19; P = 0.0003), with a moderate degree of heterogeneity (I2 = 55%). Our meta-analysis showed that 2–12 weeks of TCM could improve clinical symptoms such as overall pain, physical performance, and wellness for patients with knee OA (Fig. 3).
Adverse Events
Eight trials provided information on adverse events associated with TCM administration. Three trials reported mild gastrointestinal symptoms in the TCM groups (Kang et al., 2011; Tao et al., 2012; Zhu et al., 2013). Eight trials reported gastrointestinal symptoms, renal dysfunction, hematochezia, liver dysfunction, paresthesia of the mouth and tongue, skin allergy, and headache in the control groups (Deng, 2008; Deng and Ding, 2009; Han, 2014; Kang et al., 2011; Liu et al., 2012; Tao et al., 2012; Zhu et al., 2013). No serious adverse events occurred.
Quality Assessment
The quality (risk of bias) assessment of the trials was performed using Cochrane Collaboration’s tool with modifications. The detailed results are presented in Table 3. The overall bias quality of trials was modest. Randomization was adequate in 8 trials (34.8%) and unclear in 15 trials (65.2%). Two studies declared appropriate allocation concealment (8.7%) but 21 trials were unclear (91.3%). Blinding of the assessor occurred in 1 trial (4.3%), but was unclear in 22 (95.7%). All studies reported the similarity of study groups at the baseline (100%). No studies reported the bias of blinding to patients and the intention-to-treat items were unclear.
Discussion
This first systemic review and meta-analysis of 23 RCTs spanning 2362 individuals indicates that TCM could be a safe approach to alleviating pain, and that TCM offers potential advantages over standard western medication for individuals who suffer from knee OA-related pain.
Comparison with other Herbal Treatments
Our findings are supported by the existing evidence on other herbal treatments and their ability to reduce pain in OA. A Cochrane review of 2 RCTs involving 327 patients indicated that orally administered avocado soybean unsaponifiables (ASU) significantly decreased pain symptoms in patients with OA of the hip compared with patients on the placebo. Furthermore, the review also provided evidence that administering ASU could help patients reduce their consumption of NSAIDs (Little et al., 2000). In separate trials (Mills et al., 1996; Randall et al., 2000; Schmid et al., 1998) Reumalex (which contains willow bark) and Stinging nettle leaf were also shown to significantly reduce pain when compared to placebo-controlled groups. Two meta-analyses of 56 studies that involved 6,765 participants showed that Arnica gel and Comfrey extract gel may improve pain symptoms as effectively as NSAIDs, but with safer adverse event profiles (Cameron and Chrubasik, 2013; 2014). Furthermore, Kessler reported that the Indian therapies, Rumalaya and Shunti-Guduchi, appear to be safe and effective drugs for the treatment of OA pain (Kessler et al., 2014). Indeed, TCM is a complex constellation of medicinal ingredients combined in very specific ratios personalized to the individual’s syndrome differentiation, and it is important to note that many of the herbal treatments described above are variants of East and South Asian traditional medicine, which is deeply rooted in and significantly influenced by TCM (NCCIH, 2013).
Mechanisms of TCM for Pain Relief
A growing body of evidence is beginning to shed light on the potential biological mechanisms by which TCM works to alleviate pain in OA:
The analgesic ingredients effect: Aconite Root, also known as Monkshood Root, has been shown to produce analgesic effects in both human and animal studies (Feng et al., 2014; Zhou et al., 2015). A recent study established an animal model of acute radiation-induced esophagitis and demonstrated that White Peony Root decreased the expression level of substance P, an important element of pain perception, in esophageal tissue (Wang et al., 2013).
The activation of blood circulation to remove stasis and improve “hemorheologicals”. Several clinical and animal studies provided evidence suggesting that Twotooth Achyranthes Root, Chinese Angelica, Salviae Miltiorrhizae, Safflower and White Peony Root can promote the flow of Qi (vital energy) and blood, and can reduce swelling, remove blood stasis, and bring more nutrients and oxygen to healing tissue (Huang et al., 2014; Lin et al., 2005; Liu et al., 2013; Luo et al., 2013; Magdalou et al., 2015; Wei et al., 1999; Weng et al., 2014; Xie et al., 2001; Zhou et al., 2013).
The anti-inflammatory effect: Various ingredients from TCM have been shown to safely suppress pro-inflammatory pathways and control inflammation-associated disease (Chen et al., 2011; Pan et al., 2011; Liu et al., 2014; Meng et al., 2014; Xu et al., 2014). For example, Clematis Root extract can inhibit matrix metalloproteinase-9 expression by suppressing NF-κ B activation (Noh et al., 2011) and can protect against cytokine-induced β-cell damage (Kim et al., 2008), contributing to its significant anti-inflammatory properties (Han et al., 2013). Salvianolic acid B, which is a hydrophilic component isolated from the Chinese herb Salviae miltiorrhizae, can significantly suppress pro-inflammatory mediators such as intercellular adhesion molecule-1, interleukin(IL)-1 β, IL-6, IL-8 mRNA and protein (Xu et al., 2015).
There is accumulating evidence to support the numerous and complex effects of TCM on pain, inflammation, and circulation in knee OA patients. Further research is warranted to explore the underlying biological mechanisms of pain in OA.
Limitations of this Study
Our study has limitations. First, many of the included RCTs have a high risk of bias. No study reported double blinding, and only one admitted single blinding. There were no placebo-controlled studies and only two studies reported allocation concealment. We also found that the reporting of procedures in some trials was insufficient according to a CONSORT statement for non-pharmacologic treatments (Boutron et al., 2008). Second, we observed high heterogeneity due to diverse TCM formulations, control groups, and different pain levels of the patients enrolled in the trials. Another potential source of heterogeneity in the efficacy among TCM and control groups was the use of different drugs in extremely varied doses and regimens. Third, we did not use statistical methods to test for publication bias due to unanimous publication in Chinese academic journals (Vickers et al., 1998; Wang et al., 2014b). Fourth, despite TCM’s statistically significant effects on pain reduction in patients with knee OA, the clinically important benefits of TCM remain to be determined. Thus, many challenges still exist and the potential benefits of TCM for knee OA need to be further evaluated through clinical trials that employ more rigorous methodologies.
Conclusion
In summary, TCM may be effective for reducing pain in knee OA. Despite the limited quality of trials included in this review, our review provides new and valuable information that TCM may have potential analgesic effects for patients with knee OA. Therefore, these studies on Traditional Chinese Medication warrant further methodologically rigorous studies to determine the clinical implications of these findings for the relief of pain in knee OA.
Acknowledgments
Dr. Wang is supported by the National Center for Complementary and Integrative Health (NCCIH, K24 AT007323). Dr. Chen is supported by the National Natural Science Foundation of China (81202707), and by the action plan of the development of Traditional Chinese medicine in Shanghai (ZY3-LCPT-2-1005). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NCCIH. The investigators are solely responsible for the contents of the manuscript and the decision to submit it for publication.
Appendix. Literature Search Strategy
| SET | Terms | Results |
|---|---|---|
| Database: PubMed | ||
| 1 | (knee osteoarthritis[MeSH Terms]) AND (Drugs, Chinese Herbal[MeSH Terms]) | 18 |
| 2 | (knee osteoarthritis[MeSH Terms]) AND (Medicine, Chinese Traditional[MeSH Terms]) | 24 |
| Database: Cochrane Library | ||
| 1 | “knee osteoarthritis” AND “Chinese Herbal” [Search all text] | 11 |
| 2 | “knee osteoarthritis” AND “Traditional Chinese Medication” [Search all text] | 1 |
| 3 | “knee osteoarthritis” AND “Traditional Chinese Medicine” [Search all text] | 3 |
| Database: Springer | ||
| 1 | “knee osteoarthritis” AND “Chinese Herbal” | 12 |
| 2 | “knee osteoarthritis” AND “Traditional Chinese Medication” | 12 |
| 3 | “knee osteoarthritis” AND “Traditional Chinese Medicine” | 2 |
| Database: China National Knowledge Infrastructure | ||
| 1 | “knee osteoarthritis” AND “Chinese Herbal” [Title] | 75 |
| 2 | “knee osteoarthritis” AND “Chinese medication” [Title] | 34 |
| 3 | “knee osteoarthritis” AND “electuary” [Title] | 11 |
| 4 | “knee osteoarthritis” AND “capsule” [Title] | 12 |
| 5 | “knee osteoarthritis” AND “pill” [Title] | 27 |
| 6 | “knee osteoarthritis” AND “prescription” [Title] | 15 |
| 7 | “knee osteoarthritis” AND “decoction” [Title] | 35 |
| 8 | “knee osteoarthritis” AND “tablet” [Title] | 6 |
| 9 | “knee osteoarthritis” AND “powder” [Title] | 9 |
| 10 | “knee osteoarthritis” AND “oral paste” [Title] | 7 |
| 11 | “knee osteoarthritis” AND “pellet” [Title] | 12 |
| Database: Chongqing VIP | ||
| 1 | “knee osteoarthritis” AND “Chinese Herbal” [Title OR Keyword] | 66 |
| 2 | “knee osteoarthritis” AND “Chinese medication” [Title OR Keyword] | 23 |
| 3 | “knee osteoarthritis” AND “electuary” [Title OR Keyword] | 21 |
| 4 | “knee osteoarthritis” AND “capsule” [Title OR Keyword] | 12 |
| 5 | “knee osteoarthritis” AND “pill” [Title OR Keyword] | 13 |
| 6 | “knee osteoarthritis” AND “prescription” [Title OR Keyword] | 15 |
| 7 | “knee osteoarthritis” AND “decoction” [Title OR Keyword] | 26 |
| 8 | “knee osteoarthritis” AND “tablet” [Title OR Keyword] | 9 |
| 9 | “knee osteoarthritis” AND “powder” [Title OR Keyword] | 15 |
| 10 | “knee osteoarthritis” AND “oral paste” [Title OR Keyword] | 17 |
| 11 | “knee osteoarthritis” AND “pellet” [Title OR Keyword] | 21 |
| Database: Chinese Biomedical Literature Database | ||
| 1 | “knee osteoarthritis” AND “Chinese Herbal” [All fields] | 35 |
| 2 | “knee osteoarthritis” AND “Chinese medication” [All fields] | 25 |
| 3 | “knee osteoarthritis” AND “electuary” [All fields] | 16 |
| 4 | “knee osteoarthritis” AND “capsule” [All fields] | 27 |
| 5 | “knee osteoarthritis” AND “pill” [All fields] | 18 |
| 6 | “knee osteoarthritis” AND “prescription” [All fields] | 16 |
| 7 | “knee osteoarthritis” AND “decoction” [All fields] | 24 |
| 8 | “knee osteoarthritis” AND “tablet” [All fields] | 9 |
| 9 | “knee osteoarthritis” AND “powder” [All fields] | 5 |
| 10 | “knee osteoarthritis” AND “oral paste” [All fields] | 4 |
| 11 | “knee osteoarthritis” AND “pellet” [All fields] | 6 |
| Database: Wanfang Data | ||
| 1 | “knee osteoarthritis” AND “Chinese Herbal” [All fields] | 56 |
| 2 | “knee osteoarthritis” AND “Chinese medication” [All fields] | 32 |
| 3 | “knee osteoarthritis” AND “electuary” [All fields] | 24 |
| 4 | “knee osteoarthritis” AND “capsule” [All fields] | 26 |
| 5 | “knee osteoarthritis” AND “pill” [All fields] | 13 |
| 6 | “knee osteoarthritis” AND “prescription” [All fields] | 17 |
| 7 | “knee osteoarthritis” AND “decoction” [All fields] | 24 |
| 8 | “knee osteoarthritis” AND “tablet” [All fields] | 12 |
| 9 | “knee osteoarthritis” AND “powder” [All fields] | 15 |
| 10 | “knee osteoarthritis” AND “oral paste” [All fields] | 13 |
| 11 | “knee osteoarthritis” AND “pellet” [All fields] | 28 |
References
- Abou-Raya A, Abou-Raya S, Khadrawe T. Methotrexate in the treatment of symptomatic knee osteoarthritis: Randomised placebo-controlled trial. Ann Rheum Dis. 2014:2013–204856. doi: 10.1136/annrheumdis-2013-204856. [DOI] [PubMed] [Google Scholar]
- Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke T, Greenwald R, Hochberg M. Development of criteria for the classification and reporting of osteoarthritis: Classification of osteoarthritis of the knee. Arthriti Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Barnes PM, Bloom B, Nahin RL, National Center for Health Statistics . Complementary and alternative medicine use among adults and children: United States, 2007. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; Hyattsville, MD: 2008. [PubMed] [Google Scholar]
- Boutron I, Moher D, Altman DG, Schulz K, Ravaud P, for the CONSORT group Extending the CONSORT Statement to randomized trials of nonpharmacologic treatment: Explanation and elaboration. Ann Intern Med. 2008;148(4):295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- Brown GA. AAOS clinical practice guideline: Treatment of osteoarthritis of the kneeE Evidence-based guideline. J Am Acad Orthop Sur. 2013;21:577–579. doi: 10.5435/JAAOS-21-09-577. [DOI] [PubMed] [Google Scholar]
- Cameron M, Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Db Syst Rev. 2013;31 doi: 10.1002/14651858.CD010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron M, Chrubasik S. Oral herbal therapies for treating osteoarthritis. Cochrane Db Syst Rev. 2014;22 doi: 10.1002/14651858.CD002947.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao YL, Shi YY, Zheng YX, Shi MY, Lo SK. Blood-nourishing and hard-softening capsule costs less in the management of osteoarthritic knee pain: A randomized controlled trial. Evid Based Complement Altern Med. 2005;2:363–368. doi: 10.1093/ecam/neh104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CW, Sun J, Li YM, Shen PA, Chen Y-Q. Action mechanisms of du-huo-ji-sheng-tang on cartilage degradation in a rabbit model of osteoarthritis. Evid Based Complement Altern Med. 2011;2011 doi: 10.1093/ecam/neq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung F. TCM: Made in China. Nature. 2011;480:S82–S83. doi: 10.1038/480S82a. [DOI] [PubMed] [Google Scholar]
- Chinese Orthopaedic Association. Diagnosis and treatment of osteoarthritis. Orthop Sur. 2010;2:1–6. doi: 10.1111/j.1757-7861.2009.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W. Clinical research on Gancao Fuzi Decoction in treating osteoarthritis of knee joint. J Chinese Med Mater. 2008;31:1107–1110. [PubMed] [Google Scholar]
- Deng W, Ding MH. Effects of Fuzi Decoction on patients with osteoarthritis of knee. Chinese J Tradit Med Traumatol Orthop. 2009;17:23–25. [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC. Trends in alternative medicine use in the United States, 1990–1997: Results of a follow-up national survey. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- Fan A, Lao L, Zhang R, Zhou A, Wang L, Moudgil K, Lee D, Ma Z, Zhang W, Berman B. Effects of an acetone extract of Boswellia carterii Birdw (Burseraceae) gum resin on adjuvant-induced arthritis in lewis rats. J Ethnopharmacol. 2005;101:104–109. doi: 10.1016/j.jep.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Fan XH, Yu Y, Huang Y, Chen RG, Wang XL. Observation on theerapeutic effect of modified Danggui Sini Decoction treating 76 cases of knee osteoarthritis. Liaoning J Tradit Chinese Med. 2012;39:2184–2186. [Google Scholar]
- Felson DT. Osteoarthritis of the knee. New Engl J Med. 2006;354:841–848. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- Feng L, Liu WK, Deng L, Tian JX, Tong XL. Clinical Efficacy of Aconitum-Containing Traditional Chinese Medicine for Diabetic Peripheral Neuropathic Pain. Am J Chin Med. 2014;42:109–117. doi: 10.1142/S0192415X14500074. [DOI] [PubMed] [Google Scholar]
- Fu A, Teng HL. Clinical efficacy observation of Xitong Prescription in treatment of knee osteoarthritis. J New Chinese Med. 2014;46:93–95. [Google Scholar]
- Gao G, Wu H, Tian J, Du JF, Xie X, Gao J. Clinical efficacy of Bushen Huoxue Qubi Decoction on treatment of knee osteoarthritis and its effect on hemarheology, anti-inflammation and antioxidation. China J Chinese Materia Medica. 2012;37:390–396. [PubMed] [Google Scholar]
- Han W, Xiong Y, Li Y, Fang W, Ma Y, Liu L, Li F, Zhu X. Anti-arthritic effects of clematichinenoside (AR-6) on PI3K/Akt signaling pathway and TNF-α associated with collagen-induced arthritis. Pharm Biol. 2013;51:13–22. doi: 10.3109/13880209.2012.698287. [DOI] [PubMed] [Google Scholar]
- Han WP. Clinical study on 30 cases of knee osteoarthritis treated by Yiqi Jianpi Huoxue Tongluo Decoction. Liaoning J Tradit Chinese Med. 2014;41:958–960. [Google Scholar]
- Harbord RM, Higgins JP. Meta-regression in Stata. Stata J. 2008;8:493–519. [Google Scholar]
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions. 2011:8.1–8.53. version 5102011. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthrit Care Res. 2012;64:465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- Huang CF, Yang RS, Liu SH, Hsieh PC, Lin-Shiau SY. Evidence for improved neuro-pharmacological efficacy and decreased neurotoxicity in mice with traditional processing of Rhizoma Arisaematis. Am J Chin Med. 2011;39:981–998. doi: 10.1142/S0192415X11009354. [DOI] [PubMed] [Google Scholar]
- Huang CY, Kuo WW, Kuo CH, Tsai FJ, Liu PY, Hsieh DJY. Protective effect of Danggui (Radix Angelicae Sinensis) on angiotensin II-induced apoptosis in H9c2 cardio-myoblast cells. BMC Complement Altern Med. 2014;14:358. doi: 10.1186/1472-6882-14-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im NK, Lee SG, Lee DS, Park PH, Lee IS, Jeong GS. Spatholobus suberectus inhibits osteoclastogenesis and stimulates chondrogenesis. Am J Chin Med. 2014;42:1123–1138. doi: 10.1142/S0192415X14500700. [DOI] [PubMed] [Google Scholar]
- Ji W, Lu JQ, Yang N, Wang XD. Tong Bi Granule impact on the WOMAC index of knee osteoarthritis. J Nanjing Univ of Tradit Chinese Med. 2008;24:15–17. [Google Scholar]
- Jiang L, Li W, Wang Y, Zhang X, Yu D, Yin Y, Xie Z, Yuan Y. Effects of cichoric acid extract from Echinacea purpurea on collagen-induced arthritis in rats. Am J Chin Med. 2014;42:679–692. doi: 10.1142/S0192415X1450044X. [DOI] [PubMed] [Google Scholar]
- Jotanovic Z, Mihelic R, Sestan B, Dembic Z. Emerging pathways and promising agents with possible disease modifying effect in osteoarthritis treatment. Curr Drug Targets. 2014;15:635–661. doi: 10.2174/1389450115666140306153115. [DOI] [PubMed] [Google Scholar]
- Kang XZ, Wu QF, Jie HY, Ding ZX, Wang XC, Wang ZY, Peng JY, Gao ML, He DY, Feng FH. Clinical study on the treatment of knee osteoarthritis by Wangbi tablet. Chinese J Integr Tradit West Med. 2011;31:1205–1207. [PubMed] [Google Scholar]
- Kessler CS, Pinders L, Michalsen A, Cramer H. Ayurvedic interventions for osteoarthritis: A systematic review and meta-analysis. Rheumatol Int. 2014:1–22. doi: 10.1007/s00296-014-3095-y. [DOI] [PubMed] [Google Scholar]
- Kim E, Song M, Hwang T, Kim H, Moon W, Ryu D, So H-S, Park R, Park J, Kwon K-B. Radix clematidis extract protects against cytokine-and streptozotocininduced β-cell damage by suppressing the NF-κB pathway. Int J Mol Med. 2008;22:349–356. [PubMed] [Google Scholar]
- Li TH, Luo SF, Yang SY. Small Meridian-Activating Pill treating for 58 cases of knee osteoarthritis. Henan Tradit Chinese Med. 2012;32:489–490. [Google Scholar]
- Lin J, Liu Z, Wang S. Clinical observation of Jiawei Simiao San on treatment of knee osteoarthritis. Chinese J Tradit Med Sci Technol. 2013;20:70–71. [Google Scholar]
- Lin J, Wang S. Clinical study of Jintiange Capsule in the prevention and treatment of the knee osteoarthritis via P38-MAPK signal pathway. Chinese J Osteoporosis. 2014;20:936–947. [Google Scholar]
- Lin JJ, Jin CN, Zheng ML, Ouyang XN, Zeng JX, Dai XH. Clinical study on treatment of primary hepatocellular carcinoma by Shenqi mixture combined with microwave coagulation. Chinese J Integr Med. 2005;11:104–110. doi: 10.1007/BF02836465. [DOI] [PubMed] [Google Scholar]
- Ling W, Huang Y, Xu JH, Li Y, Huang YM, Ling HB, Sui Y, Zhao HL. Consistent efficacy of Wendan Decoction for the treatment of digestive reflux disorders. Am J Chin Med. 2015;43:893–913. doi: 10.1142/S0192415X15500524. [DOI] [PubMed] [Google Scholar]
- Little CV, Parsons T, Logan S. Herbal therapy for treating osteoarthritis. Cochrane Db Syst Rev. 2000;4 doi: 10.1002/14651858.CD002947. [DOI] [PubMed] [Google Scholar]
- Liu J, Pan J, Wang Y, Lin D, Shen D, Yang H, Li X, Luo M, Cao X. Component analysis of Chinese medicine and advances in fuming-washing therapy for knee osteoarthritis via unsupervised data mining methods. J Tradit Chinese Med. 2013;33:686–691. doi: 10.1016/s0254-6272(14)60043-1. [DOI] [PubMed] [Google Scholar]
- Liu SH, Lu TH, Su CC, Lay IS, Lin HY, Fang KM, Ho TJ, Chen KL, Su YC, Chiang WC, Chen YW. Lotus leaf (Nelumbo nucifera) and its active constituents prevent inflammatory responses in macrophages via JNK/NF-κB signaling pathway. Am J Chin Med. 2014;42:869–889. doi: 10.1142/S0192415X14500554. [DOI] [PubMed] [Google Scholar]
- Liu W, Xue B. Clinical observation on the treatment of knee osteoarthritis by Bi Qi capsule. Liaoning J Tradit Chinese Med. 2011;38:1254–1255. [Google Scholar]
- Liu WD, Bai YT, Zhang F. The clinical research on Compound Xuanju Capsule for the treatment of knee osteoarthritis. Acta Chinese Med Pharmacol. 2012;40:77–80. [Google Scholar]
- Long L, Soeken K, Ernst E. Herbal medicines for the treatment of osteoarthritis: A systematic review. Rheumatology. 2001;40:779–793. doi: 10.1093/rheumatology/40.7.779. [DOI] [PubMed] [Google Scholar]
- Luo J, Xu H, Chen K. Systematic Review of Compound Danshen Dropping Pill: A Chinese patent medicine for acute myocardial infarction. Evid Based Complement Altern Med. 2013;2013 doi: 10.1155/2013/808076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Xu FQ, Feng XH. Clinical observation of the method of nourishing the liver and kidney, promoting blood circulation for treating 118 knee osteoarthritis. Clin J Tradit Chinese Med. 2009;21:439–440. [Google Scholar]
- Magdalou J, Chen LB, Wang H, Qin J, Wen Y, Li XJ, Shang L, Li J. Angelica sinensis and osteoarthritis: A natural therapeutic link? Bio-Med Mater Engl. 2015;25:179–186. doi: 10.3233/BME-141250. [DOI] [PubMed] [Google Scholar]
- Maroon JC, Bost JW, Maroon A. Natural anti-inflammatory agents for pain relief. Surg Neurol Int. 2010;1 doi: 10.4103/2152-7806.73804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y, Hunter DJ, Kawaguchi H. OARSI guidelines for the nonsurgical management of knee osteoarthritis. Osteoarthr Cartilage. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Meng ZQ, Tang ZH, Yan YX, Guo CR, Cao L, Ding G, Huang WZ, Wang ZZ, Wang KD, Xiao W, Yang ZL. Study on the anti-gout activity of chlorogenic acid: Improvement on hyperuricemia and gouty inflammation. Am J Chin Med. 2014;42:1471–1483. doi: 10.1142/S0192415X1450092X. [DOI] [PubMed] [Google Scholar]
- Mills S, Jacoby R, Chacksfield M, Willoughby M. Effect of a proprietary herbal medicine on the relief of chronic arthritic pain: A double-blind study. Rheumatology. 1996;35:874–878. doi: 10.1093/rheumatology/35.9.874. [DOI] [PubMed] [Google Scholar]
- Nagi R, Yashoda Devi BK, Rakesh N, Reddy SS, Patil DJ. Clinical implications of prescribing nonsteroidal anti-inflammatory drugs in oral health care-a review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;119:264–271. doi: 10.1016/j.oooo.2014.12.002. [DOI] [PubMed] [Google Scholar]
- NCCIH. backgrounder. Traditional Chinese Medicine: An Introduction. National Center for Complementary and Alternative Medicine. 2013 https://nccih.nih.gov/sites/nccam.nih.gov/files/Backgrounder_TraditionaLChinese_Medicine_10-25-2013.pdf.
- Noh EM, Lee YR, Hur H, Kim JS. Radix clematidis extract inhibits TPA-induced MMP-9 expression by suppressing NF-κB activation in MCF-7 human breast cancer cells. Mol Med Report. 2011;4:879–883. doi: 10.3892/mmr.2011.532. [DOI] [PubMed] [Google Scholar]
- Pan L, Zhou J, Zhu H, Wang W, Zhang M, Tian X, Lu J, Zeng M. Study on integrated pharmacokinetics of gardenia acid and geniposide: Time-antioxidant efficacy after oral administration of Huanglian-Zhizi couplet medicine from Huang-Lian-Jie-Du-Tang in MCAO rats. Am J Chin Med. 2014;42:393–407. doi: 10.1142/S0192415X14500268. [DOI] [PubMed] [Google Scholar]
- Pan MH, Chiou YS, Tsai ML, Ho CT. Anti-inflammatory activity of traditional Chinese medicinal herbs. J Tradit Compl Med. 2011;1:8–24. doi: 10.1016/s2225-4110(16)30052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi LQ, Wang JB, Du SQ. The clinical observation of Yang Yuan Rou Gan Decoction for treating 183 knee osteoarthritis. J Hebei Tradit Chinese Med and Pharmacol. 2009;24:14–15. [Google Scholar]
- Randall C, Randall H, Dobbs F, Hutton C, Sanders H. Randomized controlled trial of nettle sting for treatment of base-of-thumb pain. J Roy Soc Med. 2000;93:305–309. doi: 10.1177/014107680009300607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid B, Tschirdewahn B, Kötter I, Günaydin I, Lüdtke R, Selbmann H, Schaffner W, Heide L. Analgesic effects of willow bark extract in osteoarthritis: Results of a clinical double-blind trial. Focus Alt Compl Ther. 1998;3:186–186. [Google Scholar]
- Sun J, Qiu ML, He YH. The clinical observation of the osteoarthritis of knee incorporated with osteoporosis treated with Jintiange Capsule. Chinese J Tradit Med Traumatol Orthop. 2012;20:19–21. [Google Scholar]
- Tang J, Wu WH, Ni WZ. Clinical effect discussion on Yanghe Decoction treating for mild to moderate pain of knee osteoarthritis. Chinese J Mod Drug Appl. 2014;8:149–150. [Google Scholar]
- Tao QW, Xu Y, Wang JM, Yan XP. Clinical research of Gubitong Recipe in treating knee osteoarthritis. Rheumatism Arthritis. 2012;1:11–14. [Google Scholar]
- Teekachunhatean S, Kunanusorn P, Rojanasthien N, Sananpanich K, Pojchamarnwiputh S, Lhieochaiphunt S, Pruksakorn S. Chinese herbal recipe versus diclofenac in symptomatic treatment of osteoarthritis of the knee: A randomized controlled trial [ISRCTN70292892] BMC Complement Altern Med. 2004;4:19. doi: 10.1186/1472-6882-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials. 1998;19:159–166. doi: 10.1016/s0197-2456(97)00150-5. [DOI] [PubMed] [Google Scholar]
- Wang CY, Bai XY, Wang CH. Traditional Chinese medicine: A treasured natural resource of anticancer drug research and development. Am J Chin Med. 2014a;42:543–559. doi: 10.1142/S0192415X14500359. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang L, Chai Q, Liu J. Positive results in randomized controlled trials on acupuncture published in Chinese journals: A systematic literature review. J Altern Complement Med. 2014b;20:A129–A129. [Google Scholar]
- Wang Z, Shen L, Li X, Shu X, Shan B, Zhang L, Gong Y, Dong Z. Pain-relieving effect of a compound isolated from white peony root oral liquid on acute radiation induced esophagitis. Mol Med Rep. 2013;7:1950–1954. doi: 10.3892/mmr.2013.1427. [DOI] [PubMed] [Google Scholar]
- Wei L, Chen B, Ye R, Li H. Treatment of complications due to peritoneal dialysis for chronic renal failure with traditional Chinese medicine. J Tradit Chinese Med. 1999;19:3–9. [PubMed] [Google Scholar]
- Weng X, Lin P, Liu F, Chen J, Li H, Huang L, Zhen C, Xu H, Liu X, Ye H. Achyranthes bidentata polysaccharides activate the Wnt/β-catenin signaling pathway to promote chondrocyte proliferation. Int J Mol Med. 2014;34:1045–1050. doi: 10.3892/ijmm.2014.1869. [DOI] [PubMed] [Google Scholar]
- Xie F, Li X, Sun K, Chu Y, Cao H, Chen N, Wang W, Liu M, Liu W, Mao D. An experimental study on drugs for improving blood circulation and removing blood stasis in treating mild chronic hepatic damage. J Tradit Chinese Med. 2001;21:225–231. [PubMed] [Google Scholar]
- Xie JH, Cui LQ, Liu MZ. Effect of Chinese herbs of resolving stasis and removing damp-pathogen on osteoarthritis of knee. Hebei J tradit Chinese Med. 2010;32:1776–1778. [Google Scholar]
- Xu S, Zhong A, Bu X, Ma H, Li W, Xu X, Zhang J. Salvianolic acid B inhibits platelets-mediated inflammatory response in vascular endothelial cells. Thromb Res. 2015;135:137–145. doi: 10.1016/j.thromres.2014.10.034. [DOI] [PubMed] [Google Scholar]
- Xu Y, Dai GJ, Liu Q, Ma XP, Li L, Chen WH, Lin N. Effect of Ermiao Recipe with medicinal guide Angelicae pubescentis Radix on promoting the homing of bone marrow stem cells to treat cartilage damage in osteoarthritis rats. Chinese J Integr Med. 2014;20:600–609. doi: 10.1007/s11655-014-1761-2. [DOI] [PubMed] [Google Scholar]
- Yang M. Oral Gancao Fuzi Decoction combined with injection of sodium hyaluronate in the treatment of 60 patients with knee osteoarthritis. Chinese J Tradit Med Traumatol Orthop. 2012;20:9–11. [Google Scholar]
- Yang RC, Chang CC, Sheen JM, Wu HT, Pang JH, Huang ST. Davallia bilabiata inhibits TNF-alpha-induced adhesion molecules and chemokines by suppressing IKK/NF-κB pathway in vascular endothelial cells. Am J Chin Med. 2014;42:1411–1429. doi: 10.1142/S0192415X1450089X. [DOI] [PubMed] [Google Scholar]
- Zhang EQ. Bi Syndrome (Arthralgia Syndrome) J Tradit Chinese Med. 2010;30:145–152. doi: 10.1016/s0254-6272(10)60032-5. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Liang ZJ, He MT, Zhang LY. The Decoction of suppling qi and activating blood circulation for treating 30 knee osteoarthritis. J Tradit Chinese Med. 2009;50:439–440. [Google Scholar]
- Zhang XL, Zhang MF, Yang ZF, Fan YK, Hui AW, Zheng J, Yao M, Xie RM, Wang DH. A study on the anti-inflammation and analgesic effects of nature of anti-rheumatism traditional Chinese medicine. Chinese Arch Tradit Chinese Med. 2008;26:2386–2396. [Google Scholar]
- Zheng XY. Guiding Principle of Clinical Research on New Drugs of Traditional Chinese Medicine. China Medical Science Press; Beijing, China: 2002. pp. 349–353. [Google Scholar]
- Zhang Y, Wang ZZ, Sun HM, Li P, Li YF, Chen NH. Systematic review of traditional Chinese medicine for depression in Parkinson’s disease. Am J Chin Med. 2014;42:1035–1051. doi: 10.1142/S0192415X14500657. [DOI] [PubMed] [Google Scholar]
- Zhong J, Ma T, Huang C, Liu H, Chen Z, Cao L, Li X, Li J. Flavonoids from Litsea coreana decreases TNF-alpha secretion from peritoneal macrophages in adjuvant-induced arthritis rats via UPR pathway. Am J Chin Med. 2014;42:905–919. doi: 10.1142/S0192415X14500578. [DOI] [PubMed] [Google Scholar]
- Zhou D, Liu B, Xiao X, Dai P, Ma S, Huang W. The effect of safflower yellow on spinal cord ischemia reperfusion injury in rabbits. Oxid Med Cell Longev. 2013;692302 doi: 10.1155/2013/692302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Tang L, Zhou X, Wang T, Kou Z, Wang Z. A review on phytochemistry and pharmacological activities of the processed lateral root of Aconitum carmichaelii Debeaux. J Ethnopharmacol. 2015;160:173–193. doi: 10.1016/j.jep.2014.11.043. [DOI] [PubMed] [Google Scholar]
- Zhou XS, Zhou K, Feng F. Effect of Radix Clematidis on IL-1β, TNF-α and PGE2 in the rabbit knee osteoarthritis. Lishizhen Med Materia Medica Res. 2011;22:1143–1144. [Google Scholar]
- Zhu FX, Zhou RH, Shi HY, Tan Y. The Clinical study of Zhengqing Fengtongning treating for knee osteoarthritis and its effect on cytokine. Contemp Med. 2013;19:1–3. [Google Scholar]
- Zou LY, Fang F, Tu GQ, Tang QZ, Cao YX, Zhu YH, Xie Y. Effect of achyranthes bidentata polysaccharides on hemorrheology of rabbit’ s knee osteoarthritis. Henan J Tradit Chinese Med. 2013;33:2083–2085. [Google Scholar]


