Abstract
Objective
To determine the effect of an acute (1 week) and chronic (3 weeks) exposure to E-cigarette (E-cig) emissions on mucociliary clearance (MCC) in murine lungs.
Methods
C57BL/6 male mice (age 10.5 ±2.4 weeks) were exposed for 20min/day to E-cigarette aerosol generated by a Joyetech 510-T® E-cig containing either 0% nicotine (N)/propylene glycol (PG) for 1 week (n = 6), or 3 weeks (n = 9), or 2.4% N/PG for one week (n = 6), or 3 weeks (n = 9), followed by measurement of MCC. Control mice (n = 15) were not exposed to PG alone, or N/PG. MCC was assessed by gamma camera following aspiration of 99mtechnetium aerosol and was expressed as the amount of radioactivity removed from both lungs over 6 hours (MCC6hrs). Venous blood was assayed for cotinine levels in control mice and in mice exposed for 3-weeks to PG alone and N/PG.
Results
MCC6hrs in control mice and in mice acutely exposed to PG alone and N/PG was similar, averaging (±1 standard deviation) 8.6±5.2%, 7.5±2.8% and 11.2±5.9%, respectively. In contrast, chronic exposure to PG alone stimulated MCC6hrs (17.2 ±8.0)% and this stimulation was significantly blunted following chronic exposure to N/PG (8.7 ±4.6)% (p < .05). Serum cotinine levels were <0.5ng/ml in control mice and in mice exposed to PG alone, whereas, N/PG exposed mice averaged 14.6 ± 12.0 ng/ml.
Conclusions
In this murine model, a chronic, daily, 20 min-exposure to N/PG, but not an acute exposure, slowed MCC, compared to exposure to PG alone and led to systemic absorption of nicotine.
Keywords: E-cigarette emissions, mucociliary clearance, murine model, chronic exposure, acute exposure
Introduction
Electronic cigarettes (E-cigarettes) have become increasingly popular in the U.S. and globally and their use is widespread among adolescents and adults (Centers for Disease and Prevention, 2013; Gravely et al., 2014; Pearson et al., 2012; Regan et al., 2013). Several population-based studies showed that the highest rate of E-cigarette users was among those who were current tobacco smokers and former smokers (Adkison et al., 2013; Dockrell et al., 2013; King et al., 2013). The major constituents of E-cigarette formulations often include nicotine (N), a carrier such as propylene glycol (PG), with or without flavorings.
The size distribution of liquid droplets generated by an E-cigarette is an important determinant of how much of the aerosol deposits in the lungs and how much is exhaled, with very small (i.e. submicrometer) particles having a higher probability of being exhaled compared to larger (i.e. micrometer) particles. Schripp et al. (2013) reported that the particle size for aerosol generated by one E-cigarette device shifted from a bimodal distribution with a maxima at 60 and 100 nm to a single-mode distribution with a maxima at 45 nm with aging over 1–3 minutes, when measured in a 10 L chamber. Zhang et al. (2013) quantified the particle size distribution (PSD) of another E-cigarette product (the Bloog MaxX Fusion E-cigarette) and estimated the regional respiratory tract deposition and the exhaled fraction of emissions from that delivery system, based on the International Commission on Radiological Protection (ICRP) deposition model. The PSD of the Fusion E-cigarette was also found to be in the nanometer range, with a volume median diameter (VMD) of 250 nm (Zhang et al., 2013). Based on the ICRP model (Human respiratory tract model for radiological protection. A report of a Task Group of the International Commission on Radiological Protection, 1994), the investigators estimated that between 10 and 18% of the particles should remain in the lungs of the user, 9–15% should be absorbed into the blood of the user and 73–78% should be exhaled. Others have also reported that significant percentages of PG and nicotine may be retained by E-cigarette users during inhalation (O’Connell et al., 2015; St Helen et al., 2016).
It is not known if the exhaled fraction is as large as predicted by the ICRP model for E-cigarette particles with this size-distribution profile. Studies with direct measurement of particles that are exhaled are needed to answer this question. In addition, the unique constituents of E-cigarette aerosols may also affect deposition. For example, the hygroscopic properties of PG could induce particle growth and increase the deposited fraction and the retention of particles generated by E-cigarettes within the respiratory tract may be high due to their chemical nature. In addition, absorption of nicotine from the respiratory tract may be greater than what is predicted for other chemicals (Armitage et al., 2004).
Although constituents of E-cigarettes deposit within the lungs and nicotine is absorbed into the systemic circulation following inhalation (Armitage et al., 2004), little is known regarding the effects of exposure to emissions from E- cigarettes on respiratory health. Flouris et al. (2013) have reported that active exposure to E-cigarette emissions is not associated with any significant impairment in lung function as measured by spirometry. Nevertheless, as the authors point out, their studies did not explore the effects of longterm exposure, or the effect of dosage. Previous studies in our laboratory showed that neonatal mice (i.e. 24 hours old) exposed to emissions from an E-cigarette once a day for 20 minutes for days one and two of life and twice/day for days three to nine of life had impaired alveolar growth, compared to control mice (McGrath-Morrow et al., 2015). These results suggest that environmental exposure to emissions from an E-cigarette, during rapid lung growth, can alter lung structure. The long-term biological effect of these alterations was not studied and is unknown.
Insoluble agents that deposit in the airway mucus are removed within hours by the mucociliary clearance (MCC) apparatus. Impairment to this major lung defense mechanism leads to prolonged exposure to irritants, bacteria and viruses, the development of infections and subsequent reinjury to the MCC apparatus (Randell et al., 2006). Although the short- and long-term effects of a number of environmental pollutants on MCC have been studied in different animal models and in vitro (Wanner et al., 1996), little is known of the effect of acute, or chronic, exposures to emissions generated by E-cigarettes on the MCC apparatus.
Propylene glycol is a major constituent of many E- cigarette solutions and appears to be an irritant to upper respiratory airways, when used in other inhalation products. For example, nasal burning, stinging and throat irritation were reported by patients suffering from allergic rhinitis, following treatment with inhalation of flunisolide nasal spray containing a high concentration of PG (Greenbaum et al., 1988; Meltzer et al., 1990; Ratner et al., 1996) and acute ocular and upper airway irritation has been reported in non-asthmatic human volunteers following short exposures to PG mist from artificial smoke generators (Wieslander et al., 2001). It is unknown if acute, or chronic, exposures to PG affects MCC.
The studies described in this article were designed to test the hypothesis that acute and chronic exposure to nicotine and PG from E-cigarette emissions significantly impair MCC in a murine model. Results from these studies will provide important information about the short- and long-term effects of exposure to emissions from E-cigarettes on MCC, a mechanism essential to lung defense and respiratory health.
Methods
Aerosol generation
Aerosol was mechanically generated from the commercially available Joyetech 510-T® E-cigarette, attached to a Masterflex 7523-80L/S Digital Peristaltic Pump Drive (Cole- Parmer, Vernon Hills, IL). Joyetech atomizers, 510-T refill- able cartridges and batteries were obtained from LITECIGUSA (Avis, PA). We obtained 0% N/PG solution and 2.4% N/PG solution with no flavorings in bulk from Johnson Creek Vapor Company (Hartland, WI). The pharmaceutical grade of these constituents was not provided by the manufacturer. Six hundred microliters of E-cigarette solution was aerosolized per treatment.
Mice population
C57BL/6 male mice were purchased from Charles River Laboratories River (Frederick, MD). Animals were maintained on an AIN 76A diet and water ad libitum and housed at a temperature range of 20–23 °C under 12-h light/dark cycles. All experiments were conducted in accordance with the standards established by the United States Animal Welfare Acts, set forth in NIH guidelines and the Policy and Procedures Manual of the Johns Hopkins University Animal Care and Use Committee.
Acute and chronic exposures
This was a whole-body exposure protocol that was designed to allow for measurements of the effect of exposure to two of the major constituents of E-cigarettes, namely PG alone and in combination with nicotine, on MCC in mice. This exposure protocol was not meant to reflect human E-cigarette use. Mice were placed in groups of two in a one liter chamber (13.5 cm ×9cm ×8.7cm). These chambers were not airtight, allowing the mice to entrain room air during the exposure. Two one liter chambers containing a total of four mice were simultaneously exposed to emissions from the E-cigarette. Temperature, humidity and air flow rate were neither monitored, nor controlled, during these exposures. Figure 1 is a photo of the various components of the exposure and delivery system, including the pump, tubing, E-cigarette and exposure chambers separated by a Y-tube connection. Between exposures, mice were kept in a clean cage to minimize crosscontamination from the environment.
Figure 1.
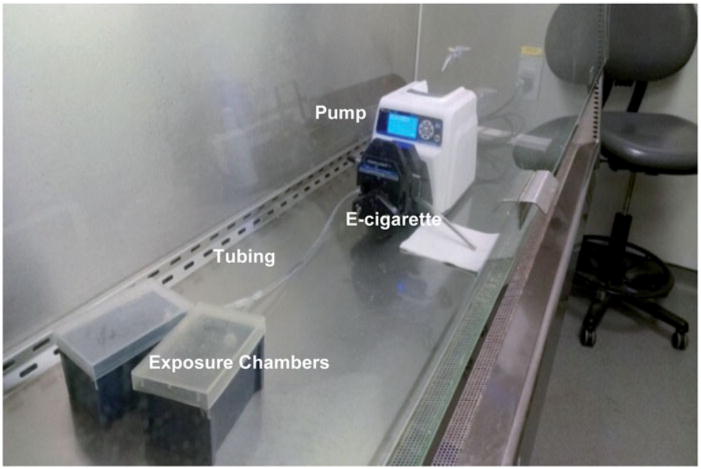
Photo of E-cigarette connected to MasterFlex Pump with tubing and Y-connector to two murine exposure chambers.
Two groups of mice were exposed to 0% N/PG aerosol for 1 week (n = 6) (acute), or for 3 weeks (n = 9) (chronic). Two additional groups of mice were exposed to 2.4% N/PG aerosol for 1 week (n = 6) (acute), or for 3 weeks (n = 9) (chronic). A fifth group of mice (n = 15) did not undergo any exposure protocol and, thereby, provided control values for the MCC measurements. Age of first exposure was 10.5 ± 2.4 weeks.
The Masterflex Pump Drive was programed to actuate E- cigarette puffs for six seconds every 15 seconds for 20 minutes, as previously described (McGrath-Morrow et al., 2015; Smith et al., 2015). Mice were exposed for 20 minutes/day. The puff volume used to generate the aerosol was not quantified. The puff duration of six seconds and puff interval of 15 seconds was chosen to provide enough time to generate the aerosol bolus, clear the aerosol from the tubing and deliver it to the exposure chambers. The 20-minute exposure time was chosen because it resulted in both physiological and behavioral responses and no fatalities in mice in previous studies (McGrath-Morrow et al., 2015; Smith et al., 2015). This puff duration was not intended to be representative of normal human use. Others have reported shorter and longer puff durations, when testing E- cigarettes during human use (Behar et al., 2015; Farsalinos et al., 2013; Talih et al., 2015).
As noted above, 600 μl of E-cigarette solution was aerosolized per treatment. For 2.4% nicotine, this meant that a maximum of 14.4 mg nicotine was aerosolized (0.6 ml × 24 mg/ml) during each 20 minute exposure. Dividing this exposure between the two chambers, we estimated that each mouse per chamber could be exposed to approximately 3.6 mg of nicotine (14.4 mg ÷ 4). Since this was a whole- body exposure occurring in a chamber, not all of the nicotine would be expected to be inhaled by the mouse, with aerosol depositing on the chamber walls, on the bodies of the animals and some aerosol escaping from the chamber, as it was not airtight.
Particle size distribution
Particle size distribution was determined for aerosol generated from the 2.4% N/PG solution and the 0% N/PG solution, from two dedicated Joyetech 510 E-cigarettes. Aerosols were sampled from the murine chambers during aerosol generation as described above. No mice were in the chambers at the time. Aerosol sampling started 5 min before generation of the E-cigarette aerosol to obtain the background particle size distribution (PSD), then continued over the 20-min E-cigarette aerosol generation period. Airborne particles with diameter between 10 and 1000 nm were monitored using a Scanning Mobility Particle Sizer (SMPS, Model 3938 with Electrostatic Classifier Model 3082 and Condensation Particle Counter Model 3787, TSI Inc., Shoreview, MN), as described previously (Hinds, 1999; Wang & Flagan, 1990). Particles in the size range of 0.5–20 μm were monitored using an Aerodynamic Particle Sizer (APS, Model 3321, TSI Inc., Shoreview, MN), as previously described (Armendariz & Leith, 2002; Hinds, 1999). The number size distribution was determined once per minute by the SMPS and once every 20 seconds by the APS with a total of 20 and 60 scans completed, respectively. The SMPS and APS were connected to the murine exposure chamber from separate ports located opposite to the aerosol inlet (Figure 2). Volume median diameter was determined from the SMPS data, assuming unit density for all particles.
Figure 2.
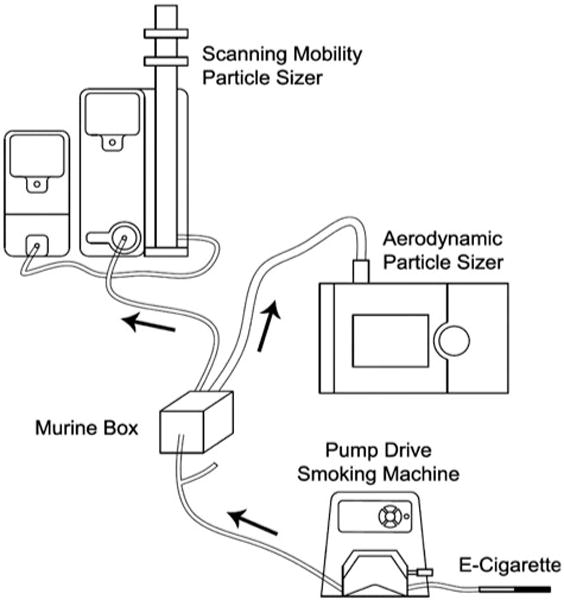
Schematic of the aerosol monitoring set-up showing E-cigarette connected to MasterFlex Pump, Y-tubing connected to one murine chamber and second tube unconnected, exit tubing connected to Scanning Mobility Particle Sizer (SMPS) and Aerodynamic Particle Sizer (APS).
MCC measurements
One group of mice (n = 15) underwent MCC measurements and served as controls. They did not undergo any E-cigarette exposures. One group of mice was exposed to 0% N/PG aerosol for 1 week (n = 6) (acute) and another group was exposed for 3 weeks (n = 9) (chronic). These mice underwent MCC measurements to provide information about the effects of PG exposure. Another group of mice was exposed to 2.4% N/PG aerosol for 1 week (n = 6) (acute) and another group was exposed for 3 weeks (n = 9) (chronic). These mice underwent MCC measurements to provide information about the combined effects of nicotine and PG exposure. Approximately, 16–18 hours following the last E- cigarette exposure, mice were anesthetized by intraperitoneal injection of ketamine (100 μg/g) and xylazine (16 μg/g) and suspended from their upper incisors by suture material at a 45° incline. With the tongue extended, mice aspirated one 50 μl aliquot of the radioisotope technetium-labeled sulfur colloid (99mTc-SC) aerosol, as described previously (Bhashyam et al., 2010, 2012; McGrath-Morrow et al., 2006). Each aliquot contained 60–80 μCi of the isotope, but no nicotine, or PG. Isotope was supplied by Cardinal Health, Inc. (Dublin, OH). The protocol was approved by the Johns Hopkins ACUC.
Following aspiration, mice underwent 2-D planer lung imaging in the prone position with an X-SPECT™ gamma camera (Gamma Medica, Inc., Northridge, CA). Retention of 99mTc-SC within the total lung (i.e. both lungs) was recorded at 0 h post aspiration (Time 0) and at 6 h post aspiration, as previously described (Bhashyam et al., 2010, 2012; McGrath-Morrow et al., 2006). Retention values at 6h were background-corrected and decay-corrected to Time 0 and subtracted from the Time 0 retention value. The difference was expressed as a percentage of the Time 0 value and represented the amount of radioactivity removed from the lungs by MCC.
Cotinine measurements
Cotinine was measured in serum as previously described (McGrath-Morrow et al., 2015). Cotinine levels were measured from venous blood in: (1) control, unexposed mice (n = 7); (2) 16–18 hours after the last 20 minute E-cigarette exposure in a group of mice that underwent 3 weeks of exposure to 2.4% N/PG (n = 12); and (3) 16–18 hours after the last 20 minute E-cigarette exposure in a group of mice that underwent 3 weeks of exposure to 0% N/PG (n = 8). Cotinine was not measured in mice that underwent a 1 week exposure to 2.4% N/PG, or 0% N/PG.
Trachea histology
Mice were sacrificed and tracheas removed. Tracheas were fixed overnight in 20% formalin (Sigma-Aldrich, St. Louis, MO). Tracheas were paraffin-embedded, cut into 5-μm sections and stained with hematoxylin and eosin (H&E).
Data analyses
The main outcome measure was percent MCC at 6 hours from the total lung (MCC6hrs). MCC6hrs was compared between: (1) untreated, control mice, mice exposed to 0% N/PG for 1 week and mice exposed to 2.4% N/PG for 1 week; and (2) untreated, control mice, mice exposed to 0% N/PG for 3 weeks and mice exposed to 2.4% N/PG for 3 weeks. Comparisons were made on-line using one-way Analysis of Variance (ANOVA) and post hoc Tukey’s HSD (Honestly Significant Difference) tests (statistica.mooo.com). Particle size distribution data are presented as mean ±1 standard error. All other data are presented as mean ±1 standard deviation. p values < .05 were considered statistically significant.
Results
Particle size distribution
Number-based plots (i.e. number of particles/cm3) indicated that aerosol particles generated from the two solutions were predominantly in the sub-micrometer range. The particle number distributions for 0% N/PG, 2.4% N/PG and chamber background for sub-micrometer particles and micron-sized particles are shown in Figures 3 and 4, respectively. There were approximately 104 times more submicrometer particles than micron-sized particles for both the 0% N/PG and 2.4% N/PG formulations. Average particle mode sizes were 200 nm and 120 nm for solutions of 2.4% N/PG and 0% N/PG, respectively, which were not statistically significantly different. VMD (±geometric standard deviation) was 264 ± 1.3 nm for 2.4% N/PG and 224 ± 1.4 nm for 0% N/PG.
Figure 3.
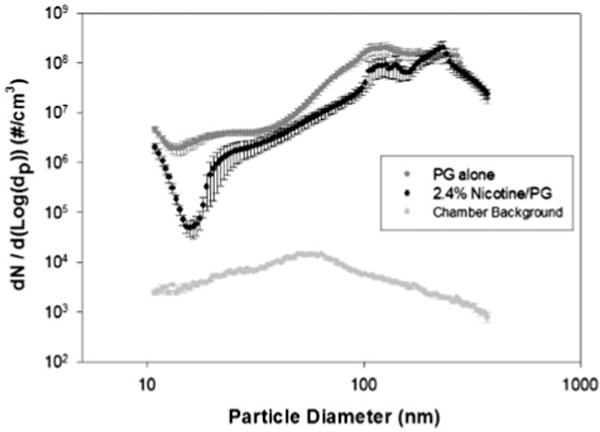
Number-based plots (i.e. number of particles/cm3) for nano-sized airborne particles tested by SMPS and averaged over the 20 min aerosol generation period (error bars indicate standard errors).
Figure 4.
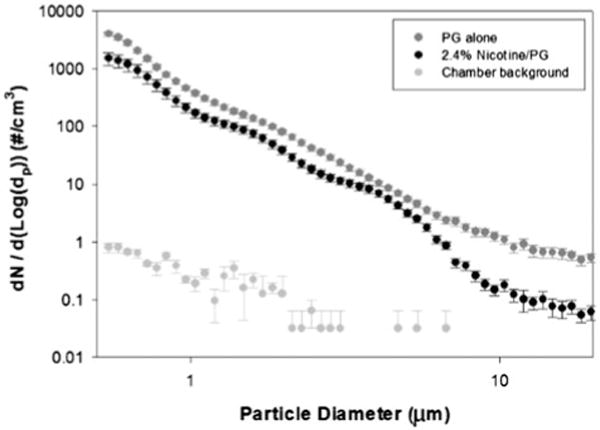
Number-based plots (i.e. number of particles/cm3) for micron-sized airborne particles for the two solutions tested by APS and averaged over the 20 min aerosol generation period (error bars indicate standard errors).
Gamma camera scans after aspiration of radioaerosol
Figure 5 shows representative 2-D images of the retention of 99mTc-SC at Time 0 and at six hours from a mouse exposed to 3 weeks of inhaled nicotine generated by the Joyetech E- cigarette. The red line delineates the right and left lung borders. The right lung is on the left. Activity below the left lung after six hours represents activity that was removed from the lung by the MCC apparatus, swallowed and retained in the stomach.
Figure 5.
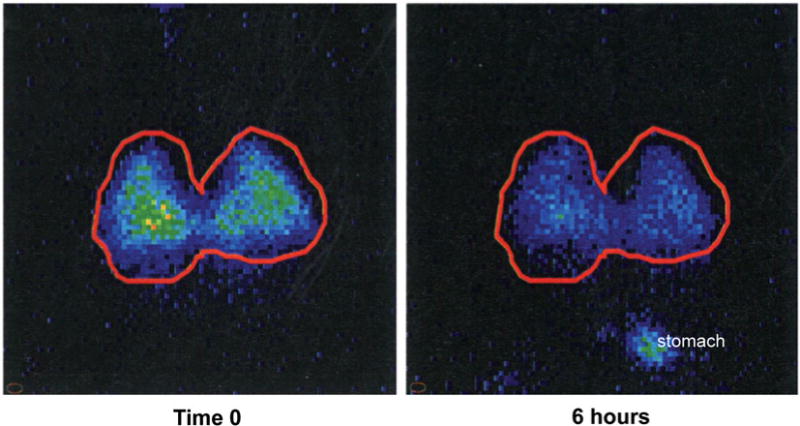
2-D images showing lung retention of radioisotope at 0 and 6 hrs post aspiration. Right and left lungs are bordered in red. A portion of the radioactivity that was cleared from the lungs over 6 hours was swallowed and deposited in the stomach. This activity appears in the bottom right of the 6 hour image. In general, regions of the images with light blue/yellow-green coloration indicate areas with higher concentration of the radioisotope, whereas, regions with dark blue coloration indicate areas with lower concentration of the radioisotope.
MCC after acute exposure to 2.4% N/PG versus 0% N/PG
Figure 6 shows that percent MCC following acute exposure to aerosol containing 2.4% N/PG was not significantly different from MCC measured after acute exposure to 0% N/PG aerosol. Mean MCC6hrs was 7.5 ± 3.2% in the 0% N/PG treated mice and 11.2 ±5.9% in the 2.4% N/PG treated mice (p = .19). None of these values was significantly different from the average control MCC value of 8.6 ± 5.2%.
Figure 6.
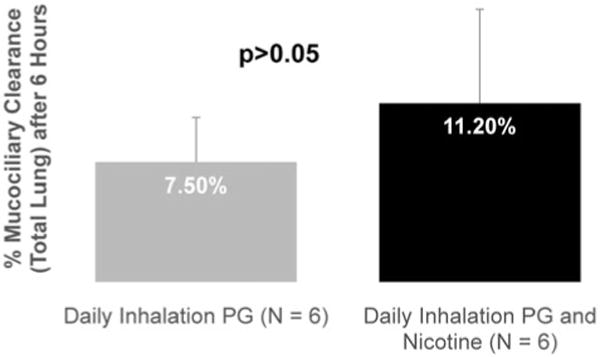
MCC was not statistically different in mice that were acutely exposed (1 week) for 20min/day to emissions from a Joyetech E-cigarette containing 2.4% N/PG, or 0% N/PG (p > .05).
MCC after chronic exposure to 2.4% N/PG versus 0% N/PG
Figure 7 shows that chronic exposure to 0% N/PG stimulated MCC in these mice and this stimulation was significantly blunted following chronic exposure to aerosol containing 2.4%N/PG. Mean MCC6hrs was 17.2 ±8.0% in the 0% N/PG treated mice and 8.7 ± 4.6% in the 2.4% N/PG treated mice (p = .01). Average MCC after chronic exposure to 0% N/PG was also significantly higher than the average control value for MCC of 8.6 ± 5.2% (p < .01).
Figure 7.
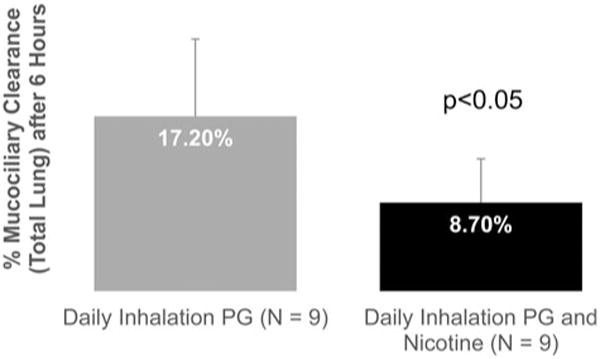
MCC was statistically significantly reduced in mice that were chronically exposed (3 weeks) for 20min/day to emissions from a Joyetech E-cigarette containing 2.4% N/PG, compared to mice that were chronically exposed to emissions containing 0% N/PG (p < .05).
Serum cotinine levels
Serum cotinine levels averaged < 0.5 ng/ml in control, unexposed, mice (n = 7) and in mice that were chronically exposed to 0% N/PG (n = 8). Serum cotinine levels averaged 14.6 ± 12.0 ng/ml in mice that were chronically exposed to 2.4% N/PG (n = 12).
Trachea histology
Figure 8 shows that there was no evidence of gross histological differences between ciliated epithelial cells, or the mucus layer, in the tracheas of mice chronically exposed (3 weeks) to emissions from the E-cigarette containing 0% N/PG, or 2.4% N/PG.
Figure 8.
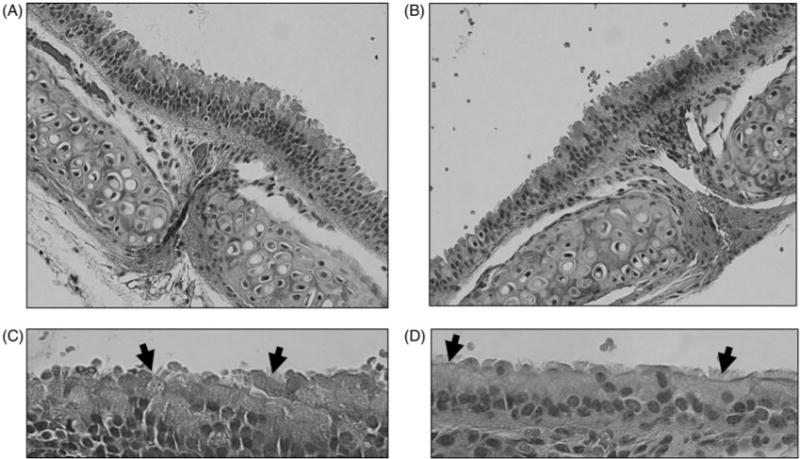
Representative examples of tracheas exposed to three weeks of daily 0% N/PG, or 2.4% N/PG. (A) Trachea from mouse exposed to 0% N/PG (20×); (B) trachea from mouse exposed to 0% N/PG (40×); (C) trachea from mouse exposed to 2.4% N/PG (20×); (D) trachea from mouse exposed to 2.4% N/PG (40×). Arrows point to cilia from ciliated epithelium.
Discussion
This is the first study to investigate the effect of acute and chronic exposure to E-cigarette emissions on MCC in mice in vivo. Results from this study showed that MCC in C57BL/6 male mice was not statistically different following an acute (1 week) exposure to emissions from the Joyetech E-cigarette containing either 0% N/PG, or 2.4% N/PG (Figure 6). In contrast, a chronic (3 weeks) exposure to 0% N/PG aerosol stimulated MCC in these mice, compared to controls, whereas PG plus 2.4% N significantly blunted the MCC response to PG plus 0% N (Figure 7).
Results from our particle sizing experiments indicated that a large fraction of particles generated by the Joyetech E-cigarette from the 2.4% N/PG solution and from the 0% N/PG solution were in the sub-micrometer range, with VMDs of 264 nm and 224 nm, respectively. These VMDs were similar to the VMD (250 nm) reported by Zhang et al. (2013), who tested the Bloog MaxX Fusion E-cigarette. This finding suggests that E-cigarettes that generate emissions using a method similar to the Joyetech and Bloog MaxX Fusion devices may produce particles with similar size characteristics. More studies are needed to confirm this finding.
The SMPS diluted the sampled aerosol with a filtered dry air at the flow rate of 6 Lpm to improve size resolution of the individual particles and to avoid interference of the high concentration particles with the charging electrode. Previous PSD measurements of E-cigarette aerosol by Ingebrethsen et al. (2012) suggested that particle sizers using dilution air for their operation (e.g. SMPS) may underestimate particle sizes of an E-cigarette aerosol up to nine times (particle mode size of 450 nm versus a particle mode size of 50 nm). The decrease in particle size is due to evaporation of the particles when exposed to dry, filtered air. Ingebrethsen et al. proposed using spectral transmission to reliably determine undiluted particle sizes. However, Ingebrethsen et al. also reported negligible changes in the total number concentration of an E-cigarette aerosol using both methods. Although there is no dilution involved with the spectral transmission technique, the use of a light scattering mechanism for ultrafine particle detection may not be accurate when number-based PSDs are desired. The SMPS (based on electrical mobility) is well characterized and its accuracy in obtaining PSD for all types of submicron aerosols have been reported elsewhere (Jeong & Evans, 2009; Sioutas et al., 1999). Moreover, the particle mode sizes obtained in the present study (200 nm and 120 nm for solutions of 2.4% N/PG and 0% N/PG, respectively) are considerably larger than the reported value by Ingebrethsen et al. (i.e. 50 nm).
Particle size distribution is challenging to measure when particles are non-steady state, are comprised of volatile and semi-volatile species and are emitted at high number concentrations. All of these conditions occur when measuring E-cigarette emissions. In the present study, the goal was to measure the exposures in the mouse chambers, which we have done. These chambers likely do not reflect all possible conditions of exposure (temperature, dilution rates, etc.).
The short, but intense, daily exposure of 20 minutes for 3 weeks was sufficient to lead to systemic absorption of nicotine in this murine model. The reported average serum cotinine levels of 14.6 ± 12.0 ng/ml were solely the result of chronic exposure to nicotine, since serum cotinine levels in control mice and in 0% N/PG averaged < 0.5 ng/ml. The pharmacological relevance of these serum cotinine levels in this murine model is unknown.
The effects of 0% N/PG and of 2.4% N/PG on MCC do not appear to cause histological changes to the MCC cellular apparatus found in the murine trachea. No gross changes to ciliated epithelial cells, or the mucus layer, were observed, following exposure to either compound (Figure 8). One explanation of the stimulatory effect of PG on MCC in this mouse model could involve bronchopulmonary C fibers that are thought to be integral to MCC function. Bronchopulmonary C fibers are quiescent at baseline, but can be activated by noxious stimuli, such as cigarette smoke, particulate matter or inflammation. Acute application of nicotine to vagal afferents has been shown to elicit C fiber activation, possibly through nicotinic acetylcholine receptors (a7nAChRs) (Lee et al., 2010).
It is likely that inhaled PG also stimulates bronchopulmonary C fibers in the respiratory tract due to its irritation effects. Nasal burning, stinging and throat irritation have been reported by patients suffering from allergic rhinitis, following treatment by inhalation of a nasal spray containing PG (Meltzer et al., 1990) and acute ocular and upper airway irritation has also been reported in non-asthmatic human volunteers following short exposures to PG mist from artificial smoke generators (Greenbaum et al., 1988).
One possible explanation for the observed impairment of MCC, following chronic exposure to nicotine emitted by E- cigarettes in this murine model, could involve an interaction, or regulatory link, between airway α7nAChRs and the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR), a regulator of chloride and water transport homeostasis. Maouche et al. (2013) reported that chronic systemic nicotine exposure can desensitize α7nAChRs, disrupt CFTR function and impair airway MCC. It is likely that impaired MCC in their model occurred as a result of disruption in the airway periciliary liquid layer, which along with the mucus layer is needed for effective airway clearance (Mall, 2008). Results from the present study suggest that exposure to aerosolized nicotine could impair MCC through similar mechanisms.
There were a few limitations to this study. The small size of our chamber (i.e. one liter) may represent a worst-case scenario for the mice in these experiments in terms of an E- cigarette exposure. Larger exposure chambers could dilute the E-cigarette emissions and, thereby, lead to a reduction in the MCC response. However, the small-size chamber used in this study may be representative of an environmental exposure to non-E-cigarette users, who are in a confined space with E-cigarette users.
We only tested one brand of E-cigarette in terms of effect on MCC. Others need to be tested to determine possible variability in the MCC response between brands with similar nicotine content.
As this was a whole-body exposure, another limitation is the possibility that the mice in our study may have absorbed nicotine through thirdhand routes. Research on this topic is just emerging. In recent studies, nicotine was readily detectable on the surfaces of laboratory exposure chambers with as little as 100 puffs generated by a machine (Goniewicz & Lee, 2015). Nicotine has also been detected on indoor surfaces in the homes of E-cigarette users (Bush & Goniewicz, 2015), but there was no difference in levels of nicotine detected in E-cigarette users’ and non-users’ homes. Any thirdhand exposure from these experiments should have been extremely low, since nicotine is not readily absorbable from the stomach, because of the acidic nature of the gastric juice (Ivey & Triggs, 1978; Travell, 1960) and the exposure chambers were wiped clean after each E-cigarette run.
The impairment in MCC that we detected was mild. This could be because three weeks of exposure to 2.4% N/PG for 20 min/day may not be sufficient to detect a severe impairment to MCC in this murine model. Increasing the number of days of exposure and/or increasing the exposure time/day may be required to detect a more severe response.
Propylene glycol can undergo decomposition when it comes in contact with the heating coil of an E-cigarette atomizer, forming volatile carbonyls including formaldehyde and acetaldehyde (Geiss et al., 2016). Generation of these carbonyls was not measured as part of this experimental protocol. Thus, it is unknown if the presence of carbonyls was associated with the significant increase in MCC observed in mice that were chronically exposed to PG alone. Nor is it known if the addition of nicotine to the PG formulation altered carbonyl generation such that MCC decreased in mice chronically exposed to 2.4% N/PG. What is known is that the heat associated with the atomizer coil was the same for both groups of mice, since the puff duration was the same, and PG was present in both formulations.
Conclusions
Little is known of the chronic health effects of E-cigarettes. Results from these experiments will help to fill-in that information-gap. These data suggest that chronic, but not acute, exposure to two of the major constituents of E-cigarettes, PG alone and in combination with nicotine, have significant effects on MCC in this murine model. Chronic exposure to emissions containing PG alone appears to stimulate MCC, whereas the addition of 2.4% nicotine appears to blunt the stimulatory response to PG alone. Additional studies are needed to confirm these findings, which could provide much needed information about potential risks to the MCC apparatus from chronic exposure to E-cigarette emissions.
Acknowledgments
This work was supported by the Flight Attendant Medical Research Institute under a Center of Excellence Grant to the American Academy of Pediatrics and by the National Institutes of Health under grant NIH RHL114800. Neither funding agency was involved in the analysis of the results, nor in the preparation of this manuscript.
Funding
This work was supported by the Flight Attendant Medical Research Institute under a Center of Excellence Grant to the American Academy of Pediatrics and by the National Institutes of Health under grant NIH RHL114800.
Footnotes
Disclosure statement
No financial interest, or benefit, has arisen from this research.
References
- Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: international tobacco control four-country survey. Am J Prev Med. 2013;44:207–15. doi: 10.1016/j.amepre.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armendariz AJ, Leith D. Concentration measurement and counting efficiency for the aerodynamic particle sizer 3320. J Aerosol Sci. 2002;33:133–48. [Google Scholar]
- Armitage AK, Dixon M, Frost BE, et al. The effect of tobacco blend additives on the retention of nicotine and solanesol in the human respiratory tract and on subsequent plasma nicotine concentrations during cigarette smoking. Chem Res Toxicol. 2004;17:537–44. doi: 10.1021/tx0340753. [DOI] [PubMed] [Google Scholar]
- Behar RZ, Hua M, Talbot P. Puffing topography and nicotine intake of electronic cigarette users. PLoS ONE. 2015;10:e0117222. doi: 10.1371/journal.pone.0117222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhashyam AR, Mogayzel PJ, Cleary JC, et al. Vagal control of mucociliary clearance in murine lungs: a study in a chronic preparation. Auton Neurosci. 2010;154:74–8. doi: 10.1016/j.autneu.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhashyam AR, Mogayzel PJ, Jr, McGrath-Morrow S, et al. A pilot study to examine the effect of chronic treatment with immunosuppressive drugs on mucociliary clearance in a vagotomized murine model. PLoS One. 2012;7:e45312. doi: 10.1371/journal.pone.0045312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D, Goniewicz ML. A pilot study on nicotine residues in houses of electronic cigarette users, tobacco smokers, and non-users of nicotine-containing products. Int J Drug Policy. 2015;26:609–11. doi: 10.1016/j.drugpo.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease C, Prevention. Tobacco product use among middle and high school students - United States, 2011 and 2012. MMWR Morbid Mortal Week Rep. 2013;62:893–7. [PMC free article] [PubMed] [Google Scholar]
- Dockrell M, Morrison R, Bauld L, McNeill A. E-cigarettes: prevalence and attitudes in Great Britain. Nicot Tobacco Res. 2013;15:1737–44. doi: 10.1093/ntr/ntt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Tsiapras D, et al. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health. 2013;10:2500–14. doi: 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flouris AD, Chorti MS, Poulianiti KP, et al. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal Toxicol. 2013;25:91–101. doi: 10.3109/08958378.2012.758197. [DOI] [PubMed] [Google Scholar]
- Geiss O, Bianchi I, Barrero-Moreno J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int J Hyg Environ Health. 2016;219:268–77. doi: 10.1016/j.ijheh.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Goniewicz ML, Lee L. Electronic cigarettes are a source of thirdhand exposure to nicotine. Nicot Tobacco Res. 2015;17:256–8. doi: 10.1093/ntr/ntu152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravely S, Fong GT, Cummings KM, et al. Awareness, trial, and current use of electronic cigarettes in 10 countries: findings from the ITC project. Int J Environ Res Public Health. 2014;11:11691–704. doi: 10.3390/ijerph111111691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum J, Leznoff A, Schulz J, et al. Comparative tolerability of two formulations of Rhinalar (flunisolide) nasal spray in patients with seasonal allergic rhinitis. Ann Allergy. 1988;61:305–10. [PubMed] [Google Scholar]
- Hinds WC. Aerosol technology: properties, behavior and measurement of airborne particles. 2nd. New York, NY, USA: Wiley Interscience; 1999. [Google Scholar]
- Human Respiratory Tract Model for Radiological Protection. A report of a Task Group of the International Commission on Radiological Protection. Ann ICRP. 1994;24:1–482. [PubMed] [Google Scholar]
- Ingebrethsen BJ, Cole SK, Alderman SL. Electronic cigarette aerosol particle size distribution measurements. Inhal Toxicol. 2012;24:976–84. doi: 10.3109/08958378.2012.744781. [DOI] [PubMed] [Google Scholar]
- Ivey KJ, Triggs EJ. Absorption of nicotine by the human stomach and its effect on gastric ion fluxes and potential difference. Am J Dig Dis. 1978;23:809–14. doi: 10.1007/BF01079790. [DOI] [PubMed] [Google Scholar]
- Jeong C-H, Evans GJ. Inter-comparison of a fast mobility particle sizer and a scanning mobility particle sizer incorporating an ultrafine water-based condensation particle counter. Aerosol Sci Technol. 2009;34:364–73. [Google Scholar]
- King BA, Dube SR, Tynan MA. Awareness and ever-use of electronic cigarettes among U.S. adults, 2010–2011. Nicot Tobacco Res. 2013;15:1623–7. doi: 10.1093/ntr/ntt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Gu Q, Lin YS. Effect of smoking on cough reflex sensitivity: basic and preclinical studies. Lung. 2010;188(Suppl 1):S2–S7. doi: 10.1007/s00408-009-9191-1. [DOI] [PubMed] [Google Scholar]
- Mall MA. Role of cilia, mucus, and airway surface liquid in mucociliary dysfunction: lessons from mouse models. J Aerosol Med Pulmon Drug Deliv. 2008;21:13–24. doi: 10.1089/jamp.2007.0659. [DOI] [PubMed] [Google Scholar]
- Maouche K, Medjber K, Zahm JM, et al. Contribution of alpha7 nicotinic receptor to airway epithelium dysfunction under nicotine exposure. Proc Natl Acad Sci USA. 2013;110:4099–104. doi: 10.1073/pnas.1216939110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath-Morrow S, Laube B, Tzou SC, et al. IL-12 overexpression in mice as a model for Sjögren lung disease. Am J Physiol Lung Cell Mol Physiol. 2006;291:L837–46. doi: 10.1152/ajplung.00134.2006. [DOI] [PubMed] [Google Scholar]
- McGrath-Morrow SA, Hayashi M, Aherrera A, et al. The effects of electronic cigarette emissions on systemic cotinine levels, weight and postnatal lung growth in neonatal mice. PLoS One. 2015;10:e0118344. doi: 10.1371/journal.pone.0118344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer EO, Orgel HA, Bush RK, et al. Evaluation of symptom relief, nasal airflow, nasal cytology, and acceptability of two formulations of flunisolide nasal spray in patients with perennial allergic rhinitis. Ann Allergy. 1990;64:536–40. [PubMed] [Google Scholar]
- O’Connell G, Colard S, Breiev K, et al. An experimental method to determine the concentration of nicotine in exhaled breath and its retention rate following use of an electronic cigarette. Int J Environ Anal Chem. 2015;2:161. [Google Scholar]
- Pearson JL, Richardson A, Niaura RS, et al. e-Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health. 2012;102:1758–66. doi: 10.2105/AJPH.2011.300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randell SH, Boucher RC, University of North Carolina Virtual Lung Group Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol. 2006;35:20–8. doi: 10.1165/rcmb.2006-0082SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner P, van Bavel J, Gross G, et al. New formulation of aqueous flunisolide nasal spray in the treatment of allergic rhinitis: comparative assessment of safety, tolerability, and efficacy. Allergy Asthma Proc. 1996;17:149–56. doi: 10.2500/108854196779165049. [DOI] [PubMed] [Google Scholar]
- Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the ‘e-cigarette’ in the USA. Tobacco Control. 2013;22:19–23. doi: 10.1136/tobaccocontrol-2011-050044. [DOI] [PubMed] [Google Scholar]
- Schripp T, Markewitz D, Uhde E, Salthammer T. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23:25–31. doi: 10.1111/j.1600-0668.2012.00792.x. [DOI] [PubMed] [Google Scholar]
- Sioutas C, Abt E, Wolfson JM. Evaluation of the measurement performance of the scanning mobility particle sizer. Aerosol Sci Technol. 1999;30:84–92. [Google Scholar]
- Smith D, Aherrera A, Lopez A, et al. Adult behavior in male mice exposed to E-cigarette nicotine vapors during late prenatal and early postnatal life. PLoS One. 2015;10:e0137953. doi: 10.1371/journal.pone.0137953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Helen G, Havel C, Dempsey DA, et al. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111:535–44. doi: 10.1111/add.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talih S, Balhas Z, Eissenberg T, et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicot Tobacco Res. 2015;17:150–7. doi: 10.1093/ntr/ntu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travell J. Absorption of nicotine from various sites. Ann NY Acad Sci. 1960;90:13–30. doi: 10.1111/j.1749-6632.1960.tb32612.x. [DOI] [PubMed] [Google Scholar]
- Wang SC, Flagan RC. Scanning electrical mobility spectrometer. Aerosol Sci Technol. 1990;13:230–40. [Google Scholar]
- Wanner A, Salathe M, O’Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- Wieslander G, Norback D, Lindgren T. Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occup Environ Med. 2001;58:649–55. doi: 10.1136/oem.58.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sumner W, Chen DR. In vitro particle size distributions in electronic and conventional cigarette aerosols suggest comparable deposition patterns. Nicot Tobacco Res. 2013;15:501–8. doi: 10.1093/ntr/nts165. [DOI] [PubMed] [Google Scholar]


