Abstract
Purpose
Reovirus type 3 Dearing (RT3D) replicates preferentially in Ras-activated cancers. RT3D shows synergistic in vitro cytotoxicity in combination with platins and taxanes. The purpose of this phase I/II study was to assess RT3D combined with carboplatin/paclitaxel in patients with advanced cancers.
Experimental Design
Patients were initially treated in a dose-escalating, phase I trial with intravenous RT3D days 1 to 5, carboplatin [area under curve (AUC) 5, day 1] and paclitaxel (175 mg/m2, day 1) 3-weekly. RT3D was escalated through three dose levels: 3 × 109, 1 × 1010, and 3 × 1010 TCID50 in cohorts of three. Primary endpoints were to define the maximum tolerated dose and dose-limiting toxicity and to recommend a dose for phase II studies. Secondary endpoints included pharmacokinetics, immune response, and antitumor activity. A subsequent phase II study using the 3 × 1010 TCID50 dose characterized the response rate in patients with head and neck cancer.
Results
Thirty-one heavily pretreated patients received study therapy. There were no dose-limiting toxicities during dose-escalation and most toxicities were grade I/II. Overall effectiveness rates were as follows: one patient had a complete response (3.8%), six patients (23.1%) had partial response, two patients (7.6%) had major clinical responses clinically evaluated in radiation pretreated lesions which are not evaluable by Response Evaluation Criteria in Solid Tumors (RECIST), nine patients (34.6%) had stable disease, and eight patients (30.8%) had disease progression. Viral shedding was minimal and antiviral immune responses were attenuated compared with previous single-agent data for RT3D.
Conclusions
The combination of RT3D plus carboplatin/paclitaxel is well tolerated with evidence of activity in cancer of the head and neck. A randomized phase III study is currently open for recruitment.
Introduction
Reovirus type 3 Dearing (RT3D, Reolysin; Oncolytics Biotech, Inc.) is a naturally occurring, nonpathogenic, double-stranded RNA virus isolated from the respiratory and gastrointestinal tracts of humans (1). Reovirus infection is essentially ubiquitous with up to 100% seropositivity rates in healthy adults (2). Ras pathway activation, either through Ras mutation or overexpression/mutational activation of EGF receptor (EGFR), has been reported to be a prerequisite for reovirus sensitivity (3, 4). Reovirus has been shown to exert significant antitumor effects in preclinical in vitro and in vivo studies. In addition, reovirus can activate both innate and adaptive antitumor immune responses against murine and human tumors (5, 6).
Both intralesional (7) and systemic (8, 9) administration of RT3D have very favorable toxicity profiles with preliminary evidence of antitumor activity. However, responses to RT3D monotherapy are modest and short-lived and this has led to clinical trials testing combinations of RT3D with either radiotherapy (10) or single-agent chemotherapy (11, 12). These studies have confirmed feasibility and safety with a number of patients showing disease responses. When RT3D was administered intravenously in a phase I study in 33 heavily pretreated patients with solid tumors, no dose-limiting toxicity (DLT) was observed up to the dose of 3 × 1010 TCID50/d for 5 consecutive days repeated every 4 weeks. Toxicity was mild including fever, fatigue, and headache (9). Dose-escalation did not proceed above the 3 × 1010 TCID50/d level because this was the manufacturing limit of RT3D at that time. As a result, none of the subsequent studies in which intravenous RT3D has been combined with cytotoxic chemotherapy has exceeded that dose limit. In the combination of RT3D and gemcitabine, 3 subjects experienced DLT (2 asymptomatic grade III liver enzyme increases and 1 asymptomatic grade III troponin increase); therefore, the recommended dose of RT3D was a single infusion of 1 × 1010 TCID50 alongside the full dose of gemcitabine (12). However, when RT3D was combined with docetaxel, one DLT of grade IV neutropenia was observed but the maximum tolerated dose (MTD) was not reached. The combination of RT3D at a dose of 3 × 1010 TCID50, days 1 to 5, and docetaxel of 75 mg/m2 every 21 days is safe and tolerable (11). Systemic rather than intralesional administration of RT3D increases the likelihood of virus reaching diffuse metastatic sites and makes the agent more generally applicable for clinical development. In our initial phase I study, pre- and posttreatment biopsy samples were collected and tested for the presence of virus. Replication-competent virus was present only in posttreatment biopsies (9). In addition, we recently confirmed virus delivery to metastatic cancer deposits by immunohistochemistry in patients treated with RT3D and docetaxel (11).
Preclinical in vitro and in vivo combinations of RT3D with platin- and/or taxane-based chemotherapy have been shown to be highly synergistic in melanoma (13), prostate (14), and non–small cell lung cancer (15) through enhanced viral replication and apoptosis. Therefore, we hypothesized that combining RT3D with carboplatin/paclitaxel combination chemotherapy would be an attractive approach for clinical testing. Here, we report a phase I trial of carboplatin/paclitaxel and reovirus in patients with relapsed/metastatic cancers. The promising initial results in patients with tumors of the head and neck (including squamous and nonsquamous histologies) led to a phase II expansion exclusively in this indication. As a result of the phase I and II experience, a phase III trial has now opened for taxane-naive patients with relapsed/metastatic squamous cell cancer of the head and neck (SCCHN) that has progressed within 6 months of first-line palliative platin-based chemotherapy.
Patients and Methods
This phase I/II, open-label trial was conducted at 2 U.K. centers. This trial was approved by Institutional Review Boards at each center and was conducted in accordance with the principles of Good Clinical Practice (GCP) as outlined in the Declaration of Helsinki and International Conference on Harmonization (ICH) guidelines.
Patients
Patients with histologically proven, incurable relapsed/metastatic solid tumors for whom combined carboplatin/paclitaxel was appropriate, palliative chemotherapy were eligible for the phase I study. Phase II was restricted to patients with incurable or relapsed/metastatic HNC. Tumor material was not collected for analysis of Ras mutation or EGFR mutation/overexpression. Additional inclusion criteria were as follows: age ≥ 18 years; measurable or evaluable disease; Eastern Cooperative Oncology Group performance status (PS) ≤ 2; life expectancy ≥ 3 months; ≥28 days from previous chemotherapy, radiotherapy, or surgery; absolute neutrophils ≥ 1.5 × 109/L, platelets ≥ 100 × 109/L, hemoglobin ≥ 9.0g/dL; and adequate renal [creatinine ≤1.5 × upper limit of the normal (ULN)] and liver function (bilirubin ≤ 1.5 × ULN, aspartate aminotransferase and alanine aminotransferase ≤ 2.5 × ULN). Exclusion criteria were as follows: pregnancy or breastfeeding; immunosuppressive therapy; concurrent therapy with another investigational anticancer agent; seropositivity for human immunodeficiency virus, hepatitis B or C; clinically significant cardiac disease; and any other serious preexisting medical illness. Patients with known brain metastases were ineligible unless stable for 6 months or more after therapy. All patients provided written informed consent.
Treatment plan
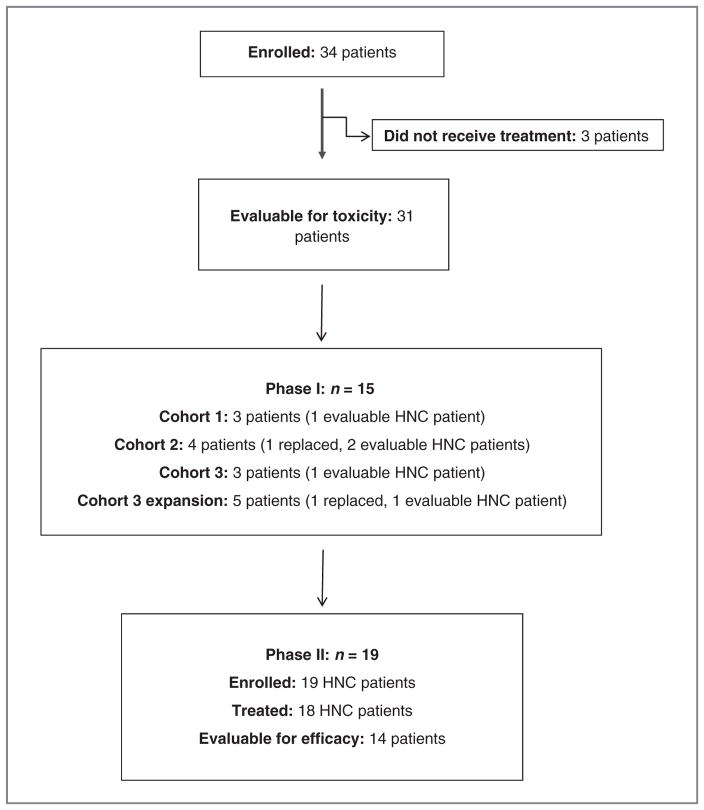
An initial dose-escalation, phase I study defined the safety profile of RT3D combined with carboplatin/paclitaxel and provided a recommended dose for phase II evaluation (Fig. 1). At the end of phase I dose-escalation, an expansion cohort of 5 patients was recruited at the 3 × 1010 TCID50 dose level. The 3 × 1010 TCID50 dose level of RT3D has been previously defined as a ceiling dose for escalation based on manufacturing limit. So, in the phase I studies of RT3D with chemotherapy for ethical, safety, and logistical reasons, we did not plan to escalate beyond the 3 × 1010 TCID50 dose level. Thereafter, all patients were recruited to the phase II study and had a diagnosis of HNC (including both squamous and nonsquamous histologies). All patients received treatment up to 8 cycles or until disease progression or unacceptable toxicity. Any patient still responding at 8 cycles had therapy discontinued.
Figure 1.
CONSORT diagram.
For phase I, patients were enrolled in groups of 3 and individually assessed for safety and DLTs. Paclitaxel (175 mg/m2 over 3 hours) and carboplatin (AUC5 over 30 minutes) were given intravenously on day 1 of a 3-week cycle. On the basis of our previous single-agent phase I study, successive cohorts received 60-minute intravenous infusions of dose-escalated reovirus (3 × 109, 1 × 1010, and 3 × 1010 TCID50 for cohorts 1, 2, and 3, respectively) on days 1 to 5 of each cycle. Intrapatient dose-escalation was not permitted. To be evaluable for dose-escalation decisions, patient must have received at least one cycle of therapy or have been withdrawn because of drug-related toxicity. Escalation was allowed when 2 patients had received 2 cycles of therapy on the previous dose level. If 1 of 3 patients in a cohort were to experience DLT in cycle 1, 3 more patients would be added to that cohort. If 2 or more patients in a cohort were to experience DLT in cycle 1, the previous lower dose would be the MTD and the recommended phase II dose (RP2D). DLT was defined as any of the following events occurring in the first cycle that were determined to be possibly or probably related to combination therapy (as determined by the investigator), irrespective of whether the toxicity resolved: absolute neutrophil count < 0.5 × 109 for >7 days, or absolute neutrophil count < 0.5 × 109 with sepsis; platelet count < 25 × 109/L; grade II neurotoxicity or cardiotoxicity; any other drug-related nonhematologic grade III/IV toxicity, with the exceptions of flu-like symptoms, nausea, and vomiting, if appropriate, prophylactic or therapeutic measures had not been administered; and inability to tolerate one course of therapy due to toxicity. All patients received standard steroids and antihistamines to prevent hypersensitivity. Prophylactic antiemetics were used. To define DLTs, growth factor support, antidiarrheals, or antipyretics were not prescribed prophylactically in cycle 1. If a patient experienced ≥grade III flu-like symptoms or nausea/vomiting, prophylactic administration of appropriate agents was indicated for subsequent cycles. All concomitant medications were recorded. In the absence of an MTD, patients in the phase II study would receive combination chemotherapy (as detailed above) and reovirus 3 × 1010 TCID50 on days 1 to 5 of a 3-weekly cycle.
Objectives
Phase I
The phase I primary objective was to determine the safety profile and MTD/RP2D of reovirus in combination with carboplatin/paclitaxel. Sample size was based on clinical and regulatory considerations for phase I dose-escalation studies designed to establish MTD and had no formal statistical basis. Secondary objectives included description of pharmacokinetics of carboplatin and paclitaxel, viral shedding, antiviral humoral immune responses, and evidence of antitumor activity and tumor responses.
Phase II
The phase II primary objective was to measure tumor response and duration by Response Evaluation Criteria in Solid Tumors (RECIST; ref. 16) or describe any evidence of antitumor activity. On the basis of the work of Gehan (17), the phase II component was evaluated by a standard 2-question Gehan design, where question 1 was to determine whether the combination is unlikely to be effective in 20% of patients or more and question 2 was to determine whether the combination could be effective in 20% of patients or more. With a rejection error of 5% or 0.05, this led to a sample size of up to 14 patients. A single response in any of up to 14 patients satisfies both questions. Secondary objectives included further documentation of the safety profile of the combination and progression-free and overall survival rates.
Safety
Safety was assessed by evaluating the type, frequency, and severity of adverse events; changes in physical examination, clinical laboratory tests (including hematology, clinical chemistry, coagulation studies, and urinalysis), and viral immunogenicity. Interval medical history and physical examination were conducted daily during virus administration and weekly thereafter. Vital signs were recorded at 15, 30, and 60 minutes after the end of RT3D infusion and electrocardiographies were conducted at baseline, after the first virus infusion of the first cycle, on day 1 of each additional cycle, and at the end of the study. Toxicity was recorded in all patients receiving at least one dose of reovirus according to National Cancer Institute Common Terminology Criteria of Adverse Events, version 3.0. Patients experiencing any DLT in any cycle had their treatment held until toxicity resolved to baseline or grade I. Upon resolution, therapy could recommence at a predefined lower dose level. Patients were evaluated 1 month after the last dose of virus and subsequently every 3 months for toxicity and disease progression.
Effectiveness evaluation
Response was assessed by RECIST (16) or by clinical evaluation where applicable. All patients were clinically evaluated on a weekly basis while on treatment and radiologically every 3 cycles (cycle 3, 6, and 8). Per protocol, effectiveness was evaluated in patients who received at least 2 cycles of combination treatment.
Laboratory analyses
Analysis of viral shedding by reverse transcription PCR
Patients in the phase I study had biologic samples (blood, urine, feces, sputum) collected for viral detection before reovirus infusion, 4 hours after the day 5 dose, on day 15 of cycles 1 and 2, and at follow-up visits. Sample processing and reverse transcription PCR (RT-PCR) methods were carried out as previously described (9).
Detection of neutralizing antireoviral antibodies
A modified neutralizing antibody assay was used as previously described (18). The neutralizing antireoviral antibody (NARA) titer of serum samples was expressed as the last dilution causing less than 80% cell killing. NARA titers were measured at baseline and weekly for the first 2 cycles of treatment and at follow-up visits for all patients in phase I.
Pharmacokinetics
Paclitaxel and carboplatin pharmacokinetics were measured during the first cycle in patients in the dose-escalation, phase I study. Sample times were as follows: 0 minutes (pre-paclitaxel); 1, 2, and 3 hours (pre-carboplatin); 3.25 and 3.5 hours (pre-reovirus); and 4.5, 6, 8, 24, and 48 hours (pre-reovirus days 2 and 3, respectively).
Results
Patient characteristics
Between May 2007 and March 2010, 34 patients were enrolled but only 31 received study treatment. The number of patients treated at the 3 × 109, 1 × 1010, and 3 × 1010 TCID50 dose levels were 3, 3, and 25, respectively. Patient demographics are outlined in Table 1.
Table 1.
Patient demographics
| Patient characteristics | Number of patients (phase I) | Number of patients (phase II) | Total number of patients |
|---|---|---|---|
| Total number | 13 | 18 | 31 |
| Median age (range), y | 55.5 (27–79) | 60.5 (48–74) | 60 (27–79) |
| Male/female | 7/6 | 17/1 | 24/7 |
| Performance status | |||
| 0 | 2 | 3 | 5 |
| 1/2 | 11 | 15 | 26 |
| Race | |||
| White | 10 | 15 | 25 |
| Asian | 3 | 2 | 5 |
| Other | 0 | 1 | 1 |
| Tumor type | |||
| SCCHN | 2 | 12 | 14 |
| HNC (other histologiesa) | 4 | 6 | 10 |
| Melanoma | 3 | 0 | 3 |
| Gynecologic cancer | 2 | 0 | 2 |
| Sarcoma | 1 | 0 | 1 |
| Unknown primary | 1 | 0 | 1 |
| Months elapsed since diagnosis (median, range) | 39.1 (1.5–137.5) | 35.5 (8.6–127.6) | 37.0 (1.5–137.5) |
Other HNC histologies include nasopharyngeal cancer, adenoid cystic carcinoma, squamous cell carcinoma of the skin, and sinonasal undifferentiated carcinoma.
Treatment-related toxicity
Treatment was well tolerated in both phase I and II studies. The commonest treatment-related toxicities included blood cytopenias, nausea and vomiting, fatigue, diarrhea, oral candidiasis/stomatitis, alopecia, muscle pain, fever and chills, influenza-like symptoms, rashes, and flushing. Grade III/IV hematologic toxicities were neutropenia (16.1%), asymptomatic lymphopenia (6.5%), and anemia (3.2%). Only one episode of grade IV neutropenia was complicated with sepsis. Nonhematologic grade III/IV toxicities included fever following reovirus infusion (9.7%), myalgia (6.5%), diarrhea (3.2%), nausea (3.2%), vomiting (3.2%), and hypotension (3.2%). There was no relationship between reovirus dose level and incidence or grade of symptoms. Adverse events and treatment-related toxicities are detailed in Table 2.
Table 2.
Adverse events and treatment-related toxicity
| Adverse event/toxicity | Intravenous reovirus combined
with carboplatin/paclitaxel, N = 31 (%) |
|||
|---|---|---|---|---|
| All–any grade | At least possibly related to treatment | Grade ≥ III | Both possibly related and ≥ grade III | |
| Blood and lymphatic system disorders | ||||
| Hemoglobin | 25 (80.6) | 13 (41.9) | 5 (16.1) | 1 (3.2) |
| WBC count | 25 (80.6) | 9 (29.0) | 13 (41.9) | 0 |
| ANC/neutrophils | 23 (74.2) | 9 (29.0) | 15 (47.9) | 5 (16.1) |
| Lymphocytes | 31 (100.0) | 14 (45.1) | 29 (93.5) | 2 (6.5) |
| Platelet count | 23 (74.2) | 7 (22.6) | 3 (9.7) | 0 |
| Alimentary/gastrointestinal disorders | ||||
| Stomatitis | 4 (12.9) | 4 (12.9) | 3 (9.7) | 0 |
| Diarrhea | 9 (29.0) | 7 (22.6) | 1 (3.2) | 1 (3.2) |
| Nausea | 11 (35.5) | 11 (35.5) | 1 (3.2) | 1 (3.2) |
| Vomiting | 8 (25.8) | 8 (25.8) | 1 (3.2) | 1 (3.2) |
| General disorders | ||||
| Chills | 8 (25.8) | 8 (25.8) | 2 (6.5) | |
| Fatigue/lethargy | 20 (64.5) | 18 (58.1) | 0 | 0 |
| Influenza-like illness | 2 (6.5) | 4 (12.9) | 0 | 0 |
| Fever | 19 (61.3) | 18 (58.1) | 5 (16.1) | 3 (9.7) |
| Infections | ||||
| Sepsis | 1 (3.2) | 1 (3.2) | 1 (3.2) | 1 (3.2) |
| Oral candidiasis | 4 (12.9) | 4 (12.9) | 0 | 0 |
| Respiratory tract | 2 (6.5) | 0 | 0 | 0 |
| UTI | 4 (12.9) | 1 (3.2) | 0 | 0 |
| Subcutaneous abscess | 1 (3.2) | 0 | 1 (3.2) | 0 |
| Investigations | ||||
| ALT increased | 21 (67.7) | 1 (3.2) | 0 | 0 |
| AST increased | 21 (67.7) | 1 (3.2) | 0 | 0 |
| ALP increased | 14 (45.2) | 0 | 0 | 0 |
| Creatinine increased | 11 (35.5) | 0 | 0 | 0 |
| Total bilirubin increased | 9 (29.0) | 1 (3.2) | 1 (3.2) | 0 |
| Metabolic/nutrition | ||||
| Anorexia | 2 (6.5) | 1 (3.2) | 0 | 0 |
| Dehydration | 2 | 0 | 0 | 0 |
| Musculoskeletal/connective tissue | ||||
| Arthralgia | 2 (6.5) | 1 (3.2) | 0 | 0 |
| Myalgia/muscle pain | 3 (9.7) | 3 (3.2) | 2 (6.5) | 2 (6.5) |
| Neurologic disorders | ||||
| Headache | 4 (12.9) | 1 (3.2) | 0 | 0 |
| Dizziness | 3 (9.7) | 0 | 0 | 0 |
| Neuropathy | 4 (12.9) | 3 (9.7) | 0 | 0 |
| Skin disorders | ||||
| Alopecia | 20 (64.5) | 20 (64.5) | 0 | 0 |
| Erythema/rash | 15 (48.4) | 8 (25.8) | 0 | 0 |
| Vascular disorders | ||||
| Flushing | 4 (12.9) | 4 (12.9) | 0 | 0 |
| Hypertension | 4 (12.9) | 1 (3.2) | 0 | 0 |
| Hypotension | 8 (25.8) | 3 (9.7) | 11 (3.2) | 1 (3.2) |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; ANC, absolute neutrophil count; AST, aspartate transaminase; WBC, white blood cell; UTI, urinary tract infection.
The mean number of cycles administered was 4.8 (range, 1–8), but this fact must be considered in light of the fact that responding patients who reached 8 cycles of treatment had their therapy discontinued per protocol. Reovirus was given at full dose in all infusions without dose reduction. Eight patients required dose reductions of carboplatin and paclitaxel: 2 patients required a 10% dose reduction; 5 patients required a 25% dose reduction; and 1 patient required a 50% dose reduction.
Effectiveness
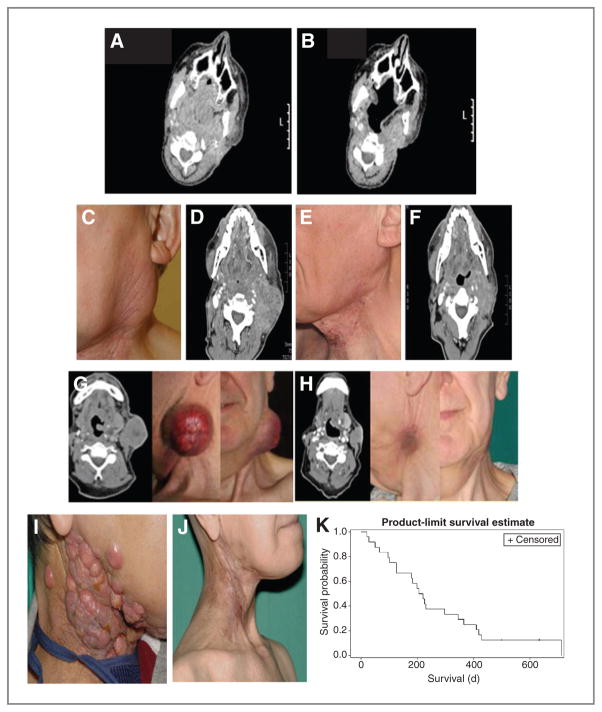
A total of 31 patients were included in the study. Twenty-six patients who received at least 2 cycles of therapy were fully evaluable for effectiveness per protocol. Of the 5 (16.1%) patients who were not fully evaluable for response, 2 died because of disease progression after receiving one cycle of treatment and three discontinued treatment after the first cycle due to a serious adverse event [sepsis (n = 1), abdominal pain and diarrhea (n =1), urinary tract infection (n = 1)]. Table 3 lists the effectiveness data by primary tumor type. One patient had a complete response (3.8%), 6 patients (23.1%) had partial response, 2 patients (7.7%) had major clinical responses evaluated in radiation pre-treated lesions which are not evaluable by RECIST, 9 patients (34.6%) had stable disease, and 8 patients (30.8%) had disease progression. Table 4 lists detailed response data for the patients with SCCHN by primary tumor, previous treatment(s), number of cycles administered, duration of response, and survival. Of note, patients with SCCHN who responded included 2 with inevaluable tumors by RECIST (due to prior treatment with radiotherapy) who had major clinical responses. These included virtually complete resolution of disfiguring lesions in 3 patients. Illustrative clinical and radiological responses from these patients are detailed in Fig. 2A–J. The median duration of objective responses (stable disease and partial response) was 6 months (range, 3–10 months). Twenty-four patients with HNC were considered in the survival observation. In an intent-to-treat analysis, median overall survival was estimated at 7.1 [confidence interval (CI), 4.2–11.5] months (mean ± SD, 8.9 + 1.4 months; Fig. 2K).
Table 3.
Overall radiological and clinical response rates in 26 evaluable patients
| Best radiological or clinical response | Primary tumor | No. of patients | Total number of evaluable patients (N = 26), n (%) |
|---|---|---|---|
| Complete | HNC (other) | 1 | 1 (3.8) |
| Partial | SCCHN | 3 | 6 (23.1) |
| HNC (other) | 3 | ||
| Major clinical response | SCCHN | 2 | 2 (7.7) |
| Stable | SCCHN | 3 | 9 (34.6) |
| HNC (other) | 3 | ||
| Gynecological cancer | 1 | ||
| Melanoma | 1 | ||
| Sarcoma | 1 | ||
| Progression | SCCHN | 2 | 8 (30.8) |
| HNC (other) | 3 | ||
| Melanoma | 2 | ||
| Gynecological cancer | 1 |
Table 4.
Details of primary tumor diagnoses, prior palliative treatments, and response data in patients with SCCHN
| Patient | Reovirus dose | Disease site | Previous treatments | Response to previous treatments | No. of cycles of trial medication | RECIST response | Clinical response |
|---|---|---|---|---|---|---|---|
| 0102 | 3 × 109 | Oropharynx | CDDP/5-FU (6 cycles) | CR | 8 | NE (assessable disease in previously irradiated region) | Major clinical response (complete resolution of cutaneous nodules) |
| 0303 | 3 × 1010 | Larynx | CDDP/Cape/Epi (1 cycle) | PD | 2 | PD | N/A |
| 2003 | 3 × 1010 | Oropharynx | CDDP/5-FU (6 cycles) | PR | 8 | PR | N/A |
| CDDP/5-FU (6 cycles) | PD | ||||||
| Zalutumumab (2 cycles) | PD | ||||||
| 2004 | 3 × 1010 | Hypopharynx | CBCDA/5-FU (6 cycles) | PD | 2 | PD | N/A |
| 2006 | 3 × 1010 | Larynx | CDDP/5-FU (4 cycles) | PR | 5 | PR | N/A |
| CDDP/5-FU (2 cycles) | PD | ||||||
| 2007 | 3 × 1010 | Hypopharynx | CDDP/5-FU (2 cycles) | PD | 1 | NE (death due to disease progression before 2 cycles) | N/A |
| 2008 | 3 × 1010 | Oral cavity | CDDP/5-FU (4 cycles) | CR | 6 | PR | N/A |
| CDDP/5-FU (4 cycles) | PR | ||||||
| CBCDA/5-FU (3 cycles) | PD | ||||||
| 2011 | 3 × 1010 | Oropharynx | CDDP/5-FU (6 cycles) | PD | 1 | NE (death due to disease progression before 2 cycles) | N/A |
| 2013 | 3 × 1010 | Oropharynx | CDDP/5-FU (3 cycles) | PD | 4 | SD | N/A |
| 2014 | 3 × 1010 | Oropharynx | CDDP/RT | PD | 1 | NE (treatment stopped because of SAE before 2 cycles) | N/A |
| 2015 | 3 × 1010 | Oral cavity | CBCDA/Cape (2 cycles) | PD | 6 | SD | N/A |
| 2016 | 3 × 1010 | Hypopharynx | CDDP/RT | PD | 6 | NE (assessable disease in previously irradiated region) | Major clinical response (complete resolution of skin nodules, Fig. 2I and J) |
| 2018 | 3 × 1010 | Oropharynx | CDDP/5-FU (6 cycles) | PR | 8 | SD | N/A |
| 2019 | 3 × 1010 | Oropharynx | CDDP (6 cycles) | CR | 1 | NE (treatment stopped because of SAE before 2 cycles) | N/A |
| Methotrexate (4 cycles) | PD |
Abbreviations: 5-FU, 5-fluorouracil; Cape, capecitabine; CBCDA, carboplatin; CDDP, cisplatin; Epi, epirubicin; N/A, not applicable; NE, nonevaluable; PD, progressive disease; PR, partial response; RT, radiotherapy; SAE, serious adverse event; SD, stable disease.
Figure 2.
A–J, Pretreatment (A) and post–cycle 3 (B) computed tomographic imaging for patient 2003. This patient had oropharyngeal cancer (tonsil) for which he had previously received chemoradiation, palliative chemotherapy with cisplatin and 5-fluorouracil, and targeted therapy with an investigational monoclonal antibody. Response was maintained through 8 cycles of treatment. C–F, pretreatment (C and D) and post–cycle 3 (E and F) photography and computed tomographic imaging for patient 2006. The patient had a supraglottic SCC which showed excellent clinical and radiological response to treatment. This patient had been previously treated with chemoradiotherapy and 2 lines of palliative chemotherapy. G and H, pretreatment (G) and post–cycle 3 (H) computed tomographic imaging and photography for patient 2008. The patient had poorly differentiated SCC of the oral cavity (tongue) and had previously received surgery, radiotherapy, and 3 lines of palliative chemotherapy. I and J, pretreatment (I) and post–cycle 3 (J) major clinical response in patient 2016 with recurrent SCC of the hypopharynx. K, Kaplan–Meier survival curve for all patients with recurrent HNCs (n = 24) expressed in days.
All 14 patients with SCCHN had received prior platinum-based chemotherapy, and in 12 of those patients, it had been used as palliative treatment for relapsed disease. In the other 2 cases, disease had progressed shortly after platin-based chemoradiotherapy. The responses of all of those patients to study medication are detailed in Table 4. Three patients (2 nasopharyngeal cancer and 1 squamous cell skin cancer) had received prior taxane-based palliative chemotherapy. As the first patient in the phase II component had a partial response, the 2-question Gehan design was satisfied, indicating the combination is likely to be effective in at least 20% of patients or more with a rejection error of 5% or 0.05.
Viral biodistribution
The presence of viral RNA in serum, urine, stool, and sputum samples was tested in all patients participating in the phase I study. All pre- and posttreatment samples were negative in 10 patients and only 3 patients had positive posttreatment signals (Supplementary Fig. S1). No patient had evidence of sustained viral excretion but one patient had positive signals in the urine at day 5 in the first 2 cycles.
NARA response
All patients in phase I had evaluable samples for NARA expressed as fold increase over pretreatment samples. All patients showed an increase in NARA titer with a range of 27- to 729-fold at cycle 1, day 15 and 27- to 2,187-fold at peak (Supplementary Fig. S2). The maximum NARA level in patients treated with carboplatin/paclitaxel and reovirus was reached later than in previous studies of single-agent reovirus. Representative examples of the neutralization curves from patients in each cohort are shown (Supplementary Fig. S3).
Paclitaxel and carboplatin pharmacokinetics
The effect of reovirus on paclitaxel and carboplatin pharmacokinetics was assessed in 8 patients (3, 3, and 2 in cohorts 1, 2, and 3, respectively). All patients had similar results (interpatient variability, 22.2%–44.5%). Total systemic clearance (CL), apparent volume of distribution (Vss), and biologic half-time were not appreciably different from parameters that would be anticipated in patients receiving chemotherapy alone (data not shown).
Discussion
We have previously shown that intravenous reovirus is safe, with the main toxicities being grade II/III flu-like illness and uncomplicated lymphopenia (8, 9). Furthermore, intravenous reovirus combined with gemcitabine was well tolerated with the expected toxicities from each drug, apart from asymptomatic, reversible grade III transaminitis and asymptomatic grade III elevation of troponin I (12). Similarly, reovirus has been combined with docetaxel in a phase I study with no toxicities other than those associated with either single agent (11). The current study is the first to add virotherapy to a combination chemotherapy regimen with a wide spectrum of activity. Treatment was well tolerated and reovirus MTD was not reached. The rate of myelosuppression (16.1%) was not greater than expected and there was only one episode of sepsis. Similarly, the occurrence of fever, flu-like illness, musculoskeletal pain, gastrointestinal disorders, fatigue, and alopecia were entirely consistent with those seen with chemotherapy alone. As with our previous studies, viral shedding was observed infrequently, suggesting that the normal rapid viral clearance from the circulation is unaffected by concomitant combination chemotherapy. These data provided the basis for outpatient treatment delivery in the phase II study. Importantly, escalating reovirus doses had no effect on the pharmacokinetics of paclitaxel or carboplatin.
As expected, NARA titers increased in all patients. However, the kinetics of NARA development were altered when compared with data for patients treated with single-agent reovirus (9, 18). Therefore, the maximum NARA titer was reached later in the treatment cycles. The role of neutralizing antiviral antibodies has been debated extensively, but there is a general perception that they may represent both an obstacle to viral delivery and a protection against virus-mediated systemic toxicity (19). Slower development of total titers may have beneficial effects on tumor seeding with virus without compromising safety. Our recent experience with reovirus combined with gemcitabine has also shown that systemic chemotherapy may be able to modulate antireoviral antibody responses in patients (12). This area is certainly worthy of further evaluation in future studies.
The phase II study was specifically designed to evaluate the antitumor activity of carboplatin/paclitaxel plus reovirus in patients with advanced or metastatic HNCs that are refractory to standard therapy or for which no curative standard therapy exists. Patients with HNC who were recruited were heavily pretreated with prior surgery, radiotherapy/chemoradiotherapy, targeted therapies and most had received at least one line of palliative chemotherapy. For 6 patients, the combination of carboplatin and paclitaxel with R3D represented third- or fourth-line treatment. All but 4 patients with HNC (83%) had received platinum-based therapy (within 6 months of study entry) and 3 patients had received both platinum and taxane therapy. A retrospective analysis of platinum-refractory SCCHN reported a 2.6% objective response rate to second-line treatment (20). Little improvement in terms of response rate was observed when this subgroup was treated with chemotherapy and cetuximab, with a response rate of 10% and a disease control rate of 53% (21). The response rate of HNCs in measurable tumors by RECIST in the evaluable patient group was 41.2% (1 complete response and 6 partial responses in 17 patients) in this study. Two of the patients who were nonevaluable by RECIST had clinically meaningful responses including virtually complete disappearance of disfiguring lesions in one patient (Fig. 2I and J), indicating an objective or clinical meaningful response in 47.4% of the patients (1 complete response, 6 partial responses, and 2 clinically meaningful responses in 19 patients). The median overall survival observed for all patients with HNC was 7.1 months and the average overall survival was 8.9 months, which is apparently higher than results of other phase II studies in the second-line setting (3.4–4.5 months; refs. 20, 22). These data provided the background for a subsequent successful application to the U.S. Food and Drug Agency (FDA) for a license under a Special Protocol Agreement to conduct a randomized, 2-arm, double-blind, multicenter 2-stage adaptive phase III study of paclitaxel and carboplatin with and without intravenous reovirus in up to 280 platinum-refractory, taxane-naive patients using survival as the primary endpoint. Study recruitment is ongoing (http://clinicaltrials.gov/ct2/show/NCT01166542).
Supplementary Material
Translational Relevance.
Reovirus type-3 Dearing (RT3D) systemic administration has shown evidence of antitumor activity with a favorable toxicity profile as monotherapy or combined with single-agent chemotherapy. We conducted a phase I trial exploring the safety and antitumor activity of reo-virus when combined with combination carboplatin/paclitaxel doublet chemotherapy in a heavily pretreated cancer population. Results of the phase I study showed tolerability and significant antitumor activity in the head and neck cancer subpopulation. Therefore, a phase II expansion study restricted to patients with advanced or metastatic head and neck cancer that was refractory to standard therapy or for which no curative treatment was available was initiated. Antiviral immune responses were attenuated compared with previous single-agent data for RT3D and viral shedding was minimal. This study proved the tolerability of adding a novel biologically targeted agent to standard combination chemotherapy, as well as yielding promising effectiveness data in head and neck cancer.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
K.J. Harrington, K. Mettinger, R.G. Vile, G.D. Hall, and A.A. Melcher have commercial research grants from Oncolytics Biotech, Inc. M. Coffey has employment in Oncolytics Biotech Inc. as the Chief Operating Officer. B. Thompson has employment in Oncolytics Biotech Inc. as the CEO. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Rosen L, Evans HE, Spickard A. Reovirus infections in human volunteers. Am J Hyg. 1963;77:29–37. doi: 10.1093/oxfordjournals.aje.a120293. [DOI] [PubMed] [Google Scholar]
- 2.Tai JH, Williams JV, Edwards KM, Wright PF, Crowe JE, Jr, Dermody TS. Prevalence of reovirus-specific antibodies in young children in Nashville, Tennessee. J Infect Dis. 2005;191:1221–4. doi: 10.1086/428911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–62. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strong JE, Lee PW. The v-erbB oncogene confers enhanced cellular susceptibility to reovirus infection. J Virol. 1996;70:612–6. doi: 10.1128/jvi.70.1.612-616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Errington F, Steele L, Prestwich R, Harrington KJ, Pandha HS, Vidal L, et al. Reovirus activates human dendritic cells to promote innate antitumor immunity. J Immunol. 2008;180:6018–26. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- 6.Prestwich RJ, Errington F, Ilett EJ, Morgan RS, Scott KJ, Kottke T, et al. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin Cancer Res. 2008;14:7358–66. doi: 10.1158/1078-0432.CCR-08-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forsyth P, Roldan G, George D, Wallace C, Palmer CA, Morris D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–32. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 8.Gollamudi R, Ghalib MH, Desai KK, Chaudhary I, Wong B, Einstein M, et al. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs. 2010;28:641–9. doi: 10.1007/s10637-009-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–37. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- 10.Harrington KJ, Karapanagiotou EM, Roulstone V, Twigger KR, White CL, Vidal L, et al. Two-stage phase I dose-escalation study of intra-tumoral reovirus type 3 dearing and palliative radiotherapy in patients with advanced cancers. Clin Cancer Res. 2010;16:3067–77. doi: 10.1158/1078-0432.CCR-10-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comins C, Spicer J, Protheroe A, Roulstone V, Twigger K, White CM, et al. REO-10: a phase I study of intravenous reovirus and docetaxel in patients with advanced cancer. Clin Cancer Res. 2010;16:5564–72. doi: 10.1158/1078-0432.CCR-10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lolkema MP, Arkenau HT, Harrington K, Roxburgh P, Morrison R, Roulstone V, et al. A phase I study of the combination of intravenous reovirus type 3 Dearing and gemcitabine in patients with advanced cancer. Clin Cancer Res. 2011;17:581–8. doi: 10.1158/1078-0432.CCR-10-2159. [DOI] [PubMed] [Google Scholar]
- 13.Pandha HS, Heinemann L, Simpson GR, Melcher A, Prestwich R, Errington F, et al. Synergistic effects of oncolytic reovirus and cisplatin chemotherapy in murine malignant melanoma. Clin Cancer Res. 2009;15:6158–66. doi: 10.1158/1078-0432.CCR-09-0796. [DOI] [PubMed] [Google Scholar]
- 14.Thirukkumaran CM, Nodwell MJ, Hirasawa K, Shi ZQ, Diaz R, Luider J, et al. Oncolytic viral therapy for prostate cancer: efficacy of reovirus as a biological therapeutic. Cancer Res. 2010;70:2435–44. doi: 10.1158/0008-5472.CAN-09-2408. [DOI] [PubMed] [Google Scholar]
- 15.Sei S, Mussio JK, Yang QE, Nagashima K, Parchment RE, Coffey MC, et al. Synergistic antitumor activity of oncolytic reovirus and chemo-therapeutic agents in non-small cell lung cancer cells. Mol Cancer. 2009;8:47. doi: 10.1186/1476-4598-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Gehan EA. The determination of the number of patients required in a preliminary and a follow-up trial of a new chemotherapeutic agent. J Chronic Dis. 1961;13:346–53. doi: 10.1016/0021-9681(61)90060-1. [DOI] [PubMed] [Google Scholar]
- 18.White CL, Twigger KR, Vidal L, De Bono JS, Coffey M, Heinemann L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. 2008;15:911–20. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- 19.Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–69. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leon X, Hitt R, Constenla M, Rocca A, Stupp R, Kovacs AF, et al. A retrospective analysis of the outcome of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck refractory to a platinum-based chemotherapy. Clin Oncol (R Coll Radiol) 2005;17:418–24. doi: 10.1016/j.clon.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Baselga J, Carbonell X, Castaneda-Soto NJ, Clemens M, Green M, Harvey V, et al. Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol. 2005;23:2162–71. doi: 10.1200/JCO.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Specenier P, Rasschaert M, Vroman P, Van den Brande J, Dyck J, Schrijvers D, et al. Weekly docetaxel in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2011;34:472–7. doi: 10.1097/COC.0b013e3181ec5f16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.