Abstract
p53 plays an essential role in the regulation of cell death in dopaminergic (DA) neurons and its activation has been implicated in the neurotoxic effects of methamphetamine (MA). However, how p53 mediates MA neurotoxicity remains largely unknown. In this study, we examined the effect of DA specific p53 gene deletion in DAT-p53KO mice. Whereas in vivo MA binge exposure reduced locomotor activity in wild type (WT) mice, this was significantly attenuated in DAT-p53KO mice, and associated with significant differences in the levels of the p53 target genes BAX and p21 between WT and DAT-p53KO. Notably, DA specific deletion of p53 provided protection of substantia nigra pars reticulata (SNpr) tyrosine hydroxylase (TH) positive fibers following binge MA, with DAT-p53KO mice having less decline of TH protein levels in striatum versus WT mice. Whereas DAT-p53KO mice demonstrated a consistently higher density of TH fibers in striatum compared to WT mice at 10 days after MA exposure, DA neuron counts within the substantia nigra pars compacta (SNpc) were similar. Finally, supportive of these results, administration of a p53 specific inhibitor (PFT-α) provided a similarly protective effect on MA binge-induced behavioral deficits. Neither DA specific p53 deletion nor p53 pharmacological inhibition affected hyperthermia induced by MA binge. These findings demonstrate a specific contribution of p53 activation in behavioral deficits and DA neuronal terminal loss by MA binge exposure.
Introduction
Methamphetamine (MA) is a pyschostimulant drug with high abuse potential. Prolonged drug exposure can lead to long-lasting damage of the dopaminergic (DA) system. Some studies have reported that MA-induced neuronal apoptosis contributes to the transition to a pathological state (Krasnova and Cadet, 2009), whereas others have in contrast have reported that MA selectively injures the neurites of DA neurons without generally inducing cell death (Ricaurte et al., 1982, Larsen et al., 2002). Immunocytochemistry analysis has revealed a marked increase in cytochrome c release from mitochondria in rat brain after MA exposure, which is correlated with caspase-9, caspase-6, and caspase-3 activation. However, DA neuronal death has been reported to be absent after MA binge (Jimenez et al., 2004). It has recently been suggested that distinct pathways mediate axonal degeneration without initiating apoptosis of the neuronal body (Cusack et al., 2013), and involve a BAX-dependent mechanism(Schoenmann et al., 2010). These findings suggest a major role of apoptotic or axonal degeneration pathways in the neurotoxic effects resulting from MA exposure. However, the precise molecular mechanisms underpinning MA neurotoxicity remain to be elucidated.
The tumor suppressor gene p53 plays an essential role in the regulation of cell death in DA neurons (Trimmer et al., 1996, Porat and Simantov, 1999, Perier et al., 2007, Qi et al., 2016). The possibility for p53 involvement in MA-induced toxicity is supported by the observations that MA caused marked increases in p53-like immunoreactivity in wild-type mice (Hirata and Cadet, 1997) and that the p53 downstream target genes, BAX and p21, were demonstrated upregulated by MA exposure (Pereira et al., 2006, Astarita et al., 2015). In contrast, traditional p53-Knockout (p53KO) mice are protected against the long-term effects of MA on DA terminals and cell bodies (Hirata and Cadet, 1997). It has also been demonstrated that MA exposure-induced cell apoptosis is attenuated by silencing PUMA (p53 upregulated modulator of apoptosis) in PC12 and SH-SY5Y cells (Chen et al., 2016). Moreover, Melatonin ameliorates MA-induced inhibition of proliferation of adult rat hippocampal progenitor cells by down-regulating the cell cycle regulators p53/p21, and decreasing the accumulation of p21 in the nucleus (Ekthuwapranee et al., 2015). Whereas these studies provide evidence for a role of p53 in the neurotoxic actions of MA, whether or not p53 mediates such MA neurotoxicity in dopaminergic neurons remains to be elucidated.
Due to widespread inhibition of p53 genes by pharmacological inhibitors and the loss of p53 function across all cell types in traditional p53 KO mice, such pharmacological inhibitor and traditional genetic studies do not address the question as to whether p53 directly regulates DA neuronal survival or regulates the microenvironment in the brain by actions on other cell types. To specifically address this, we generated DA neuron-specific p53 gene deletion mice (Qi et al., 2016) and examined the role of p53 in MA neurotoxicity. The focus of our studies was to determine the specific role of DA neuronal p53 in MA mediated neurotoxicity and to identify target genes that are differentially regulated in DA specific p53KO mice subjected to MA binge exposure.
Materials and Methods
Animals and Treatment
Animal protocols in this study (conducted under National Institutes of Health [NIH] guidelines as outlined in Animals in Research) were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University (CWRU) and mice were housed in the on-site animal facility on a 12hr light/dark diurnal cycle with food provided ad libitum. The transgenic line Slc6a3Cre provided the system for the conditional inactivation of p53 in DA neurons in which Cre recombinase is activated by the DA transporter promoter starting around embryonic day 16 (Backman et al., 2006). TRP53loxP/loxP mice (Jonkers et al., 2001) were crossed with Slc6a3Cre knock-in mice to obtain the DA neuronal specific p53 KO mice lines, DATcre(WT/+)/TRP53loxP/loxP (DAT-p53KO) and DATcre(+/−)/TRP53WT/WT (DAT-p53WT) mice as described in our recent papers (Filichia et al., 2015, Qi et al., 2016). The method for genotyping and characterizing the specific deletion of p53 in DA neurons was also described in a recent study of ours (Qi et al., 2016) and all mice strains in this study were maintained in a C57BL/6 genetic background. For the MA binge exposure (MA binge group), mice were administered binge injections (MA 10 mg/kg, x4, per 2hr, s.c.) and saline was injected as the control vehicle in both WT and KO mice.
Open Field Locomotion
MA binge exposure has been reported to lead to a decrease in open field locomotion activity in mice (Grace et al., 2010, Shen et al., 2011, Reiner et al., 2014). Therefore, we used the same methods in these past studies to evaluate motor deficits in DAT-p53KO and WT mice subjected to MA binge in the present study; evaluating locomotion function both before and 1 day after MA administration (as described previously (Luo et al., 2009, Shen et al., 2011)). Briefly, automated infra-red locomotor activity chambers were used to quantify spontaneous locomotor function. A grid of photobeams (VersaMax system by AccuScan Instruments, Columbus, OH) in an open field was used to conduct 120 min trials of locomotor function. Counts of the number of continuous photobeams broken at 5 min intervals during the trial were obtained and, from this data, total vertical (rearing) activity and total vertical (rearing) movement numbers were calculated by the VersaMax software.
Quantitative Reverse Transcription (qRT-PCR)
Brain tissue was obtained for qRT-PCR analysis 48 hours following saline or MA binge exposure. After euthanization, brains were immediately harvested from the mice and chilled on ice. A ventral midbrain tissue punch was obtained using the Paxinos Atlas and total RNA was extracted following the manufacturer’s instructions (RNAqueous, Ambion). Specifically, 1ug of total RNA was treated with RQ-1 RNase-free DNase I and was reverse transcribed into cDNA by Superscript III reverse transcriptase (Life Sciences) using random hexamers. cDNA levels for HPRT1 (hypoxanthine phosphoribosyltransferase 1), HMBS (hydroxymethylbilane synthase), and various target genes were acquired by quantitative RT-PCR using specific universal probe library primer probe sets (Roche) and a Roche Light Cycler II 480. Relative expression levels were calculated using the double delta Ct analysis method, which were compared to HMBS as a reference gene (n=8–10 for each group), as previously described (Filichia et al., 2016). Primers and FAM-labeled probes used in the qRT-PCR for each gene are listed in Table 1.
Table 1.
Primer/probe sets used in qRT-PCR.
| Primer/probe set: | ||
|---|---|---|
| Hmbs: | Forward Primer: | 5′-TCC CTG AAG GAT GTG CCT AC-3′ |
| Reverse Primer: | 5′-ACA AGG GTT TTC CCG TTT G-3′ | |
| Probe: | Universal Probe Library: Probe 79 - Roche | |
| HPRT1: | Forward Primer: | 5′-TGA TAG ATC CAT TCC TAT GAC TGT AGA-3′ |
| Reverse Primer: | 5′-AAG ACA TTC TTT CCA GTT AAA GTT GAG-3′ | |
| Probe: | Universal Probe Library: Probe 22 - Roche | |
| P53: | Forward Primer: | 5′-GCA ACT ATG GCT TCC ACC TG-3′ |
| Reverse Primer: | 5′-GTA CGT GCA CAT AAC AGA CTT GG-3′ | |
| Probe: | Universal Probe Library: Probe 4 - Roche | |
| Bcl2: | Forward Primer: | 5′-GTA CCT GAA CCG GCA TCT G-3′ |
| Reverse Primer: | 5′-GGG GCC ATA TAG TTC CAC AA-3′ | |
| Probe: | Universal Probe Library: Probe 75 - Roche | |
| BAX: | Forward Primer: | 5′-GTG AGC GGC TGC TTG TCT-3′ |
| Reverse Primer: | 5′-GGT CCC GAA GTA GGA GAG GA-3′ | |
| Probe: | Universal Probe Library: Probe 83 - Roche | |
| PUMA: | Forward Primer: | 5′-CAT GGG ACT CCT CCC CTT AC-3′ |
| Reverse Primer: | 5′-CAC CTA GTT GGG CTC CAT TT-3′ | |
| Probe: | Universal Probe Library: Probe 1 - Roche | |
| p21: | Forward Primer: | 5′-ACA TCT CAG GGC CGA AAA C-3′ |
| Reverse Primer: | 5′-GCG CTT GGA GTG ATA GAA AT-3′ | |
| Probe: | Universal Probe Library: Probe 16 - Roche | |
| Iba1: | Forward Primer: | 5′-GGA TTT GCA GGG AGG AAA A-3′ |
| Reverse Primer: | 5′-TGG GAT CAT CGA GGA ATT G-3′ | |
| Probe: | Universal Probe Library: Probe 3 - Roche | |
| GFAP: | Forward Primer: | 5′-TGG AGG AGG AGA TCC AGT TC-3′ |
| Reverse Primer: | 5′-AGC TGC TCC CGG AGT TCT-3′ | |
| Probe: | Universal Probe Library: Probe 69 - Roche |
Western Blot Analysis
Harvested tissues obtained from the striatum at 72 hour post-MA or saline exposure were lysed by sonication following their suspension in RIPA lysis buffer containing a protease inhibitor cocktail, as previously described (Filichia et al., 2016). The resulting homogenate was centrifuged at 14,000 RPM for 20 minutes in a chilled chamber, and the supernatant was removed and handled separately. Protein concentrations were determined using the Bradford absorbance assay. Equal amounts of protein were then resuspended with 5x sample buffer (with beta-mercaptoethanol), and were separated by electrophoresis on SDS-PAGE gels (Bio-Rad) and transferred onto a PVDF membrane (Bio-Rad). Thereafter, membranes were probed with primary antibodies in 1:1000 dilution (rabbit anti-tyrosine hydroxylase [Millipore AB152], rabbit anti-b-actin [Abcam ab8227]) overnight in a cold room and subsequently with HRP-labelled secondary antibodies in 1:2000 dilution (goat anti-rabbit [Jackson 111-035-003]) for 1 hour at room temperature. Protein bands were visualized using an ECL kit (Thermo Fisher) and analysis was performed using NIH ImageJ software.
Immunostaining
Animals from different treatment groups were anesthetized and transcardially perfused with saline followed by 4% paraformaldehyde (PFA) in phosphate buffer (PB, 0.1M, pH 7.2). Brains were then removed, dissected, post-fixed in PFA for 16 hours, and sequentially transferred to 20% and 30% sucrose in 0.1M PB. Serial sections of the full-length of the brain were cut at 30um or 40um thickness on a cryotome, and one series from every 4th section was stained for each antibody used. To control for variability in staining, specimens from all experimental groups were included in each staining batch and reacted together within a netted well tray under the same conditions as described previously (Luo et al., 2010, Zhou et al., 2016). Sectioned groups were rinsed in 0.1M PB and blocked with 4% bovine serum albumin (BSA) and 0.3% Trion X-100 in 0.1M PB. They were then incubated in the primary antibody (rabbit anti-TH, 1:1000, Chemicon, Temecula, CA) diluted in the previously described blocking buffer for 24 hours in a chilled room. Sections were then rinsed in 0.1M PB and incubated in either biotinylated goat anti-rabbit IgG (for TH) in buffer (1:200, Vector Laboratories, Burlingame, CA) for 1 hour, followed by a subsequent 1 hour incubation with avidin-biotin-horseradish peroxidase complex. Development of the stain was undertaken with 2,3′-diaminobenzidine tetrahydrochloride (0.5 mg/mL in 50 mM Tris-HCl buffer, pH 7.4). Control group sections were incubated without the primary antibody. Finally, all sections were mounted onto slides and cover-slipped, and histological images were then obtained using an Infinity3 camera and Olympus microscope. TH immunoreactivity within the striatum was visualized using a Nikon Super Coolscan 9000 Scanner. Analysis of the optical density of TH immunoreactivity was performed using Scion Image (v4.02) and averaged from 3 sections with a visualized anterior commissure (AP: +0.26mm, +0.14mm, +0.02mm to bregma), as described previously (Luo et al., 2010, Zhou et al., 2016). TH fiber optical density in the SNpr and TH neuronal density in SNpc were quantified by Nikon NIS-Elements software, and averaged from 3 sections (AP: 3.28 mm, 3.40 mm, 3.52 mm to bregma) as previously described (Luo et al., 2010). All immunohistochemical measurements were performed by observers blinded to the experimental groups. Minor variations in background staining were corrected by use of the background density of cortical regions from striatal density measurements.
P53 inhibitor (PFT-α) treatment during MA binge exposure
C57bl6 male mice (3 month old) received MA binge exposure (MA 10 mg/kg, 4X with 2 hour intervals, s.c.) during which time they also received either 10% DMSO or PFT-α (2mg/kg, 2X, 4 mg/10 ml in 10 % DMSO, i.p.) following the third and fourth MA injections. Tests to evaluate locomotion function were, likewise, conducted across all mice both before and 1 day after MA binge exposure (as described previously (Shen et al., 2011)). Total rearing activity and rearing movement numbers were determined by use of the Versamax software.
Hyperthermia induced by MA binge in WT and DAT-p53 KO or PFT-α treated mice
DAT p53 KO and DAT p53 WT mice were placed in acrylic plastic chambers that isolated the mice from each other to prevent huddling-associated alterations of body temperature. They were allowed to acclimate for 1 h. Core body temperature was recorded 30 min before the first injection and 1 h after each of the four subsequent injections of saline or MA. For saline or PFT-α injected mice, mice received either 10% DMSO or PFT-α 30 min after the third or fourth MA injections. Temperature was measured rectally by use of a digital thermometer (Oakton Instrument, IL, USA).
Statistics
Statistical analyses were performed using the Student’s t test and one-/two-way ANOVA (analysis of variance), with Newman-Keuls post hoc tests (as appropriate). P values of equal to or less than 0.05 are considered of significance.
Results
To achieve DA specific p53 deletion we used the Slc6a3Cre line to generate DAT-p53KO mice and, thereby, provide DA neuron-specific cre expression with minimal interference of endogenous DAT gene expression (Backman et al., 2006). Our previous studies have demonstrated DA specific deletion of the p53 gene in this mouse line. They have additionally shown that p53 ablation within the DA system does not alter either the DA system or locomotor activity under normal (control) conditions (Qi et al., 2016). This model is thus ideally suited to evaluate the role of the p53 gene under neurotoxic challenge or stress conditions.
Deletion of the p53 gene results in attenuated motor deficits in DAT-p53KO mice after MA binge exposure
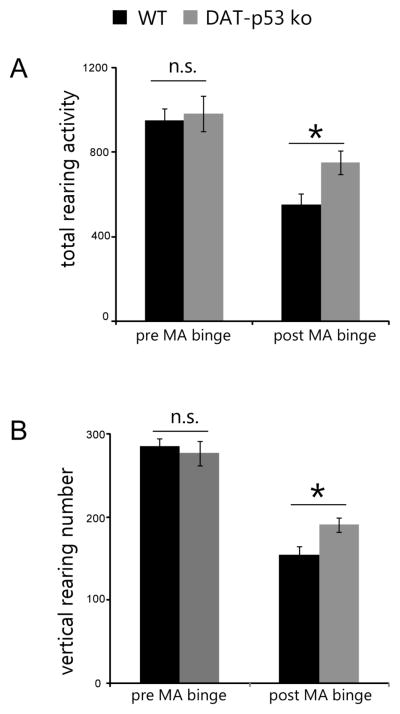
As the measured baseline nigrostriatal parameters were similar between the DAT-p53KO and -WT mice (Qi et al., 2016), we evaluated the response of these mice to MA binge exposure (4X 10 mg/kg s.c. with 2 hour intervals). We and others have previously shown that MA binge exposure results in decreased locomotion activity both in mice and rats, when evaluated 24 hours after the administration of MA (Grace et al., 2010, Shen et al., 2011, Reiner et al., 2014). In the present study, 36 mice (DA-p53KO=17, -WT=19) were subjected to locomotion testing before MA exposure to establish a baseline (Pre MA). At 24 hours after the initial injection of MA binge exposure, animals were placed in the activity chambers and rearing activity over a 30 min period was measured in automatic locomotion chambers. Two variables (genotypes and treatment with MA: pre or post MA binge) were used to analyze changes in behaviors using a two way ANOVA. For total rearing activity, there was a trend of difference of genotype effect (p=0.064, F1,69=3.560), treatment effect (Pre vs after MA, p<0.001, F1,69=27.611) and no genotype and treatment interaction (p=0.167, F1,69=1.953). For rearing numbers, overall effects of treatment and genotype × treatment were observed (treatment, p<0.001, F1,69=103.142 and genotype × treatment, p=0.041, F1,69=4.350). For post hoc analysis, no significant difference was found between DA-p53KO and -WT mice before MA exposure in either total rearing activity (Fig. 1, p=0.734) or rearing numbers (Fig. 1, p=0.585). These behavioral parameters were significantly reduced by MA binge exposure (Fig. 1, total rearing activity: p<0.001, F1,69=27.611; rearing number: p<0.001, F1,69=103.142), consistent with previous studies. Notably, there was a significantly lower decrease for DA-p53KO mice in both parameters, as compared to -WT mice post MA binge exposure (Fig. 1, p=0.022 DAT-p53KO vs. -WT in the MA challenged groups, for total rearing activity and p=0.017 for rearing numbers, post-hoc Student-Newman-Keuls test). In synopsis, these data indicate that repeated high exposure to MA reduces rearing activity in WT mice, and that DAT-p53KO mice are partially protected.
Figure 1.
p53 gene deletion in dopaminergic neurons results in attenuated behavioral deficits in rearing activities induced by MA binge exposure. (A) total rearing activities (sum of time in rearing positions) and (B) total rearing numbers are both decreased after MA binge exposure, but DA-p53KO mice showed less decrease compared to -WT mice. Data are mean ± SE. *P<0.05 for WT vs DA-p53KO after MA, Two way ANOVA. n=17–19 per group.
Dopaminergic neuronal-specific deletion of p53 suppresses upregulation of BAX, p21, and GFAP gene transcription in substantia nigra (SN) after MA binge exposure
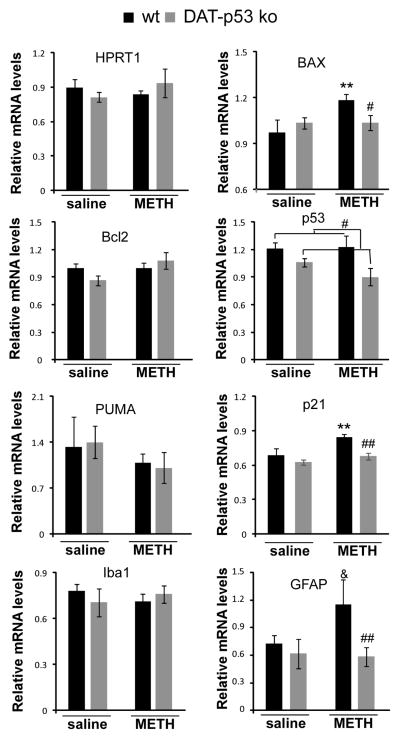
We next examined changes in gene expression in the nigrostriatal system after saline or MA binge exposure to evaluate the effects of DA-specific p53 deletion. SN tissue was obtained 48 hours after saline or MA administration in both DAT-p53KO (n=8 for saline and n=10 for MA) and WT (n=8 for saline and n=10 for MA) mice, and gene expression was analyzed by qRT-PCR. mRNA levels for appraised genes were normalized to the housekeeping gene Hmbs (Fig 2). To validate our methods, we evaluated the expression of a second housekeeping gene, HPRT1, which, in line with our expectations, was unchanged between the animal groups. Two variables (genotypes and treatment with MA: pre or post MA binge) were used to analyze changes in gene expression using a two way ANOVA analysis. For p53 gene expression, there was a significant main effect for genotype (p=0.009, F1,34=7.893) but no effect for treatment or genotype x treatment, possibly due to the acute and transient p53 upregulation after MA binge that occurs before 48 hours (Asanuma et al., 2002). For post hoc analysis, there was no significant difference between WT and KO in saline treated mice (p=0.109). However, p53 transcripts were significantly lower in the SN of DAT-p53KO mice vs. WT mice after MA binge exposure (Fig 2, p=0.023, post hoc Student-Newman-Keuls test). MA binge exposure induced BAX upregulation (effect of treatment: p=0.048, F1,34=4.452; effect of treatment x genotype: p=0.05, F1,34=4.017). WT mice had significantly upregulated BAX gene expression after MA exposure (p=0.008, post hoc analysis) and DA-p53KO mice had significantly lower BAX gene expression compared to WT mice (p=0.038, post-hoc analysis) that was not different from DA-p53KO receiving saline injections. Similarly, p21 and GFAP gene expressions also showed a significant effect of genotype (for p21, p=0.008, F1,34=8.282; GFAP, p=0.025, F1,34=4.028). MA binge exposure led to a significant upregulation of p21 (p=0.006, post hoc analysis) and a trend for GFAP gene upregulation in WT mice (p=0.08, post hoc analysis). DA-p53KO mice demonstrated a significantly lower p21 (p=0.005, post hoc analysis) and GFAP gene (p=0.006, post hoc analysis) expression compared to MA binged WT mice, which was not different from DA-p53KO that received saline injections. There were no significant differences among the four groups of animals in the expression of Bcl2, PUMA or Iba1 gene expression levels (Fig 2). Together, this suggests that BAX and p21 are involved in the p53-mediated neurotoxicity that is instigated by MA binge exposure in vivo, which are abolished in DA-p53KO mice.
Fig 2.
Dopaminergic neuronal-specific p53 deletion results in differential gene transcriptional regulation in SN after MA binge exposure. Brain tissues were obtained 48 hours after MA binge challenge. qPCR analysis was conducted to determine the expression level of HPRT, p53, Bcl2, BAX, PUMA, p21, Iba1 and GFAP in DAT-p53KO and -WT mice. At this 48 hour time point, MA binge exposure induced upregulation of BAX, p21 and GFAP genes and these upregulations were attenuated in DA-p53KO mice. Data are shown as mean ± SEM. * and ** indicates p<0.05 or p<0.01, & indicates p=0.08 in MA binge exposed WT mice compared to saline injected mice. #, or ## indicates p<0.05 or p<0.01 in MA injected DAT-p53KO vs. -WT mice, n=8–10 for each group.
Dopaminergic neuronal-specific deletion of p53 results in protected neuronal terminals after MA binge exposure
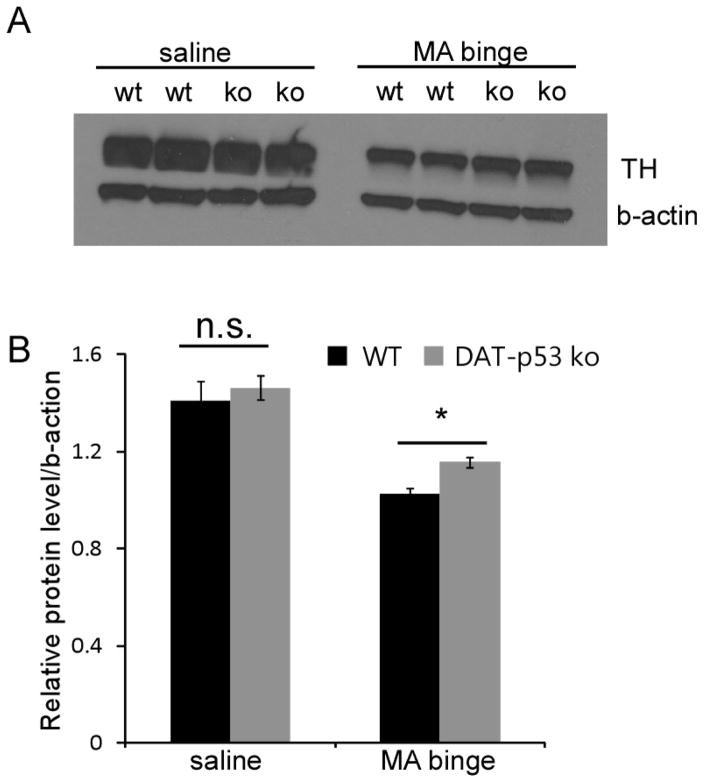
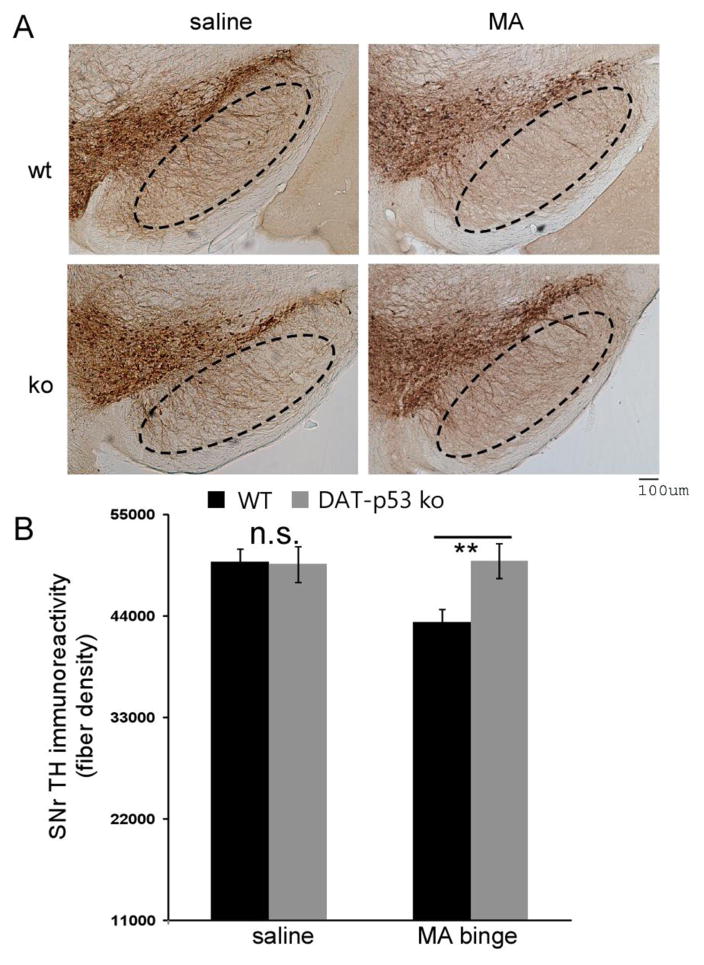
We next examined the effect of MA binge exposure on the protein expression TH both in the striatum and the substantia nigra (SN). Previous studies from our group and others have shown that MA binge exposure does not lead to a decrease in the number of TH-positive neurons within the SNpc (Ricaurte et al., 1982, Larsen et al., 2002, Boger et al., 2007, Luo et al., 2010), whereas decreased TH protein levels have been consistently reported within the striatum (Raineri et al., 2012, Reiner et al., 2014). Therefore, we first examined TH protein levels in the striatum of both saline and MA binged exposed DAT-p53KO and WT mice at 72 hours after administration (n=4 for saline/WT or saline/KO and n=6 for MA/WT or MA/KO per group). Evaluation by Western blot analysis demonstrated that MA binge exposure significantly decreased TH levels in striatum in both WT and DAT-p53KO mice (Fig. 3, saline vs. MA, p<0.001, F(1,19) = 65.552, Two-way ANOVA). There was also an effect of genotype (p=0.043, F1,19= 4.856, Two-way ANOVA) but not treatment x genotype interaction (p=0.370). Notably, DAT-p53KO mice showed less of a decline in TH protein levels in striatum compared to WT mice (Fig. 3, p=0.025, post-hoc Student-Newman-Keuls Analysis, ANOVA, n=6 each groups). In light of evidence demonstrating that changes in TH fiber density in the SNpr reflect neuronal terminal damage in MA challenged mice in the absence of loss of DA neuronal numbers (Luo et al., 2010), we therefore next analyzed for differences in SNpr TH fiber density between DAT-p53KO and -WT mice after MA binge exposure. There was no effect of genotype (p=0.222, F1,66= 1.22) or treatment alone (p=0.247, F1,66= 1.367) but a significant effect of genotype x treatment was evident (p=0.01, F1,66= 7.104). In addition, consistent with previous results, we found that MA binge exposure significantly reduced the density of TH fibers within the SNpr in WT mice (Fig. 4, p<0.01, saline vs MA in WT mice, post hoc Newman-Keuls test, n=8 for saline and n=10 for MA). Of note, DA-specific deletion of p53 provided protection of TH-positive fibers within SNpr following MA binge treatment. (Fig. 4, MA binge groups, DAT-p53KO vs. -WT p=0.003, post hoc Newman-Keuls test, n=10; saline vs. MA in DAT-p53KO groups, p=0.320, n=8 for saline and n=10 for MA). In summary, MA exposure significantly decreased (i) TH protein levels in striatum, (ii) TH-immunoreactivity in SNpr, and (iii) DA p53 deletion rendered partial to complete protection of the axonal and dendritic terminals of the DA system.
Fig 3.
p53 gene deletion in dopaminergic neurons results in moderate protection of TH protein levels in striatum after MA binge exposure in DAT-p53KO mice. Brain tissue (striatum) was collected at 72 hours after MA binge challenge. Protein levels of TH in striatum (A) were examined by Western blot analysis. Actin was used as a loading control. (B) Quantification of data in (A) shows that MA binge exposure leads to a decrease in TH protein levels in striatum in WT animals, and this decrease in TH protein level is attenuated in DA-p53KO mice. Data are mean ± SEM. * p<0.05 DAT-p53KO vs. -WT mice after MA binge exposure. The data was analyzed by two-way ANOVA, with Newman–Keuls post hoc tests. n=4–6 for each group.
Figure 4.
p53 gene deletion in dopaminergic neurons results in protection against a decline in TH-immunoreactivity in SNpr following MA binge exposure, as evaluated at 72 hours. (A) TH immunostaining indicates that MA exposure led to a reduction in TH-immunoreactivity within the SNpr in WT mice but not DAT-p53KO. Calibration bar =100 um. (B) TH-immunoreactivity fiber density analysis indicated MA exposure reduced TH-immunoreactive fiber density within the SNpr in WT but not DAT-p53KO mice following MA binge exposure. (MA binge exposure groups, DAT-p53KO vs. -WT **, p=0.003), n=8 for saline groups and n=10 for MA exposed groups.
Protection of DA terminals is sustained for up to 10 days after MA binge exposure
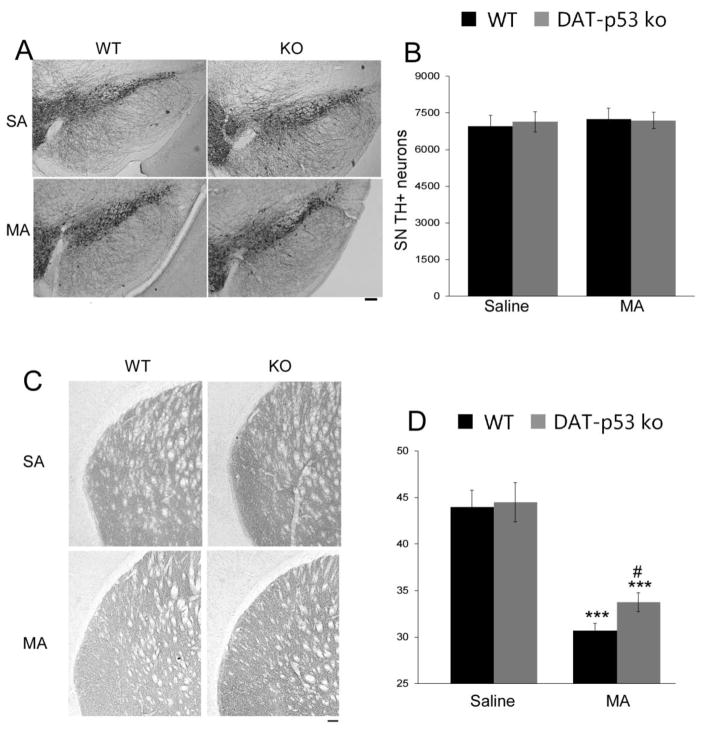
Repeated MA binge exposure during 8 hours could have a sustained subacute or chronic effect on the DA system. To evaluate this, we examined the survival of DA neurons at 10 days following MA binge exposure using unbiased stereological quantification. Consistent with previous reports (Ricaurte et al., 1982, Boger et al., 2007), we did not observe a loss of DA neurons within the SN after MA binge exposure, nor a difference between WT and DA-p53KO mice (Treatment: p=0.685, F1, 24=0.685), nor an effect of genotype (p=0.886, F1, 24 =0.021) or interaction of treatment x genotype (p=0.782, F1, 24=0.0789). Of note, however, we observed a consistently lower density of TH fibers within the striatum at 10 days after MA binge (Effect of treatment, p<0.001, F1, 43 =79.833) with no effect of genotype or genotype x treatment. Post hoc analysis demonstrates higher density of TH fibers within the striatum in DAT-p53KO as compared to -WT mice at 10 days after MA binge exposure (Fig. 5, p<0.05, post hoc analysis); thereby confirming the protection that we observed at an earlier time (72 hours) in DAT-p53KO mice using a different analysis, and demonstrating that this was sustained to at least 10 days.
Figure 5.
Dopaminergic specific p53 deletion sustained the protection of DA terminals within the striatum, as evaluated at 10 days following MA binge exposure. (A) No loss of TH positive neurons was evident within the SN of MA injected WT or DA-p53KO mice, (B) which was confirmed by unbiased stereological counts. (C) MA binge led to a decrease in striatal TH immunoreactivity density in both WT and DA-p53KO mice when evaluated 10 days after MA binge. Notable, however, DA-p53KO mice continue to demonstrate a higher density of TH-immunoreactivity within the striatum vs. -WT mice at 10 days after MA binge exposure (D). *** indicates p<0.001 compared to saline injected mice and # indicates p<0.05 WT vs KO mice=7–8 for each group). (Calibration bar =100 um).
Treatment with a small molecule p53 inhibitor rescued the behavioral deficits induced by MA binge exposure in mice
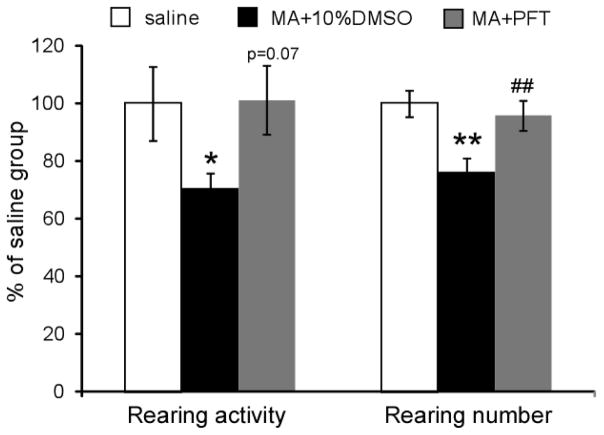
As DAT-p53KO mice demonstrated protection against MA-induced rearing behaviors, we next evaluated whether administration of a p53 specific inhibitor (PFT-α) had any effect on MA binge-induced behavioral deficits. Vehicle (10% DMSO) treated mice challenged with MA binge exposure exhibited a decrease in rearing activity (70.7± 5.3% of the pre-MA exposure score, p<0.05, one way ANOVA, n=8–9) and rearing numbers (76.2± 4.7% of the pre-MA exposure score, p<0.01, one way ANOVA, n=8–9). In contrast, PFT-α treated animals demonstrated a higher rearing activity (101.2 ± 12.1% of their pre-MA exposure score) and rearing numbers (95.8± 5.3% of their pre-MA exposure score); indicating that pharmacological inhibition of p53 function by PFT-α protected mice from MA binge-induced rearing activity deficits (p=0.07 for total rearing activity and p=0.005 for rearing number comparing MA±10% DMSO to MA+PFT-α treated mice, one way ANOVA, n=8–9, Fig. 6).
Figure 6.
Treatment with the p53 inhibitor PFT-α rescued the behavioral deficits evident in MA binge exposed mice. Vehicle (10% DMSO) treated mice that received MA binge exposure demonstrated a reduction in both rearing activity and rearing number (*p<0.05 or ** p<0.0, ANOVA, n=8–9). PFT-α treated animals exhibited higher rearing activity (p=0.07 vs. MA +10%DMSO) and rearing number (##, p=0.005 vs. MA +10% DMSO. One way ANOVA, n=8–9 for each group).
DA specific p53 deletion or PFT-α treatment did not alter hyperthermia induced by MA
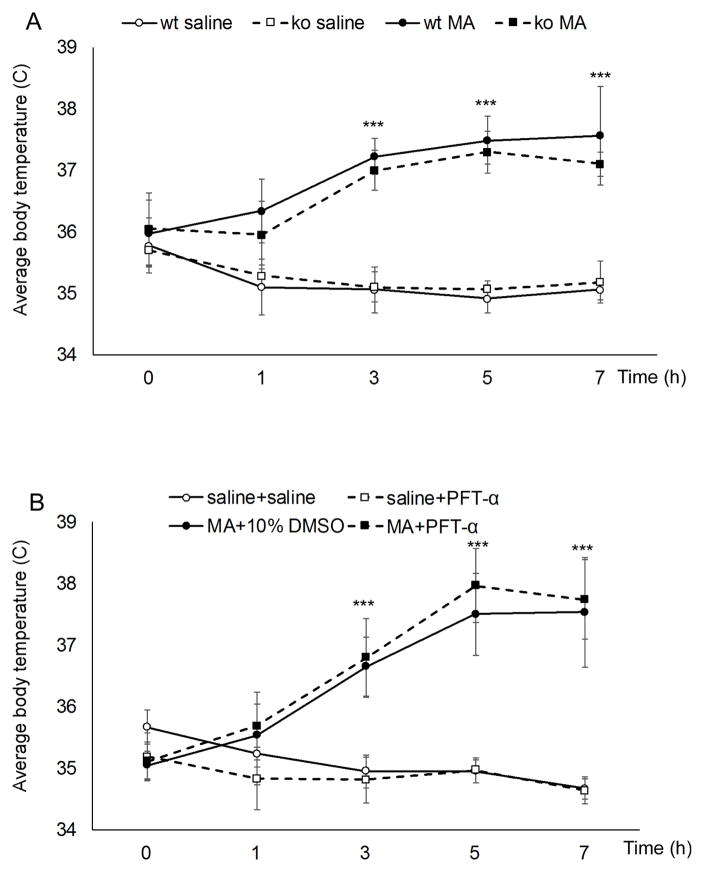
We next examined whether protective effects of DA specific p53 gene deletion or PFT-α treatment are due to alterations in hyperthermia induced by MA binge, which is known to be necessary for MA-induced neurotoxicity (Miller and O’Callaghan, 1994). Core body temperature was measured before and at different time intervals after each MA injection (Fig. 7). Two-way ANOVA (Genotype and MA or saline injection) at each timepoint within data sets showed that MA binge exposure produced a significant hyperthermic response in both the WT and DA-p53KO mice (Effect: MA, p<0.001,, F1,27=281.312 for second injection, p<0.001, F1,27=446.410 for 3rd injection treatments and p<0.001, F1,27=146.299 for last injection). However, there was no effect of genotype or genotype x MA treatment at any of the time points, suggesting that DA specific p53 knockout does not affect MA induced hyperthermia. Post hoc tests confirmed that MA binge induced hyperthermia in both WT and DA-p53KO mice at all of the post injection times (p<0.05) and that there was no difference between WT and DA-p53KO mice at any of these times (p>0.05). To determine whether PFT-α treatment could influence MA induced hyperthermia, PFT-α was administered at 30 min after the third and fourth MA injection, and body temperature was measured at 1hour after each of these MA injections. Two way ANOVA analysis for treatment and time showed that treatments and time demonstrated a significant change [F(treatment) (3,105) =142.307, p<0.001, F(time) (3,105) =19.083, p<0.001], and that there was an interaction between the two factors [F(treatment × time) (9,105) = 9.694, p<0.001]. Post hoc tests showed that A) MA-treated mice demonstrated an hyperthermic response, which was significantly different from the saline group after the 2nd, 3rd and 4th MA injections (p<0.001), B) PFT-α administration alone had no effect on body temperature at any time (p>0.05), and C) PFT-α co-administration with MA (MA+PFT-α group) did not significantly alter MA-induced hyperthermia at the 3rd MA injection (p>0.05) or 4th MA injection (p>0.05).
Figure 7.
DA specific p53 deletion or PFT-α treatment did not alter hyperthermia induced by MA. (A) Repeated MA injection led to hyperthermia in both WT and DA-p53KO mice and there was no significance between the temperature in these WT and KO mice. (B) treatment with PFT-α at 30 min after the third and fourth MA injections did not significantly alter MA-induced hyperthermia. Data is presented as mean core temperature (°C) at the indicated times ± SD. Repeated measures two-way ANOVA followed by Bonferroni post hoc analysis, ***p<0.001 MA vs. saline (n=7–8 for each group).
In summary, taken as a whole our data demonstrates the occurrence of significant DA neuronal terminal loss in a repeated MA binge paradigm, which was partially protected by DA-specific p53 deletion. Behavioral benefits in vulnerability to MA binge exposure was also observed in DAT-p53KO as well as p53 inhibitor-treated mice, which are potentially mediated by p53 downstream genes that include BAX and p21.
Discussion
The p53 gene has been demonstrated to be essential for the natural neuronal apoptotic processes that occur within the central nervous system. For example, when p53 levels are reduced or absent in heterozygous and homozygous p53 KO mice, naturally occurring sympathetic neuronal death is inhibited (Aloyz et al., 1998). Although useful in evaluating the function of p53 under physiological and pathological conditions, such mouse models are potentially disadvantaged by abnormalities for example incomplete neural tube closure and other defects that have been reported to occur in embryos from p53 KO mice and result in their premature death in utero (Armstrong et al., 1995). In Adult p53 KO mice, p53 gene deletion has been described to lead to learning deficits and behavioral alterations (Amson et al., 2000). Hence, traditional knockout of p53 can introduce confounding factors into the interpretation of the results of studies elucidating mechanisms, such as tumor formation and altered general metabolisms. Therefore, to precisely evaluate p53 function in different neural systems and to evaluate p53’s role under different toxicological insults, it is critical to utilize cell type-specific p53 conditional knockout that we have recently generated and characterized (Filichia et al., 2015, Qi et al., 2016). In our DAT-p53KO mice, the p53 gene is deleted in DA neurons during the late developmental stage (starting from E16) and DA-specific p53 deletion does not affect the general structure of the nigrostrial dopamine system or the general behavior of these mice, making them an ideal model to evaluate the role of p53 in DA neuronal survival and function under various pathological conditions as well as neurotoxic challenges (Qi et al., 2016). Our recent study using this mouse model has demonstrated that the p53 gene is critical in the death of DA neurons that occurs in the MPTP mouse model of Parkinson’s disease, but whether or not MA binge exposure induces apoptosis of DA neurons and the role that p53 plays in this process has not been examined in DA-specific p53 KO mice.
It is well established that MA can induce dopamine release and cause neurotoxicity of DA neurons both in vitro and in vivo. Whereas terminal damage in both the striatum and the SNr has been reported in many previous studies (Luo et al., 2010, Reiner et al., 2014, Ekthuwapranee et al., 2015), whether or not MA induces DA neuronal apoptosis or neuronal loss remains controversial. Studies have reported transient decreases of TH expression in both the striatum and SN. But this may be followed by a spontaneous recovery that then results in an apparent lack of dopaminergic neuronal loss within the SN in rodents (Ricaurte et al., 1982, Krasnova and Cadet, 2009, Luo et al., 2010). As the p53 gene is a master regulator of apoptosis and neuronal terminal damage, we therefore examined whether p53 affects the neurotoxicity of MA and whether regulation of apoptosis or neuronal terminal damage through p53 is involved in MA neurotoxicity in dopaminergic neurons. Our data shows that dopaminergic-specific p53 gene inactivation protects the dopaminergic system in mice subjected to binge MA exposure. In WT mice, MA binge exposure resulted in a significant decline in rearing activity and rearing numbers, as assessed at 24 hours. In contrast, DAT-p53KO mice showed attenuated deficits in rearing activities when compared to WT mice. In accord with this behavioral data, both TH protein levels in the striatum and TH-positive dentritic processes in the SNr of WT mice were reduced by MA binge exposure, whereas DAT-p53KO mice showed alleviated terminal damage in both the striatum and SNr that was maintained for up to 10 days after MA binge exposure. Notably, although p53-related pathway genes were upregulated by MA binge exposure, we did not observe loss of TH-positive neurons at 10 days following MA binge, consistent with previous studies (Ricaurte et al., 1982, Krasnova and Cadet, 2009, Luo et al., 2010). Specifically, our data shows that MA binge exposure in WT mice upregulated the p53 gene and select p53 downstream targets (BAX and p21). However, the lack of a TH-positive neuron loss at later stages suggests that rather than mediating neuronal cell death, p53 may instead have a role in regulating the neuronal terminal damage evident in MA-induced toxicity. In support of this, studies by Gliman et al., (Gilman et al., 2003) and Merlo et al. (Merlo et al., 2014) have demonstrated that p53 is present in synaptic terminals, has the ability to regulate synaptosome survival, and plays a role in synaptic plasticity and function. Additionally, our data shows that unlike MPTP-induced DA neuronal death, in which the PUMA gene plays an essential role (as evident in our recent study (Qi et al., 2016)), MA binge exposure induced the upregulation of BAX but not the PUMA gene. This suggests that, as recently described, p53 and BAX are involved in mediating either neuronal terminal degeneration or cell body apoptosis (Cusack et al., 2013) that is selectively regulated through distinct pathways. This can be considered essential to support the extensive neuronal apoptosis and axon pruning that are each separately required when establishing specific neuronal circuits during development, as well as to the selective pruning of axons that is continuously necessary to permit plasticity in the adult nervous system (Dorszewska, 2013, Merlo et al., 2014). Notably, our data shows that DAT-p53KO mice demonstrate a reduced up regulation of distinct sets of p53 target genes (BAX and p21), in line with a role of p53 in axon-specific terminal degeneration.
Alterations in p53 mRNA and protein expression have been associated with neuronal damage in a variety of in vivo and in vitro model systems that include ischemia, Parkinson’s disease and TBI (Greig et al., 2004, Raghupathi, 2004, Gudkov and Komarova, 2010, Checler and Alves da Costa, 2014). The role of p53 in the pathophysiology of central nervous system injuries remains complex and warrants further elucidation, particularly in light of a non-apoptotic role for p53 in the promotion of neuronal and axonal regeneration after cellular insults (Tedeschi and Di Giovanni, 2009). A number of these different effects likely relate to multiple factors that include the cell types involved, their microenvironment and both the form and severity of injury incurred, in addition to when p53 activity and its involved signaling pathways are evaluated (Tedeschi and Di Giovanni, 2009, Dorszewska, 2013, Jebelli et al., 2014, Merlo et al., 2014, Wang et al., 2014). In relation to MA neurotoxicity, p53 is implicated based on the finding of attenuated MA neurotoxicity in traditional p53 KO mice (Hirata and Cadet, 1997). MA binge exposure has been reported to induce the expression of multiple genes related to cell death or neuronal terminal damage (Jayanthi et al., 2001, Jayanthi et al., 2005), including p53 (Asanuma et al., 2002). In a previous study, MA binge exposure increased p53-DNA binding activity in the nigrostriatal system, which was markedly attenuated in Cu, Zn-superoxide dismutase transgenic mice but not affected by treatment with N-methyl-D-aspartate or D1 receptor antagonists (Asanuma et al., 2002). These results suggest that oxidative stress might be involved in p53 activation following MA binge exposure; but whether or not this is related to dopamine D1 receptor dependent endoplasmic reticulum stress mediated gene expression regulation warrants further investigation (Jayanthi et al., 2009). Recently, the BAX gene has been reported to be involved in axonal protection (Schoenmann et al., 2010) and the p21 gene described upregulated by MA exposure and involved in cellular senescence induced by MA(Astarita et al., 2015). Hence, our results indicating that both BAX and p21 gene expression are diminished in our DAT-p53KO mice following MA binge exposure supports the hypothesis that these genes are key regulators of MA-induced neurotoxicity through the p53 pathway. Validating our DAT-p53KO studies, treatment with a small chemical p53 inhibitor (PFT-α), known to be effective in neuronal cells in culture and in vivo studies (Zhu et al., 2002), attenuated the behavioral deficits induced by MA binge exposure. It is known that p53 can induce its biological response through the transcriptional transactivation of specific target genes (Greig et al., 2004, Gudkov and Komarova, 2010, Wang et al., 2014). PFT-α is reported to affect both the transcriptional and mitochondrial activity of p53 (Culmsee et al., 2001, Endo et al., 2006), which makes it difficult to distinguish between the two distinct mechanisms when utilizing this agent. In contrast, treatment with the small molecular weight compound PFT-μ can completely inhibit such mitochondrial p53-dependent apoptosis without impacting p53 transcriptional activity (Strom et al., 2006, Gudkov and Komarova, 2010). Models that could permit the separation of these factors to allow the characterization of p53 actions in neurons are thus important. In future studies, it would be interesting to compare the efficacy of specific inhibitors (such as PFT-α or PFT-μ to differentiate transcriptional and mitochondrial mechanisms underpinning p53 action) both in characterizing mechanisms that underlie MA-induced neurotoxicity and developing strategies to protect against it.
Acknowledgments
This research was supported in part by a grant from (i) The National Institutes of Health NINDS grant RO1NS094152, and (ii) the Intramural Research Program of the National Institute on Aging, NIH
References
- Aloyz RS, Bamji SX, Pozniak CD, Toma JG, Atwal J, Kaplan DR, Miller FD. p53 is essential for developmental neuron death as regulated by the TrkA and p75 neurotrophin receptors. The Journal of cell biology. 1998;143:1691–1703. doi: 10.1083/jcb.143.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amson R, Lassalle JM, Halley H, Prieur S, Lethrosne F, Roperch JP, Israeli D, Gendron MC, Duyckaerts C, Checler F, Dausset J, Cohen D, Oren M, Telerman A. Behavioral alterations associated with apoptosis and down-regulation of presenilin 1 in the brains of p53-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5346–5350. doi: 10.1073/pnas.97.10.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JF, Kaufman MH, Harrison DJ, Clarke AR. High-frequency developmental abnormalities in p53-deficient mice. Current biology: CB. 1995;5:931–936. doi: 10.1016/s0960-9822(95)00183-7. [DOI] [PubMed] [Google Scholar]
- Asanuma M, Miyazaki I, Higashi Y, Cadet JL, Ogawa N. Methamphetamine-induced increase in striatal p53 DNA-binding activity is attenuated in Cu, Zn-superoxide dismutase transgenic mice. Neuroscience letters. 2002;325:191–194. doi: 10.1016/s0304-3940(02)00291-4. [DOI] [PubMed] [Google Scholar]
- Astarita G, Avanesian A, Grimaldi B, Realini N, Justinova Z, Panlilio LV, Basit A, Goldberg SR, Piomelli D. Methamphetamine accelerates cellular senescence through stimulation of de novo ceramide biosynthesis. PloS one. 2015;10:e0116961. doi: 10.1371/journal.pone.0116961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, Tomac AC. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Patrick KS, Ramamoorthy S, Denehy ED, Zhu H, Pacchioni AM, Granholm AC, McGinty JF. Long-term consequences of methamphetamine exposure in young adults are exacerbated in glial cell line-derived neurotrophic factor heterozygous mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:8816–8825. doi: 10.1523/JNEUROSCI.1067-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checler F, Alves da Costa C. p53 in neurodegenerative diseases and brain cancers. Pharmacology & therapeutics. 2014;142:99–113. doi: 10.1016/j.pharmthera.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Chen C, Qincao L, Xu J, Du S, Huang E, Liu C, Lin Z, Xie WB, Wang H. Role of PUMA in methamphetamine-induced neuronal apoptosis. Toxicology letters. 2016;240:149–160. doi: 10.1016/j.toxlet.2015.10.020. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Zhu X, Yu QS, Chan SL, Camandola S, Guo Z, Greig NH, Mattson MP. A synthetic inhibitor of p53 protects neurons against death induced by ischemic and excitotoxic insults, and amyloid beta-peptide. Journal of neurochemistry. 2001;77:220–228. doi: 10.1046/j.1471-4159.2001.t01-1-00220.x. [DOI] [PubMed] [Google Scholar]
- Cusack CL, Swahari V, Hampton Henley W, Michael Ramsey J, Deshmukh M. Distinct pathways mediate axon degeneration during apoptosis and axon-specific pruning. Nature communications. 2013;4:1876. doi: 10.1038/ncomms2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorszewska J. Cell biology of normal brain aging: synaptic plasticity-cell death. Aging clinical and experimental research. 2013;25:25–34. doi: 10.1007/s40520-013-0004-2. [DOI] [PubMed] [Google Scholar]
- Ekthuwapranee K, Sotthibundhu A, Govitrapong P. Melatonin attenuates methamphetamine-induced inhibition of proliferation of adult rat hippocampal progenitor cells in vitro. Journal of pineal research. 2015;58:418–428. doi: 10.1111/jpi.12225. [DOI] [PubMed] [Google Scholar]
- Endo H, Saito A, Chan PH. Mitochondrial translocation of p53 underlies the selective death of hippocampal CA1 neurons after global cerebral ischaemia. Biochemical Society transactions. 2006;34:1283–1286. doi: 10.1042/BST0341283. [DOI] [PubMed] [Google Scholar]
- Filichia E, Hoffer B, Qi X, Luo Y. Inhibition of Drp1 mitochondrial translocation provides neural protection in dopaminergic system in a Parkinson’s disease model induced by MPTP. Scientific reports. 2016;6:32656. doi: 10.1038/srep32656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filichia E, Shen H, Zhou X, Qi X, Jin K, Greig N, Hoffer B, Luo Y. Forebrain neuronal specific ablation of p53 gene provides protection in a cortical ischemic stroke model. Neuroscience. 2015;295:1–10. doi: 10.1016/j.neuroscience.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman CP, Chan SL, Guo Z, Zhu X, Greig N, Mattson MP. p53 is present in synapses where it mediates mitochondrial dysfunction and synaptic degeneration in response to DNA damage, and oxidative and excitotoxic insults. Neuromolecular medicine. 2003;3:159–172. doi: 10.1385/NMM:3:3:159. [DOI] [PubMed] [Google Scholar]
- Grace CE, Schaefer TL, Herring NR, Graham DL, Skelton MR, Gudelsky GA, Williams MT, Vorhees CV. Effect of a neurotoxic dose regimen of (+)-methamphetamine on behavior, plasma corticosterone, and brain monoamines in adult C57BL/6 mice. Neurotoxicology and teratology. 2010;32:346–355. doi: 10.1016/j.ntt.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig NH, Mattson MP, Perry T, Chan SL, Giordano T, Sambamurti K, Rogers JT, Ovadia H, Lahiri DK. New therapeutic strategies and drug candidates for neurodegenerative diseases: p53 and TNF-alpha inhibitors, and GLP-1 receptor agonists. Annals of the New York Academy of Sciences. 2004;1035:290–315. doi: 10.1196/annals.1332.018. [DOI] [PubMed] [Google Scholar]
- Gudkov AV, Komarova EA. Pathologies associated with the p53 response. Cold Spring Harbor perspectives in biology. 2010;2:a001180. doi: 10.1101/cshperspect.a001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Cadet JL. p53-knockout mice are protected against the long-term effects of methamphetamine on dopaminergic terminals and cell bodies. Journal of neurochemistry. 1997;69:780–790. doi: 10.1046/j.1471-4159.1997.69020780.x. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Bordelon M, McCoy MT, Cadet JL. Methamphetamine causes differential regulation of pro-death and anti-death Bcl-2 genes in the mouse neocortex. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2001;15:1745–1752. doi: 10.1096/fj.01-0025com. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:868–873. doi: 10.1073/pnas.0404990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, McCoy MT, Beauvais G, Ladenheim B, Gilmore K, Wood W, 3rd, Becker K, Cadet JL. Methamphetamine induces dopamine D1 receptor-dependent endoplasmic reticulum stress-related molecular events in the rat striatum. PloS one. 2009;4:e6092. doi: 10.1371/journal.pone.0006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebelli J, Hooper C, Pocock JM. Microglial p53 activation is detrimental to neuronal synapses during activation-induced inflammation: Implications for neurodegeneration. Neuroscience letters. 2014;583:92–97. doi: 10.1016/j.neulet.2014.08.049. [DOI] [PubMed] [Google Scholar]
- Jimenez A, Jorda EG, Verdaguer E, Pubill D, Sureda FX, Canudas AM, Escubedo E, Camarasa J, Camins A, Pallas M. Neurotoxicity of amphetamine derivatives is mediated by caspase pathway activation in rat cerebellar granule cells. Toxicology and applied pharmacology. 2004;196:223–234. doi: 10.1016/j.taap.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nature genetics. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain research reviews. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:8951–8960. doi: 10.1523/JNEUROSCI.22-20-08951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Kuo CC, Shen H, Chou J, Greig NH, Hoffer BJ, Wang Y. Delayed treatment with a p53 inhibitor enhances recovery in stroke brain. Annals of neurology. 2009;65:520–530. doi: 10.1002/ana.21592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Wang Y, Kuang SY, Chiang YH, Hoffer B. Decreased level of Nurr1 in heterozygous young adult mice leads to exacerbated acute and long-term toxicity after repeated methamphetamine exposure. PloS one. 2010;5:e15193. doi: 10.1371/journal.pone.0015193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo P, Frost B, Peng S, Yang YJ, Park PJ, Feany M. p53 prevents neurodegeneration by regulating synaptic genes. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:18055–18060. doi: 10.1073/pnas.1419083111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. The Journal of pharmacology and experimental therapeutics. 1994;270:752–760. [PubMed] [Google Scholar]
- Pereira FC, Lourenco ES, Borges F, Morgadinho T, Ribeiro CF, Macedo TR, Ali SF. Single or multiple injections of methamphetamine increased dopamine turnover but did not decrease tyrosine hydroxylase levels or cleave caspase-3 in caudate-putamen. Synapse. 2006;60:185–193. doi: 10.1002/syn.20285. [DOI] [PubMed] [Google Scholar]
- Perier C, Bove J, Wu DC, Dehay B, Choi DK, Jackson-Lewis V, Rathke-Hartlieb S, Bouillet P, Strasser A, Schulz JB, Przedborski S, Vila M. Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8161–8166. doi: 10.1073/pnas.0609874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porat S, Simantov R. Bcl-2 and p53: role in dopamine-induced apoptosis and differentiation. Annals of the New York Academy of Sciences. 1999;893:372–375. doi: 10.1111/j.1749-6632.1999.tb07858.x. [DOI] [PubMed] [Google Scholar]
- Qi X, Davis B, Chiang YH, Filichia E, Barnett A, Greig NH, Hoffer B, Luo Y. Dopaminergic neuron-specific deletion of p53 gene is neuroprotective in an experimental Parkinson’s disease model. Journal of neurochemistry. 2016;138:746–757. doi: 10.1111/jnc.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain pathology. 2004;14:215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineri M, Gonzalez B, Goitia B, Garcia-Rill E, Krasnova IN, Cadet JL, Urbano FJ, Bisagno V. Modafinil abrogates methamphetamine-induced neuroinflammation and apoptotic effects in the mouse striatum. PloS one. 2012;7:e46599. doi: 10.1371/journal.pone.0046599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner DJ, Yu SJ, Shen H, He Y, Bae E, Wang Y. 9-Cis retinoic acid protects against methamphetamine-induced neurotoxicity in nigrostriatal dopamine neurons. Neurotoxicity research. 2014;25:248–261. doi: 10.1007/s12640-013-9413-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain research. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- Schoenmann Z, Assa-Kunik E, Tiomny S, Minis A, Haklai-Topper L, Arama E, Yaron A. Axonal degeneration is regulated by the apoptotic machinery or a NAD+-sensitive pathway in insects and mammals. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:6375–6386. doi: 10.1523/JNEUROSCI.0922-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Luo Y, Yu SJ, Wang Y. Enhanced neurodegeneration after a high dose of methamphetamine in adenosine A3 receptor null mutant mice. Neuroscience. 2011;194:170–180. doi: 10.1016/j.neuroscience.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom E, Sathe S, Komarov PG, Chernova OB, Pavlovska I, Shyshynova I, Bosykh DA, Burdelya LG, Macklis RM, Skaliter R, Komarova EA, Gudkov AV. Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nature chemical biology. 2006;2:474–479. doi: 10.1038/nchembio809. [DOI] [PubMed] [Google Scholar]
- Tedeschi A, Di Giovanni S. The non-apoptotic role of p53 in neuronal biology: enlightening the dark side of the moon. EMBO reports. 2009;10:576–583. doi: 10.1038/embor.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer PA, Smith TS, Jung AB, Bennett JP., Jr Dopamine neurons from transgenic mice with a knockout of the p53 gene resist MPTP neurotoxicity. Neurodegeneration: a journal for neurodegenerative disorders, neuroprotection, and neuroregeneration. 1996;5:233–239. doi: 10.1006/neur.1996.0031. [DOI] [PubMed] [Google Scholar]
- Wang DB, Kinoshita C, Kinoshita Y, Morrison RS. p53 and mitochondrial function in neurons. Biochimica et biophysica acta. 2014;1842:1186–1197. doi: 10.1016/j.bbadis.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Pace J, Filichia E, Lv T, Davis B, Hoffer B, Selman W, Luo Y. Effect of the sonic hedgehog receptor smoothened on the survival and function of dopaminergic neurons. Experimental neurology. 2016;283:235–245. doi: 10.1016/j.expneurol.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Yu QS, Cutler RG, Culmsee CW, Holloway HW, Lahiri DK, Mattson MP, Greig NH. Novel p53 inactivators with neuroprotective action: syntheses and pharmacological evaluation of 2-imino-2,3,4,5,6,7-hexahydrobenzothiazole and 2-imino-2,3,4,5,6,7-hexahydrobenzoxazole derivatives. Journal of medicinal chemistry. 2002;45:5090–5097. doi: 10.1021/jm020044d. [DOI] [PubMed] [Google Scholar]