Abstract
Cobalt(II)-based metalloradical catalysis has been successfully applied to radical bicyclization of allyl azidoformates to construct aziridine/oxazolidinonefused bicyclic structures. The Co(II) complex of D2-symmetric chiral amidoporphyrin 3,5-DitBu-QingPhyrin has been identified as an effective metalloradical catalyst for the intramolecular radical aziridination of this type of carbonyl azides, allowing for high-yielding formation of synthetically useful chiral [3.1.0]-bicyclic aziridines with high diastereo- and enantioselectivity.
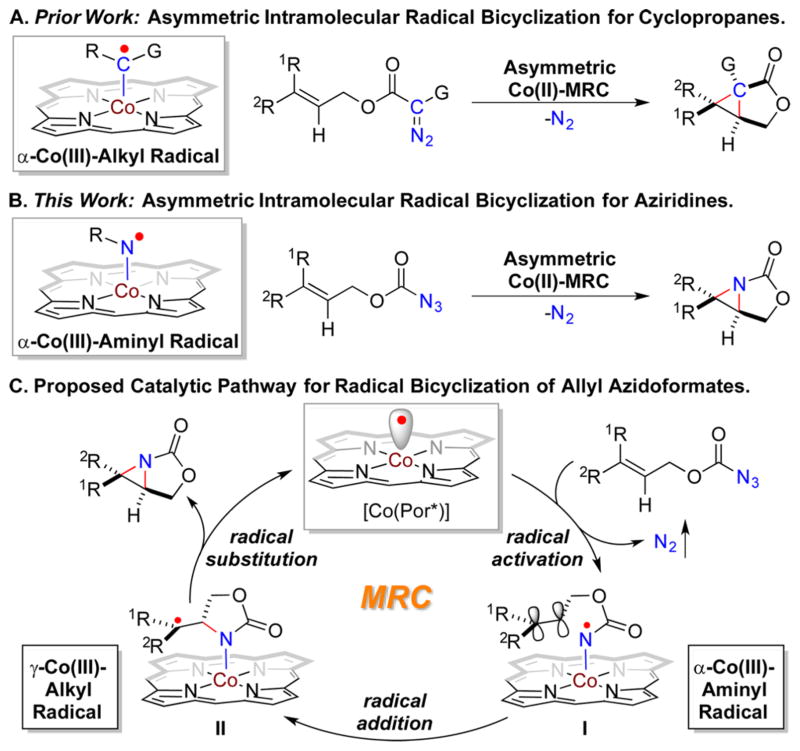
Radical chemistry has been increasingly explored for the development of new synthetic tools to construct molecular structures.1,2 Despite tremendous advances, formidable challenges, such as control of enantioselectivity, remain largely unaddressed for many radical reactions. Among recent strategies,3 metalloradical catalysis (MRC) presents a conceptually new approach in that metal-centered radicals are exploited as open-shell catalysts for initiating as well as controlling homolytic radical processes.4,5 As stable 15e-metalloradicals, Co(II) porphyrin complexes have recently been demonstrated with the unusual ability in activating diazo compounds and organic azides to generate the unprecedented α-Co(III)-alkyl radicals (also known as Co(III)-carbene radicals)6 and α-Co(III)-aminyl radicals (also known as Co(III)-nitrene radicals), 7 respectively. These metal-stabilized organic radicals are competent for both H atom abstraction and radical addition, leading to new catalytic radical processes for C–H amination,8 C–H alkylation,9 C=C aziridination,10 and C=C cyclopropanation. 11 With D2-symmetric chiral porphyrins as supporting ligands,11a,c the Co(II)-based MRC (Co(II)-MRC) enables control of reactivity as well as stereochemistry of these radical processes, including enantioselectivity.9–11 In particular, through α-Co(III)-alkyl radicals as intermediates, asymmetric intramolecular radical bicyclization of allyl diazoacetates was achieved to afford bicyclic cyclopropanes (Scheme 1A).11c By parallel thinking, we were attracted to the possibility of the equivalent process through α-Co(III)-aminyl radicals as intermediates (Scheme 1B). In addition to the prerequisite for metalloradical activation of this type of carbonyl azides, this proposed catalytic process would require the control of stereochemistry of two consecutive radical cyclization steps: (i) enantioselective 5-exotrig cyclization of the α-Co(III)-aminyl radical I and (ii) diastereoselective 3-exo-tet cyclization of the γ-Co(III)-alkyl radical II (Scheme 1C). If achieved, this type of asymmetric intramolecular aziridination would be synthetically useful since the resulting chiral [3.1.0]-bicyclic aziridines are versatile intermediates for the synthesis of chiral oxazolidinone and vicinal amino alcohol derivatives, which are key structural motifs in many biologically important molecules (see Figure S1 in Supporting Information (SI)).
Scheme 1.
Asymmetric Intramolecular Radical Bicyclization Processes via Co(II)-Based Metalloradical Catalysis
Catalytic intramolecular olefin aziridination represents one of the most attractive approaches for the construction of fused bicyclic aziridines.12 While diastereoselective catalytic systems have been developed,13 allyl azidoformates have not been successfully employed for catalytic intramolecular aziridination. 14 The challenge is attributed to the inertness of this type of carbonyl azides toward catalytic activation.13a,14a–d,15 As a novel alternative involving the catalytic activation of N-tosyloxy-carbamates, Lebel and co-workers developed a nonoxidative approach for the intramolecular aziridination process.14a–d In all the previous systems, however, the control of enantioselectivity has not been addressed. As a new application of Co(II)-MRC, we wish to report herein the development of the first catalytic system for asymmetric intramolecular aziridination of allyl azidoformates through a stepwise radical bicyclization pathway without the need for bases or oxidants. Using D2-symmetric chiral amidoporphyrin as the ligand, the Co(II)-catalyzed process allows for efficient construction of 3-oxa-1-azabicyclo[3.1.0]-hexan-2-one structures in high yields with excellent diastereo- and enantioselectivity. The resulting optically active bicyclic aziridines can serve as useful intermediates for the preparation of chiral oxazolidinone and vicinal amino alcohol derivatives with retention of the original enantiopurity.
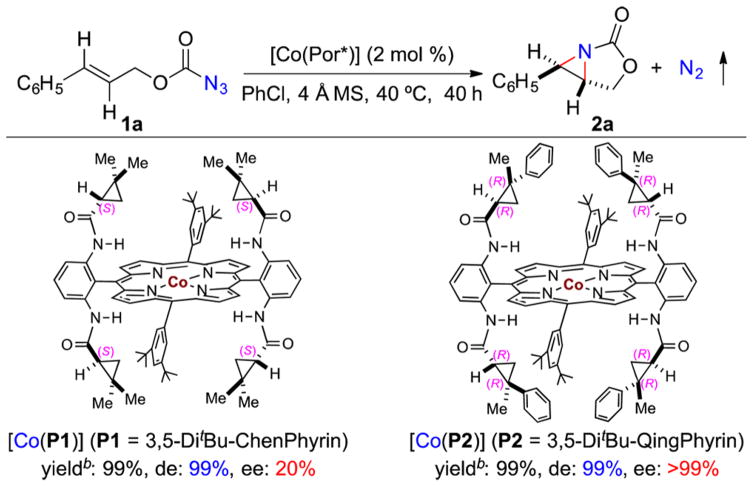
At the outset of this project, cinnamyl azidoformate (1a) was selected as the model substrate to explore both the reactivity and stereoselectivity of intramolecular radical aziridination via Co(II)-MRC (Scheme 2). After optimization of reaction conditions, the first-generation Co(II) complex of D2-symmetric chiral amidoporphyrin [Co(P1)] (P1 = 3,5-DitBu-ChenPhyrin) 11a was shown to be effective in catalyzing the reaction, affording the desired bicyclic aziridine 2a in quantitative yield. While the diastereoselectivity was excellent, it displayed a low but significant level of enantioselectivity. When the second-generation catalyst [Co(P2)] (P2 = 3,5-DitBu-QingPhyrin) was used,11c the enantioselectivity was increased dramatically, affording 2a as essentially a single enantiomer while maintaining the excellent yield and diastereoselectivity (Scheme 2).
Scheme 2. Ligand Effect on Co(II)-Catalyzed Asymmetric Radical Bicyclization of Cinnamyl Azidoformatea.
aPerformed in PhCl at 40 °C for 40 h using 2 mol % [Co(Por*)] under N2 in the presence of 4 Å MS; [azide 1a] = 0.1 M. bYields were based on 1H NMR.
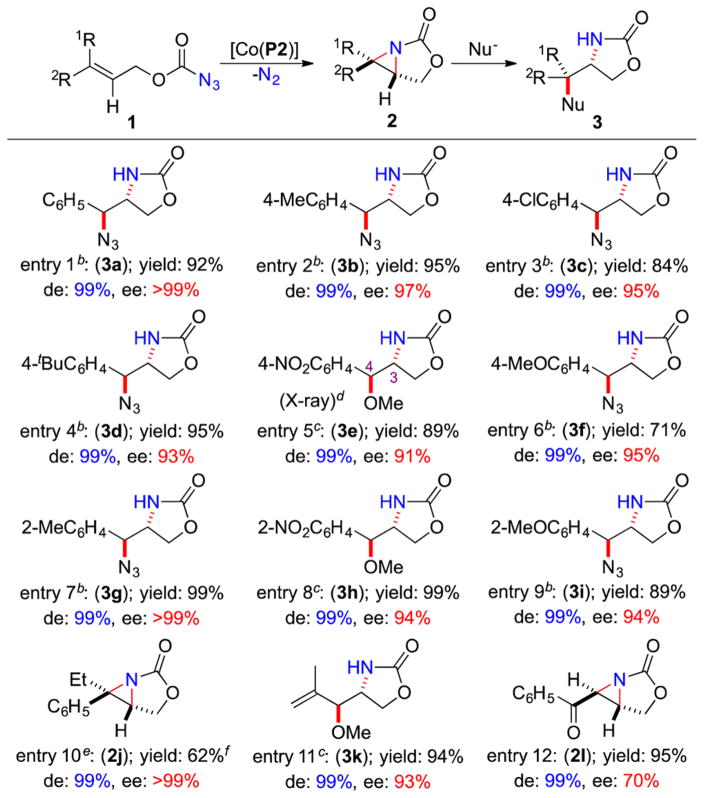
Under the same conditions, the [Co(P2)]-based system could effectively catalyze asymmetric intramolecular radical aziridination of a broad range of allyl azidoformates 1 (Table 1). Due to the high reactivity associated with the strain of 3-oxa-1-azabicyclo[3.1.0]hexan-2-one structures, some of the resulting bicyclic aziridines 2 were difficult to isolate in high yields. Taking advantage of the reactivity, aziridines 2 could be directly transformed to the corresponding 2-oxazolidinones 3 via in situ ring-opening reactions by nucleophiles. For example, although 2a was not stable enough for high-yielding isolation (Scheme 2), it could be directly converted, upon subsequent in situ reaction with TMSN3, to oxazolidinone 3a in 92% yield while preserving the original excellent stereochemistry (entry 1). Similarly, allyl azidoformates containing aryl substituents with varied electronic and steric properties were all suitable substrates for the aziridination process and the subsequent in situ ring-opening reaction, forming 2-oxazolidinones 3b–3f in high overall yields for the two steps with excellent diastereo- and enantioselectivity (entries 2–6). The absolute configuration of 3e (entry 5) was established as (3R, 4S). In addition, this combined aziridination and ring-opening system could tolerate substrates with sterically more hindered 2-substituted groups, leading to high-yielding formation of 3g–3i with excellent diastereo- and enantioselectivity (entries 7–9). Furthermore, the Co(II)-catalyzed bicyclization could even be applied for trisubstituted alkenes as demonstrated for the reaction of 1j, affording 2j in full conversion but in 62% isolated yield due to its instability during purification (entry 10). It is remarkable that both the relative and absolute configurations of the two newly generated contiguous chiral centers in the ring structure, including one quaternary stereogenic center, could be completely controlled. In addition, the aziridination/ring-opening protocol worked equally well with vinyl-substituted allyl azidoformates as exemplified with the high-yielding transformation of 1k to 3k as a single diastereomer with high enantioselectivity (entry 11). Likewise, acyl-substituted allyl azidoformates such as 1l could be bicyclized to 2l in an excellent yield with complete control of diastereoselectivity but with lower enantioselectivity (entry 12). It is noted that the olefin, ketone, and other functionalities were well tolerated by the catalytic radical process.
Table 1.
Asymmetric Intramolecular Radical Bicyclization of Allyl Azidoformates by [Co(P2)]a
Performed in PhCl at 40 °C for 40 h using 2 mol % [Co(P2)] under N2 in the presence of 4 Å MS; [azide 1] = 0.1 M; isolated yields.
In situ addition of TMSN3 (1.1 equiv) and TBAF (1.1 equiv).
In situ addition of MeOH (2.0 mL) and H2SO4 (30 mol %).
Absolute configuration was determined by X-ray as (3R, 4S).
At 80 °C for 20 h.
100% conversion; >90% NMR yield.
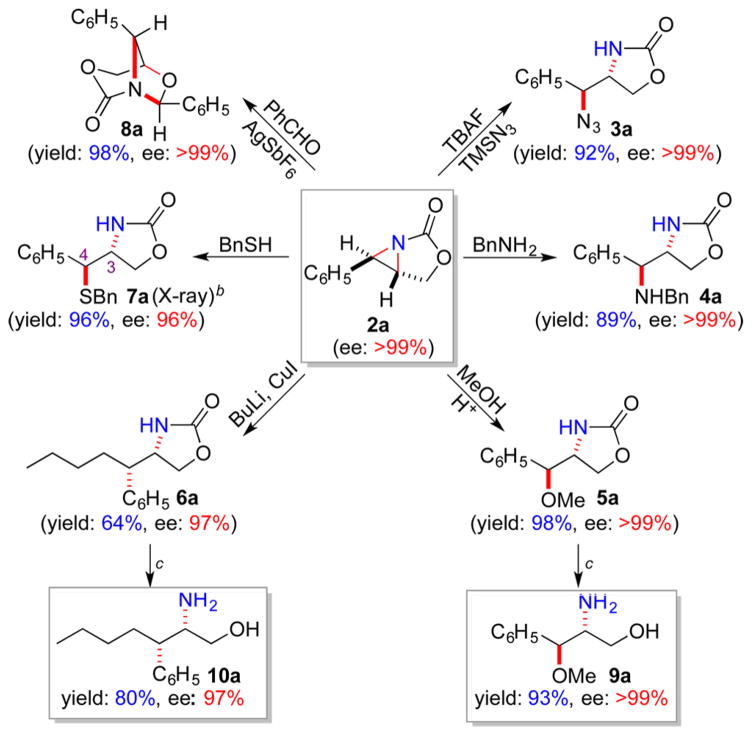
To further demonstrate the versatility of the resulting optically active [3.1.0]-bicyclic aziridines as chiral building blocks for stereoselective organic synthesis, ring-opening reactions by different types of nucleophiles were performed with the use of the enantiopure aziridine 2a as the representative reactant (Scheme 3). In addition to stereoselective formation of oxazolidinone 3a with TMSN3, the aziridine ring in 2a could be regioselectively opened at the exo-position by N-, O-, C-, and S-based nucleophiles, leading to high-yielding formation of 3a–7a, respectively, as single diastereomers without erosion of the original enantiopurity.15b,c In addition to the exo-ring openings, the endo-ring opening of aziridine 2a was achieved selectively via 1,3-dipole addition with benzaldehyde in the presence AgSbF6 as a Lewis acid.16 The [3 + 2] cycloaddition product 8a with a bridged bicyclic structure was formed in quantitative yield with full retention of the original enantiopurity. Moreover, the resulting oxazolidinones from the regioselective ring-opening reactions could be further transformed to the corresponding vicinal amino alcohol derivatives through simple decarbonylation (Scheme 3). For example, upon treatment with 1,3-diaminopropane under reflux, 5a and 6a were readily converted to chiral vicinal amino alcohols 9a and 10a, respectively, in high yields with complete retention of the original optical activity.
Scheme 3. Regioselective Ring-Opening of Enantiopure [3.1.0]-Bicyclic Aziridine and Further Decarbonylationa.
aIsolated yields. bAbsolute configuration was determined by X-ray as (3R, 4S). cRefluxed in NH2(CH2)3NH2 for 0.5 h.
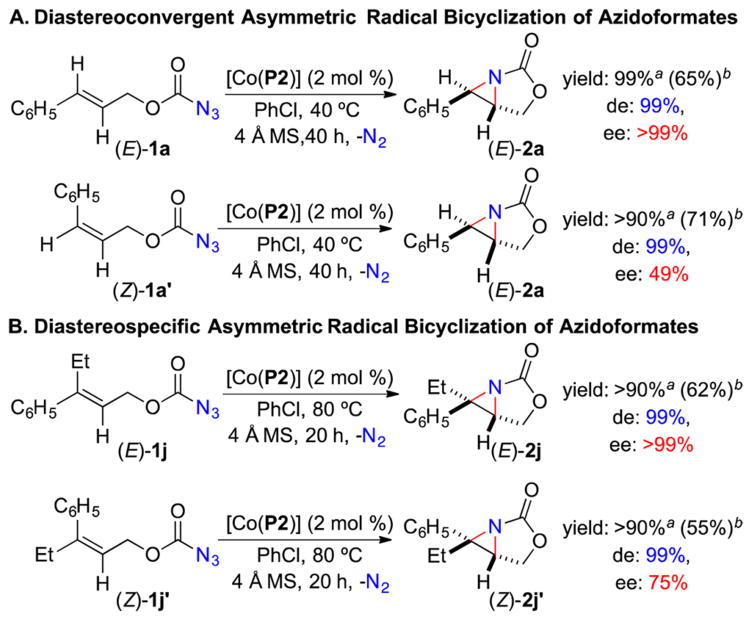
To probe the underlying stepwise radical mechanism, two pairs of (E)- and (Z)-allyl azidoformates with different olefin substitution patterns were employed as the substrates for the Co(II)-based intramolecular aziridination (Scheme 4). Under the standard conditions, both reactions of the disubstituted allyl azidoformates (E)-1a and (Z)-1a′ were found to produce the same (E)-aziridine 2a as the single diastereomer in similar yields but with different enantioselectivities (Scheme 4A). While the difference in enantioselectivity indicates that the first radical cyclization (Scheme 1C: I→II) is likely the enantio-determining step, the observed diastereoconvergence is attributed to the facile interconversion between the corresponding intermediates II generated from 1a and 1a′, respectively, due to the low-barrier rotation of the α-C–C bond of the C-centered radical (Scheme 1C), leading to the formation of the more stable (E)-aziridine 2a after the second radical cyclization. In contrast, when the sterically more congested trisubstituted allyl azidoformates (E)-1j and (Z)-1j′ were used as the substrates, the stereospecific formation of aziridines (E)-2j and (Z)-2j′, respectively, were observed (Scheme 4B), indicating restricted rotation of the α-C–C bond of the corresponding intermediates II even at 80 °C as a result of the increased steric hindrance. Furthermore, as detailed in the SI, the α-Co(III)-aminyl radical I with a dangling olefin moiety from the reaction of (E)-1a with [Co(P2)] could be directly detected by both EPR spectroscopy (Figure S2)7a,d and HRMS (Figure S3).7d Collectively, these observations convincingly support the proposed stepwise radical bicyclization mechanism (Scheme 1C).
Scheme 4. Catalytic Reactions of (E)- and (Z)-Allyl Azidoformates to Probe Radical Bicylization Mechanism.
aNMR yields. bIsolated yields. Note: Aziridines were unstable toward workup.
In summary, metalloradical catalysis (MRC) has been successfully applied to develop the first catalytic system that is highly stereoselective for asymmetric intramolecular olefin aziridination of allyl azidoformates through a unique stepwise radical bicyclization pathway. The Co(II)-based metalloradical system can effectively activate this type of carbonyl azides, which are known to be inert toward activation by existing close-shell metal systems, under mild conditions without the need for bases or oxidants. The [Co(P2)]-catalyzed intramolecular radical aziridination has a broad scope and can be applicable to various allyl azidoformates, leading to stereoselective construction of 3-oxa-1-azabicyclo[3.1.0]hexan-2-one derivatives in high yields with both high diastereo- and enantioselectivity. Through both exo- and endo-regioselective ring-opening reactions, the resulting enantioenriched oxazolidinone-fused bicyclic aziridines have been demonstrated to be versatile synthetic intermediates for the preparation of the biologically important chiral oxazolidinone and vicinal amino alcohol derivatives with preservation of the original enantiopurity.
Supplementary Material
Acknowledgments
We are grateful for financial support by NIH (R01-GM102554).
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.7b05778.
Experimental details and analytical data for all new compounds (PDF)
Crystallographic data for 3e (CIF)
Crystallographic data for 7a (CIF)
References
- 1.Select reviews: Jasperse CP, Curran DP, Fevig TL. Chem Rev. 1991;91:1237.Dowd P, Zhang W. Chem Rev. 1993;93:2091.Quiclet-Sire B, Zard SZ. Pure Appl Chem. 2010;83:519.Studer A, Curran DP. Angew Chem, Int Ed. 2016;55:58. doi: 10.1002/anie.201505090.
- 2.Recent examples: Pirnot MT, Rankic DA, Martin DBC, MacMillan DWC. Science. 2013;339:1593. doi: 10.1126/science.1232993.Lo JC, Gui J, Yabe Y, Pan CM, Baran PS. Nature. 2014;516:343. doi: 10.1038/nature14006.Hoyt JM, Schmidt VA, Tondreau AM, Chirik PJ. Science. 2015;349:960. doi: 10.1126/science.aac7440.Brill ZG, Grover HK, Maimone TJ. Science. 2016;352:1078. doi: 10.1126/science.aaf6742.
- 3.Reviews and select examples: Sibi MP, Manyem S, Zimmerman J. Chem Rev. 2003;103:3263. doi: 10.1021/cr020044l.Brimioulle R, Bach T. Angew Chem, Int Ed. 2014;53:12921. doi: 10.1002/anie.201407832.Du J, Skubi KL, Schultz DM, Yoon TP. Science. 2014;344:392. doi: 10.1126/science.1251511.Hashimoto T, Kawamata Y, Maruoka K. Nat Chem. 2014;6:702. doi: 10.1038/nchem.1998.Huo H, Shen X, Wang C, Zhang L, Röse P, Chen LA, Harms K, Marsch M, Hilt G, Meggers E. Nature. 2014;515:100. doi: 10.1038/nature13892.Kainz QM, Matier CD, Bartoszewicz A, Zultanski SL, Peters JC, Fu GC. Science. 2016;351:681. doi: 10.1126/science.aad8313.Lin JS, Dong XY, Li TT, Jiang NC, Tan B, Liu XY. J Am Chem Soc. 2016;138:9357. doi: 10.1021/jacs.6b04077.
- 4.Reviews and highlights on Co(II)-based MRC: Fantauzzi S, Caselli A, Gallo E. Dalton Trans. 2009:5434. doi: 10.1039/b902929j.Driver TG. Org Biomol Chem. 2010;8:3831. doi: 10.1039/c005219c.Che CM, Lo VKY, Zhou CY, Huang JS. Chem Soc Rev. 2011;40:1950. doi: 10.1039/c0cs00142b.Pellissier H, Clavier H. Chem Rev. 2014;114:2775. doi: 10.1021/cr4004055.Doyle MP. Angew Chem, Int Ed. 2009;48:850. doi: 10.1002/anie.200804940.Miyabe H, Kawashima A, Yoshioka E, Kohtani S. Chem - Eur J. 2017;23:6225. doi: 10.1002/chem.201603124.
- 5.Reviews and select examples on Ti(III)-based MRC: Gansäuer A, Hildebrandt S, Vogelsang E, Flowers RA., II Dalton Trans. 2016;45:448. doi: 10.1039/c5dt03891j.Gansäuer A, Rinker B, Pierobon M, Grimme S, Gerenkamp M, Mück-Lichtenfeld C. Angew Chem, Int Ed. 2003;42:3687. doi: 10.1002/anie.200351240.Gansäuer A, Fleckhaus A, Lafont MA, Okkel A, Kotsis K, Anoop A, Neese F. J Am Chem Soc. 2009;131:16989. doi: 10.1021/ja907817y.Hildebrandt S, Gansäuer A. Angew Chem, Int Ed. 2016;55:9719. doi: 10.1002/anie.201603985.
- 6.Detailed studies on the radical mechanism of [Co(Por)]-catalyzed radical cyclopropanation including EPR observation of α-Co(III)-alkyl radical (also known as Co(III)-carbene radical) intermediates: Dzik WI, Xu X, Zhang XP, Reek JNH, de Bruin B. J Am Chem Soc. 2010;132:10891. doi: 10.1021/ja103768r.Belof JL, Cioce CR, Xu X, Zhang XP, Space B, Woodcock HL. Organometallics. 2011;30:2739. doi: 10.1021/om2001348.Lu H, Dzik WI, Xu X, Wojtas L, de Bruin B, Zhang XP. J Am Chem Soc. 2011;133:8518. doi: 10.1021/ja203434c.
- 7.Detailed studies on the radical mechanism of [Co(Por)]-catalyzed C–H amination and aziridination, including EPR observation of α-Co(III)-aminyl radical (also known as Co(III)-nitrene radical) intermediates: Lyaskovskyy V, Suarez AIO, Lu H, Jiang H, Zhang XP, de Bruin B. J Am Chem Soc. 2011;133:12264. doi: 10.1021/ja204800a.Olivos Suarez AI, Jiang H, Zhang XP, de Bruin B. Dalton Trans. 2011;40:5697. doi: 10.1039/c1dt10027k.Hopmann KH, Ghosh A. ACS Catal. 2011;1:597.Goswami M, Lyaskovskyy V, Domingos SR, Buma WJ, Woutersen S, Troeppner O, Ivanović-Burmazović I, Lu H, Cui X, Zhang XP, Reijerse EJ, DeBeer S, van Schooneveld MM, Pfaff FF, Ray K, de Bruin B. J Am Chem Soc. 2015;137:5468. doi: 10.1021/jacs.5b01197.. A recent review on nitrogen-centered radical ligands: Suarez AIO, Lyaskovskyy V, Reek JNH, van der Vlugt JI, de Bruin B. Angew Chem, Int Ed. 2013;52:12510. doi: 10.1002/anie.201301487.
- 8.Select examples of radical C–H amination via Co(II)-MRC: Lu H, Jiang H, Wojtas L, Zhang XP. Angew Chem, Int Ed. 2010;49:10192. doi: 10.1002/anie.201005552.Villanueva O, Weldy NM, Blakey SB, MacBeth CE. Chem Sci. 2015;6:6672. doi: 10.1039/c5sc01162k.Lu H, Lang K, Jiang H, Wojtas L, Zhang XP. Chem Sci. 2016;7:6934. doi: 10.1039/c6sc02231f.Kuijpers PF, Tiekink MJ, Breukelaar WB, Broere DLJ, van Leest NP, van der Vlugt JI, Reek JNH, de Bruin B. Chem - Eur J. 2017;23:7945. doi: 10.1002/chem.201700358.
- 9.An example of radical C–H alkylation via Co(II)-MRC: Cui X, Xu X, Jin LM, Wojtas L, Zhang XP. Chem Sci. 2015;6:1219. doi: 10.1039/c4sc02610a.. Synthesis of β-ester-γ-amino ketones involving α-Co(III)-alkyl radical intermediates: Zhang J, Jiang J, Xu D, Luo Q, Wang H, Chen J, Li H, Wang Y, Wan X. Angew Chem, Int Ed. 2015;54:1231. doi: 10.1002/anie.201408874.
- 10.Select examples of radical olefin aziridination via Co(II)-MRC: Subbarayan V, Ruppel JV, Zhu S, Perman JA, Zhang XP. Chem Commun. 2009:4266. doi: 10.1039/b905727g.Jin LM, Xu X, Lu H, Cui X, Wojtas L, Zhang XP. Angew Chem, Int Ed. 2013;52:5309. doi: 10.1002/anie.201209599.Jiang H, Lang K, Lu H, Wojtas L, Zhang XP. Angew Chem, Int Ed. 2016;55:11604. doi: 10.1002/anie.201605238.
- 11.Select examples of radical olefin cyclopropanation via Co(II)-MRC: Chen Y, Fields KB, Zhang XP. J Am Chem Soc. 2004;126:14718. doi: 10.1021/ja044889l.Fantauzzi S, Gallo E, Rose E, Raoul N, Caselli A, Issa S, Ragaini F, Cenini S. Organometallics. 2008;27:6143.Xu X, Lu H, Ruppel JV, Cui X, Lopez de Mesa S, Wojtas L, Zhang XP. J Am Chem Soc. 2011;133:15292. doi: 10.1021/ja2062506.Reddy AR, Hao F, Wu K, Zhou CY, Che CM. Angew Chem, Int Ed. 2016;55:1810. doi: 10.1002/anie.201506418.Wang Y, Wen X, Cui X, Wojtas L, Zhang XP. J Am Chem Soc. 2017;139:1049. doi: 10.1021/jacs.6b11336.Goswami M, de Bruin B, Dzik WI. Chem Commun. 2017;53:4382. doi: 10.1039/c7cc01418j.
- 12.Reviews on catalytic aziridination: Müller P, Fruit C. Chem Rev. 2003;103:2905. doi: 10.1021/cr020043t.Pellissier H. Adv Synth Catal. 2014;356:1899.Degennaro L, Trinchera P, Luisi R. Chem Rev. 2014;114:7881. doi: 10.1021/cr400553c.
- 13.Metal-catalyzed diastereoselective intramolecular aziridination of allyl carbamates: Levites-Agababa E, Menhaji E, Perlson LN, Rojas CM. Org Lett. 2002;4:863. doi: 10.1021/ol025634k.Padwa A, Stengel T. Org Lett. 2002;4:2137. doi: 10.1021/ol0259490.Padwa A, Flick AC, Leverett CA, Stengel T. J Org Chem. 2004;69:6377. doi: 10.1021/jo048990k.. A metal-free system: Deng QH, Wang JC, Xu ZJ, Zhou CY, Che CM. Synthesis. 2011;2011:2959.
- 14.Lebel H, Huard K, Lectard S. J Am Chem Soc. 2005;127:14198. doi: 10.1021/ja0552850.Lebel H, Lectard S, Parmentier M. Org Lett. 2007;9:4797. doi: 10.1021/ol702152e.Liu R, Herron SR, Fleming SA. J Org Chem. 2007;72:5587. doi: 10.1021/jo0705014.Lebel H, Leogane O, Huard K, Lectard S. Pure Appl Chem. 2006;78:363.. A related intramolecular aminohydroxylation process: Liu GS, Zhang YQ, Yuan YA, Xu H. J Am Chem Soc. 2013;135:3343. doi: 10.1021/ja311923z.
- 15.Thermal and photolytic intramolecular aziridination of azidoformates: Rhouati S, Bernou A. J Chem Soc, Chem Commun. 1989:730.Bergmeier SC, Stanchina DM. J Org Chem. 1997;62:4449. doi: 10.1021/jo970473x.Bergmeier SC, Stanchina DM. J Org Chem. 1999;64:2852. doi: 10.1021/jo9823893.Kan C, Long CM, Paul M, Ring CM, Tully SE, Rojas CM. Org Lett. 2001;3:381. doi: 10.1021/ol0069002.Yoshimitsu T, Ino T, Tanaka T. Org Lett. 2008;10:5457. doi: 10.1021/ol802225g.Chang YJ, Hsuan YC, Lai ACY, Han YC, Hou DR. Org Lett. 2016;18:808. doi: 10.1021/acs.orglett.6b00090.. Photolytic intermolecular aziridination of azidoformates: Scholz SO, Farney EP, Kim S, Bates DM, Yoon TP. Angew Chem, Int Ed. 2016;55:2239. doi: 10.1002/anie.201510868.
- 16.(a) Wender PA, Strand D. J Am Chem Soc. 2009;131:7528. doi: 10.1021/ja901799s. [DOI] [PubMed] [Google Scholar]; (b) Dauban P, Malik G. Angew Chem, Int Ed. 2009;48:9026. doi: 10.1002/anie.200904941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.