Abstract
Aims
To project the impact of scaling up oral antiviral therapy and harm reduction on chronic hepatitis C (CHC) prevalence and incidence among people who inject drugs (PWID) in Greece, to estimate the relationship between required treatment levels and expansion of harm reduction programs to achieve specific targets and to examine whether hepatitis C viruse (HCV) elimination among PWID is possible in this high prevalence setting.
Design
A dynamic discrete time, stochastic individual-based model was developed to simulate HCV transmission among PWID incorporating the effect of HCV treatment and harm reduction strategies, and allowing for reinfection following treatment.
Setting/Participants
The population of 8,300 PWID in Athens Metropolitan area
Measurements
Reduction in HCV prevalence and incidence in 2030 compared with 2016.
Findings
Moderate expansion of HCV treatment (treating 4%–8% of PWID/year), with simultaneous increase of 2%/year in harm reduction coverage (from 44% to 72% coverage over 15 years), was projected to reduce CHC prevalence among PWID in Athens by 46%–90% in 2030, compared with 2016. CHC prevalence would reduce below 10% within the next 4–5 years if annual HCV treatment numbers were increased up to 16%–20% PWID/year. The effect of harm reduction on incidence was more pronounced under lower treatment rates.
Conclusions
Based on theoretical model projections, scaled-up hepatitis C virus (HCV) treatment and harm reduction interventions could achieve major reductions in HCV incidence and prevalence among people who inject drugs (PWID) in Athens, Greece by 2030. Chronic hepatitis C could be eliminated in the next 4–5 years by increasing treatment to more than 16% of PWID per year combined with moderate increases in harm reduction coverage.
Introduction
Hepatitis C (HCV) virus affects about 170 million persons (3% of world population) worldwide (1). In the developed world, mortality attributed to HCV is higher than HIV (2, 3) and the most common route of transmission is unsafe injection of drugs e.g. receptive sharing (4, 5). It is estimated that around 10 million or 60% of people who inject drugs (PWID) have been infected by HCV worldwide (6). Harm reduction strategies such as high-coverage needle and syringe programs (HCNSP) and opiate substitution therapy (OST) have been often used to prevent the spread of HCV among PWID. Empirical studies have shown that these interventions can substantially reduce the risk of HCV virus acquisition among PWID (7, 8), but modeling work has shown they have slow impact on the reduction of HCV prevalence if not combined with antiviral therapy (9, 10).
Over recent years, significant developments in the treatment of chronic hepatitis C (CHC) have occurred. New therapeutic regimens targeting specific HCV genome regions have been developed (direct acting antivirals [DAAs]). These therapies offer higher efficacy (sustained virological response (SVR) ≥90%), shorter duration and simplified dosing in contrast to the traditional therapies involving pegylated interferon with ribavirin (11, 12). Due to these recent clinical advances, HCV has now become curable in the great majority of cases. The concept of using HCV treatment for preventing onward transmission (treatment as prevention [TasP]) to achieve the goal of HCV elimination is under discussion. Recently, WHO released the Global Health Sector Strategy on viral hepatitis targeting elimination by 2030 (13).
Mathematical modeling studies have shown that HCV treatment for PWID could be an effective and cost-effective strategy to reduce CHC prevalence and incidence among PWID (14–19). More specifically, treating PWID could be a more cost-effective strategy than treating ex- or non- PWID, due to prevention of secondary infections, if chronic CHC prevalence among PWID is less than 60% (15). Non treatment-based prevention strategies (like OST and HCNSP), coupled with antiviral therapy, could contribute significantly to decreasing CHC prevalence and incidence (20).
Prior to 2002, guidelines in the US recommended against treating active PWID due to considerations about treatment adherence and the possibility of re-infection (21). Recent studies have shown that PWID, under Peg-IFN+RBV therapy, exhibit similar SVR compared to non-PWID (22–24), and have low rates of re-infection (1–5% per year) (25–28). Revised guidelines for HCV now recommend treatment for PWID (29), and new European guidelines on treatment recommend prioritizing DAA therapy for those with an ongoing risk of transmission (30).
Athens faces serious public health problems among PWID. Apart from the HIV outbreak in 2011 (31–33), the anti-HCV prevalence among PWID was 80% in 2014 (34). A substantial increase in the burden of CHC is anticipated to occur in the next 15–20 years (35). Treatment could be an effective way to inhibit the continuing transmission in this population.
The aims of this study were to: 1) Estimate the potential impact of scaling up rates of HCV DAA treatment and harm reduction strategies on CHC prevalence and incidence among PWID in Athens until 2030; 2) Estimate the degree to which scaling up harm reduction strategies reduces the required CHC treatment levels needed to achieve a specified impact on HCV prevalence and incidence; and 3) Assess if HCV elimination among PWID is a feasible goal in Athens over the next few years.
Methods
Design of the Mathematical Model
In infectious disease epidemiology, mathematical models are a common way to explain the spread of diseases, to highlight the key factors that influence the course of infection, to predict the impact of potential intervention policies and to help inform public health interventions. In those models the population is stratified into compartments corresponding to different states of the infection process (susceptible, infected, recovered, etc.) and individuals are assumed to move between states according to annual transition rates. The impact of various interventions can be simulated by modifying these rates, e.g. the effect of harm reduction can be simulated by reducing the probability of a PWID becoming infected. More details about the methodology and the application of mathematical modeling could be found elsewhere (36).
Description of the Mathematical Model
A discrete time, stochastic, individual based model of HCV transmission among PWID was developed in C++ (v.5.6.3) and parameterized to data from Athens (Table 1), with the model structure shown in Figure 1. The model describes the transitions between four groups of PWID: susceptible people including those who either never been HCV infected, have cleared infection or have had successful treatment; HCV chronically infected untreated individuals; patients who failed HCV treatment; and PWID being currently treated. The PWID population was additionally stratified according to risk of infection (high or low-risk), and whether the person participates in harm reduction programmes, i.e. on OST and/or HCNSP (Yes/No). Individuals could transition between risk states and on/off harm reduction. We defined as high-risk those PWID who experience unstable housing, as homelessness is a risk factor for HIV infection in this population and both HIV and HCV are spread through injecting drug use (31). The transition from low-risk to high-risk was balanced so that the proportion of PWID in the high-risk group remained constant over time.
Table 1.
Model Parameters and references
| Parameter | Estimate | Reference |
|---|---|---|
| PWID population size in Athens (95% CI) | 8300 (6392–10985) | (34) |
| Anti-HCV prevalence among PWID (95% CI) | 80% (77.5%, 82.5%) | (34) |
| Proportion acutely infected spontaneously clearing infection | 20% | (37) |
| Duration of injecting carrier among PWID in Athens | 12 years | (31) |
| Overall PWID mortality | 2% | (50–52) |
| Proportion of PWID at high risk (η)* | 23% | (31) |
| Proportion participating in harm reduction programmes (OST or high coverage HCNSP) (β) | 44% | (32) |
| Relative risk for HCV infection for high risk PWID (Π) | 2.3 | (31) |
| Relative risk for HCV infection while in a harm reduction programme (Z) | 0.41 | (7) |
| Duration in the high risk group (12/κ) | 12 months | (32) |
| Duration of DAA therapy (52/T) | 12 weeks | (40) |
| Proportion achieving SVR IFN-free DAAs |
90% until 2017 95% until 2030 |
(41–43) |
Defined as proportion of PWID experiencing unstable housing
Figure 1.
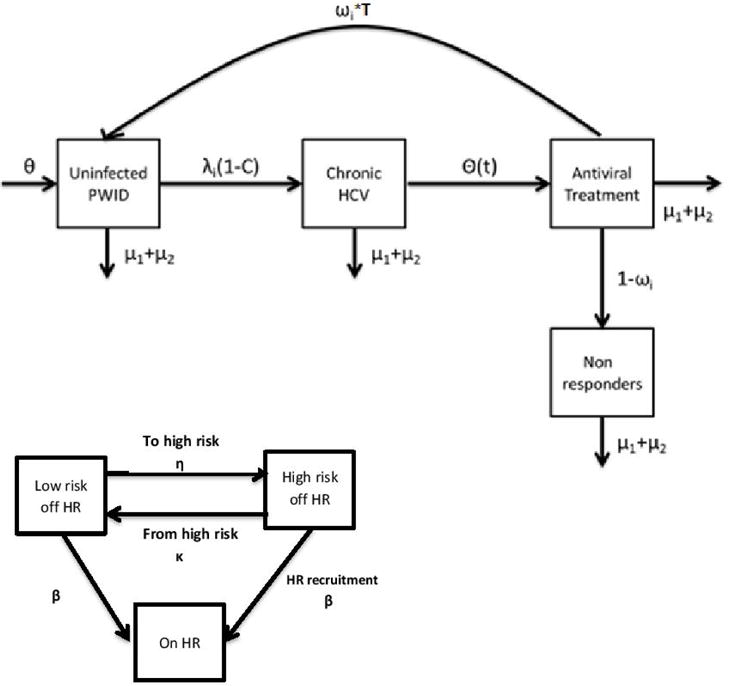
Schematic outline of the mathematical model. Parameters: θ represents the new injectors flow rate, λi the infection rate per year which depends on PWID status (i.e. whether they are high risk or not and/or whether they participate in a harm reduction programme), C the proportion that spontaneously clear the infection, Θ(t) the annual treatment rate, ωi the proportion of PWID achieving SVR, μ1 (mortality in the population of PWID), μ2 (rate of leaving PWID population due to injecting cessation). Θ(t) depend on whether the injector participates in a harm reduction programme.
Every year, PWID enter in the susceptible group at rate θ and exit the various states through death (μ1) or cessation of drug use (μ2), equal to the entry rate to keep the population size at constant levels. The force of infection for susceptible PWID depends on HCV prevalence and whether they are high-risk, participates in harm reduction programmes, or both. In that case, the force of infection is multiplied by a factor Ζ, Π and ΖΠ (7, 31), respectively. Initially, new injectors are not participating in harm reduction programs.
After infection, PWID have a probability of spontaneously clearing the disease and are then at risk of re-infection; to be conservative we assumed that the risk of re-infection was equal to the initial infection rate. Those who do not clear the infection progress to the chronically infected stage. Every year, a fixed number of PWID (Θ(t)) are treated. If the number of chronic infections is lower than Θ(t) then all PWID receive treatment. We assumed that the probability of treatment depends on whether the patient participates in a harm reduction programme or not, i.e. PWID who participate in OST or HCNSP have higher probability of initiating therapy with DAAs that those who do not. In the evaluated scenarios with high treatment coverage, if the number of available treatments exceeded the number of previously untreated PWID participating in harm reduction programmes, then it was assumed that they were allocated to PWID who were not in harm reduction programmes.
If PWID achieve SVR, then they become susceptible again and are at risk of re-infection, (assuming risk of re-infection equal to the initial infection rate). Otherwise, they progress to the chronically infected non-SVR component of the model. PWID in this group are assumed to be infectious and are not allowed to be re-treated.
The model was run until it achieved steady state, which is the level of prevalence in the population of PWID in Athens without use of treatment, by varying the infection rate. Due to the small proportion (<5‰) of PWID who have been treated for CHC in Greece, and for simplicity reasons, we assumed that the treatment rate before 2016 was negligible. After reaching steady state, we examined intervention scenarios involving scaled up treatment coverage and/or increased proportions of PWID on harm reduction programmes, and assessed their impact on CHC prevalence. We have also assessed under which intervention strategies HCV elimination is possible, which we define as reaching a prevalence of chronic HCV of less than 10% or reducing incidence by 90%.
For each scenario, 1000 runs were performed and the results were summarized. In order to include the appropriate uncertainty (stochastic variability), the 2.5 and 97.5 percentiles of the simulations were also shown.
Model parameterization
The total population size of PWID in Athens in 2015 is about 8300 individuals (Table 1) (34). HCV antibody prevalence among PWID in Athens was 80% in 2014 (34). Assuming that 20% of the infected individuals spontaneously clear the infection (37), the corresponding prevalence of chronic hepatitis was 64%. We accounted for the increased mortality among PWID by firstly assuming that the majority of PWID are between 15 and 44 years old, which have an annual death rate according to the United Nations Statistics Division for the Greek population of 1% (38). Then a standardized mortality ratio of 2 was applied (39), resulting in an estimated 2% annual death rate among PWID. The average duration of injecting till cessation was assumed to be 12 years (31, 32).
The proportion of PWID in the high-risk group, i.e. those experiencing unstable housing, is estimated to be 23% in Athens (32). In our projections, we have assumed that this proportion remained constant over time. In 2013, 44% of PWID participated in harm reduction programmes (26% in OST, 15% in HCNSP and 3% in both (32)). In Greece, harm reduction programmes include maintenance programmes, where OST delivery can be provided indefinitely, meaning that PWID may stay under substitution indefinitely. The Greek Organization Against Drugs aims at expanding OST programs in Greece by 2% each year from 2015 to achieve the goal of 75% OST coverage by 2030.
We assumed that IFN-free DAAs have 90% SVR until 2017 and 95% in subsequent years with a treatment duration of 12 weeks (40), irrespective of genotype (41–43). Assuming that PWID who participate in OST or HCNSP have a higher probability of initiating DAA therapy than those that do not, we hypothesized that the proportion of annual treatments delivered in the two groups are 66.6% and 33.3%, respectively. The impact of this assumption was evaluated in the sensitivity analysis.
Estimation of the relationship between the required treatment coverage and expansion of harm reduction programmes to achieve specific targets in CHC prevalence
Through the model, we estimated the required treatment coverage needed to reduce CHC prevalence or HCV incidence among PWID by 50%, 75% or 85% by 2030 assuming either harm reduction coverage levels similar to 2016 (44%) or an increase to 60% or 70% coverage from 2017 onwards. Then a linear regression analysis was used to estimate the relationship between the required treatments and harm reduction to achieve specific targets in CHC prevalence or incidence. This regression relationship was used to estimate the reduction in the number of required treatments for increases in harm reduction coverage.
Sensitivity analysis
To examine the impact of different model assumptions, we undertook a univariate sensitivity analysis on the anticipated CHC prevalence achieved in 2030 for the intervention scenario that assumed 332 annual CHC treatments (4% of PWID). We explored the impact of shorter/longer average duration of injecting carrier (6 or 18 versus 12 years), lower or higher SVR under treatment with DAAs (80%/100% vs. 95%), greater or lesser difference in risk between PWID in harm reduction programmes vs. those who are not (0.2/0.8 vs. 0.41), greater difference in risk between PWID in the high-risk group vs. those in the low-risk group (5 times higher relative risk vs. 2.3), longer duration in the high-risk group (10 years vs. 12 months), equal/unequal allocation of treatments among those engaging in harm reduction prorgammes vs. those who do not (50%/50%, 80%/20% vs. 66%/33%), the influence of changes in risk behavior after successful treatment (50% lower/higher probability of re-infection vs. no change in risk behavior), shorter duration in harm reduction programmes (12 months vs. always), and the impact of delaying the interventions by 3 years (2019 vs. 2016).
Results
Baseline annual HCV incidence and number of CHC among PWID in Athens
From the model, it was estimated that about 700 PWID are newly infected with HCV in Athens in 2015 (annual incidence: 22.7 (17.3, 27.4) new HCV infections per 100 person-years). Concerning the prevalent number of PWID with CHC, we estimate that there are 5312 PWID with CHC in Athens in 2015 (based on the estimate of 80% anti-HCV prevalence and that 80% are viremic).
Model projections under various scenarios concerning annual treatment coverage
Figure 2a shows the reduction in CHC prevalence over time for different annual treatment rates and assuming a simultaneous annual increase of 2% in harm reduction programmes coverage (from 44% in 2015 to 72% in 2030). Moderate expansion in treatment levels, i.e. treating 166 PWID /year (2% of PWID) or 332 PWID /year (4% of PWID), results in a relative reduction in CHC prevalence of 26.5% (18.6%–31.5%) and 46.2% (38.7%–53.8%) in 2030, respectively, compared to 2016 (CHC prevalence of 64%). When the number of antiviral treatments/year was increased to 664 PWID /year (8% of PWID), the projected CHC prevalence in 2030 would fall by almost 94.8% (92.3%–97.0%). To reduce CHC prevalence to less than 10% in the next 4–5 years, DAAs should be administered to more than 1370 PWID /year (16% of PWID). More specifically, treating 1370 PWID /year (16% of PWID) or 1660 PWID /year (20% of PWID) would reduce the prevalence below 10% (more than 85% relative reduction) by 2021 and 2020, respectively.
Figure 2.
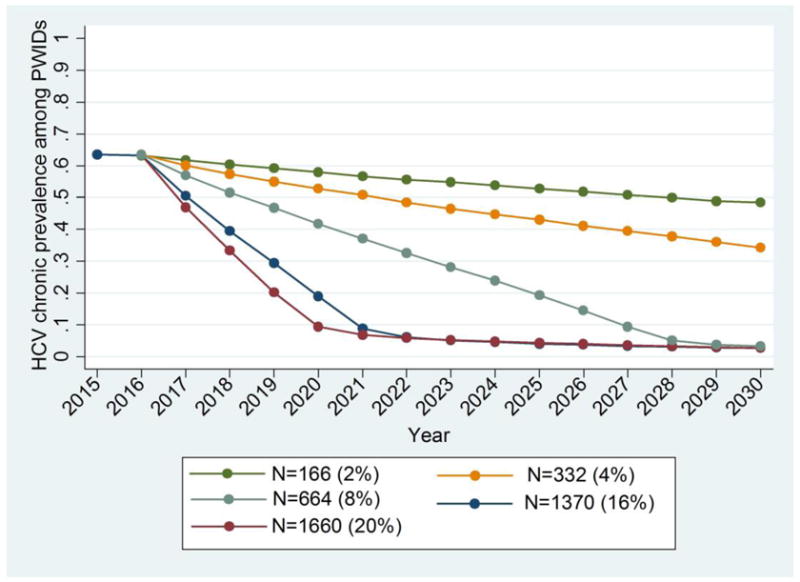
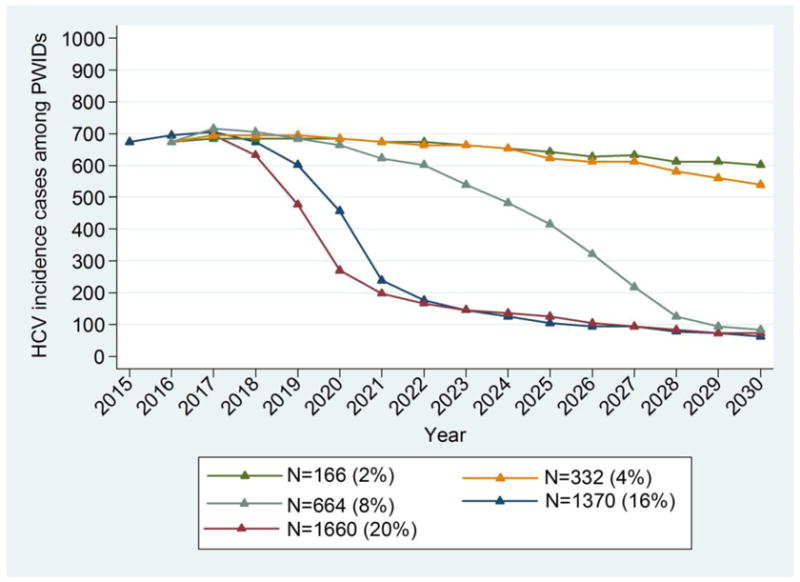
Model predictions for chronic HCV prevalence and the number of new infections among PWID in Athens for different annual treatment rates (N) while assuming 2% annual increase in harm reduction programmes coverage (from 44% in 2015 to 72% in 2030). The number of treated PWID per year and the corresponding percentage over the whole population of PWID in Athens are shown in parentheses.
a. Chronic HCV Prevalence among PWID
b. New infections among PWID
Annual HCV incidence would be reduced by 88% (75.5%–97%) in 2030 if more than 664 PWID received treatment per year. Under moderate treatment coverage i.e. treating 166 PWID/year or 332 PWID/year, the annual incidence would be reduced by 14% (1%–34%) and 21% (1%–42%) in 2030 compared to 2015, respectively (Figure 2b).
Model projections under various scenarios concerning harm reduction programmes
Figure 3 shows the contribution of harm reduction programmes in reducing CHC prevalence and incidence among PWID, coupled with antiviral therapy. Under moderate treatment coverage (332 PWID/year), an increase of harm reduction coverage of 2%/year would contribute an additional 5.1% (1.5%, 11.7%) absolute decrease in CHC prevalence and 19.2% (1%, 42.8%) lower incidence in 2030 compared to constant harm reduction coverage levels.
Figure 3.
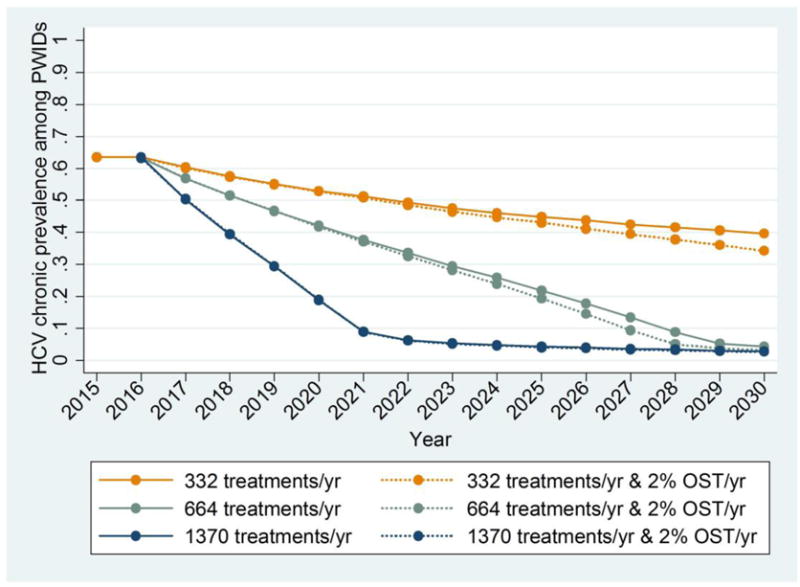
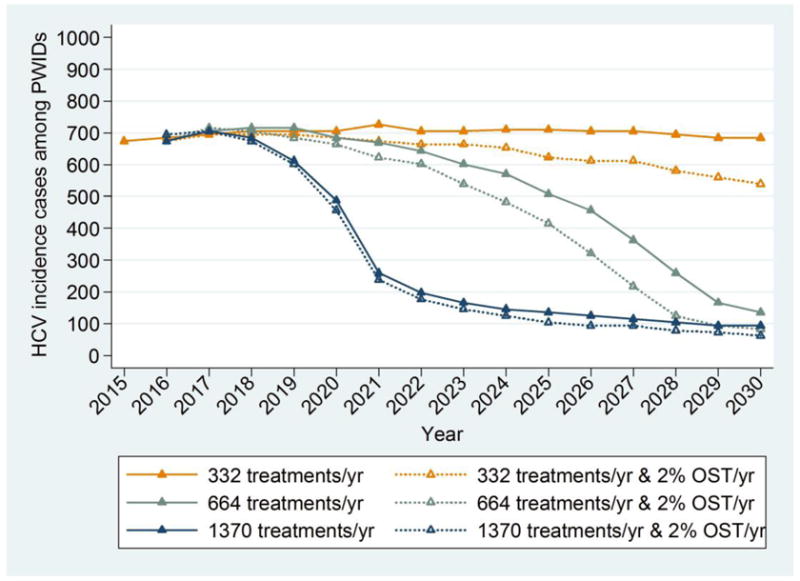
Model predictions for chronic HCV prevalence and incident cases among PWID in Athens for different annual increases in harm reduction coverage and annual antiviral treatments rates (solid line: harm reduction coverage same as in 2015, i.e. 44%, dotted line: increase in harm reduction coverage by 2% per year).
a. Chronic HCV Prevalence among PWID
b. New infections among PWID
Under high treatment coverage (664 PWID/year), an increase in harm reduction coverage of 2% every year would contribute an additional 1% (0.3%, 4.2%) absolute decrease in CHC prevalence and 38% (5%, 71.1%) lower incidence in 2030 compared to constant harm reduction coverage levels. Thus, under aggressive HCV antiviral treatments strategies (1370 PWID/year), the added contribution of harm reduction in reducing prevalence is minimal. However, it has a significant impact in the reduction of HCV incidence.
Coverage of treatment and harm reduction to reach fixed targets concerning CHC prevalence
A reduction in CHC prevalence or HCV incidence could be reached either by increasing treatment coverage, or harm reduction coverage, or both. Using a combined intervention, the necessary number of treatments for reaching specific targets in CHC prevalence or HCV incidence could be reduced.
The relationship between the annual treatment rates needed to reach a specific target in CHC prevalence and harm reduction coverage is approximately linear (within the range of 44%–70% harm reduction coverage): for example, from the model we have estimated that in order to halve CHC prevalence by 2030, the required antiviral treatment rate is 5%, 4.25% and 3.8% per year assuming a harm reduction coverage of 44% or immediate increase to 60% and 70% coverage, respectively. Using linear regression, it was estimated that, increasing the coverage of harm reduction by 10% reduces the required annual HCV treatment rate by about 0.46% (N=40 less HCV treatments per year) (Figure 4a) for halving prevalence by 2030. Similar results were found for the goal of a 75% reduction or decreasing CHC prevalence to less than 10% by 2030. No significant changes in the required treatment rates were observed if the target is to reduce CHC prevalence to less than 10% in the next five years (data not shown).
Figure 4.
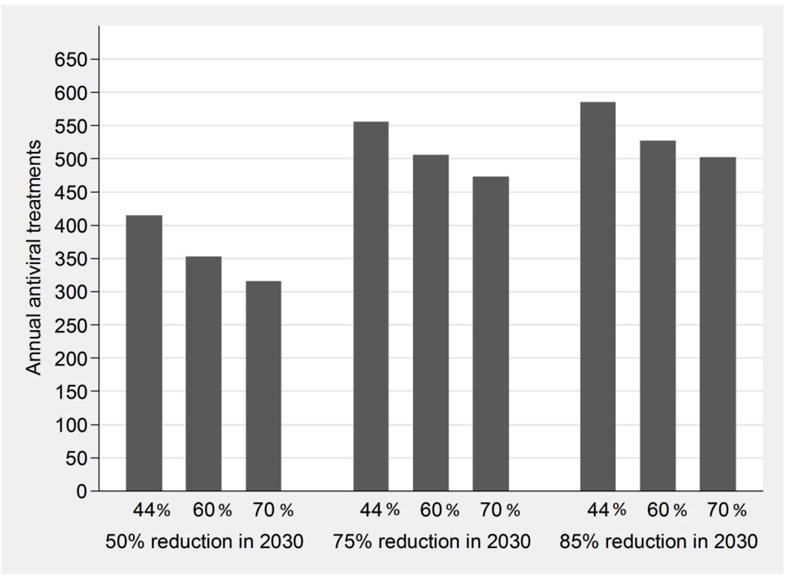
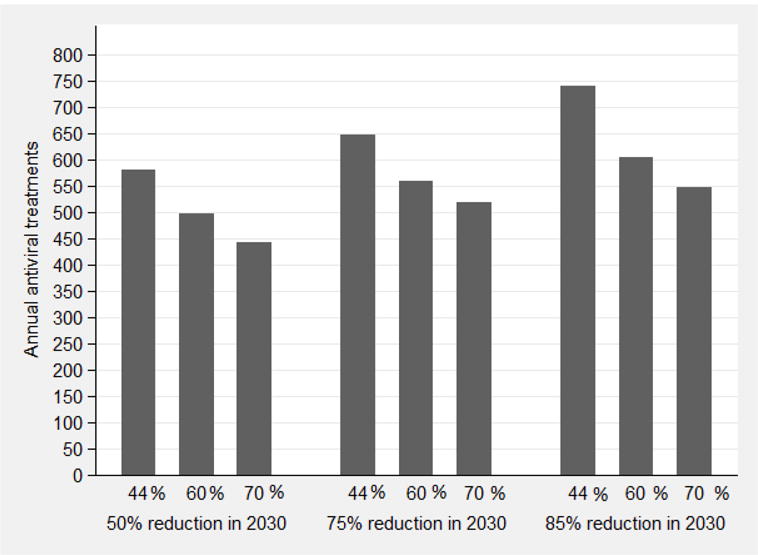
The impact of combining interventions with various annual antiviral treatment rates and increases in harm reduction coverage to achieve fixed targets in the reduction of CHC prevalence (4a) or HCV incidence (4b) among PWID by 2030 (50%, 75% or 85% reduction by 2030 compared to 2016). The bars correspond to scenarios of harm reduction coverage at 44%, 60% and 70% of PWID.
a. Chronic HCV Prevalence among PWID
b. New infections among PWID
The impact of harm reduction programmes was more pronounced for reducing incidence than CHC prevalence. Linear regression suggests that increasing the coverage of harm reduction by 10% would reduce the required annual HCV treatment rate by about 0.73% (N=60 less HCV treatments per year) (Figure 4b).
Sensitivity analysis
The sensitivity analysis shows that the variation in the average injecting duration substantially affected the forecasted impact of treatment on CHC prevalence in 2030 (Figure 5). Specifically, for shorter injecting duration (6 years instead of 12 years) the intervention impact decreased relatively by 24% while for longer injection duration (18 years instead of 12 years) impact increased by 17%.
Figure 5.
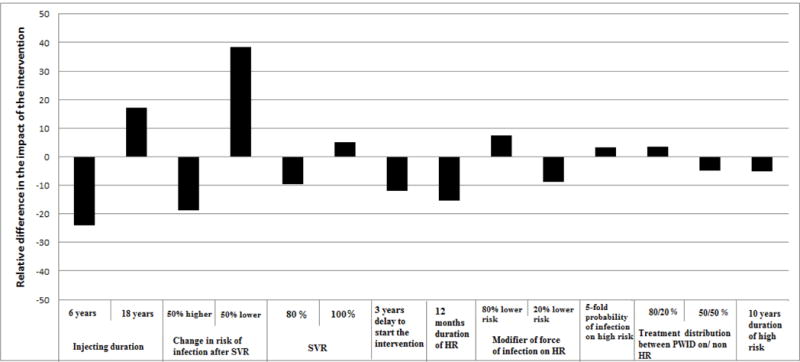
Results of one-way sensitivity analysis showing the relative difference in CHC prevalence projected in 2030 for varying parameters of the model compared to the base parameter values in Table 1. A value of zero describes no change from the projected CHC prevalence in 2030 compared to the base scenario. A positive or a negative value means that the projected CHC prevalence is lower or higher, respectively, compared to that projected under the base scenario. The scenario of 332 annual antiviral treatments was used.
Furthermore, potential changes in the risk behavior following successful treatment also affected the predictions of the model. Specifically, if risk behavior halved after treatment, the potential impact increased by 38.3% compared to the base case. Conversely, if the risk of infection increased after treatment - e.g. due to complacency caused by improvements in antiviral therapy - the anticipated impact was be 18.7% lower than the base case. Decreasing the duration of harm reduction programmes to 12 months, results in 14.7% lower impact of the intervention (Figure 5, Figure S1).
Delaying the initiation of the intervention (i.e. scale-up of treatment and harm reduction coverage) by 3 years resulted in 11.8% less impact. Variability in SVR also affected the intervention’s impact — if the SVR of DAAs was 80% (95% in base case), the estimated intervention impact would be 10% lower, whereas if SVR was 100%, the impact would increase by 5%. Other assumptions had <10% impact on the projections.
Discussion
This study examines the feasibility of HCV elimination among PWID in Athens using an integrated healthcare strategy, which considers a scale up in both antiviral treatment and harm reduction coverage. Using combined interventions, the benefits are optimized with the expansion of harm reduction programmes acting directly to reduce incidence while antiviral treatment acting directly on chronic prevalence (and subsequently incidence) of HCV infection. Our results support current recommendations from WHO (13) which suggest that HCV elimination among PWID is possible with large increases in HCV treatment coupled with modest increases in harm reduction levels, even in PWID populations with very high prevalence of HCV infection. More specifically, elimination is achievable by 2030 under treatment coverage rates of more than 644 PWID/year with simultaneous annual increase of 2% in harm reduction coverage. However, under aggressive treatment strategies (coverage of more than 1370 PWID/year), elimination would be possible in the next 4–5 years.
Although a 2% increase in harm reduction programs may seem optimistic in the context of austerity in Greece, it has to be pointed out that, during the first years of economic crisis, the Greek Organization Against Drugs substantially increased the number of centers providing opioid substitution treatment by 50% during 2011–2012 and, in cooperation with non-governmental organizations and the Hellenic Centre for Diseases Control and Prevention scaled up needle and syringe programmes (from 7 to 45 syringes per estimated PWID per year between 2010 and 2012) (44).
The assumption concerning HCV re-infection is an important part of the modeling procedure. In this paper, we have assumed that the risk of re-infection was equal to that of initial infection. Page et al (45) have shown similar incidence rates concerning initial infection and reinfection after spontaneous clearance. However, PWID completing treatment successfully may also exhibit reduced risk behavior, which would provide a greater prevention effect than we predict. As a result, our hypothesis of similar risk of infection and reinfection after treatment might be relatively conservative.
Moreover, in our model we do not allow for re-treatment of PWID in whom treatment fails. Based on the assumed SVR (90%–95%), we do not anticipate this to have a substantial impact on our prevalence/incidence impact estimates. Furthermore, based on a recent review (46), next-generation DAAs are anticipated to reduce resistance-associated substitutions.
Comparison with other studies
Our results are consistent with a previous modeling analysis showing that combination prevention with HCV DAA therapy and harm reduction could have a substantial impact on chronic prevalence and incidence among PWID populations (20). Importantly, this analysis explores an even higher prevalence setting than previously examined (64% chronic prevalence among PWID in Athens, compared to a maximum of 60% explored in Martin et al. (20). Notably, although our analysis may be seen as slightly more optimistic as we assume a long duration of participation in harm reduction programmes, we assume about a 60% efficacy of harm reduction programs in reducing an individual’s risk of acquiring HCV, whereas Martin’s analysis assumes approximately 50–60% on OST or HCNSP, and 80% combined. Overall, however, both these studies underline the significance of both expanding harm reduction programmes as well as ensuring PWID are retained on these interventions.
Strengths and Limitations
This paper is useful as it provides theoretical support that HCV elimination is possible under high treatment rates combined with harm reduction, even in high-prevalence settings like Athens. The strength of our model is that based on reliably epidemiological parameters which were obtained from a large sample of the PWID population in Athens (31, 32).
However, our analysis has also limitations. First, the model ignores the impact of social networks on HCV transmission and assumes that the population is totally mixed i.e. injectors have equal contact with all other injectors in the population. This hypothesis may lead to underestimation of the forecasted reduction of prevalence (47). Second, the model did not take into account HCV/HIV coinfection. The effect of coinfection on our projections is likely to be marginal because HIV/HCV coinfected PWID achieve similar SVR to HCV monoinfected PWID under DAA therapy (48) and the additional mortality risk amongst HIV infected PWID is likely to be small, over the duration of our projections, because most HIV-infected PWID were infected recently. A third limitation is that the model does not take into account issues related to identifying HCV-infected PWID. The majority of infected PWID are undiagnosed and unlinked to care, so the success of treatment as prevention interventions is inseparable with the success of case-finding of infected PWID. Furthermore, the high cost of antiviral treatment today is the main barrier for increasing the coverage of treatment. In this paper, we have not addressed this problem since currently, there is a considerable uncertainty concerning cost estimations as price negotiations on DAAs have been initiated in Greece and the costs should reduce in the near future. In addition, in assessing the impact of harm reduction and HCV treatment we have not taken into account the different costs associated with these interventions. Future work examining the relative cost-effectiveness and optimal allocation from a budgetary standpoint is needed. The cost of treating 664 or 1370 PWID per year with the current prices, i.e. before negotiations, would range between 22–31 and 45–63 million euros, respectively. Clearly, this cost is very high and underlines the need for negotiating to further reduce the cost of HCV care. Encouragingly, we show that increasing the coverage of harm reduction can reduce HCV incidence as well as the required treatments needed to reach a specific decline in prevalence and, thus, the cost of the strategies.
Finally, the model projections are sensitive to uncertainty in a number of underlying parameters. Sensitivity analyses provide evidence that under shorter injecting durations, i.e. if the population of PWID was renewed more rapidly, the effect of treatments would shrink due to less time to accrue treatment benefits. On the other hand, if injecting duration was longer than 12 years, the impact of treatment would be more substantial. More accurate estimation of injecting duration would reduce the uncertainty in the model projections. Our estimate of 12 years was obtained from a very large sample of PWID in Athens. According to data collected from PWID in treatment, the mean duration of injecting drug use was 10.1 years33. Adherence to therapy is a significant parameter that affects the efficacy of the intervention. More specifically, under low adherence e.g. if the assumed SVR is 80% instead of 95%, the impact of the intervention would be reduced by 10%. Another significant factor that contributes to the optimization of the intervention is any change in risk behavior after a successful treatment. Under the treatment as prevention strategy, treatment expansion to more recent PWID with higher infection risk behavior or younger PWID without major concerns about HCV reinfection could decrease the potential impact of the intervention(6, 49). Conversely, if the risk of reinfection following treatment is 50% lower than the primary risk of infection, then the impact of the intervention would be substantially higher. Therefore, treatment should be coupled with risk reduction counseling and behavior change interventions.
Conclusion
Overall, our results are based on a complex model with uncertainties concerning e.g. reinfection and re-treatment, new medications, possible changes in injection careers, network effects etc. However, in the absence of empirical data, modeling is useful in establishing the possibility and plausibility of the results, and indicates which parameters are most crucial in influencing the likely outcome. Our results, based on these theoretical modelling projections, show that it is plausible to observe substantial reduction in HCV incidence and prevalence in the high prevalence population of PWID in Athens using an integrated healthcare strategy. Elimination in the next 4–5 years is feasible by increasing treatments rates to over 1370 PWID per year. Using integrated strategies, the necessary treatments to achieve specific goals are reduced.
Supplementary Material
Acknowledgments
AH: Receipt of grants/research support: Gilead, Novartis, Co-Chair of Hepatitis B and C Public Policy Association funded by Gilead, Abbvie, BMS. Receipt of honoraria or consultation fees: Gilead, BMS.
Footnotes
Conflict of interest:
IG: No conflict of interest
VS: Receipt of honoraria or consultation fees: Gilead and Abbvie
OA: No conflict of interest
NM: Received research grants from Gilead, and honoraria from Merck, Abbvie, and Janssen
PV: Received grants from Gilead.
EK: No conflict of interest
References
- 1.Hatzakis A, Van Damme P, Alcorn IK, Gore C, Benazzouz M, Berkane S, et al. The state of hepatitis B and C in the Mediterranean and Balkan countries: report from a summit conference. Journal of viral hepatitis. 2013;20(Suppl 2):1–20. doi: 10.1111/jvh.12120. [DOI] [PubMed] [Google Scholar]
- 2.Ly ΚΝ, Xing J, Klevens RΜ, Jiles RΒ, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Annals of internal medicine. 2012;156:271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 3.Nelson PΚ, Mathers ΒΜ, Cowie Β, Hagan H, Des Jarlais D, Horyniak D, et al. Global epidemiology of hepatitis Β and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aceijas C, Rhodes T. Global estimates of prevalence of HCV infection among injecting drug users. The International journal on drug policy. 2007;18:352–358. doi: 10.1016/j.drugpo.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Vickerman P, Hickman Μ, May Μ, Kretzschmar Μ, Wiessing L. Can hepatitis C virus prevalence be used as a measure of injection-related human immunodeficiency virus risk in populations of injecting drug users? An ecological analysis, Addiction. 2010;105:311–318. doi: 10.1111/j.1360-0443.2009.02759.x. [DOI] [PubMed] [Google Scholar]
- 6.Grebely J, Dore GJ. Can hepatitis C virus infection be eradicated in people who inject drugs? Antiviral research. 2014;104:62–72. doi: 10.1016/j.antiviral.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Turner ΚM, Hutchinson S, Vickerman P, Hope V, Craine Ν, Palmateer Ν, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011;106:1978–1988. doi: 10.1111/j.1360-0443.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 8.Vickerman P, Page Κ, Maher L, Hickman Μ, Commentary on Nolan et al. Opiate substitution treatment and hepatitis C virus prevention: building an evidence base? Addiction. 2014;109:2060–2061. doi: 10.1111/add.12750. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vickerman P, Martin Ν, Turner Κ, Hickman Μ. Can needle syringe programmes opiate substitution therapy achieve substantial reductions in hepatitis C virus prevalence? Model projections for different epidemic settings. Addiction. 2012;107:1984–1995. doi: 10.1111/j.1360-0443.2012.03932.x. [DOI] [PubMed] [Google Scholar]
- 10.Martin ΝΚ, Vickerman P, Foster GR, Hutchinson SJ, Goldberg DJ, Hickman M. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modeling analysis of its prevention utility. Journal of hepatology. 2011;54:1137–1144. doi: 10.1016/j.jhep.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Sulkowski ΜS, Gardiner DF, Rodriguez-Torres Μ, Reddy ΚR, Hassanein Τ, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. The New England journal of medicine. 2014;370:211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 12.Feld JJ, Kowdley ΚV, Coakley Ε, Sigal S, Nelson DR, Crawford D, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. The New England journal of medicine. 2014;370:1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Global Health Strategy Sector on viral hepatitis, 2016–2021. 2015 [Google Scholar]
- 14.Martin ΝΚ, Vickerman P, Hickman M. Mathematical modelling of hepatitis C treatment for injecting drug users. Journal of theoretical biology. 2011;274:58–66. doi: 10.1016/j.jtbi.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 15.Martin ΝΚ, Vickerman P, Miners A, Foster GR, Hutchinson SJ, Goldberg DJ, et al. Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology. 2012;55:49–57. doi: 10.1002/hep.24656. [DOI] [PubMed] [Google Scholar]
- 16.Martin ΝΚ, Vickerman P, Grebely J, Hellard Μ, Hutchinson SJ, Lima VD, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58:1598–1609. doi: 10.1002/hep.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin ΝΚ, Foster GR, Vilar J, Ryder S, Cramp ME, Gordon F, et al. HCV treatment rates and sustained viral response among people who inject drugs in seven UK sites: real world results and modelling of treatment impact. Journal of viral hepatitis. 2015;22:399–408. doi: 10.1111/jvh.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellard ME, Jenkinson R, Higgs P, Stoove MA, Sacks-Davis R, Gold J, et al. Modelling antiviral treatment to prevent hepatitis C infection among people who inject drugs in Victoria, Australia. The Medical journal of Australia. 2012;196:638–641. doi: 10.5694/mja11.10981. [DOI] [PubMed] [Google Scholar]
- 19.Cousien A, Tran VC, Deuffic-Burban S, Jauffret-Roustide M, Dhersin JS, Yazdanpanah Y. Hepatitis C treatment as prevention of viral transmission and liver-related morbidity in persons who inject drugs. Hepatology. 2016;63:1090–1101. doi: 10.1002/hep.28227. [DOI] [PubMed] [Google Scholar]
- 20.Martin ΝΚ, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy, Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57(Suppl 2):S39–45. doi: 10.1093/cid/cit296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institutes of Health Consensus Development Conference Panel statement: management of hepatitis C. Hepatology. 1997;26:2S–10S. doi: 10.1002/hep.510260701. [DOI] [PubMed] [Google Scholar]
- 22.Hellard Μ, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence, Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;49:561–573. doi: 10.1086/600304. [DOI] [PubMed] [Google Scholar]
- 23.Dore GJ, Hellard M, Matthews GV, Grebely J, Haber PS, Petoumenos Κ, et al. Effective treatment of injecting drug users with recently acquired hepatitis C virus infection. Gastroenterology. 2010;138:123–135. e121–122. doi: 10.1053/j.gastro.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Backmund Μ, Meyer Κ, Von Zielonka Μ, Eichenlaub D. Treatment of hepatitis C infection in injection drug users. Hepatology. 2001;34:188–193. doi: 10.1053/jhep.2001.25882. [DOI] [PubMed] [Google Scholar]
- 25.Reimer J, Schulte Β, Castells X, Schafer I, Polywka S, Hedrich D, et al. Guidelines for the treatment of hepatitis C virus infection in injection drug users: status quo in the European Union countries. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;40(Suppl 5):S373–378. doi: 10.1086/427456. [DOI] [PubMed] [Google Scholar]
- 26.Foster GR. Injecting drug users with chronic hepatitis C: should they be offered antiviral therapy? Addiction. 2008;103:1412–1413. doi: 10.1111/j.1360-0443.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- 27.Dalgard Ο. Follow-up studies of treatment for hepatitis C virus infection among injection drug users. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;40(Suppl 5):S336–338. doi: 10.1086/427449. [DOI] [PubMed] [Google Scholar]
- 28.Midgard Η, Bjoro Β, Maeland A, Konopski Z, Kileng Η, Damas JK, et al. Hepatitis C Reinfection Following Sustained Virological Response - Seven-Year Follow-Up Of Patients Infected Through Injecting Drug Use. Journal of hepatology . 2016 [Google Scholar]
- 29.European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. Journal of hepatology. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 30.European Association for Study of L. EASL Recommendations on Treatment of Hepatitis C 2015. Journal of hepatology. 2015;63:199–236. doi: 10.1016/j.jhep.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 31.Sypsa V, Paraskevis D, Malliori Μ, Nikolopoulos GΚ, Panopoulos A, Kantzanou Μ, et al. Homelessness and Other Risk Factors for HIV Infection in the Current Outbreak Among Injection Drug Users in Athens, Greece. American journal of public health. 2015;105:196–204. doi: 10.2105/AJPH.2013.301656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatzakis A, Sypsa V, Paraskevis D, Nikolopoulos G, Tsiara C, Micha Κ, et al. Design and baseline findings of a large-scale rapid response to an HIV outbreak in people who inject drugs in Athens, Greece: the ARISTOTLE programme. Addiction. 2015;110:1453–1467. doi: 10.1111/add.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paraskevis D, Nikolopoulos G, Tsiara C, Paraskeva D, Antoniadou A, Lazanas Μ, et al. HIV-1 outbreak among injecting drug users in Greece, 2011: a preliminary report. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2011:16. doi: 10.2807/ese.16.36.19962-en. [DOI] [PubMed] [Google Scholar]
- 34.Drugs Ν. C O D A I O Annual Report. 2014 [Google Scholar]
- 35.Hatzakis A, Chulanov V, Gadano AC, Bergin C, Ben-Ari Ζ, Mossong J, et al. The present and future disease burden of hepatitis C virus (HCV) infections with today’s treatment paradigm - volume 2. Journal of viral hepatitis. 2015;22(Suppl 1):26–45. doi: 10.1111/jvh.12351. [DOI] [PubMed] [Google Scholar]
- 36.Cousien A, Tran VC, Deuffic-Burban S, Jauffret-Roustide Μ, Dhersin JS, Yazdanpanah Υ. Dynamic modelling of hepatitis C virus transmission among people who inject drugs: a methodological review. Journal of viral hepatitis. 2015;22:213–229. doi: 10.1111/jvh.12337. [DOI] [PubMed] [Google Scholar]
- 37.Sypsa V, Touloumi G, Tassopoulos ΝC, Ketikoglou I, Vafiadis I, Hatzis G, et al. Reconstructing and predicting the hepatitis C virus epidemic in Greece: increasing trends of cirrhosis and hepatocellular carcinoma despite the decline in incidence of HCV infection. Journal of viral hepatitis. 2004;11:366–374. doi: 10.1111/j.1365-2893.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 38.ECDC. Migrant health: Access to HIV prevention, treatment and care for migrant populations in EU/EEA countries. 2009 [Google Scholar]
- 39.Mathers ΒM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman Μ. Mortality among people who inject drugs: a systematic review and meta-analysis. Bulletin of the World Health organization. 2013;91:102–123. doi: 10.2471/BLT.12.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dore GJ. The changing therapeutic landscape for hepatitis C. The Medical journal of Australia. 2012;196:629–632. doi: 10.5694/mja11.11531. [DOI] [PubMed] [Google Scholar]
- 41.Gane ΕJ, Stedman CA, Hyland RΗ, Ding X, Svarovskaia E, Symonds WΤ, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. Ν Engl J Med. 2013;368:34–44. doi: 10.1056/NEJMoa1208953. [DOI] [PubMed] [Google Scholar]
- 42.Poordad F, Lawitz Ε, Kowdley ΚV, Cohen DΕ, Podsadecki Τ, Siggelkow S, et al. Exploratory study of oral combination antiviral therapy for hepatitis C. Ν Engl J Med. 2013;368:45–53. doi: 10.1056/NEJMoa1208809. [DOI] [PubMed] [Google Scholar]
- 43.Poordad F, Mccone J, Bacon ΒR, JR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. Ν Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malliori Μ, Golna C, Souliotis K, Hatzakis A. Managing opioid dependence treatment and controlling for HIV incidence among injecting drug users in Greece: a case study of optimism in the face of adversity. Addiction. 2013;108:1174–1175. doi: 10.1111/add.12179. [DOI] [PubMed] [Google Scholar]
- 45.Page Κ, Hahn JA, Evans J, Shiboski S, Lum P, Delwart Ε, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. The Journal of infectious diseases. 2009;200:1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forton DM. How much of a problem is resistance in treating hepatitis C? Current opinion in infectious diseases. 2016 doi: 10.1097/QCO.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 47.Hellard M, McBryde Ε, Sacks Davis R, Rolls DA, Higgs P, Aitken C, et al. Hepatitis C transmission and treatment as prevention - The role of the injecting network. The International journal on drug policy. 2015;26:958–962. doi: 10.1016/j.drugpo.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Shafran SD. HIV Coinfected Have Similar SVR Rates as HCV Monoinfected With DAAs: It’s Time to End Segregation and Integrate HIV Patients Into HCV Trials. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;61:1127–1134. doi: 10.1093/cid/civ438. [DOI] [PubMed] [Google Scholar]
- 49.Valerio Η, Goldberg DJ, Lewsey J, Weir A, Allen S, Aspinall ΕJ, et al. Evidence of continued injecting drug use after attaining sustained treatment-induced clearance of the hepatitis C virus: Implications for reinfection. Drug and alcohol dependence. 2015;154:125–131. doi: 10.1016/j.drugalcdep.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 50.Cornish R, Macleod J, Strang J, Vickerman P, Hickman M. Risk of death during and after opiate substitution treatment in primary care: prospective observational study in UK General Practice Research Database. Bmj. 2010;341:c5475. doi: 10.1136/bmj.c5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoove ΜA, Dietze PΜ, Aitken CK, Jolley D. Mortality among injecting drug users in Melbourne: a 16-year follow-up of the Victorian Injecting Cohort Study (VICS) Drug and alcohol dependence. 2008;96:281–285. doi: 10.1016/j.drugalcdep.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Jarrin I, Pantazis Ν, Gill ΜJ, Geskus R, Perez-Hoyos S, Meyer L, et al. Uptake of combination antiretroviral therapy and HIV disease progression according to geographical origin in seroconverters in Europe, Canada, and Australia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54:111–118. doi: 10.1093/cid/cir814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


