Abstract
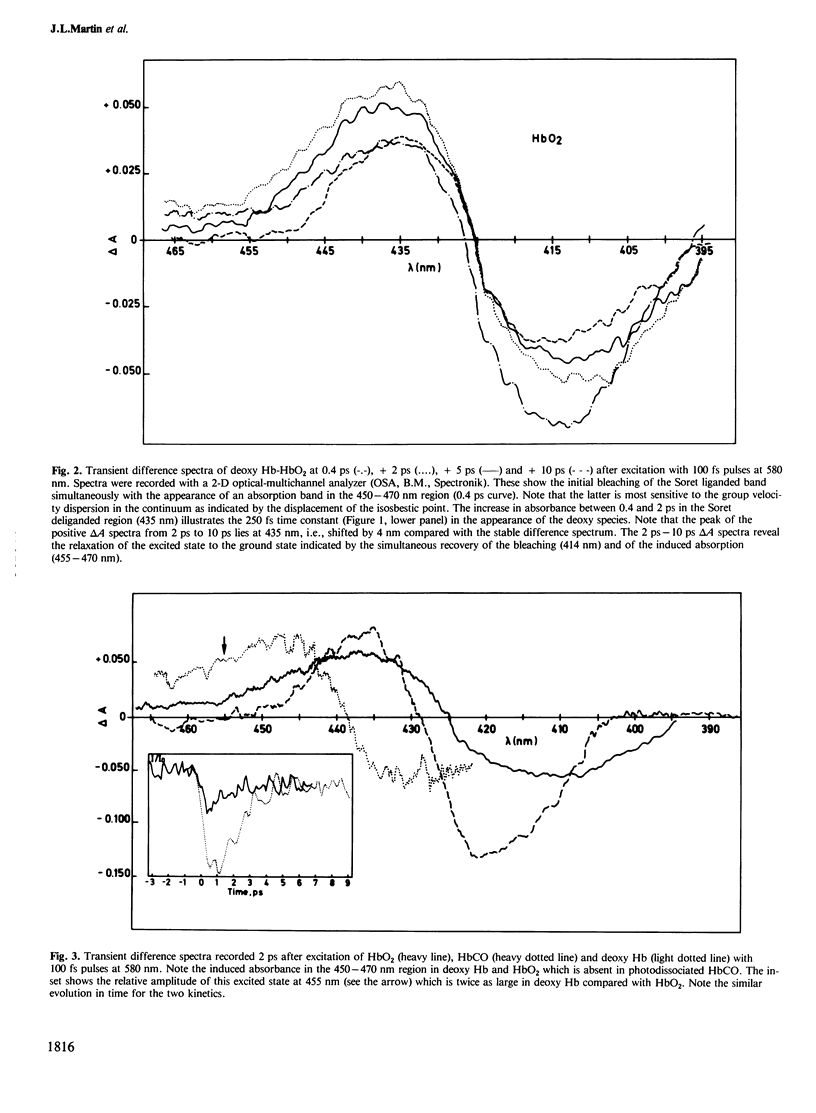
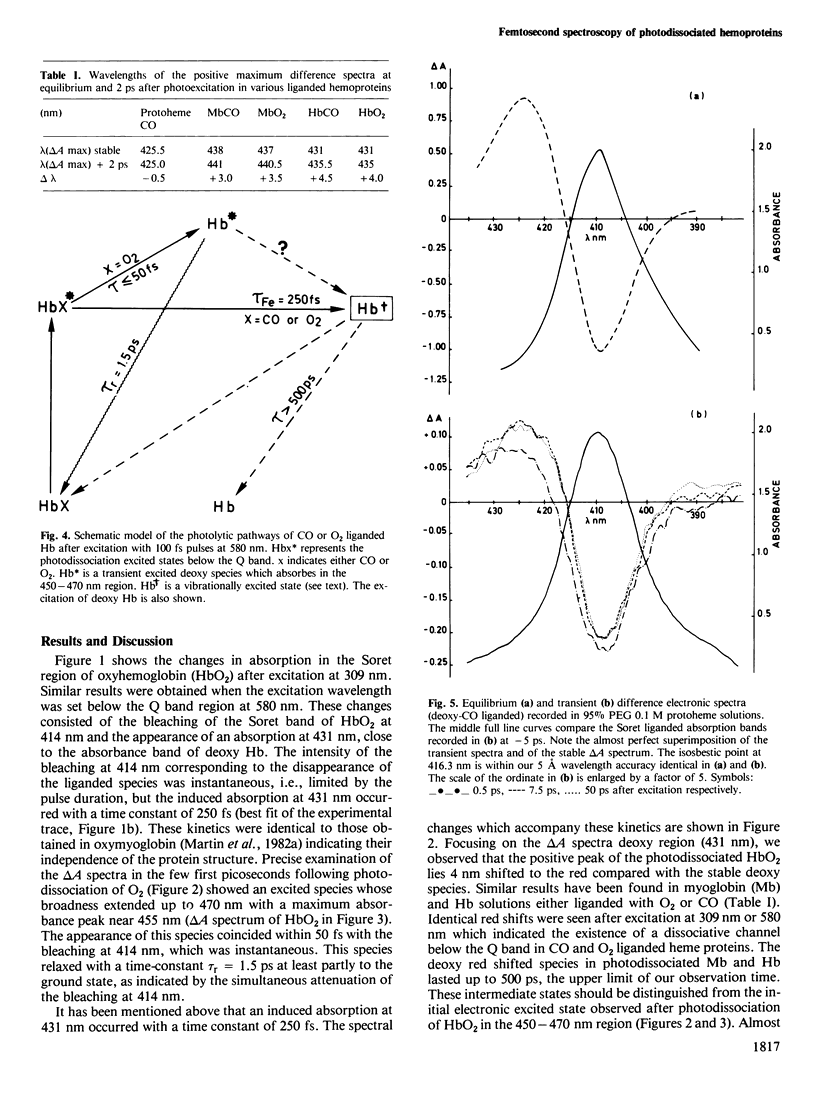
We have measured spectral and kinetic differences in protoheme, sperm whale or horse heart myoglobin and human hemoglobin following photodissociation induced by optical pulses of 80 fs duration. Full ligation was performed with oxygen or carbon monoxide. Femtosecond kinetics and transient difference spectra revealed the appearance of a deoxy species with tau approximately equal to 250-300 fs. The transient deoxy species in myoglobin and hemoglobin evidenced a 3-4 nm red shift of their delta A spectra compared with the equilibrium delta A spectrum. This shift was not observed after photodissociation of the carbon monoxide liganded protoheme. We proposed that the 250 fs time constant corresponding to the appearance of the deoxy-like species is related to the displacement of the ferrous iron out of the heme plane. Consequently, the small red shift of the delta A spectra observed in photodissociated hemoproteins may be tentatively attributed to changes in the vibrational modes of either the proximal histidine-Fe2+ bond and/or of the N4 porph-Fe-N epsilon His (F8) bent.
Full text
PDF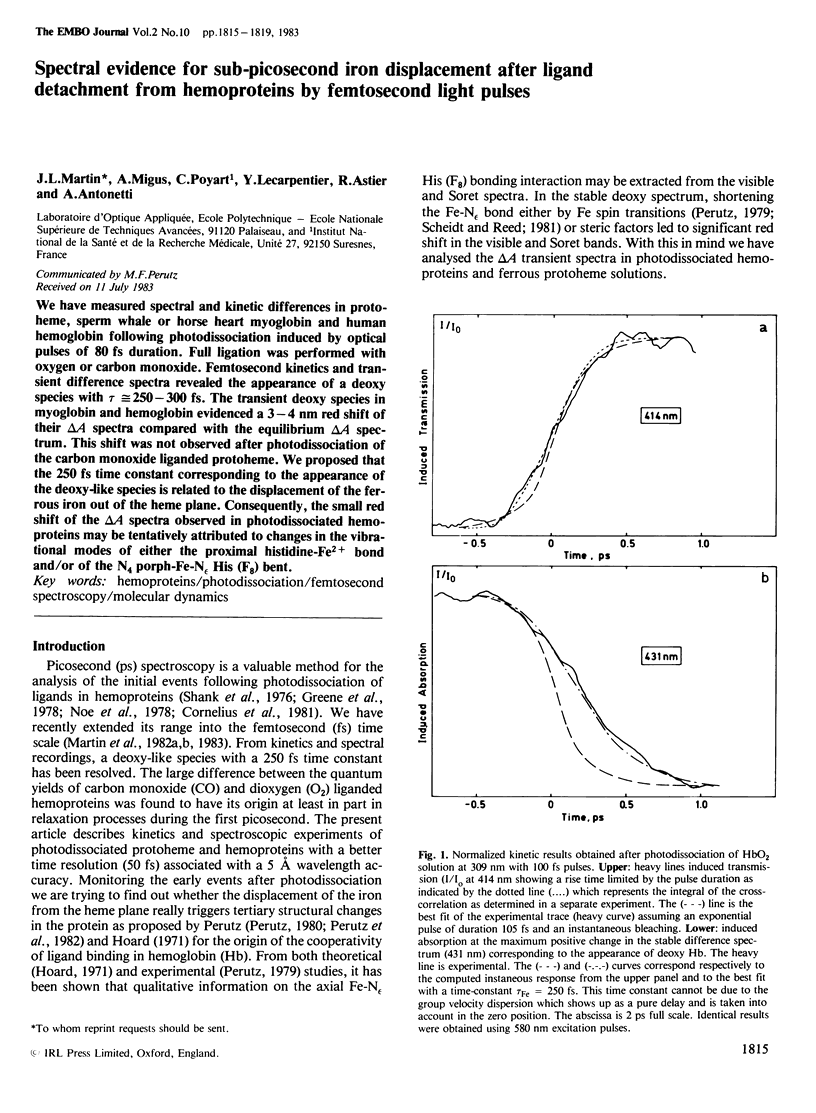


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornelius P. A., Steele A. W., Chernoff D. A., Hochstrasser R. M. Different dissociation pathways and observation of an excited deoxy state in picosecond photolysis of oxy- and carboxymyoglobin. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7526–7529. doi: 10.1073/pnas.78.12.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoard J. L. Stereochemistry of hemes and other metalloporphyrins. Science. 1971 Dec 24;174(4016):1295–1302. doi: 10.1126/science.174.4016.1295. [DOI] [PubMed] [Google Scholar]
- Irwin M. J., Atkinson G. H. Low-frequency resonance Raman spectroscopy of the deoxyhaemoglobin transient of photolysed carboxyhaemoglobin. Nature. 1981 Sep 24;293(5830):317–318. doi: 10.1038/293317a0. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Migus A., Poyart C., Lecarpentier Y., Antonetti A., Orszag A. Femtosecond photodissociation and picosecond recombination of O2 in myoglobin: a plausible explanation for the low quantum yield in MbO2. Biochem Biophys Res Commun. 1982 Aug;107(3):803–810. doi: 10.1016/0006-291x(82)90594-0. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Migus A., Poyart C., Lecarpentier Y., Astier R., Antonetti A. Femtosecond photolysis of CO-ligated protoheme and hemoproteins: appearance of deoxy species with a 350-fsec time constant. Proc Natl Acad Sci U S A. 1983 Jan;80(1):173–177. doi: 10.1073/pnas.80.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Kitagawa T., Morimoto H. Quaternary structures and low frequency molecular vibrations of haems of deoxy and oxyhaemoglobin studied by resonance raman scattering. J Mol Biol. 1980 Jan 25;136(3):271–289. doi: 10.1016/0022-2836(80)90374-5. [DOI] [PubMed] [Google Scholar]
- Noe L. J., Eisert W. G., Rentzepis P. M. Picosecond photodissociation and subsequent recombination processes in carbon monoxide hemoglobin. Proc Natl Acad Sci U S A. 1978 Feb;75(2):573–577. doi: 10.1073/pnas.75.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe L. J., Eisert W. G., Rentzepis P. M. Picosecond photodissociation and subsequent recombination processes in carbon monoxide hemoglobin. Proc Natl Acad Sci U S A. 1978 Feb;75(2):573–577. doi: 10.1073/pnas.75.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondrias M. R., Rousseau D. L., Shelnutt J. A., Simon S. R. Quaternary-transformation-induced changes at the heme in deoxyhemoglobins. Biochemistry. 1982 Jul 6;21(14):3428–3437. doi: 10.1021/bi00257a028. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Hasnain S. S., Duke P. J., Sessler J. L., Hahn J. E. Stereochemistry of iron in deoxyhaemoglobin. Nature. 1982 Feb 11;295(5849):535–538. doi: 10.1038/295535a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Regulation of oxygen affinity of hemoglobin: influence of structure of the globin on the heme iron. Annu Rev Biochem. 1979;48:327–386. doi: 10.1146/annurev.bi.48.070179.001551. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Phillips S. E. Structure and refinement of oxymyoglobin at 1.6 A resolution. J Mol Biol. 1980 Oct 5;142(4):531–554. doi: 10.1016/0022-2836(80)90262-4. [DOI] [PubMed] [Google Scholar]
- Rothgeb T. M., Gurd F. R. Physical methods for the study of myoglobin. Methods Enzymol. 1978;52:473–486. doi: 10.1016/s0076-6879(78)52052-1. [DOI] [PubMed] [Google Scholar]
- Shaanan B. The iron-oxygen bond in human oxyhaemoglobin. Nature. 1982 Apr 15;296(5858):683–684. doi: 10.1038/296683a0. [DOI] [PubMed] [Google Scholar]
- Shank C. V., Ippen E. P., Bersohn R. Time-resolved spectroscopy of hemoglobin and its complexes with subpicosecond optical pulses. Science. 1976 Jul 2;193(4247):50–51. doi: 10.1126/science.935853. [DOI] [PubMed] [Google Scholar]
- Terner J., Stong J. D., Spiro T. G., Nagumo M., Nicol M., El-Sayed M. A. Picosecond resonance Raman spectroscopic evidence for excited-state spin conversion in carbonmonoxy-hemoglobin photolysis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1313–1317. doi: 10.1073/pnas.78.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. M., Brinigar W. S. A correlation of the visible and Soret spectra of dioxygen- and carbon monoxide-heme complexes and five-coordinate heme complexes with the spectra of oxy-, carboxy-, and deoxyhemoglobins. Biochemistry. 1979 Oct 30;18(22):4960–4977. doi: 10.1021/bi00589a026. [DOI] [PubMed] [Google Scholar]



