Abstract
The genus Allium is a rich source of steroidal saponins, and its medicinal properties have been attributed to these bioactive compounds. The saponin compounds with diverse structures play a pivotal role in Allium’s defense mechanism. Despite numerous studies on the occurrence and chemical structure of steroidal saponins, their biosynthetic pathway in Allium species is poorly understood. The monosomic addition lines (MALs) of the Japanese bunching onion (A. fistulosum, FF) with an extra chromosome from the shallot (A. cepa Aggregatum group, AA) are powerful genetic resources that enable us to understand many physiological traits of Allium. In the present study, we were able to isolate and identify Alliospiroside A saponin compound in A. fistulosum with extra chromosome 2A from shallot (FF2A) and its role in the defense mechanism against Fusarium pathogens. Furthermore, to gain molecular insight into the Allium saponin biosynthesis pathway, high-throughput RNA-Seq of the root, bulb, and leaf of AA, MALs, and FF was carried out using Illumina's HiSeq 2500 platform. An open access Allium Transcript Database (Allium TDB, http://alliumtdb.kazusa.or.jp) was generated based on RNA-Seq data. The resulting assembled transcripts were functionally annotated, revealing 50 unigenes involved in saponin biosynthesis. Differential gene expression (DGE) analyses of AA and MALs as compared with FF (as a control) revealed a strong up-regulation of the saponin downstream pathway, including cytochrome P450, glycosyltransferase, and beta-glucosidase in chromosome 2A. An understanding of the saponin compounds and biosynthesis-related genes would facilitate the development of plants with unique saponin content and, subsequently, improved disease resistance.
Introduction
Allium is an enormous genus (850 species) that stretches broadly across the northern hemisphere from the boreal zone to the dry subtropics [1–3]. A region with diverse ecological niches led to the development of an astonishing number of Allium species with different morphological and physiological traits [1]. Due to their culinary and medicinal properties, many plants of this genus [A. cepa (onion), A. cepa Aggregatum group (shallot), A. fistulosum (Japanese bunching onion), A. sativum (garlic), A. ampeloprasum (leek), and A. tuberosum (Chinese chives)] have significant economic importance worldwide as vegetables or medicinal plants [4–6]. However, genetic shifts and drastic unbalance selection by farmers and breeders have caused the loss of many useful agronomic traits in Allium [1]. Therefore, to develop disease-resistant Allium germplasm, novel alleles with desirable physiological attributes can be introduced by crossing with disease-resistant cultivars or wild relatives [1, 3, 4, 7].
The shallot is a species of subtropical origin that has been recognized as a potential genetic resource for Allium crop improvements because of their adaptability to environmental stresses [8, 9]. However, the molecular and physiological architecture underlying this tolerability is still unclear. The utilization of monosomic addition lines (MALs) as valuable genetic resources for understanding physiological traits has been reported in several plant species, including the Beta vulgaris L. genome with the addition of chromosome 9 from B. corolliflora to improve salt stress [10, 11] and the Brassica napus genome mediated by one alien chromosome from Orychophragmus violaceus for understanding the metabolism pathways regulating brassinosteroid (BR) biosynthesis and the role of auxin signaling in gynoecium development [12]. Our previous studies have revealed the significance of utilizing a shallot (AA) chromosomal engineering technique to improve A. fistulosum (FF) physiological traits that exhibited interesting phenotypes [13, 14]. MALs of A. fistulosum with an extra chromosome from the shallot enhanced the flavonoid [15], carbohydrate [16], cysteine sulfoxide [17] and saponin [18] contents. The increased saponin content in FF2A line was positively correlated with increased Fusarium disease resistance index [18]. These findings give insight into the significant role of shallot saponin in the disease resistance improvement of A. fistulosum despite the fact that the casual genes regulating this biosynthesis process in Allium are still unknown.
Steroidal saponins are synthesized via the mevalonic acid (MVA) pathway in the cytoplasm [19] and/or through the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway in plastids [20]. The cyclization of precursor compound 2, 3-oxidosqualene by oxidosqualene cyclase (OSC) combined with steroidal skeleton modifications through hydroxylation and glycosylation leads to the formation of various saponin compounds [21]. Several OSC-related genes, such as cycloartenol synthase (CAS), lupeol synthase (LS), and beta-amyrin synthase (β-AS), have been isolated from various plant systems [22–24]. According to the proposed pathway, some specific cytochrome P450, UDP-glucosyltransferases (UGT), and beta-glucosidase protein encoding genes are involved in the cyclization of the downstream pathway of the saponin biosynthesis [21, 25–27]. Despite many studies on the chemical structure and pharmaceutical activities of steroidal saponins, little is known about the molecular mechanism of the cyclization process involved at the downstream level.
For large-scale transcriptome analysis, next-generation sequencing (NGS) has rapidly evolved into an expedient technique for providing huge expression data in a much shorter time and has accelerated our understanding of metabolic pathways as well as contributing to gene discovery [9, 26, 28]. Transcriptome analysis, followed by the identification of prospective candidate genes involved in the secondary metabolic pathway, will lead to further understanding of biosynthesis and the diversity of secondary metabolites [29]. In the present study, we have performed phytochemical analyses to identify the shallot-specific saponin compound detected in FF2A, using column chromatography and two-dimensional nuclear magnetic resonance (2D NMR) spectroscopy. Phytochemical analyses resulted in the isolation and identification of a spirostanol saponin compound named Alliospiroside A. The antifungal activity of Alliospiroside A, the furostanol saponin fraction, and the root crude saponin extracts of AA, MALs (MALs = FF1A, FF2A, FF3A, FF4A, FF5A, FF6A, FF7A, and FF8A), and FF was examined against different Fusarium pathogens. Furthermore, to identify the candidate genes involved in saponin biosynthesis, high-throughput transcriptome analyses of the root, bulb, and leaf of AA, MALs, and FF were performed using NGS technology based on Illumina’s HiSeq 2500 platform. The resulting assembled transcripts were functionally annotated and used for DGE analyses of AA and MALs compared with FF (as a control). The DGE data were further used for saponin pathway analyses. The RNA-Seq data set including contigs length, nucleotide sequences, amino acid sequences, annotation, and expression values has been submitted to the open access Allium Transcript Database (Allium TDB, http://alliumtdb.kazusa.or.jp). Our ultimate goal is to discover the candidate genes that encode enzymes in the steroidal saponin biosynthetic pathway and to provide an overview of transcriptome dynamics in the different tissues as well as the role of shallot saponin in the defense mechanism against Fusarium pathogens. Our transcriptome dataset is a valuable and unique resource that will facilitate future functional genetics studies and molecular marker development for Allium breeding.
Materials and methods
Plant materials
Allium cepa Aggregatum group, monosomic lines, and A. fistulosum were grown in clay pots filled with sand (one plant per pot) under the same conditions at the Yamaguchi University greenhouse. The average temperature was 20 ± 2°C, relative humidity 78% and 10 h daylight length. Water and fertilizers were applied equally for all genotypes on weekly base. Plants were collected from each line separately in biological replica (n = 3). After cleaning, the root, bulb, and leaf [3 replicates × 3 tissues × 10 genotypes (AA, eight MALs, and FF)] were cut separately and immediately frozen in liquid nitrogen for RNA extraction and phytochemical analyses.
AA, MALs, and FF saponin profiling
The extraction of the saponin from AA, MALs, and FF root was carried out in accordance with the procedures of Mostafa et al. [4]. Freeze-dried root (2 g) was exhaustively extracted at room temperature with the following solvents: 20 ml of n-hexane and 50 ml of 70% MeOH. Each solvent extraction step was conducted for one day and was repeated three times with 30 min of sonication and filtration. The MeOH extract was dried in a rotary evaporator with a vacuum pump (v-700; BUCHI, Rotavapor R-3, BÜCHI Labortechnik AG Postfach, Switzerland) under reduced pressure at 50°C and then partitioned between n-Butanol (BuOH) and H2O (1:1, v/v). The BuOH layer was filtered and concentrated under vacuum to afford saponin crude extracts. A small amount of the extracts (10–20 μl) were chromatographed using thin-layer chromatography (TLC) silica gel plates (60 F254; Merck KgaA, Darmstadt, Germany). The chromatogram was developed with CHCl3:MeOH:H2O (30:15:2.5, v/v/v). The plates were dried, and the spots were visualized using a p-anisaldehyde reagent for total saponins and Ehrlich's reagent for furostanol saponins.
Determination of total saponin contents in the root, bulb and leaf of AA, MALs and FF
The extraction of the saponin from AA, MALs, and FF root, bulb and leaf was carried out as described above [4]. Total saponin contents were determined spectrophotometrically at 473 nm [3]. Saponin concentrations were calculated based on disogenin standard. All chemical extractions consisted of three replications.
Extraction and isolation of shallot-specific saponin compounds
The extraction of saponins from FF2A root (25 g) was carried out as mentioned above. Aliquot of the crude extract was chromatographed by C300 silica gel column chromatography (3 cm × 60 cm; AG Tokyo, Japan). The column was developed using a gradient solvent system, starting with CHCl3, CHCl3:MeOH (9:1–1:9), MeOH, and MeOH:H2O (9:1–7:4) as eluents to give 8 fractions after the evaporation of solvents (F1–F8). Each fraction was rechromatographed using TLC silica gel plates. The chromatogram was developed with CHCl3:MeOH:H2O (30:15:2.5, v/v/v). Fraction 2 yielded a 10 mg pure compound that was subjected to 2D NMR analysis.
2D NMR spectroscopy
The structure of isolated saponin compound was elucidated using 2D NMR. Optical rotations were determined with the JASCO DIP-1000 digital polarimeter. 13C and 1H NMR spectra were recorded in a pyridine-d5 solution at 500 and 125 MHz, respectively, using the JEOL ECA 500 spectrometer. The J values were expressed in hertz, and chemical shifts (δ) in parts per million (ppm), using pyridine-d5 for 13C NMR (123.5 ppm) and 1H NMR (7.20 ppm). The high-resolution electrospray ionization mass spectrometry was recorded using the JEOL JMS-T100LP spectrometer.
Biological assays
The antifungal activity of saponin extracts from AA, MALs, and FF root was examined against F. oxysporum f. sp. cepa strains TA and AF22. Pathogens were obtained from the Laboratory of Plant Molecular Pathology, Yamaguchi University, Japan. Antifungal activity was evaluated with the agar plate diffusion method, using 3.2-cm diameter Perspex plates with potato dextrose agar (PDA). Crude saponins were added to obtain the final concentration of 1000 μg ml-1, and the plates were inoculated with a 5-mm plug that contained the fungi grown on PDA for 5 days. The plates were incubated at 25 ± 2°C and the fungal radical growth was measured after one week. The antifungal activity of the furostanol saponin fraction and Alliospiroside A was examined against the respective strains as mentioned above at final concentration of 200 μg ml-1. All experiments were conducted in three replicates (n = 3).
Construction of unigenes and functional annotation
Unigene sets were constructed by assembling the Illumina RNA-Seq sampled from the bulb of AA. The cDNA library was prepared in accordance with Illumina's protocol, and sequencing was performed using Illumina’s HiSeq 2500 platform. The reads including adapter sequence and unknown nucleotides more than 5% and low-quality nucleotides (QV≤10) more than 20% in length were respectively excluded. The remained reads were assembled into contigs by Trinity r20121005 [30] with parameters,—seqType fq—min_contig_length 100—group_pairs_distance 250—path_reinforcement_distance 85—min_kmer_cov 2. In each sample, the contigs were clustered by TGI Clustering Tool (TGICL) v2.1 [31] with parameters, -l 40 -c 10 -v 20, and further assembled into unigenes by Phrap 23.0 [32, 33] with parameters, -repeat stringency 0.95 -minmatch 35 -minscore 35. A total of 56,161 obtained unigene sequences were searched against the databases, the Arabidopsis Information Resource (TAIR10; http://www.arabidopsis.org), RAP-DB (International Rice Genome Sequencing Project (IRGSP-1.0; http://rgp.dna.affrc.go.jp/IRGSP/), and the NCBI’s non-redundant proteins (nr) database (http://www.ncbi.nlm.nih.gov), using BLASTX [34] program with an E-value cutoff of 1E-10. The functional categories of these unigene sets were assigned using Gene Ontology (GO) database (http://geneontology.org).
RNA sequencing and read mapping of AA, MALs and FF
Total RNA was isolated using RNeasy Plant Mini Kit (QIAGEN Sciences, Germantown, Maryland, USA). Total RNA was assessed using BioSpec-nano (Shimadzu, Kyoto, Japan), and an additional RNA quality check was completed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Samples with RNA integrity number (RIN) values of more than 8.0 were selected for further use. The cDNA library was generated using TruSeq™ RNA Sample Preparation Kit (Illumina, San Diego, CA, USA) in accordance with the manufacturer's instructions. Briefly, oligo (dT) beads were used to purify poly (A) mRNA from total RNA. mRNA was fragmented using an RNA fragmentation kit (Ambion Life Technologies, USA). First-strand cDNA was synthesized from the fragmented mRNA using random hexamer primers and reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA, USA). The cDNA library was prepared in accordance with Illumina's protocol, and sequencing was performed using Illumina’s HiSeq 2500 platform. The trimmed RNA-Seq reads of each sample (AA, MALs, and FF) were respectively mapped onto the unigene sequences by Bowtie 2 ver. 2.2.0 with the end-to-end mode. The paired-end reads were treated as single-end reads. Based on the number of reads mapped onto the unigene sequences, the RPKM (Reads Per Kilobase per Million mapped reads) value of each gene was calculated by an in-house Perl script. We tested for differences between the normalized means of AA and MALs compared with FF as a control. Comparisons were accepted to be significant at adjusted-P value ≤ 0.05 with RPKM fold change ≥ 2 (up-regulated) and ≤ 0.5 (down-regulated) using R v3.2.2 (https://www.r-project.org).
Saponin pathway analyses
Saponin pathway assignments were carried out using online KEGG mapper (http://www.genome.jp/kegg/tool/map_pathway2.html).
Submitting RNA-Seq data
The obtained RNA-Seq data from AA, MALs and FF were submitted in DNA Data Bank of Japan (DDBJ) under the following accession numbers:
Submission: DRA005096 (hirakawa-0068_Submission)
BioProject: PRJDB3595 (PSUB004388)
BioSample: SAMD00059523-SAMD00059582 (SSUB006662)
Experiment: DRX062890-DRX062949 (hirakawa-0068_Experiment_0001–0060)
Run: DRR068940-DRR068999 (hirakawa-0068_Run_0001–0060)
Results
Genetic effects of the A. cepa Aggregatum group on the saponin profile of A. fistulosum
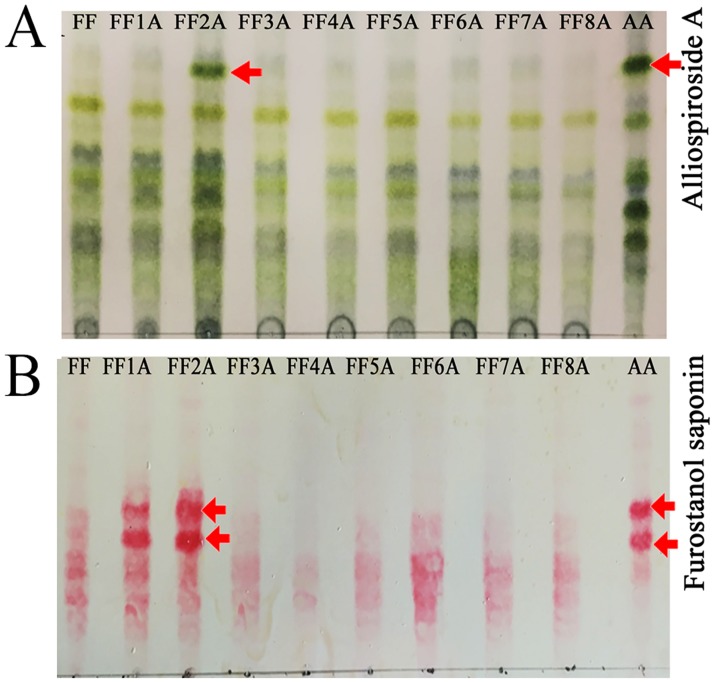
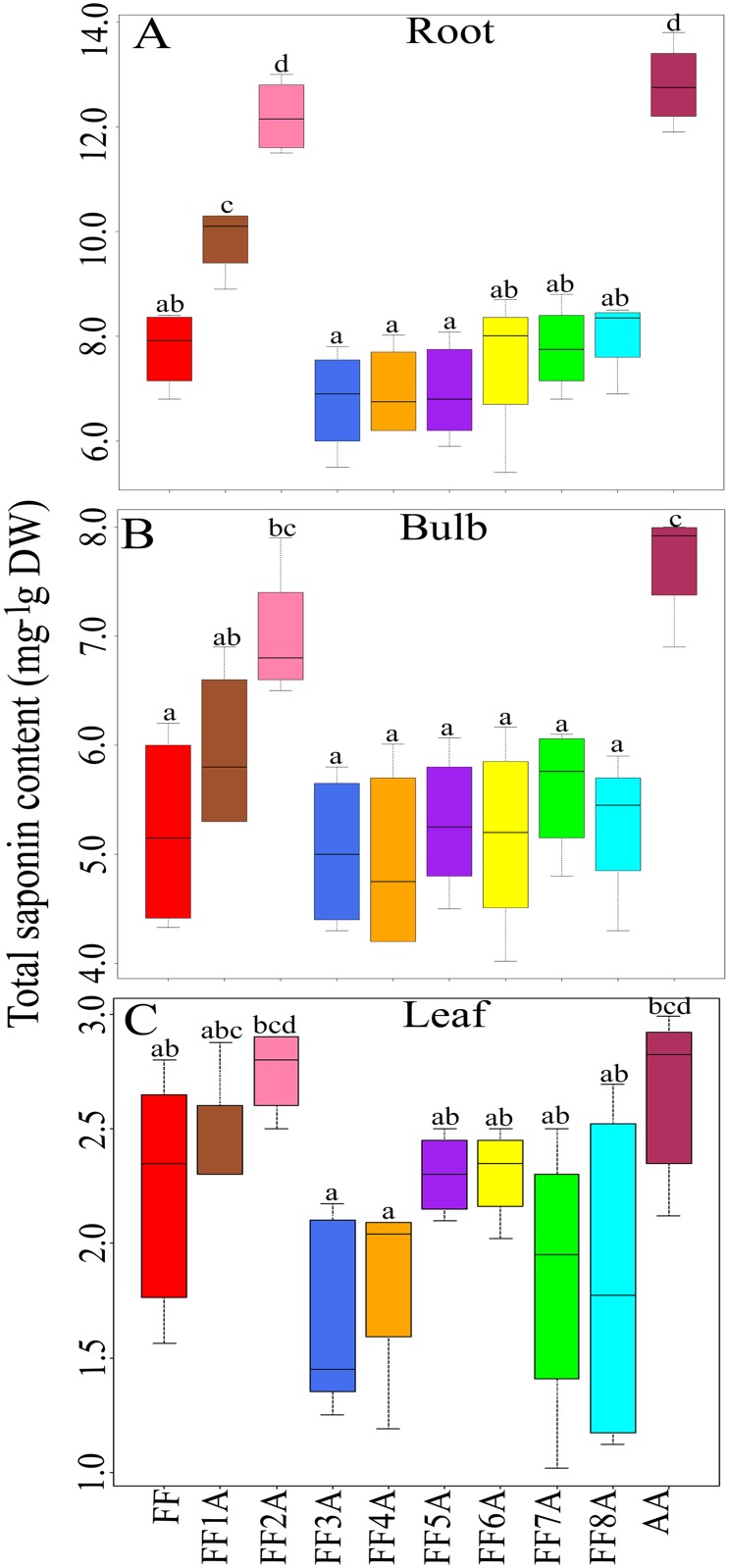
To understand the functional role of the A. cepa Aggregatum group (AA) saponin in the improved disease resistance of A. fistulosum (FF) against Fusarium pathogens, the crude saponin extracts from the roots of AA, MALs, and FF were subjected to TLC analyses. The saponin TLC profile revealed a distinctive saponin spot in the AA and FF2A (Fig 1A); however, this saponin compound was missing in other MALs and FF. In addition, two furostanol saponin compounds were clearly accumulated in the AA, FF1A, and FF2A relative to other MALs and FF (Fig 1B). Total saponin contents were highly abundant in the root followed by bulb and leaf tissues (Fig 2), and the highest saponin accumulation was detected in AA, FF1A and FF2A root relative to FF and other MALs root (Fig 2A). Based on the observed results, we hypothesized that a set of saponin biosynthesis-related genes could be allocated in chromosome 2A of the shallot, and these genes are responsible for the distinctive saponin compound biosynthesis. In addition, a set of furostanol saponin biosynthesis-related genes could be also allocated in chromosomes 1A and 2A. To identify this shallot-specific saponin compound in FF2A, crude saponin extract from roots was subjected to column chromatography using a gradient solvent system that yielded a partially purified fraction F2 (18 mg), which was further purified by TLC to obtain 10 mg of the pure compound. The structure of the isolated pure compound was elucidated by 600 MHz NMR analyses (Fig 3D). The 1H NMR and 13C NMR data of the pure compound was identical to that of Alliospiroside A [35] (S1 Table). The Alliospiroside A is a spirostanol saponin with two glycoside units [[(25S)-3β-hydroxyspirost-5-en-1β-yl] 2-O-(6-deoxy-α-L-mannopyranosyl)-α-L-arabinopyranoside] and a molecular formula of C38H60O12.
Fig 1. Allium cepa Aggregatum group (AA), monosomic addition lines (MALs = FF1A, FF2A, FF3A, FF4A, FF5A, FF6A, FF7A, and FF8A) and A. fistulosum (FF) saponin TLC profile.
(A) Total saponin profile visualized by p-anisaldehyde reagent; arrow indicates the Alliospiroside A accumulation in AA and FF2A. (B) Furostanol saponin profile visualized by Ehrlich's reagent; arrow indicates the furostanol saponin accumulation in AA, FF1A, and FF2A.
Fig 2. Box plot diagram showing the changes in (A) root, (B) bulb (C) and leaf saponin contents (mg g−1 DW) in Allium cepa Aggregatum group (AA), monosomic addition lines (MALs = FF1A, FF2A, FF3A, FF4A, FF5A, FF6A, FF7A, and FF8A) and A. fistulosum (FF).
Values represent the maximum, third quartile, median, first quartile and minimum of three independent replicates (n = 3). Different letters indicate statistically significant difference at P < 0.05 according to Tukey’s honest significant difference (HSD) post-hoc test.
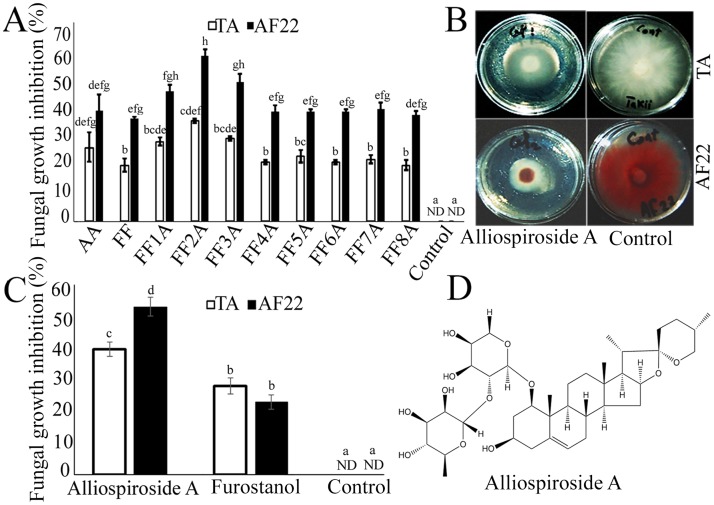
Fig 3. The biological assays of Allium cepa Aggregatum group (AA), monosomic addition lines (MALs = FF1A, FF2A, FF3A, FF4A, FF5A, FF6A, FF7A, and FF8A) and A. fistulosum (FF) crude saponin extracts and Alliospiroside A against Fusarium oxysporum f.sp. cepa.
(A) AA-, MALs- and FF-root saponin extracts antifungal activity against F. oxysporum f.sp. cepa strain TA and AF22. (B) Potato dextrose agar (PDA) plates of Alliospiroside A antifungal activity against F. oxysporum f.sp. cepa strain TA and AF22. (C) Alliospiroside A and furostanol saponin antifungal activity against F. oxysporum f.sp. cepa strain TA and AF22. (D) Alliospiroside A chemical structure. Antifungal activity values are means of three independent replication (n = 3) ± standard errors (SEs). Different letters indicate statistically significant differences according to Tukey’s honestly significant difference (HSD) post-hoc test.
To validate the functional role of Alliospiroside A in disease resistance against Fusarium pathogens, crude saponin extracts from AA, MALs, and FF roots were examined against F. oxysporum f. sp. cepa strain TA and AF22 using agar diffusion method. The highest significant (P ≤ 0.05) fungal growth inhibition percentage was detected in FF2A-root saponin extract with 35.41 and 58.33% against F. oxysporum f. sp. cepa strains TA and AF22, respectively (Fig 3A). In addition, Alliospiroside A antifungal activity was revealed to be 39.58 and 53.12%; however, the furostanol saponin fraction showed 28.12 and 22.91% fungal growth inhibition against the respective strains (Fig 3B and 3C).
Analysis of differential gene expression in the root, bulb, and leave of AA and MALs as compared with those of FF
The abundance of a transcript in a cDNA library from specific tissues usually corresponds to its expression level, which can indicate the enduring biological processes [36]. In the present study, we accumulated RNA-Seq data from 30 cDNA libraries of root, bulb, and leave of AA, MALs, and FF. The obtained transcriptomic data were deposited in the open access Allium Transcript Database (Allium TDB, http://alliumtdb.kazusa.or.jp). To identify genes with different expression levels in the root, bulb, and leave of AA and MALs as compared with those of FF (as a control), we initially used the RPKM fold change and false discovery rate (FDR < 0.05) to determine the differential expression. The obtained DGE data are summarized (S2 Table). AM scatter plots of the DGE data of the root, bulb, and leave of AA and MALs versus those of FF (as a control) were carried out (S1–S3 Figs) using average counts and log2 fold changes of RPKM values. The DGE data showed strong up-regulation in AA genotypes as compared with FF at the constative level; 8760, 12354, and 8773 contigs were up-regulated in AA root, bulb, and leave, respectively, and 1910, 1697, and 2321 contigs were up-regulated in FF2A root, bulb, and leave, respectively, as compared with those of FF (S2 Table). The overview of the transcriptome level in each tissue per genotype and its cross-linked expression with AA revealed 1899, 1163, and 1170 contigs were commonly up-regulated in AA and FF2A root, bulb, and leave, respectively; 375, 467, and 413 contigs were commonly down-regulated in AA and FF2A root, bulb, and leave, respectively (S4 Fig).
Candidate genes involved in the steroidal saponin biosynthesis pathway
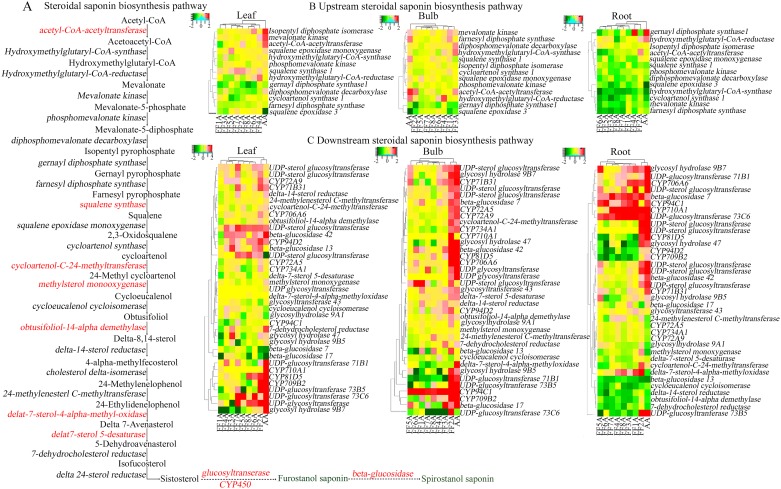
The Kyoto Encyclopedia of Genes and Genomes (KEGG) assignments provide functional annotation of gene-associated biochemical pathways with their corresponding enzyme commission (EC) [37]. Based on similar searches in the KEGG database and our Allium unigene sequences, multiple transcript-encoding enzymes involved in the MVA pathway and saponin biosynthesis pathway were identified. We found 50 unigenes in the root, bulb, and leaf of AA and MALs that were functionally involved in saponin biosynthesis (Table 1). In some cases, more than one unique sequence was annotated on the same gene. These unique sequences may represent either different fragments of a single transcript or different parts of a gene or both. Clustering analysis of the unigene dataset based on their expression levels in the AA and MALs revealed a clear clustering of AA and FF2A from other MALs in the saponin downstream pathway (Fig 4A and 4C). Acetyl-CoA-acetyltransferase (CL6820.Contig3) and squalene synthase (Unigene26049) are two highly expressed saponin-related transcripts in FF2A at the upstream level (Fig 4B). However, most of the up-regulated genes were located at the downstream level, including cycloartenol-C-24-methyltransferase (Unigene11966), methylsterol monooxygenase (Unigene21326), Obtusifoliol-14-alpha-demethylase (Unigene27267), delta-7-sterol-4-alpha-methyl-oxidase (Unigene39702), and delta-7-sterol-5-desaturase (CL1225.Contig2) (Fig 4C). Further hydroxylation, oxidation and glycosylation steps of the saponin compound via cytochrome P450 and UGT family transcripts, respectively were remarkably up-regulated in the FF2A line (Fig 4C). The final cyclization of the saponin compound via glucosidase family transcripts including beta-glucosidase 7, 13, 17, and 42 (Unigene27678, CL5385.Contig1, Unigene27758, and CL534, respectively), and glycosyl hydrolase 9A1, 9B5, 9B7, and 47 (HG9A1, GH9B5, GH9B7, and GH47, respectively) (CL28.Contig2, CL1599.Contig3, CL1599.Contig2, and Unigene34243, respectively) was also up-regulated in the FF2A line. The transcript data of the downstream saponin biosynthesis pathway revealed up-regulation in the FF2A root and bulb when cross-linked with the AA genotype (Fig 4C).
Table 1. List of identified genes involved in the saponin biosynthesis pathway with their unigene.
Kyoto Encyclopedia of Genes and Genomes (KEGG) enzyme commission (EC) number and read length (base pair, bp).
| Gene name | Unique sequence | KEGG enzyme commission (EC) | Read length (bp) |
|---|---|---|---|
| acetyl-CoA acetyltransferase | CL6820.Contig3 | [EC:2.3.1.9] | 685 |
| hydroxymethylglutaryl-CoA-synthase | CL2608.Contig3 | [EC:2.3.3.10] | 1827 |
| hydroxy methylglutaryl-CoA-reductase | Unigene29227 | [EC:1.1.1.34] | 1926 |
| mevalonate kinase | CL3170.Contig2 | [EC:2.7.1.36] | 1256 |
| phosphomevalonate kinase | Unigene11622 | [EC:2.7.4.2] | 2283 |
| diphosphomevalonate decarboxylase | Unigene27463 | [EC:4.1.1.33] | 1584 |
| isopentenyl diphosphate isomerase 1 | Unigene3676 | [EC:5.3.3.2] | 871 |
| geranyl diphosphate synthase 1 | Unigene37026 | [EC:2.5.1.1 2.5.1.10 2.5.1.29] | 206 |
| farnesyl diphosphate synthase | Unigene27333 | [EC:2.5.1.1 2.5.1.10] | 1394 |
| squalene synthase 1 | Unigene26049 | [EC:2.5.1.21] | 524 |
| squalene epoxidase monooxygenase 1 | CL5543.Contig2 | [EC:1.14.14.17] | 1922 |
| squalene epoxidase 3 | CL5543.Contig3 | [EC:1.14.14.17] | 302 |
| cycloartenol synthase 1 | CL2408.Contig1 | [EC:5.4.99.8] | 2718 |
| cycloartenol-C-24-methyltransferase | Unigene11966 | [EC:2.1.1.41] | 1406 |
| methylsterol monooxygenase | Unigene21326 | [EC:1.14.13.72] | 1259 |
| cycloeucalenol cycloisomerase | CL6464.Contig1 | [EC:5.5.1.9] | 1252 |
| obtusifoliol (Sterol) 14-alpha demethylase | Unigene27267 | [EC:1.14.13.70] | 1826 |
| delta(14)-sterol reductase | Unigene28058 | [EC:1.3.1.70] | 1358 |
| 7-dehydrocholesterol reductase | Unigene27272 | [EC:1.3.1.21] | 1669 |
| delta-7-sterol 4-alpha-methyl-oxidase | Unigene39702 | [EC:1.14.13.72] | 152 |
| 24-methylenesterol C-methyltransferase 2 | CL4881.Contig1 | [EC:2.1.1.143] | 168 |
| delta-7-sterol-C5(6)-desaturase | CL1225.Contig2 | [EC:1.14.19.20] | 639 |
| UDP-sterol-glucosyltransferase | Unigene10659 | [EC:2.4.1.173] | 224 |
| UDP-sterol-glucosyltransferase | CL141.Contig10 | [EC:2.4.1.173] | 1000 |
| UDP-sterol-glucosyltransferase | Unigene26213 | [EC:2.4.1.173] | 736 |
| glycosyltransferase, family 43 | CL1481.Contig2 | [EC:2.4.1.17] | 1568 |
| UDP-sterol-glucosyltransferase | CL141.Contig4 | [EC:2.4.1.173] | 1657 |
| UDP-glycosyltransferase | Unigene24555 | [EC:2.4.1.17] | 441 |
| UDP-glycosyltransferase | Unigene15513 | [EC:2.4.1.17] | 294 |
| UDP-glucosyltransferase73B5 | Unigene27343 | [EC:2.4.1.17] | 1509 |
| UDP-glucosyltransferase71B1 | CL2556.Contig3 | [EC:2.4.1.17] | 227 |
| UDP-glucosyltransferase 73C6 | Unigene18419 | [EC:2.4.1.-] | 535 |
| CYP72A5 | CL2624.Contig2 | [EC:1.14.-.-] | 1430 |
| CYP709B2 | Unigene21995 | [EC:1.14.-.-] | 1794 |
| CYP734A1 | CL2624.Contig3 | [EC:1.14.-.-] | 1392 |
| CYP72A9 | CL2624.Contig4 | [EC:1.14.-.-] | 1177 |
| CYP94D2 | CL3985.Contig1 | [EC:1.14.-.-] | 1364 |
| CYP71B31 | Unigene25910 | [EC:1.14.-.-] | 430 |
| CYP81D5 | CL5820.Contig2 | [EC:1.14.-.-] | 736 |
| CYP94C1 | Unigene12533 | [EC:1.14.-.-] | 1867 |
| CYP706A6 | Unigene416 | [EC:1.14.-.-] | 1024 |
| CYP710A1 | Unigene30597 | [EC:1.14.19.41] | 467 |
| beta-glucosidase 7 | Unigene27678 | [EC:3.2.1.58] | 1402 |
| beta glucosidase 13 | CL5385.Contig1 | [EC:3.2.1.21] | 193 |
| beta-glucosidase 17 | Unigene27758 | [EC:3.2.1.58] | 1674 |
| beta glucosidase 42 | CL534.Contig1 | [EC:3.2.1.21] | 841 |
| glycosyl hydrolase 9A1 | CL28.Contig2 | [EC:3.2.1.-] | 2243 |
| glycosyl hydrolase 9B5 | CL1599.Contig3 | [EC:3.2.1.-] | 282 |
| glycosyl hydrolase 9B7 | CL1599.Contig2 | [EC:3.2.1.-] | 744 |
| glycosyl hydrolase family 47 | Unigene34243 | [EC:3.2.1.113] | 432 |
Fig 4. Transcriptomic profiling of steroidal saponin pathway in the root, bulb and leaf of Allium cepa Aggregatum group (AA) and monosomic addition lines (MALs = FF1A, FF2A, FF3A, FF4A, FF5A, FF6A, FF7A and FF8A) as compared with A. fistulosum (FF) as control.
(A) Schematic representation of the steroidal saponin biosynthesis pathway and heatmap clustering of the 50 unigene detected in this study which functionally involved in the upstream (B) and downstream (C) saponin biosynthesis pathway. Heatmap constructed using FPKM log2 fold change.
Discussion
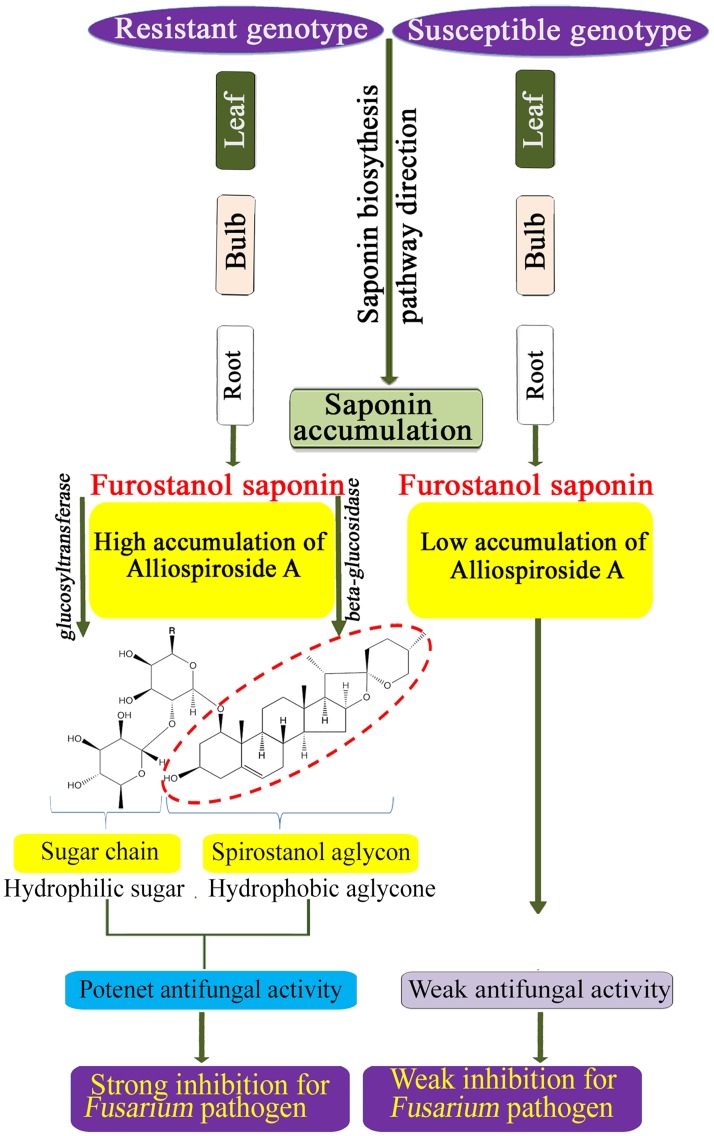
In our previous studies, we were able to illustrate the potential role of crude saponins of shallots for improving the disease resistance of Japanese bunching onion through chromosomal engineering techniques with a certain focus on the phenotypic characters [18]. This study extends the previous work to identify the shallot-specific saponin compound involved in the mechanism of disease resistance as well as candidate genes regulating the steroidal saponin biosynthesis pathway in FF2A using high-throughput RNA-Seq analyses. As far as we know, this is the first study addressing the high-throughput RNA-Seq analysis of saponin pathways in Allium species. A more detailed understanding of the saponin compounds and biosynthetic-related genes would facilitate the development of plants with unique saponin contents, either via classical plant breeding or by gene transformation.
Recently, interest in the biosynthesis of steroidal saponins has increased due to their scientific importance in the biomedical application as well as their pivotal role in plant defenses [38, 39]. Allium species are rich sources of steroidal saponins, and various saponin compounds isolated from Allium species with cytotoxic and antifungal activity have been reported [4, 40, 41]. Previous studies have reported that the initial steps of saponin biosynthesis occur in the leaves, while the later steps of the modification and storage of saponins occur in the roots [19, 42]. Our recent studies have revealed high accumulations of saponin contents in the bulb basal stem and roots of different Allium species [3, 4]. The accumulation of saponins in these organs could be related to the physiological role of saponins as a chemical barrier against soil-born fungal pathogens [3, 4, 43]. In the present study, the root saponin TLC profile of AA, MALs, and FF revealed a shallot-specific saponin compound in FF2A (Fig 1A). The observed compound was isolated and identified using column chromatography and 2D NMR spectroscopy. The compound’s identification and structure elucidation were identical to those of spirostanol saponin Alliospiroside A [35] (S1 Table). The saponin extract of FF2A roots revealed significant antifungal activity in comparison with saponin extracts of AA, MALs, and FF roots against Fusarium pathogens (Fig 3A). These results indicate the importance of Alliospiroside A as a major bioactive compound against Fusarium pathogens and the significant role of spirostanol saponins in disease resistance in comparison with the furostanol type. The obtained results give a better physiological explanation of the phenotypic observation of Fusarium basal rot disease resistance in FF2A [18]. Our results were in accordance with a recent report that showed higher fungal growth inhibition of different phytopathogens treated with Alliospiroside A than of those treated with other saponin compounds [35]. The accumulation of Alliospiroside A and two furostanol saponin compounds in the FF2A line was probably due to the saponin genes allocated in chromosome 2A. To validate this hypothesis, a high-throughput RNA-Seq was performed using NGS technology.
RNA-Seq for transcriptome profiling using the NGS technique has shown great potential for functional gene mining and can help in gene discovery, due to its great sequencing depth [44]. Squalene monooxygenase/epoxidase catalyzes the conversion of squalene into 2, 3-oxidosqualene acting as a precursor in the biosynthesis of both triterpenes and steroidal saponins in plants [39, 45]. The cyclization of 2, 3-oxidosqualene by the activity of OSC is the branch point for sterol and triterpenoid biosynthesis in many plants [39, 45, 46]. One OSC gene, encoding CAS1, was detected in our database. The cyclization of 2, 3-oxidosqualene through the activity of CAS leads to the production of cycloartenol and subsequent methylation by cycloartenol-C-24-methyltransferase [45, 46, 47, 48]. Steroidal saponins are thought to be derived from cycloartenol formation through the downstream phytosterol pathway. However, the steps at which steroidal saponins and phytosterol biosynthesis diverge have not been clarified, although cholesterol has been suggested as a candidate precursor of steroidal saponins [49, 50, 51]. Our results revealed the up-regulation of cycloartenol-C-24-methyltransferase in FF2A bulb and root which could be a candidate gene involved in Alliospiroside A accumulation (Fig 4A and 4C). Additional hydroxylation and oxidation steps are catalyzed by P450 family genes; this step contributes to increasing structural diversity [39; 51, 52]. P450 is one of the largest and most diverse gene families in plants, and only a few P450s have been identified in saponin-involved biosynthesis [39, 53]. In the present study, CYP51G1 (obtusifoliol-14-alpha-demethylase) revealed up-regulation in FF2A bulbs (Fig 4A and 4C). Cytochrome P450 family 51 is essential for sterol and steroidal saponin biosynthesis pathway [27]. CYP734A1 (CL2624.Contig2) and CYP72B1 (CL2624.Contig3) were up-regulated in FF2A bulb. These P450s have been reported in brassinosteroid catabolism via C-26 hydroxylation [54]; however, no information has been reported previously about their role in steroidal saponins. In addition, CYP71B31 was up-regulated in FF2A bulb and leaf, which has been reported in terpenoid biosynthesis [55]. CYP94C1, which was involved in Jasmonoyl-isoleucine oxidation [56], was up-regulated in FF2A bulb. The unigene dataset of the P450 family (Table 1) generated in this study provides a significant resource for further molecular and biochemical studies regarding the functional role of these candidates in the biosynthesis of steroidal saponins.
Saponins have one or more sugar chains attached to their aglycone structure through glycoside linkages, and these saccharide moieties can be linear or branched [25]. Sugar moieties are a determining factor in the antifungal activity and hydrophilic properties of saponin compounds [40, 41]. The UGT family catalyzes the transfer of glycosyl residues to precursors that are decorated by P450s. Similarly to the P450, the UGT gene family is large and diverse. Few reports have characterized the UGT family’s role in saponin glycosylation, including the role of SaGT4A in the steroidal saponins of Solanum aculeatissimum [25], of UGT71G1 and UGT73K1 in the triterpenoid saponins of Medicago truncatula [57], and of GmSGT2 in soyasaponins of Glycine max [58]. In the present study, SGT, UGT, GT43, UGT73B5, UGT71B1, and UGT73C6 were up-regulated in FF2A root and bulb (Fig 4A and 4C). UGT73C6 was functionally reported in the glycosylation of flavonoids [59], and UGT73B5 was reported in the hyper-responsive mechanism of Arabidopsis against the Pseudomonas syringae pv. tomato [60]. The SGT family catalyzes the transfer of sugar molecules into diverse sterol molecules and secondary metabolites [61]. Previous studies of the functionality of UGTs revealed a dual nature, and the oligosaccharide extension step in saponin glycosylation is catalyzed by multiple UGTs rather than by a single enzyme [25, 60].
In the present study, the spirostanol saponin Alliospiroside A was shown to play a pivotal role in disease resistance as compared with furostanol saponin (Fig 3C). Similar reports have addressed the importance of spirostanol saponins over the furostanol type [40, 41]. Therefore, increasing spirostanol saponin through classical breeding or gene transformation techniques would be a useful approach for achieving plant resistance against diseases. Beta-glucosidase is the key enzyme that catalyzes the conversion of furostanol saponins into spirostanol by the cleavage of the C-26-bound glucose moiety of furostanol glycosides [62]. Several recent reports have discussed the functional role of beta-glucosidase in the formation of spirostanol saponins, including several spirostanol saponins obtained from seeds of Trigonella foenum-graecum after the enzymatic hydrolysis of the furostanol saponin fraction by beta-glucosidase [63], and garlic furostanol proto-eruboside-B(1) conversion into spirostanol saponin eruboside-B(2) [64]. In the present study, beta-glucosidases 7, 13, 17, and 42, and GH9A1, GH9B5, GH9B7, and GH47 revealed strong up-regulation in FF2A bulb (Fig 4A and 4C). These candidate genes might be involved in the enzymatic conversion of furostanol saponins into the spirostanol saponin Alliospiroside A in FF2A and its subsequent disease resistance against Fusarium pathogens.
Conclusion
In this study, phytochemical analyses resulted in the isolation and identification of the spirostanol saponin Alliospiroside A in A. fistulosum with additional chromosome 2A from shallot (FF2A). Alliospiroside A was the major bioactive compound against Fusarium pathogens and a potential chemical marker for disease-resistant genotype selection (Fig 5). The transcriptome of the root, bulb, and leaf of AA, MALs, and FF was obtained using RNA-Seq. The DGE data revealed many up-regulated genes in the biosynthetic pathway of steroidal saponins in the FF2A line. A series of candidate genes involved in the downstream level of the saponin pathway was identified including Cytochrome P450, glycosyltransferases, and beta-glucosidase. Furthermore, clustering analysis of the saponin transcriptional genes revealed a distinctive segregation of the AA and FF2A in the saponin downstream pathway. The obtained results imply the genetic effect of the A. cepa Aggregatum group on A. fistulosum saponin physiology and bioactivity. The unigene dataset that was generated in this study provides a significant resource for further molecular and biochemical studies of steroidal saponins.
Fig 5. Representative model of Alliospiroside A biosynthesis and defense mechanism in resistance and susceptible Allium genotypes against Fusarium pathogens.
Supporting information
(PDF)
(PDF)
Log2 fold change of AA/FF and MALs/FF on the y-axis and average count of RPKM (Reads Per Kilobase of exon per Million mapped reads) values on the x-axis. Up-regulated genes (Red, fold change > 2 and adjusted-P < 0.05), down-regulated genes (green, fold change < 0.5 and adjusted-P < 0.05), and differential expressed genes (Blue, adjusted-P < 0.05). Non differential expressed genes (black).
(PDF)
Up-regulated genes (Red, fold change > 2 and adjusted-P < 0.05), down-regulated genes (green, fold change < 0.5 and adjusted-P < 0.05), and differential expressed genes (Blue, adjusted-P < 0.05). Non differential expressed genes (black).
(PDF)
Up-regulated genes (Red, fold change > 2 and adjusted-P < 0.05), down-regulated genes (green, fold change < 0.5 and adjusted-P < 0.05), and differential expressed genes (Blue, adjusted-P < 0.05). Non differential expressed genes (black).
(PDF)
(PDF)
Data Availability
1-Allium Transcript Database: http://alliumtdb.kazusa.or.jp 2-The obtained RNA-Seq data from AA, MALs and FF were submitted in DNA Data Bank of Japan (DDBJ) under the following accession numbers: Submission: DRA005096 (hirakawa-0068_Submission) BioProject: PRJDB3595 (PSUB004388) BioSample: SAMD00059523-SAMD00059582 (SSUB006662) Experiment: DRX062890-DRX062949 (hirakawa-0068_Experiment_0001-0060) Run: DRR068940-DRR068999 (hirakawa-0068_Run_0001-0060).
Funding Statement
This research topic was fully supported by JSPS KAKENHI Grant Number 16H06279. The RNA-Sequencing experiments were supported by Cooperative Research Grant of the Genome Research for BioResource, NODAI Genome Research Center, Tokyo University of Agriculture in FY2014 and FY2015, respectively for leaf sheath and leaf blade. Another RNA-Sequencing experiment in FY2014 for root was supported by JSPS KAKENHI Grant Number 16H06279.
References
- 1.Kamenetsky R, Rabinowitch HD. The Genus Allium: A developmental and horticultural analysis. Horticult Rev. 2006;32: 329–378. [Google Scholar]
- 2.Fritsch RM, Blattner FR, Gurushidze M. New classification of Allium L. subg. Melanocrommyum (Webb & Berthel) Rouy (Alliaceae) based on molecular and morphological characters. Phyton. 2010;49: 145–220. [Google Scholar]
- 3.Abdelrahman M, Hirata S, Ito S-I, Yamauchi N, Shigyo M. Compartmentation and localization of bioactive metabolites in different organs of Allium roylei. Biosci Biotechnol Biochem. 2014;7: 1112–1122. [DOI] [PubMed] [Google Scholar]
- 4.Mostafa A, Jogaiah S, El-Sayed M, Ito S, Ikeda T, Yamauchi N, et al. Aginoside saponin, a potent antifungal compound, and secondary metabolite analyses from Allium nigrum L. Phytochem Lett. 2013;6: 274–280. [Google Scholar]
- 5.Caruso G, Conti S, Villari G, Borrelli C, Melchionna G, Mintutolo M, et al. Effects of transplanting time and plant density on yield, quality and antioxidant content of onion (Allium cepa L.) in southern Italy. Sci Hortic. 2014;166: 111–120. [Google Scholar]
- 6.Abdelrahman M, Abdel-Motaal F, El-Sayed M, Jogaiah S, Shigyo M, Ito S-I, et al. Dissection of Trichoderma longibrachiatum induced-defense in onion (Allium cepa L.) against Fusarium oxysporum f. sp. cepa by target metabolite profiling. Plant Sci. 2016;246: 128–138. doi: 10.1016/j.plantsci.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 7.Kofoet A, Kik C, Wietsma WA, Vries JN. Inheritance of resistance to downy mildew (Peronospora destructor (Berk) Casp.) from Allium roylei Stearn in the backcross Allium cepa L. x (A. roylei x A. cepa). Plant Breed. 1990;105: 144–149. [Google Scholar]
- 8.Currah L. Onions in the tropics: cultivars and country reports In: Rabinowitch HD, Currah L, editors. Allium crop science: recent advances. CABI Publishing: Wallingford; 2002. pp. 379–407. [Google Scholar]
- 9.Abdelrahman M, Sawada Y, Nakabayashi R, Sato S, Hirakawa H, El-Sayed M, et al. Integrating transcriptome and target metabolome variability in doubled haploids of Allium cepa for abiotic stress protection. Molecular Breeding. 2015;35: 195. [Google Scholar]
- 10.Yang L, Zhang Y, Zhu N, Koh J, Ma C, Pan Y, et al. Proteomic analysis of salt tolerance in sugar beet monosomic addition line M14. J Proteome Res. 2013;12: 4931−4950. doi: 10.1021/pr400177m [DOI] [PubMed] [Google Scholar]
- 11.Li H, Pan Y, Zhang Y, Wu C, Ma C, Yu B, et al. Salt stress response of membrane proteome of sugar beet monosomic addition line M14. J Proteomics. 2015;127: 18–33. doi: 10.1016/j.jprot.2015.03.025 [DOI] [PubMed] [Google Scholar]
- 12.Fu WQ, Zhao ZG, Ge XG, Ding L, Li ZY. Anatomy and transcript profiling of gynoecium development in female sterile Brassica napus mediated by one alien chromosome from Orychophragmus violaceus. BMC Genomics. 2014; 15: 61 doi: 10.1186/1471-2164-15-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shigyo M, Tashiro Y, Iino M, Terahara N, Ishimaru K, Isshiki S. Chromosomal locations of genes related to flavonoid and anthocyanin production in leaf sheath of shallot (Allium cepa L. Aggregatum group). Genes Genet Syst. 1997; 72: 149–152. [Google Scholar]
- 14.Shigyo M, Tashiro Y, Isshiki S, Miyazaki S. Establishment of a series of alien monosomic addition lines of Japanese bunching onion (Allium fistulosum L.) with extra chromosomes from shallot (A. cepa L. Aggregatum group). Genes Genet Syst. 1996;71: 363–371. [DOI] [PubMed] [Google Scholar]
- 15.Masuzaki S, Shigyo M, Yamauchi N. Complete assignment of structural genes involved in flavonoid biosynthesis influencing bulb color to individual chromosomes of shallot (Allium cepa L.). Genes Genet Syst. 2006a;81: 255–262. [DOI] [PubMed] [Google Scholar]
- 16.Yaguchi S, McCallum J, Shaw M, Pither-Joyce M, Onodera S, Shiomi N, et al. Biochemical and genetic analysis of carbohydrate accumulation in Allium cepa L. Plant Cell Physiol. 2008;49: 730–739. doi: 10.1093/pcp/pcn048 [DOI] [PubMed] [Google Scholar]
- 17.Masamura N, Yaguchi S, Ono Y, Nakajima T, Masuzaki SI, Imai S, et al. Characterization of amino acid and S-alk(en)yl-L-cysteine sulfoxide production in Japanese bunching onion carrying extra chromosome of shallot. J Jpn Soc Hortic Sci. 2011;80: 322–333. [Google Scholar]
- 18.Vu HQ, El-Sayed MA, Ito S, Yamauchi N, Shigyo M. Discovery of a new source of resistance to Fusarium oxysporum, cause of Fusarium wilt in Allium fistulosum, located on chromosome 2 of Allium cepa Aggregatum group. Genome. 2012;55: 797–807. doi: 10.1139/g2012-065 [DOI] [PubMed] [Google Scholar]
- 19.Haralampidis K, Trojanowska M, Osbourn AE. Biosynthesis of triterpenoid saponins in plants. Adv Biochem Eng Biotechnol. 2002;75: 31–49. [DOI] [PubMed] [Google Scholar]
- 20.Rohdich F, Kis K, Bacher A, Eisenreich W. The nonmevalonate pathway of isoprenoids: genes, enzymes and intermediates. Curr Opin Chem Biol. 2001;5: 535–540. [DOI] [PubMed] [Google Scholar]
- 21.Kalra S, Puniya PL, Kulshreshtha D, Kumar S, Kaur J, Ramachandran S, et al. De Novo transcriptome sequencing reveals important molecular networks and metabolic pathways of the plant, Chlorophytum borivilianum. PLoS ONE. 2013;8: e83336 doi: 10.1371/journal.pone.0083336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corey EJ, Matsuda SPT, Bartel B. Isolation of an Arabidopsis thaliana gene encoding cycloartenol synthase by functional expression in a yeast mutant lacking lanosterol synthase by the use of a chromatographic screen. Proc Natl Acad Sci USA. 1993;90: 11628–11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrera JB, Bartel B, Wilson WK, Matsuda SP. Cloning and characterization of the Arabidopsis thaliana lupeol synthase gene. Phytochem. 1998;49: 1905–11. [DOI] [PubMed] [Google Scholar]
- 24.Kushiro T, Shibuya M, Ebizuka Y. Beta-amyrin synthase—cloning of oxidosqualene cyclase that catalyzes the formation of the most popular triterpene among higher plants. Eur J Biochem. 1998; 256: 238–244. [DOI] [PubMed] [Google Scholar]
- 25.Kohara A, Nakajima C, Hashimoto K, Ikenaga T, Tanaka H, Shoyama Y, et al. A novel glucosyltransferase involved in steroid saponin biosynthesis in Solanum aculeatissimum. Plant Mol Biol. 2005;57: 225–239. doi: 10.1007/s11103-004-7204-2 [DOI] [PubMed] [Google Scholar]
- 26.Luo H, Sun C, Sun Y, Wu Q, Li Y, Song J, et al. Analysis of the transcriptome of Panax notoginseng root uncovers putative triterpene saponin-biosynthetic genes and genetic markers. BMC Genomics. 2011;12: S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geisler K, Hughesc RK, Sainsburyc F, Lomonossoffc GP, Rejzekc M, Fairhurstc S, et al. Biochemical analysis of a multifunctional cytochrome P450 (CYP51) enzyme required for synthesis of antimicrobial triterpenes in plants. Proc Natl Acad Sci USA. 2013;110: 3360–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morozova O, Hirst M, Marra MA. Applications of new sequencing technologies for transcriptome analysis. Annu Rev Genom. Hum Genet. 2009;10: 135–151. [DOI] [PubMed] [Google Scholar]
- 29.Upadhyay S, Phukan UJ, Mishra S, Shukla RK. De novo leaf and root transcriptome analysis identified novel genes involved in steroidal sapogenin biosynthesis in Asparagus racemosus. BMC Genomics. 2014;15: 746 doi: 10.1186/1471-2164-15-746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011; 29: 644–652. doi: 10.1038/nbt.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pertea G, Huang X, Liang F, Antonescu V, Sultana R, Karamycheva S, et al. TIGR Gene indices clustering tools (TGICL): a software system for fast clustering of large EST datasets. Bioinformatics. 2003;19: 651–652. [DOI] [PubMed] [Google Scholar]
- 32.Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998;8: 175–185. [DOI] [PubMed] [Google Scholar]
- 33.Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998;8: 186–194. [PubMed] [Google Scholar]
- 34.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215: 403–410. doi: 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 35.Teshima Y, Ikeda T, Imada K, Sasaki K, El-Sayed M, Shigyo M, et al. Identification and Biological activity of antifungal saponins from shallot (Allium cepa L. Aggregatum Group). J Agric Food Chem. 2013;61: 7440−7445. doi: 10.1021/jf401720q [DOI] [PubMed] [Google Scholar]
- 36.Audic S, Claverie JM. The significance of digital gene expression profiles. Genome Res. 1997;7: 986–995. [DOI] [PubMed] [Google Scholar]
- 37.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moses T, Papadopoulou KK, Osbourn A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 2014;49: 439–462. doi: 10.3109/10409238.2014.953628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miettinen K, Pollier J, Buyst D, Arendt P, Csuk R, Sommerwerk S, et al. The ancient CYP716 family is a major contributor to the diversification of eudicot triterpenoid biosynthesis. Nat. Comm. 2017;8: 14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanzotti V, Scala F, Bonanomi G. Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochem Rev. 2014;13: 769–791. [Google Scholar]
- 41.Sobolewska D, Michalska K, Podolak I, Grabowska K. Steroidal saponins from the genus Allium. Phytochem Rev. 2016;15: 1–35. doi: 10.1007/s11101-014-9381-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osbourn AE, Haralampidis K. Triterpenoid saponin biosynthesis in plants. Recent Adv Phytochem. 2002;75: 31–49. [DOI] [PubMed] [Google Scholar]
- 43.Lanzotti V. Bioactive polar natural compounds from garlic and onions. Phytochem Rev. 2012;11: 179–196. [Google Scholar]
- 44.Abdelrahman M, Suzumura N, Mitoma M, Matsuo S, Ikeuchi T, Mori M, et al. Comparative de novo transcriptome profiles in Asparagus officinalis and A. kiusianus during the early stage of Phomopsis asparagi infection. Sci. Rep. 2017; 7: 2608 doi: 10.1038/s41598-017-02566-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuhr CA, Radykewicz T, Sagner S, Latzel C, Zenk MH, Arigoni D, et al. Quantitative assessment of crosstalk between the two isoprenoid biosynthesis pathways in plants by NMR spectroscopy. Phytochem Rev. 2003;2: 3–16. [Google Scholar]
- 46.Mylona P, Owatworakit A, Papadopoulou K, Jenner H, Qin B, Findlay K, et al. Sad3 and Sad4 are required for saponin biosynthesis and root development in oat. Plant Cell. 2008;20: 201–212. doi: 10.1105/tpc.107.056531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohyama K, Suzuki M, Kikuchi J, Saito K, Muranaka T. Dual biosynthetic pathways to phytosterol via cycloartenol and lanosterol in Arabidopsis. Proc Nat Acad Sci USA. 2009;106: 725–730. doi: 10.1073/pnas.0807675106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faizal A, Geelen D. Saponins and their role in biological processes in plants. Phytochem Rev. 2013;12: 877–893. [Google Scholar]
- 49.Phillips DR, Rasbery JM, Bartel B, Matsuda SPT. Biosynthetic diversity in plant triterpene cyclization. Curr Opin Plant Biol. 2006;9: 305–314. doi: 10.1016/j.pbi.2006.03.004 [DOI] [PubMed] [Google Scholar]
- 50.Vincken JP, Heng L, de Groot A, Gruppen H. Saponins, classification and occurrence in the plant kingdom. Phytochem. 2007;68: 275–297. [DOI] [PubMed] [Google Scholar]
- 51.Sonawane Pd, Pollier J, Panda S, Szymanski J, Massalha H, Yona M, et al. Plant cholesterol biosynthetic pathway overlaps with phytosterol metabolism. Nat. Plants. 2017;3. [DOI] [PubMed] [Google Scholar]
- 52.Fukushima EO, Seki H, Ohyama K, Ono E, Umemoto N, Mizutani M, et al. CYP716A subfamily members are multifunctional oxidases in triterpenoid biosynthesis. Plant Cell Physiol. 2011; 52: 2050–2061. doi: 10.1093/pcp/pcr146 [DOI] [PubMed] [Google Scholar]
- 53.Carelli M, Biazzi E, Panara F, Tava A, Scaramelli L, Porceddu A, et al. Medicago truncatula CYP716A12 is a multifunctional oxidase involved in the biosynthesis of hemolytic saponins. Plant Cell. 2011;23: 3070–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohnishi T, Nomura T, Watanabe B, Ohta D, Yokota T, Miyagawa H, et al. Tomato cytochrome P450 CYP734A7 functions in brassinosteroid catabolism. Phytochem. 2006;67: 1895–1906. [DOI] [PubMed] [Google Scholar]
- 55.Ginglinger JF, Boachon B, Höfer R, Paetz C, Köllner TG, Miesch L, et al. Gene coexpression analysis reveals complex metabolism of the monoterpene alcohol linalool in Arabidopsis flowers. Plant Cell. 2013;25: 4640–4657. doi: 10.1105/tpc.113.117382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heitz T, Widemann E, Lugan R, Miesch L, Ullmann P, Désaubry L, et al. Cytochromes P450 CYP94C1 and CYP94B3 catalyze two successive oxidation steps of plant hormone jasmonoyl-isoleucine for catabolic turnover. J Chem Biol. 2012;287: 6296–6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Achnine L, Huhman DV, Farag MA, Sumner LW, Blount JW, Dixon RA. Genomics-based selection and functional characterization of triterpene glycosyltransferases from the model legume Medicago truncatula. Plant J. 2005;41: 875–887. doi: 10.1111/j.1365-313X.2005.02344.x [DOI] [PubMed] [Google Scholar]
- 58.Shibuya M, Nishimura K, Yasuyama N, Ebizuka Y. Identification and characterization of glycosyltransferases involved in the biosynthesis of soyasaponin I in Glycine max. FEEBS Lett. 2010;584: 2258–2264. [DOI] [PubMed] [Google Scholar]
- 59.Saito K, Yonekura-Sakakibara K, Nakabayashi R, Higashi Y, Yamazaki M, Tohge T, et al. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol Biochem. 2013;72: 21–34. doi: 10.1016/j.plaphy.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 60.Simon C, Langlois-Meurinne M, Didierlaurent L, Chaouch S, Bellvert F, Massoud K, et al. The secondary metabolism glycosyltransferases UGT73B3 and UGT73B5 are components of redox status in resistance of Arabidopsis to Pseudomonas syringae pv. tomato. Plant Cell Environ. 2014;37: 1114–29. doi: 10.1111/pce.12221 [DOI] [PubMed] [Google Scholar]
- 61.Pankaj C, Manoj M, Nehal A, Parul G, Pratibha M, Rakesh T. Sterol glycosyltransferases-identification of members of gene family and their role in stress in Withania somnifera. Mol Biol Rep. 2012;39: 9755–9764. doi: 10.1007/s11033-012-1841-3 [DOI] [PubMed] [Google Scholar]
- 62.Inoue K, Ebizuka Y. Purification and characterization of furostanol glycoside 26-O-beta-glucosidase from Costus speciosus rhizomes. FEBS Lett.1996;378: 157–60. [DOI] [PubMed] [Google Scholar]
- 63.Pang X, Cong Y, Yu HS, Kang LP, Feng B, Han BX, et al. Spirostanol saponins derivated from the seeds of Trigonella foenum-graecum by β-glucosidase hydrolysis and their inhibitory effects on rat platelet aggregation. Planta Med. 2012;78: 276–285. doi: 10.1055/s-0031-1280373 [DOI] [PubMed] [Google Scholar]
- 64.Matsuura H. Saponins in garlic as modifiers of the risk of cardiovascular disease. J Nutr. 2001;131: 1000S–5S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Log2 fold change of AA/FF and MALs/FF on the y-axis and average count of RPKM (Reads Per Kilobase of exon per Million mapped reads) values on the x-axis. Up-regulated genes (Red, fold change > 2 and adjusted-P < 0.05), down-regulated genes (green, fold change < 0.5 and adjusted-P < 0.05), and differential expressed genes (Blue, adjusted-P < 0.05). Non differential expressed genes (black).
(PDF)
Up-regulated genes (Red, fold change > 2 and adjusted-P < 0.05), down-regulated genes (green, fold change < 0.5 and adjusted-P < 0.05), and differential expressed genes (Blue, adjusted-P < 0.05). Non differential expressed genes (black).
(PDF)
Up-regulated genes (Red, fold change > 2 and adjusted-P < 0.05), down-regulated genes (green, fold change < 0.5 and adjusted-P < 0.05), and differential expressed genes (Blue, adjusted-P < 0.05). Non differential expressed genes (black).
(PDF)
(PDF)
Data Availability Statement
1-Allium Transcript Database: http://alliumtdb.kazusa.or.jp 2-The obtained RNA-Seq data from AA, MALs and FF were submitted in DNA Data Bank of Japan (DDBJ) under the following accession numbers: Submission: DRA005096 (hirakawa-0068_Submission) BioProject: PRJDB3595 (PSUB004388) BioSample: SAMD00059523-SAMD00059582 (SSUB006662) Experiment: DRX062890-DRX062949 (hirakawa-0068_Experiment_0001-0060) Run: DRR068940-DRR068999 (hirakawa-0068_Run_0001-0060).