Abstract
Hypomethylating agents (HMA) are the most commonly used therapeutic intervention in chronic myelomonocytic leukemia (CMML). Due to the lack of CMML-specific clinical trials, the impact of these agents in the natural history of CMML is not fully understood. We present the largest retrospective series of CMML (n=151) treated with HMA. Mean age at diagnosis was 69 years (range 50–88). According to the CMML-specific prognostic scoring system (CPSS): 17 (15%) were low-risk, 45 (39%) intermediate-1 risk, 42 (36%) intermediate-2, and 12 (10%) high-risk. 35 (23%) patients received single agent azacitidine, 73 (48%) single agent decitabine, and 43 (29%) combinations. With a median follow-up of 17 months, overall response rate (ORR) was 75%, with 41% achieving complete response (CR). Median overall survival (OS) was 24 months (95%CI: 20–28) and event-free survival 14 months (95%CI: 11–17). By multivariate analysis, age < 70 years, higher levels of hemoglobin, absence of blast in peripheral blood and lower CPSS cytogenetic risk predicted for better OS. CR was significantly higher in those patients treated with decitabine (58.3%) when compared with azacitidine (20.6%) (p<0.001). 13 patients (9%) received allo-SCT after a median of 4 cycles of HMA. 66 patients (50%) had HMA failure: 26 primary (34%) and 50 secondary (66%), including 35 (46%) that transformed to AML. Outcomes after HMA failure were poor with OS of 7 months (95%CI: 3–12). In conclusion, HMA are effective in CMML but new agents and combinations are needed. This data could be a benchmark for further drug development in CMML.
Keywords: chronic myelomonocytic leukemia, hypomethylating agents, decitabine, azacitidine, failure
INTRODUCTION
Chronic myelomonocytic leukemia (CMML) is a clonal hematopoietic stem cell disorder characterized by the presence of persistent peripheral blood (PB) monocytosis, together with the presence of myelodysplastic and myeloproliferative alterations in the bone marrow (BM). Considered initially by the French-American-British (FAB) classification as a myelodysplastic syndrome (MDS), the World Health Organization (WHO) in 2001 included it in a new group called myelodysplastic/myeloproliferative disorders, updated in 2016[1–3].
Median age at diagnosis of CMML is between 72–76 years with a male preponderance[4, 5]. CMML patients usually presents with cytopenia, monocytosis, and splenomegaly. The FAB system distinguishes two subtypes: proliferative subtype (WBC ≥13 × 109/L) and dysplastic subtype (WBC ≤13 × 109/L); while the 2016 WHO update identifies three categories (CMML-0 for patients with <2% blast in PB and <5% blast in BM; CMML-1 for patients with 2–4% blast in PB and/or 5–9% blast in BM; and CMML-2 for patients with 5–19% in PB, 10–19% in BM and/or when Auer rods are present). Both classifications have an impact in prognosis[2, 6]. Cytogenetic alterations are present in 20–30% of patients and molecular abnormalities are also common, with TET2, SRSF2, ASXL1, RAS mutations being the most frequent[7–9].
Although allogeneic stem cell transplantation (allo-SCT) is potentially the only curative option for patients with CMML[10], advanced age at diagnosis make a majority of the patients unlikely candidates for transplantation[11–15]. Historically, HMAs were approved under the umbrella of MDS trials[16–18] and therefore, due to the lack of CMML-specific clinical trials, the impact of these agents in the natural history of CMML is not fully understood. Most of the evidence of the impact of HMA therapy in CMML is from small studies, with reported response rates ranging from 25 to 70%, complete remission (CR) rates of 10 to 58% and median overall survival (OS) of 12 to 37 months[16–26]. To further understand the impact of HMA based therapy on the natural history of CMML, we analyzed the single institution experience with azacitidine or decitabine in the treatment of patients with CMML.
METHODS
Patients and methods
We retrospectively reviewed the electronic records of patients with a diagnosis of CMML treated with HMA (azacitidine or decitabine) as initial therapy between March 2004 and July 2015 at the University of Texas MD Anderson Cancer Center (MDACC). The study was performed following institutional guidelines. One hundred and fifty-one patients with peripheral monocyte count > 1 × 109/L and up to 19% peripheral and bone marrow blast met the definition of CMML according 2016 WHO classification[2].
CMML-specific prognostic scoring system (CPSS), the Mayo Clinic Prognostic Model (MCPM), International Prognostic Score System (IPSS) and the MD Anderson Prognostic Scoring System (MDAPS) were used to estimate the prognosis of these patients [27–31].
HMA was initiated at the time patients presented disease related complications such as: progressive disease with increasing blasts, complications related to cytopenias, worsening constitutional symptoms, or transfusion dependency.
Cytogenetic analysis and target gene sequencing analysis
Routine cytogenetic analysis was conducted in the Clinical Cytogenetics laboratory at MDACC following standard protocols. Cytogenetic results were interpreted and reported according to the International System for Human Cytogenetic Nomenclature (ISCN, 2005)[32]. Baseline cytogenetic was classified into 3 groups: low (normal karyotype, –Y, der(3q)), intermediate (all abnormalities not in the high or low risk groups) and high (abnormalities of chromosome 7 and complex karyotypes with more than 3 cytogenetic abnormalities) based on the modified Spanish cytogenetic risk stratification [7, 21, 33].
Genomic DNA was extracted from whole bone marrow aspirate samples at the time of diagnosis and was subject to targeted PCR-based sequencing using a next generation sequencing (NGS) platform covering 28 or 53 genes, in patients evaluated from 2012 to 2016. This analysis was performed at the MDACC CLIA-compliant Molecular Diagnostics Laboratory (additional details in Supplemental data). The limit of detection for SNVs was a tumor allele frequency of at least 5%. Previously described somatic mutations registered at the Catalogue of Somatic Mutations in Cancer (COSMIC: http://cancer.sanger.ac.uk/cosmic) were considered as potential driver mutations.
Response criteria
Response was assessed by 2015 International Consortium Response Criteria for Myelodysplastic/Myeloproliferative neoplasm in adults[34] and the 2006 International Working Group (IWG) modified response criteria in MDS [35]. A summary is showed in supplemental information.
ORR was defined as the sum of CR, partial response (PR), optimal marrow response (mCR), partial marrow response (mPR) and clinical benefit (CB) following the 2015 International Consortium Response Criteria for Myelodysplastic/Myeloproliferative neoplasm in adults or the sum of CR, PR, marrow CR and hematological improvement (HI) when 2006 International Working Group (IWG) modified response criteria in MDS was used. OS and leukemia free survival (LFS) were defined as the time between treatment onset and death or leukemia (or last contact), respectively. Event free survival (EFS) was defined as the time between treatment onset and leukemia, relapse or death (or last contact). A failure to respond to HMA was defined as primary in patients with progression or lack of response after at least 4 cycles of either decitabine or azacitidine, or secondary in patients who experienced loss of response during therapy with HMA [36, 37].
Patients with clonal abnormalities at baseline who underwent follow-up cytogenetic evaluations were evaluable for a cytogenetic response. Complete cytogenetic response (CCyR) was defined as the achievement of a diploid karyotype among at least 20 metaphases analyzed. Partial cytogenetic response (PCyR) was defined as the reduction of 50% of the abnormal metaphases without the acquisition of any new abnormality among at least twenty metaphases analyzed[35].
Statistical analysis
Statistical analyses were performed with the IBM SPSS Statistics 23.0 software. Data were summarized using median, range, and percentage as needed. Categorical variables are presented as number (percentage) and were compared using χ2 testing or Fisher exact tests, as appropriate. Continuous variables were examined for normality before analysis, are presented as mean (SD) or as median (interquartile range) and were compared using t tests or Wilcoxon rank sum tests, as appropriate.
To account for differences in baseline risk profiles, a propensity score (PS)–matched analysis was performed. Logistic regression modeling calculated the PS for each patient, representing the probability of receiving azacitidine or decitabine conditional on the following covariate set: age, sex, level of hemoglobin (Hgb), number of platelets, white blood cells (WBC), neutrophils, monocytes, lymphocytes, PB and BM blast at diagnosis, FAB classification, WHO classification, CPSS cytogenetic risk group and CPSS[2, 6, 21, 27]. Patients were matched 1:1 using nearest-neighbor matching based on therapy (5-azacitine vs decitabine). Adequacy of matching was tested using the standard differences method, and values less than 10% reflect more balanced matched groups.
Logistic regression analyses were used to calculate effect sizes in both the original and PS-matched cohorts; the effect sizes are expressed as odds ratios (ORs) and associated 95% confident intervals (CI). Censored endpoints were estimated by the nonparametric Kaplan-Meier method and compared using the log-rank test. Cox regression analyses were used to calculate effect sizes expressed as hazard ratios (HRs) and associated 95% CI. All statistical tests were 2-tailed with a level of significance of p < 0.05.
RESULTS
Patient characteristics
Baseline characteristics are shown in Table I. Forty-four (29%) patients were female. Mean age at diagnosis was 69 years (range 50–88). Overall, 40 patients (26%) had CMML-0; 58 patients (39%) CMML-1 and 52 patients (35%) CMML-2, according to WHO classification[2]. Sixty-five patients (43%) were considered to have dysplastic subtype and 86 (57%) were considered proliferative subtype according to FAB classification[38]. The majority of patients were transfusion-dependent (67%). Therapy related CMML was present in 45 patients (30%).
Table I.
Characteristics of the study group (N=151)
| Median [Range] | n (%) | |
|---|---|---|
| Age (years). Mean (95%CI) | 69 (67–70) [50–88] | |
|
| ||
| Female | 44 (29.1) | |
|
| ||
| Hgb (g/dl). Mean (95%CI) | 10.9 (10.6–11.2) | |
|
| ||
| Platelets (×109/L) | 85.5 [11.0–795.0] | |
|
| ||
| WBC (×109/L) | 16.0 [2.7–211.8] | |
|
| ||
| ANC (×109/L) | 8.4 [0.2–108.0] | |
|
| ||
| Monocytes (×109/L) | 2.9 [0.4–48.7] | |
|
| ||
| Lymphocytes (×109/L) | 2.3 [0.2–18.8] | |
|
| ||
| BM Blast (%) | 7 [0–18] | |
|
| ||
| PB Blast (%) | 0 [0–16] | |
|
| ||
| WHO CMML classification | ||
| CMML-0 | 40 (26.5) | |
| CMML-1 | 58 (38.7) | |
| CMML-2 | 52 (34.7) | |
|
| ||
| FAB classification | ||
| Dysplastic subtype | 65 (43.0) | |
| Proliferative subtype | 86 (57.0) | |
|
| ||
| AML transformation | 49 (32.5) | |
|
| ||
| Modified Spanish cytogenetic risk | ||
| Low | 105 (71.4) | |
| Intermediate | 27 (18.4) | |
| High | 15 (10.2) | |
|
| ||
| Therapy related | 45 (29.8) | |
|
| ||
| CPSS | ||
| Low | 17 (14.7) | |
| Intermediate-1 | 45 (38.8) | |
| Intermediate-2 | 42 (36.2) | |
| High | 12 (10.3) | |
|
| ||
| MDAPS | ||
| Low | 61 (40.9) | |
| Intermediate-1 | 47 (31.5) | |
| Intermediate-2 | 30 (20.1) | |
| High | 11 (7.4) | |
|
| ||
| MCPS | ||
| Low | 22 (17.6) | |
| Intermediate | 68 (54.4) | |
| High | 35 (28.0) | |
|
| ||
| IPSS | ||
| Low | 30 (20.3) | |
| Intermediate-1 | 67 (45.3) | |
| Intermediate-2 | 44 (29.7) | |
| High | 7 (4.7) | |
|
| ||
| Treatment | ||
| 5-Azacitidine | 35 (23.2) | |
| Decitabine | 73 (48.3) | |
| Azacitidine-based combinations | 30 (19.9) | |
| Decitabine-based combinations | 13 (8.6) | |
CMML: chronic myelomonocytic leukemia; Hgb: Hemoglobin; WBC: white blood cells; ANC: absolute neutrophil count; BM: bone marrow; PB: peripheral blood; AML: acute myeloblastic leukemia; CPSS: CMML-specific prognostic scoring system; MDAPS: MD Anderson Cancer Center Prognostic Score System; MCPS: Mayo Clinic Prognostic Score; IPSS: international prognostic score system.
Karyotype was available in 147 patients (abnormal in 103 [70%]). According to the modified Spanish cytogenetic risk stratification, 105 (71%) belonged to the low-risk category, 27 (18%) to the intermediate-risk category, and 15 (10%) to the high-risk category.
Gene sequencing data were available in 28 patients. At least one mutation was identified among 86% of patients. The median number of mutations was 1 (range 0–3). The frequency of identified mutations included were TET2 (n=9, 32%), ASXL1 (n=6, 21%), NRAS (n=5, 18%), IDH2 (n=3, 11%), KRAS (n=3, 11%), NPM1 (n=2, 7%), RUNX1 (n=2, 7%), TP53 (n=2, 7%), BRAF (n=1, 4%) and KIT (n=1, 4%).
According to CPSS, 17 patients (15%) belonged to the low risk group, 45 (39%) to the intermediate-1 risk group, 42 (36%) to the intermediate-2 risk group, and 12 (10%) to the high-risk group. CPSS was not available in 35 patients: in 31 patients, no data of transfusion was available; in 2 patients, cytogenetic data was not available at diagnosis and in 2 patients, both are missing.
Treatment modalities
A total of 35 patients (23%) received azacitidine as single agent and 73 patients (48%) received decitabine as a single agent. The remaining 43 patients (29%) received combinations consisting in either azacitidine (30 patients) or decitabine (13 patients) with different agents (Figure 1). The median time to treatment initiation from diagnosis was 1.8 months (range 0–71), and the median number of cycles of therapy received was 8 cycles (range 1–71). Thirteen patients (9%) underwent allo-SCT after treatment with HMA. No differences in the number of patients going to transplant between patients treated with azacitidine-based therapies (n=5; 8%) or those treated with decitabine-based therapies (n=8; 9%) were observed (p=0.727).
Figure 1. Flow chart of CMML patients receiving hypomethylating agents.
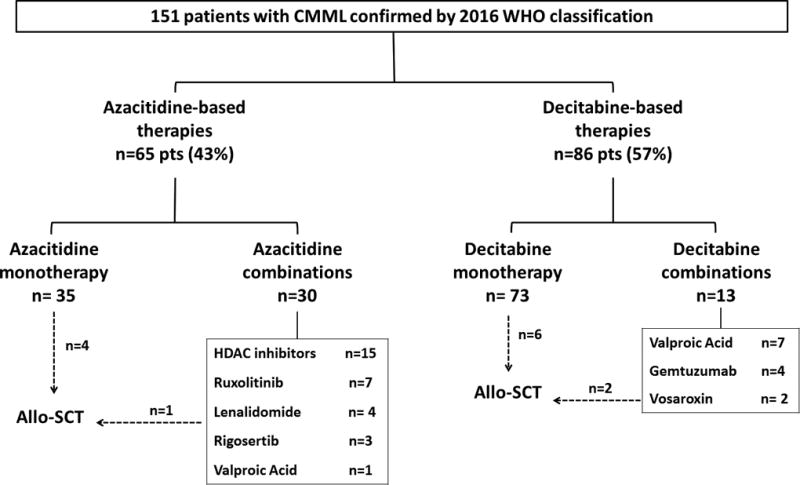
CMML: chronic myelomonocytic leukemia; allo-SCT: allogeneic stem cell transplant; HDAC: histone deacetylase.
Response to HMA
With a median follow up of 18 months (range 1–108), response was available in 145 patients (96%). The overall response rate (ORR) was 75% (109 of 145 patients), including 59 with CR (41%), 42 mCR (29%), 2 mPR (1%), 1 PR (0.7%) and 5 CB (3%) (Supplemental figure 1A). The median number of cycles to achieve a response was 3 (range 1–24) and the median duration of response was 9 months (range 0–68). Twenty-two patients (15%) had stable disease without clinical benefit and 14 (10%) progressed during treatment.
In the univariate analysis, no predictors associated with CR were observed. WHO classification was the only predictive value associated with ORR (CMML-0: p=0.008; CMML-1: p=0.008, OR [95% CI] = 3.92 [1.43–10.82]; CMML-2: p=0.007, OR [95% CI] = 4.21 [1.49–11.96]). No other factors predicting ORR were found (Supplemental table I).
Following the IWG 2006 response criteria for MDS and CMML, response was available in 145 patients including: 64 patients CR (44%), 37 marrow CR (26%), 3 PR (2%), 27 SD (19%) and 14 failures (10%). 64 patients were evaluable for HI, 22 (34.4%) achieving HI (Supplemental figure 1B).
Survival
The median OS was 24 months (95% CI: 20–28), median LFS was 39 months (95% CI: 11–67), and the median EFS was 14 months (95% CI: 11–17) (Figure 2). Responders had a 2-fold improvement in OS compared with non-responders (median OS responders 25 months vs 13 months (p=0.001; HR [95% CI] = 0.413 [0.27–0.64]). By univariate analysis, age > 70 years, lower levels of hemoglobin, the presence of blast in PB, proliferative subtype by FAB classification and the higher-risk in the modified Spanish Cytogenetic Risk stratification had an impact in OS. By multivariate analysis, age > 70 years (p=0.005; HR [95%CI] = 1.84 [1.21–2.84]), lower levels of hemoglobin (<8 g/dL: p=0.002; 8–10 g/dL: p=0.022, HR [95%CI] = 0.37 [0.16–0.87]; >10 g/dL: p=0.001, HR [95%CI] = 0.25 [0.11–0.56];), the presence of blast in PB (p<0.001; HR [95%CI] = 2.92 [1.75–4.85]) and higher-risk modified Spanish Cytogenetic Risk stratification (low: p=0.009; Intermediate: p=0.008, HR [95%CI] = 2.06 [0.20–3.52]; High: p=0.024, HR [95%CI] = 2.15 [1.11–4.22];), predicted for lower likelihood of OS (Supplemental table II).
Figure 2. Overall survival (OS), leukemia-free survival (LFS) and event-free survival (EFS) for patients with CMML treated with hypomethylating agents.
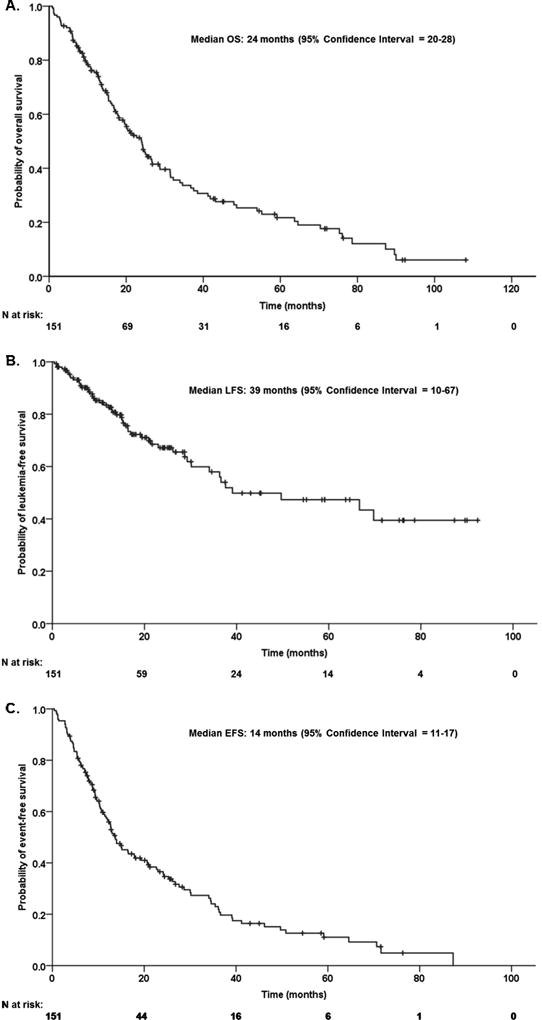
(A) OS, (B) LFS and (C) EFS of the 151 CMML patients treated with hypomethylating agents.
For patients who underwent allo-SCT, median number of cycles received before transplant was 4 (range 2–9). All patients achieved a response before transplant: 5 (38%) in CR and 8 (62%) in mCR. Median overall survival after transplant was not reached (estimated 13 months) and the cumulative incidence of treatment-related mortality (TRM) at day 100 post-transplant was 24%.
Effect of choice of hypomethylating agent
We then studied specific outcomes in patients treated with azacitidine vs decitabine. After considering only patients treated with single agent azacitidine (n=35) or decitabine (n=73), CR as well as the combination of CR+mCR was significantly higher in patients treated with decitabine when compared with azacitidine (CR: 54.8% vs 23.0%, p<0.001; CR+mCR: 77.8% vs 52.9%, p=0.009). Although no statistical differences were observed, patients treated with decitabine had a trend toward a better ORR (81.0% vs. 67.2%, respectively; p=0.059) and a longer OS (median 24 months vs. 20 months, p=0.484) compared with patients treated 5-azacitidine.
To try to minimize the differences between groups, we generated a propensity score matching including hemoglobin, platelets, white blood cells, neutrophils, monocytes, lymphocytes, number of BM and PB blast, 2016 WHO classification, FAB classification and CPSS, to match patients previously treated with azacitidine or decitabine. Patients without a matching partner were excluded. Considering all patients (including combinations), a total of 72 patients from both groups remained in the study for comparison. CR and CR+mCR was significantly higher in patients treated with decitabine when compared with patients treated with azacitidine (CR: 55.6% vs 22.2%, p=0.004; CR+mCR 80.6% vs 58.3%, p=0.041). No differences in term of ORR (decitabine 86.1% vs. azacitidine 66.7%; p=0.052) or OS (median OS: decitabine 19 months (95% CI=11–28) vs. azacitidine 21 months (95% CI=19–23); were observed based on therapy (p=0.969). Similar results were found when we generated a propensity score matching among patients treated in monotherapy (n=28) (Supplemental table III).
Cytogenetic response
Among, the 109 responders to HMAs, 26 patients (24%) with clonal abnormalities at baseline were evaluable for a cytogenetic response. 6 patients (25%) achieved a complete cytogenetic response (including 3 patients with +8, 2 patients with del(20q), 1 patient with –Y and 1 patient with complex karyotype), 1 patient (2%) with a −7 achieved a partial cytogenetic response and 18 patients (69%) did not achieve any response. In 1 patient, response was not evaluable. Median time to acquire cytogenetic response was 4 cycles (range 2–6). No statistical differences were observed in terms of OS for patients with and without complete cytogenetic response (Median OS [95% CI]: cytogenetic response, 18 months [11–29] vs no cytogenetic response, 20 months [7–29]; p=0.756).
Prognostic models
Regarding the prognostic models to estimate the risk in patients with CMML, MDAPS (median OS [95% CI]: low risk, 38 months [21–53]; Intermediate-1, 22 months [14–29]; Intermediate-2, 9 months [5–12]; and High risk, 6 months [0–16]; p<0.001), CPSS (median OS [95% CI]: low risk, 75 months [18–132]; Intermediate-1, 24 months [9–39]; Intermediate-2, 18 months [12–23]; and High risk, 3 months [2–4]; p<0.001) and MCPS (median OS [95% CI]: low risk, 43 months [29–58]; Intermediate, 24 months [17–31]; and High risk, 15 months [10–21]; p<0.001) were significantly associated with OS. Although CPPS was capable to segregate the patients into four prognostic categories for OS, the MDAPS was not capable to significantly discriminate between low and intermediate-1 group (p=0.152) and the MCPS could not differentiate between low and intermediate-risk group (p=0.375). No statistical differences were observed between low, intermediate-1 and intermediate-2 groups using the IPSS prognostic model (Table II).
Table II.
Impact of the different risk prognostic models in Overall Survival in CMML patients treated with hypomethylating agents.
|
|
||||
|---|---|---|---|---|
| Kaplan-Meier for OS (months) | Cox-Regression for OS | |||
| Median (95%CI) | p | HR (95%CI) | p | |
| CPSS | ||||
| Low | 75.3 (0.0–165.5) | <0.001 | ||
| Intermediate-1 | 24.4 (13.7–35.0) | <0.001 | 1.93 (0.85–4.40) | <0.001 |
| Intermediate-2 | 18.1 (11.8–24.4) | 2.64 (1.17–5.94) | <0.001 | |
| High | 6.1 (0.0–13.1) | 8.51 (3.15–22.99) | 0.02 | |
|
| ||||
| MDAPS | ||||
| Low | 41.2 (31.9–50.1) | <0.001 | ||
| Intermediate-1 | 23.9 (18.9–28.9) | 1.42 (0.88–2.28) | 0.152 | |
| Intermediate-2 | 9.8 (6.7–12.9) | <0.001 | 4.16 (2.41–7.17) | <0.001 |
| High | 11.7 (2.6–20.8) | 5.08 (2.46–10.49) | <0.001 | |
|
| ||||
| MCPS | ||||
| Low | 43.2 (28.7–57.7) | <0.001 | ||
| Intermediate | 26.5 (14.7–38.0) | <0.001 | 1.37 (0.68–2.74) | 0.375 |
| High | 15.4 (9.3–21.5) | 3.40 (1.64–7.05) | <0.001 | |
|
| ||||
| IPSS | ||||
| Low | 16.0 (13.2–18–8) | 0.188 | ||
| Intermediate-1 | 26.7 (21.9–31.4) | 0.181 | 0.59 (0.35–0.99) | 0.049 |
| Intermediate-2 | 20.4 (11.3–29.5) | 0.80 (0.46–1.40) | 0.436 | |
| High | 9.0 (5.0–13.1) | 1.05 (0.36–3.05) | 0.935 | |
CMML: chronic myelomonocytic leukemia; OS: Overall survival; OR: Odds ratio; CPSS: CMML-specific prognostic scoring system; MDAPS: MD Anderson Cancer Center Prognostic Score System; MCPS: Mayo Clinic Prognostic Score; IPSS: international prognostic score system.
HMA failure CMML
Seventy-six patients (50%) had HMA failure at the time of last follow up. Median time for relapse was 17 months (range 1–72). Clinical characteristics at relapse are shown in supplemental table IV. 26 patients relapsed as primary HMA failure (34%) and 50 patients as secondary HMA failure (66%). Karyotype at the time of CMML diagnosis and relapse was available in 64 patients including: 49 (76.6%) maintained karyotype observed at diagnosis, 4 (5.3%) acquired a trisomy 8, 2 (2.6%) acquired a complex karyotype, and 9 (11.8%) acquired other karyotype abnormalities at the moment of relapse.
Thirty-five patients (46%) transformed to AML during therapy and were included in AML treatment protocols. For the remaining 41 patients (54%), the treatment choice immediately after HMA failure included allogeneic stem cell transplant without prior re-induction in 1 patient, supportive care in 7 patients and salvage therapy in 28, including: change to another HMA in 5 patients, standard chemotherapy in 17 patients, and investigational agents in 6 patients. Data on subsequent therapy was not available in 5 patients.
CMML patients with HMA failure had poor outcomes with a median OS of 7 months from the time of relapse (95% CI: 3–12) (Supplemental figure 2A). Although no statistical differences were observed, patients with primary HMA failure had a trend toward a worse OS compared with patients with secondary HMA failure (median OS form failure [95% CI]: primary HMA failure, 5 [3–6] vs secondary HMA failure, 11 [6–15]; p=0.096) (Supplemental figure 2B).
Regarding the 5 patients who switched to another HMA after failure, 2 patients achieved mCR as best response, 2 patients did not achieve any response and one patient was not evaluable because he died before complete the first cycle. OS for those patients was extremely poor (median OS from failure [95% CI]: 4 months [3–4]).
DISCUSSION
CMML is a hematopoietic stem cell disorder with both myeloproliferative and myelodysplastic features. It affects mainly elderly patients and is associated with poor outcomes[39]. The approval of HMAs for the treatment of MDS, resulted in the wide spread use of these compounds for patients with CMML, at least in the United States. However, because the clinical trials that led to the approval of these drugs were not CMML specific, and the number of CMML patients enrolled was small (10 to 75 patients), the exact impact that HMAs have in the natural history of CMML is not well understood.
We retrospectively review the records of 151 patients treated with HMA at our Institution. It represents the largest series of CMML patients treated with HMA so far reported. The distribution of patient population was comparable with previous published studies[22–26, 40]. Notably, 70% of the patients in our cohort presented with a cytogenetic abnormality, clearly greater than the 30% expected in CMML patients[7]. As expected, most of them belonged to the good-risk category (71%) according to the CPSS cytogenetic risk group. Moreover, therapy-related CMML was noted in 30% of patients, a number slightly higher than the 8–16% observed in other studies [16, 19, 21].
Response was assessed with both, 2015 International Consortium Response Criteria for Myelodysplastic/Myeloproliferative neoplasm in adults[34] and the 2006 International Working Group (IWG) modified response criteria in MDS [35]. 2015 International Consortium Response Criteria for Myelodysplastic/Myeloproliferative neoplasm in adults was used to compare azacitidine vs decitabine because although we know that this response criteria have never been validated before, we considered that they are more accurate to evaluate the effect of therapy in the myeloproliferative components of the disease as leukocytosis, monocytosis or splenomegaly.
As previously published, HMA are clinically effective in the treatment of CMML patients, providing an ORR of 75% including CR rate of 41% and a OS of 20 months, comparable with other series[19–25, 40]. Clinical response was associated with a 2-fold increase in OS[20–22, 25]. Age < 70 years, higher levels of hemoglobin, the absence of blast in PB, as well as, low CPSS cytogenetic risk were associated with improved survival. In contrast to previous studies, no statistical differences in terms of OS were observed between the different 2016 WHO subgroups[41, 42]. Of interest, achieving a cytogenetic response with HMA did not predict for a better OS, probably due to the small number of patients with evaluable cytogenetic response and in contrast with a recent report in MDS population[43].
There has not been direct previous comparison between azacitidine and decitabine. In this analysis, CR rates were higher in patients treated with decitabine (58.3%) than 5-azacitdine (20.6%). This resulted in a trend towards better ORR and OS (81.9% vs 64.7% and 26 vs 20 months, respectively). However, this analysis is not powered to conclude a superiority of decitabine vs 5-azaictidine, maybe because the small number of patients in each group.
Only thirteen patients underwent a stem cell transplant after treatment with HMA. The median number of cycles received before transplant was 4, and all underwent transplant in response. Median OS was not reached, but the cumulative incidence of TRM at day 100-post transplant is high (24%), similar to previously published data[44].
Regarding the prognostic models to estimate the risk in patients with CMML, MDAPS, CPSS and MCPS were significantly associated with OS, but only CPPS was capable to segregate the patients into the 4 groups [45]. On the other hand, IPSS seems to be a poor prognostic model for survival in patients with CMML[29], supporting the importance of using CMML specific models.
CMML patients with HMA failure had poor outcomes with median OS of 7 months, particularly those with primary HMA failure (5 months), similar to previous reports in HMA failure in myelodysplastic syndromes[36, 37, 46, 47]. Although, relapsing with the same abnormalities observed in karyotype at diagnosis is the most frequent (76.6%), the most frequent abnormalities acquired during treatment was the trisomy 8, observed in 5% of the patients. Almost 50% of the patients relapse developing an AML, higher than previously published (~22%)[37, 46]. The remaining patients, relapse as a CMML, and the treatment choice was heterogeneous, including patients who received another HMA, investigational agents or allo-SCT. Switching to another HMA does not seem to be the best option in patients with HMA failure CMML, confirming the urgent need for improve salvage therapies for these patients.
Our data have several limitations. First of all, our data represent a single-institution retrospective analysis. Moreover, because we included patients with CMML diagnosed between 2004 and 2015, gene sequencing data were available only in 28 patients (platform available since 2012). However, in these patients, at least one mutation was identified among 86% of patients, similar to other published studies[8, 9] but the frequencies of the presence of different molecular alterations were slightly different with those observed from other groups. It should be noted that none of the mutations of the spliceosome components were included in the 28 or 53 NGS platform performed.
In conclusion, we present the largest series of patients with CMML treated with HMA. As previously published, HMAs has a significant activity in CMML patients, with responses associated with better outcomes. However, outcomes after HMA failure are poor, evidencing the urgency of new therapies for this type of patients.
Supplementary Material
Acknowledgments
A.A. is supported by the scholarship FEHH-Janssen from the Spanish Foundation of Haematology. Support for this project was provided by the following sources: the MD Anderson Cancer Center Support Grant P30 CA016672., the Dr. Kenneth B. McCredie Chair in Clinical Leukemia Research endowment, the Edward P. Evans Foundation, the Fundacion Ramon Areces, the Cancer Prevention & Research Institute of Texas (CPRIT) award RP140500, and by generous philanthropic contributions to MD Anderson’s MDS/AML Moon Shot Program.
References
- 1.Jaffe ES. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. [Google Scholar]
- 2.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 3.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 4.Osca-Gelis G, Puig-Vives M, Saez M, et al. Population-based incidence of myeloid malignancies: fifteen years of epidemiological data in the province of Girona, Spain. Haematologica. 2013;98:e95–97. doi: 10.3324/haematol.2013.084061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rollison DE, Howlader N, Smith MT, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 6.Bennett JM, Catovsky D, Daniel MT, et al. The chronic myeloid leukaemias: guidelines for distinguishing chronic granulocytic, atypical chronic myeloid, and chronic myelomonocytic leukaemia. Proposals by the French-American-British Cooperative Leukaemia Group. Br J Haematol. 1994;87:746–754. doi: 10.1111/j.1365-2141.1994.tb06734.x. [DOI] [PubMed] [Google Scholar]
- 7.Tang G, Zhang L, Fu B, et al. Cytogenetic risk stratification of 417 patients with chronic myelomonocytic leukemia from a single institution. Am J Hematol. 2014;89:813–818. doi: 10.1002/ajh.23751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papaemmanuil E, Gerstung M, Malcovati L, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122:3616–3627. doi: 10.1182/blood-2013-08-518886. quiz 3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itzykson R, Kosmider O, Renneville A, et al. Clonal architecture of chronic myelomonocytic leukemias. Blood. 2013;121:2186–2198. doi: 10.1182/blood-2012-06-440347. [DOI] [PubMed] [Google Scholar]
- 10.Bennett JM. Chronic myelomonocytic leukemia. Curr Treat Options Oncol. 2002;3:221–223. doi: 10.1007/s11864-002-0011-6. [DOI] [PubMed] [Google Scholar]
- 11.Kroger N, Zabelina T, Guardiola P, et al. Allogeneic stem cell transplantation of adult chronic myelomonocytic leukaemia. A report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Br J Haematol. 2002;118:67–73. doi: 10.1046/j.1365-2141.2002.03552.x. [DOI] [PubMed] [Google Scholar]
- 12.Eissa H, Gooley TA, Sorror ML, et al. Allogeneic hematopoietic cell transplantation for chronic myelomonocytic leukemia: relapse-free survival is determined by karyotype and comorbidities. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17:908–915. doi: 10.1016/j.bbmt.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park S, Labopin M, Yakoub-Agha I, et al. Allogeneic stem cell transplantation for chronic myelomonocytic leukemia: a report from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. European journal of haematology. 2013;90:355–364. doi: 10.1111/ejh.12073. [DOI] [PubMed] [Google Scholar]
- 14.Itonaga H, Iwanaga M, Aoki K, et al. Impacts of graft-versus-host disease on outcomes after allogeneic hematopoietic stem cell transplantation for chronic myelomonocytic leukemia: A nationwide retrospective study. Leukemia research. 2016;41:48–55. doi: 10.1016/j.leukres.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Symeonidis A, van Biezen A, de Wreede L, et al. Achievement of complete remission predicts outcome of allogeneic haematopoietic stem cell transplantation in patients with chronic myelomonocytic leukaemia. A study of the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Br J Haematol. 2015 doi: 10.1111/bjh.13576. [DOI] [PubMed] [Google Scholar]
- 16.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 17.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 18.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aribi A, Borthakur G, Ravandi F, et al. Activity of decitabine, a hypomethylating agent, in chronic myelomonocytic leukemia. Cancer. 2007;109:713–717. doi: 10.1002/cncr.22457. [DOI] [PubMed] [Google Scholar]
- 20.Braun T, Itzykson R, Renneville A, et al. Molecular predictors of response to decitabine in advanced chronic myelomonocytic leukemia: a phase 2 trial. Blood. 2011;118:3824–3831. doi: 10.1182/blood-2011-05-352039. [DOI] [PubMed] [Google Scholar]
- 21.Such E, Cervera J, Costa D, et al. Cytogenetic risk stratification in chronic myelomonocytic leukemia. Haematologica. 2011;96:375–383. doi: 10.3324/haematol.2010.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fianchi L, Criscuolo M, Breccia M, et al. High rate of remissions in chronic myelomonocytic leukemia treated with 5-azacytidine: results of an Italian retrospective study. Leukemia & lymphoma. 2013;54:658–661. doi: 10.3109/10428194.2012.719617. [DOI] [PubMed] [Google Scholar]
- 23.Thorpe M, Montalvao A, Pierdomenico F, et al. Treatment of chronic myelomonocytic leukemia with 5-Azacitidine: a case series and literature review. Leukemia research. 2012;36:1071–1073. doi: 10.1016/j.leukres.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 24.Wijermans PW, Ruter B, Baer MR, et al. Efficacy of decitabine in the treatment of patients with chronic myelomonocytic leukemia (CMML) Leukemia research. 2008;32:587–591. doi: 10.1016/j.leukres.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Ades L, Sekeres MA, Wolfromm A, et al. Predictive factors of response and survival among chronic myelomonocytic leukemia patients treated with azacitidine. Leukemia research. 2013;37:609–613. doi: 10.1016/j.leukres.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Pleyer L, Germing U, Sperr WR, et al. Azacitidine in CMML: matched-pair analyses of daily-life patients reveal modest effects on clinical course and survival. Leukemia research. 2014;38:475–483. doi: 10.1016/j.leukres.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Such E, Germing U, Malcovati L, et al. Development and validation of a prognostic scoring system for patients with chronic myelomonocytic leukemia. Blood. 2013;121:3005–3015. doi: 10.1182/blood-2012-08-452938. [DOI] [PubMed] [Google Scholar]
- 28.Patnaik MM, Padron E, LaBorde RR, et al. Mayo prognostic model for WHO-defined chronic myelomonocytic leukemia: ASXL1 and spliceosome component mutations and outcomes. Leukemia. 2013;27:1504–1510. doi: 10.1038/leu.2013.88. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 30.Kantarjian H, O'Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113:1351–1361. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onida F, Kantarjian HM, Smith TL, et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood. 2002;99:840–849. doi: 10.1182/blood.v99.3.840. [DOI] [PubMed] [Google Scholar]
- 32.Shaffer LG, Tommerup N. International Standing Committee on Human Cytogenetic Nomenclature. Basel. Farmington, CT: Karger; 2005. ISCN 2005 : an international system for human cytogenetic nomenclature (2005) : recommendations of the International Standing Committee on Human Cytogenetic Nomenclature; p. 130. [Google Scholar]
- 33.Wassie EA, Itzykson R, Lasho TL, et al. Molecular and prognostic correlates of cytogenetic abnormalities in chronic myelomonocytic leukemia: a Mayo Clinic-French Consortium Study. Am J Hematol. 2014;89:1111–1115. doi: 10.1002/ajh.23846. [DOI] [PubMed] [Google Scholar]
- 34.Savona MR, Malcovati L, Komrokji R, et al. An international consortium proposal of uniform response criteria for myelodysplastic/myeloproliferative neoplasms (MDS/MPN) in adults. Blood. 2015;125:1857–1865. doi: 10.1182/blood-2014-10-607341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 36.Prebet T, Gore SD, Esterni B, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3322–3327. doi: 10.1200/JCO.2011.35.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jabbour E, Garcia-Manero G, Batty N, et al. Outcome of patients with myelodysplastic syndrome after failure of decitabine therapy. Cancer. 2010;116:3830–3834. doi: 10.1002/cncr.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–199. [PubMed] [Google Scholar]
- 39.Germing U, Kundgen A, Gattermann N. Risk assessment in chronic myelomonocytic leukemia (CMML) Leukemia & lymphoma. 2004;45:1311–1318. doi: 10.1080/1042819042000207271. [DOI] [PubMed] [Google Scholar]
- 40.Wong E, Seymour JF, Kenealy M, et al. Treatment of chronic myelomonocytic leukemia with azacitidine. Leukemia & lymphoma. 2013;54:878–880. doi: 10.3109/10428194.2012.730615. [DOI] [PubMed] [Google Scholar]
- 41.Schuler E, Schroeder M, Neukirchen J, et al. Refined medullary blast and white blood cell count based classification of chronic myelomonocytic leukemias. Leukemia research. 2014;38:1413–1419. doi: 10.1016/j.leukres.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Storniolo AM, Moloney WC, Rosenthal DS, et al. Chronic myelomonocytic leukemia. Leukemia. 1990;4:766–770. [PubMed] [Google Scholar]
- 43.Jabbour E, Strati P, Cabrero M, et al. Impact of Achievement of Complete Cytogenetic Response on Outcome in Patients with Myelodysplastic Syndromes Treated with Hypomethylating Agents. Am J Hematol. 2017 doi: 10.1002/ajh.24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kongtim P, Popat U, Jimenez A, et al. Treatment with Hypomethylating Agents before Allogeneic Stem Cell Transplant Improves Progression-Free Survival for Patients with Chronic Myelomonocytic Leukemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2016;22:47–53. doi: 10.1016/j.bbmt.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calvo X, Nomdedeu M, Santacruz R, et al. Comparison of three prognostic scoring systems in a series of 146 cases of chronic myelomonocytic leukemia (CMML): MD Anderson prognostic score (MDAPS), CMML-specific prognostic scoring system (CPSS) and Mayo prognostic model. A detailed review of prognostic factors in CMML. Leukemia research. 2015 doi: 10.1016/j.leukres.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 46.Duong VH, Lin K, Reljic T, et al. Poor outcome of patients with myelodysplastic syndrome after azacitidine treatment failure. Clin Lymphoma Myeloma Leuk. 2013;13:711–715. doi: 10.1016/j.clml.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Manero G, Fenaux P, Al-Kali A, et al. Rigosertib versus best supportive care for patients with high-risk myelodysplastic syndromes after failure of hypomethylating drugs (ONTIME): a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17:496–508. doi: 10.1016/S1470-2045(16)00009-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


