Abstract
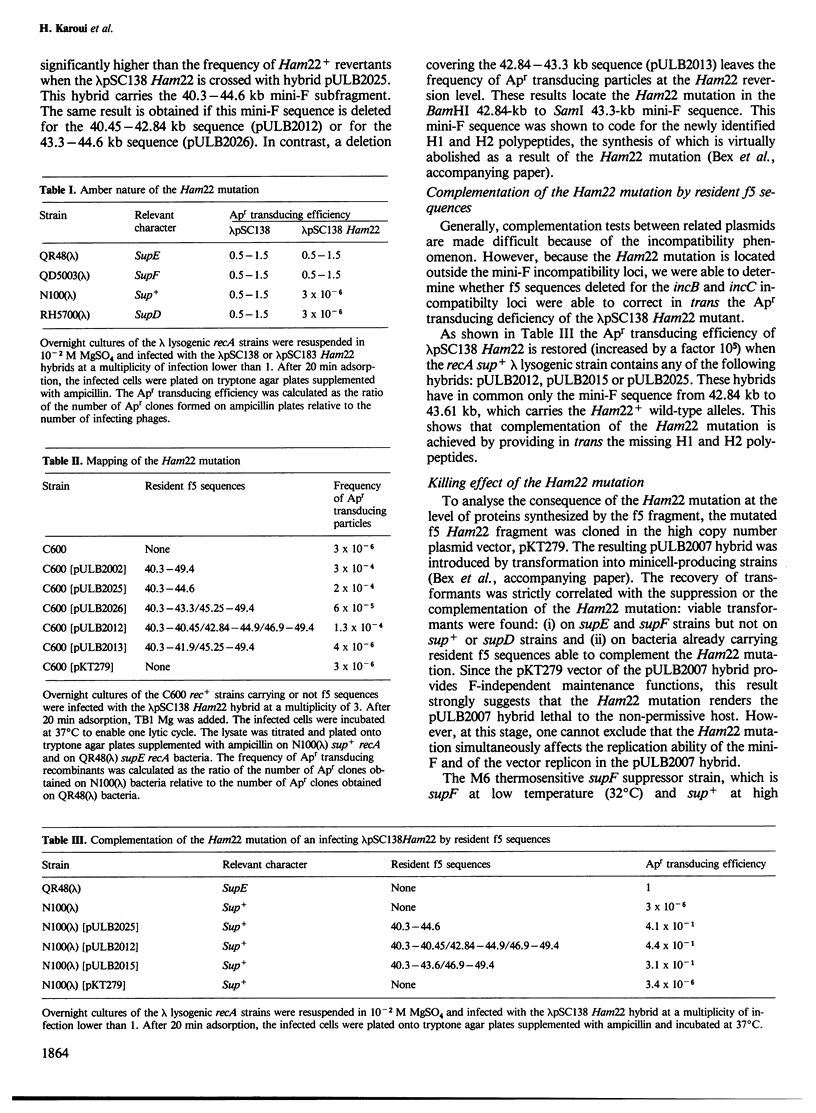
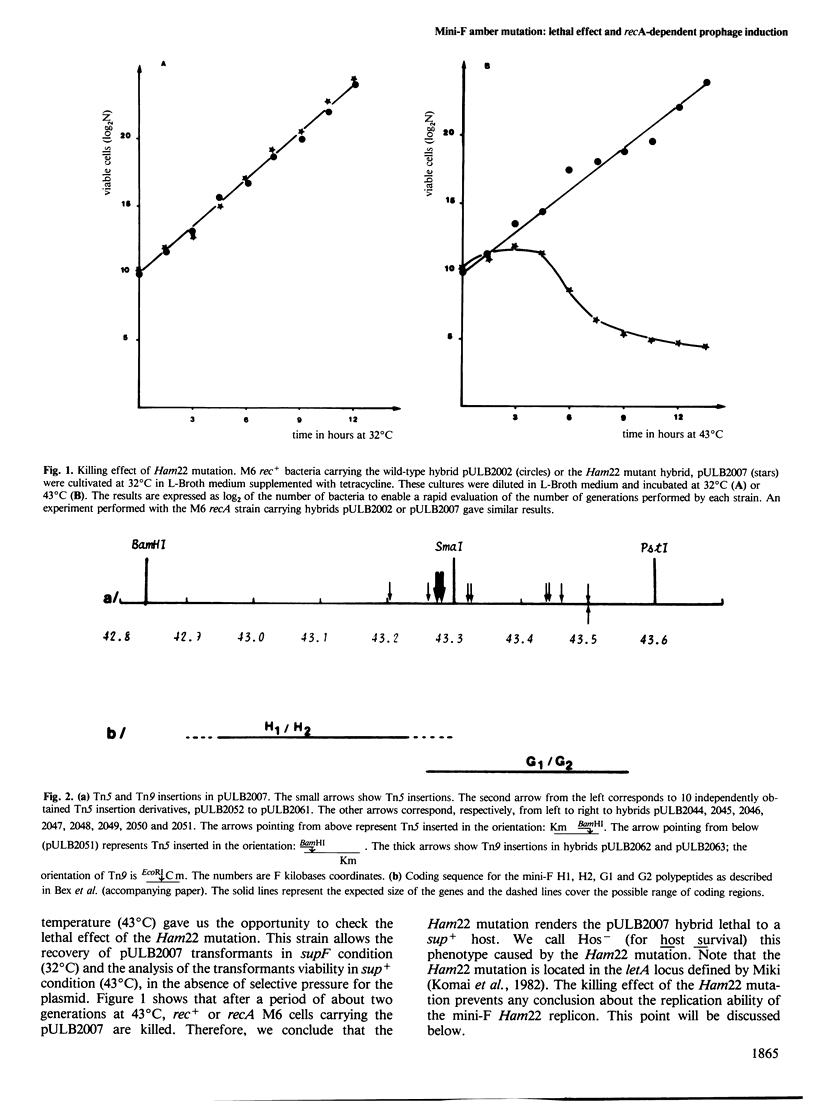
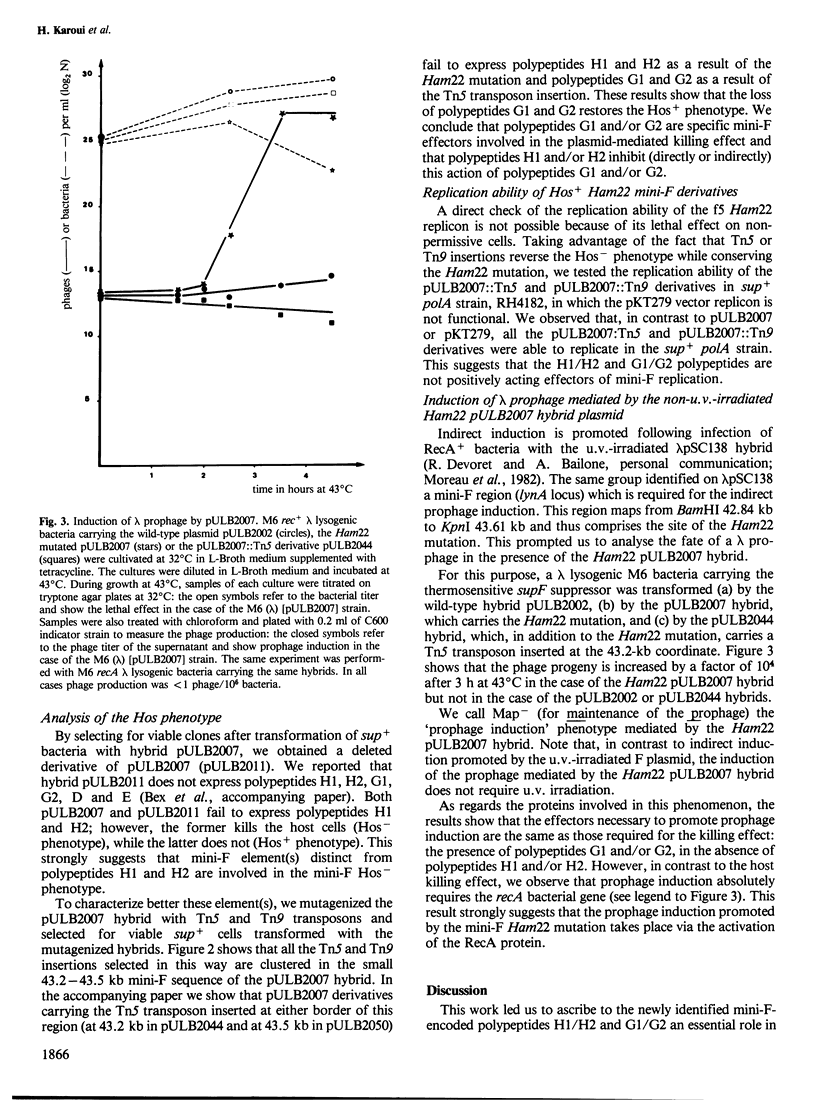
A mini-F region 800 bp long, located between the two F origin sites, plays an essential role in the relationship between the F plasmid and its host. This region comprises two sets of overlapping coding sequences: the first set codes for the newly identified H1 and H2 polypeptides; the second set codes for polypeptides G1 and G2. A mini-F amber mutation (Ham22) causes the virtual disappearance of polypeptides H1 and H2 but only slightly reduces synthesis of polypeptides G1 and G2. This mutation: (i) renders mini-F hybrids lethal to the host cells (conditional Hos- phenotype for host survival) and (ii) causes the induction of a resident prophage in recA+ strains (conditional Map- phenotype for maintenance of the prophage). When an additional mutation prevents the synthesis of polypeptides G1 and G2, both the lethal character and the induction of the prophage are abolished. We conclude: (i) that polypeptides G1 and/or G2 are specific mini-F polypeptides involved in the plasmid-mediated killing effect and in the recA-dependent induction of the resident prophage and (ii) that, in normal conditions, polypeptides H1 and/or H2 negatively control (directly or indirectly) the action of polypeptides G1 and/or G2. In relation to the analysis of indirect induction mediated by u.v.-irradiated lambda mini-F hybrids, we propose that polypeptides G1 and/or G2 are specific mini-F products involved in the activation of the bacterial SOS pathway. The H1/H2 and G1/G2 polypeptides could constitute the controlled mini-F signal enabling the coordination between cell division and F plasmid replication.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOREK E., RYAN A. The transfer of a biologically active irradiation product from cell to cell. Biochim Biophys Acta. 1960 Jun 17;41:67–73. doi: 10.1016/0006-3002(60)90369-3. [DOI] [PubMed] [Google Scholar]
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek E., Ryan A. THE TRANSFER OF IRRADIATION-ELICITED INDUCTION IN A LYSOGENIC ORGANISM. Proc Natl Acad Sci U S A. 1958 May;44(5):374–377. doi: 10.1073/pnas.44.5.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier M., Janssens J., Bex F., Desmyter A., Bonnevalle I. Construction in vitro of "phage-plasmid" chimerae: a new tool to analyse the mechanism of plasmid maintenance. Mol Gen Genet. 1979 Jan 16;169(1):113–116. doi: 10.1007/BF00267552. [DOI] [PubMed] [Google Scholar]
- D'Ari R., Huisman O. DNA replication and indirect induction of the SOS response in Escherichia coli. Biochimie. 1982 Aug-Sep;64(8-9):623–627. doi: 10.1016/s0300-9084(82)80100-4. [DOI] [PubMed] [Google Scholar]
- Delcuve G., Cabezón T., Ghysen A., Herzog A., Bollen A. Amber mutations in Escherichia coli essential genes: isolation of mutants affected in the ribosomes. Mol Gen Genet. 1977 Nov 29;157(2):149–153. doi: 10.1007/BF00267392. [DOI] [PubMed] [Google Scholar]
- Devoret R., George J. Induction indirecte du prophage lambda par le rayonnement ultraviolet. Mutat Res. 1967 Nov-Dec;4(6):713–734. doi: 10.1016/0027-5107(67)90081-4. [DOI] [PubMed] [Google Scholar]
- Goldberg A. R., Howe M. New mutations in the S cistron of bacteriophage lambda affecting host cell lysis. Virology. 1969 May;38(1):200–202. doi: 10.1016/0042-6822(69)90148-2. [DOI] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. The integration and excision of the bacteriophage lambda genome. Cold Spring Harb Symp Quant Biol. 1968;33:735–747. doi: 10.1101/sqb.1968.033.01.084. [DOI] [PubMed] [Google Scholar]
- Gottesman S. Genetic control of the SOS system in E. coli. Cell. 1981 Jan;23(1):1–2. doi: 10.1016/0092-8674(81)90261-0. [DOI] [PubMed] [Google Scholar]
- Huisman O., D'Ari R. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature. 1981 Apr 30;290(5809):797–799. doi: 10.1038/290797a0. [DOI] [PubMed] [Google Scholar]
- Komai N., Nishizawa T., Hayakawa Y., Murotsu T., Matsubara K. Detection and mapping of six miniF-encoded proteins by cloning analysis of dissected miniF segments. Mol Gen Genet. 1982;186(2):193–203. doi: 10.1007/BF00331850. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Monk M. Induction of phage lambda by transferred irradiated colI DNA. Mol Gen Genet. 1969;106(1):14–24. doi: 10.1007/BF00332817. [DOI] [PubMed] [Google Scholar]
- Moreau P. L., Pelico J. V., Devoret R. Cleavage of lambda repressor and synthesis of RecA protein induced by transferred UV-damaged F sex factor. Mol Gen Genet. 1982;186(2):170–179. doi: 10.1007/BF00331847. [DOI] [PubMed] [Google Scholar]
- Murgola E. J., Yanofsky C. Structural interactions between amino acid residues at positions 22 and 211 in the tryptophan synthetase alpha chain of Escherichia coli. J Bacteriol. 1974 Feb;117(2):444–448. doi: 10.1128/jb.117.2.444-448.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L., Kass L. R., Yarmolinsky M. B. Parallel behavior of F and Pl in causing indirect induction of lysogenic bacteria. Cold Spring Harb Symp Quant Biol. 1968;33:785–789. doi: 10.1101/sqb.1968.033.01.090. [DOI] [PubMed] [Google Scholar]
- Signer E. R., Weil J. Recombination in bacteriophage lambda. I. Mutants deficient in general recombination. J Mol Biol. 1968 Jul 14;34(2):261–271. doi: 10.1016/0022-2836(68)90251-9. [DOI] [PubMed] [Google Scholar]
- Talmadge K., Gilberg W. Construction of plasmid vectors with unique PstI cloning sites in a signal sequence coding region. Gene. 1980 Dec;12(3-4):235–241. doi: 10.1016/0378-1119(80)90105-5. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


