Abstract
Mesodiencephalic dopaminergic (mdDA) neurons are located in the ventral midbrain. These neurons form the substantia nigra (SNc) and the ventral tegmental area (VTA). Two transcription factors that play important roles in the process of terminal differentiation and subset-specification of mdDA neurons, are paired-like homeodomain transcription factor 3 (Pitx3), and homeobox transcription factor Engrailed 1 (En1). We previously investigated the single Pitx3KO and En1KO and observed important changes in the survival of mdDA neurons of the SNc and VTA as well as altered expression of pivotal rostral- and caudal-markers, Ahd2 and Cck, respectively. To refine our understanding of the regional-specific relationships between En1 and Pitx3 and their (combined) role in the programming mdDA neurons on the rostral-to-caudal axis, we created double En1tm1Alj/tm1Alj;Pitx3gfp/gfp (En1KO;Pitx3GFP/GFP) animals. Here we report, that in absence of En1 and Pitx3, only a limited number of mdDA neurons are present at E14.5. These mdDA neurons have a rudimentary dopaminergic cell fate, as they express Nurr1, Pbx3 and Otx2 but have lost their rostral or caudal subset identity. Furthermore, we report that the expression of Cck depends on En1 expression, while (in contrast) both Pitx3 and En1 are involved in the initiation of Ahd2 expression. Thus we reveal in this manuscript that regulated levels of Pitx3 and En1 control the size and rostral/caudal-identity of the mdDA neuronal population.
Introduction
The neurons of the substantia nigra (SNc) and the ventral tegmental area (VTA) originate in from the di- and mesencephalon, and as such are called mesodiencephalic dopaminergic (mdDA) neurons. These neurons are important enforcers of movement and motivation, and are targeted in neurodegenerative pathologies, such as Parkinson's Disease (PD). Interestingly, post-mortem tissue of PD patients revealed that mdDA neurons of the SNc are more vulnerable to cell loss (~80% of the SNc neurons are lost), while the mdDA neurons of the VTA are spared more (~50% loss) [1,2]. In order to enhance the understanding of subset specific vulnerability of mdDA neurons, many efforts have been made to understand the molecular similarities and differences between SNc and VTA neurons. Large rodent-based micro-array studies determined that the difference in molecular profile between the SNc and VTA is smaller than 3% [3–5]; reviewed in [6]. Several studies from our group contributed to the quest to define molecular profiles for different mdDA subsets already during embryonic development [7–10]. Recently, elegant transcriptomic studies identified these molecular profiles in single-cellular resolution in early post-natal tissue [11], as well as during embryonic development of both murine and human tissue [12].
Embryonic mdDA neurons that mature into the SNc can be distinguished from mdDA neurons that develop into the VTA, based on their anatomical position and molecular profile [10]. An important marker of the rostrolateral mdDA neurons is aldehyde dehydrogenase family 1 (Ahd2/Aldh1a1) [11,13] which is involved in the metabolism of retinoic acid (RA) out of retinol, and as such has a role during neuronal development in processes of differentiation, and survival [14,15]. Moreover, aldehyde dehydrogenases are required for the metabolism of 3,4-dihydroxyphenylacetaldehyde (DOPAL), which is a toxic metabolyte of dopamine [16]. The relevance of Ahd2 as a marker for an mdDA neuronal subset was strengthened by the report that the ventral tier of the human SNc, which consists of Ahd2-positive mdDA neurons, is the most sensitive to premature cell death in PD [1,17]. Remarkably, a recent study using a mouse model for over-expressing α-synuclein showed that Ahd2-negative neurons of the mdDA neurons were more vulnerable for neurodegeneration, suggesting a protective role for Ahd2 [17]. In contrast, mdDA neurons that will develop into the VTA are characterized by the expression of Cholecystokinin (Cck) and are located caudomedially at embryonic day (E)14.5 [13]. Cck is a neuropeptide, that has been linked to dopaminergic-mediated pathologies such as schizophrenia and addiction, and its receptors are expressed in the nucleus accumbens and the VTA [18]. Furthermore, Cck exerts a neuroprotective role on cholinergic neurons after a basal-forebrain lesion was introduced in rats [19]. Recently, such a neuroprotective role for Cck was also described in rat hippocampal cultured neurons. A 14-day supplementation of Cck-8S to adult rats significantly reduced TUNEL-activity and increased the number of KI67-positive neurons in the granular layer of the hippocampus [20]. Whether Cck also fulfills a neuroprotective role in mdDA neurons is yet unclear.
Three transcription factors that play important roles in the process of terminal differentiation and subset-specification of mdDA neurons, are the orphan nuclear hormone receptor Nurr1 (Nr4a2), paired-like homeodomain transcription factor 3 (Pitx3), and homeobox transcription factor Engrailed 1 (En1). Nurr1 expression starts in the midbrain at E10.5 and continues to be expressed in mdDA neurons into adulthood [21]. Loss of Nurr1 results in the ablation of Th, Vmat2, Dat, but Pitx3 and En1 expression remain unaffected [21–24]. Nurr1 initiates the expression of most of the DA genes in cooperation with Pitx3, as both transcription factors interact with similar dopaminergic gene regulatory transcription complexes [22]. Pitx3 is selectively expressed in all mdDA neurons from E11.5 onward [25], but in Pitx3-ablated animals mainly the mdDA neurons of the SNc are affected. Furthermore, Pitx3 is pivotal for the expression of Ahd2 (through binding to a promoter region of Ahd2), as its expression is lost in Pitx3-deficient animals [26]. Importantly, in absence of Pitx3, Nurr1 expression remains unchanged, while En1 is upregulated [22,27]. The homeobox transcription factor En1 is expressed in mid- and hindbrain from E8 onwards [28], and En1-null mice are characterized by cell loss of the entire SNc, as well as the majority of the VTA [13]. Nurr1 expression is unaltered in En1-ablated animals, but the expression of Pitx3 is diminished, suggesting that Pitx3 and En1 modulate each others expression levels. Interestingly, the regulation of En1 and Pitx3 upon each other is markedly different between the rostral and the caudal subsets, and the absence of either Pitx3 or En1 affects each subset differently [13].
To refine our understanding of the regional-specific relationships between En1 and Pitx3 and their (combined) role in mdDA neuronal programming, we created the double En1tm1Alj/tm1Alj;Pitx3gfp/gfp (En1KO;Pitx3GFP/GFP) animal. Here we report that in absence of En1 and Pitx3, only a limited number of mdDA neurons are present at E14.5, underlining the necessity of Pitx3 and En1 in the generation and/or survival of embryonic mdDA neurons. The remaining mdDA neurons still express Nurr1, Pbx3, and Otx2 but have lost their rostral (Ahd2+) or caudal (Cck+) subset identity. Additionally, the diminished expression of Cck illustrates its dependence on En1 activation, while (in contrast) both Pitx3 and En1 are involved in the initiation of Ahd2 expression. Thus, these data further substantiate the notion that En1 and Pitx3 determine the size and subset-specificity of the mdDA neuronal population.
Methods
Animals
All animals experimentation was supported and granted by the Animals experimentation committee of the University of Amsterdam according national and international legislation. Embryos were isolated at embryonic day (E)14.5, considering the morning of detection of the vaginal plug as E0.5. Tissue was isolated at post-natal day (P)0 (day of birth), immediately after birth, before lethality set in.
Pitx3gfp/gfp animals, in which the Pitx3 gene is substituted by a GFP allele [29], were inter-crossed with En1tm1Alj/+ animals to breed the 'intermediate genotype' En1tm1Alj/+;Pitx3gfp/gfp. These animals were bred to generate litters that included En1+/+;Pitx3gfp/gfp, En1tm1Alj/+;Pitx3gfp/gfp and En1tm1Alj/tm1Alj;Pitx3gfp/gfp. Genotyping on the Pitx3gfp allele and the En1-mutant allele were performed as described previously [30]. All mice are readily present in our lab.
Male and female mice a separately housed in IVC units (“Tecniplast Smartflow”) in type 2 cages. Breeding was performed in regular type 2 cages. The mice are housed with “Lignocel” bedding with soap-less tissues as cage-enrichment (“Kleenex”) with ad-lib access to “SDS-special diet” for food and ad-lib water. Animals are cared for on a daily basis according to rules and regulation of dutch and EU law. Animals were sacrificed by exposure to CO2/O2: 70%/30% for 3 minutes and then by cervical dislocation or decapitation according rules and regulation of Dutch and EU law.
Fluorescence-activated cell sorting (FACS) and dissection
Midbrains and rostral hindbrains were dissection in L15-5% Fetal Calf Serum (Gypko). Dissociation and sorting of mid-hindbrains were performed as described previously [13,27]. In short, freshly isolated tissue were dissociated using a Papain dissociation system (Worthington). Cells were sorted on a BD FACS Aria III using previously described settings [27] and collected in Trizol-LS (Invitrogen).
Quantitative PCR (qPCR)
Relative expression levels were determined by qPCR real-time PCR (Lightcycler) using the QuantiTect SYBR Green PCR LightCycler Kit (QIAGEN) according to the manufacturer’s instructions. For each reaction 0.1 ng (FAC-sorted neurons) total RNA was used as input. Primer pairs were previously published [27].
Fluorescent immunohistochemistry
Embryos were fixed in 4% paraformaldehyde in PBS, cryoprotected in 30% sucrose in PBS and subsequently stored at -80°C. Sagittal sections (16 μm) were cut on a cryostat, after which they were washed with PBS and blocked in 4% Fetal Calf Serum (FCS) in THZT (50 mM Tris-HCl pH 7.6, 0.5 M NaCl, 0.5% Triton) or PBS-T (0.5% Triton). After another wash treatment with TBS, sections were incubated overnight at 4°C with primary antibody in THZT. Sections were washed three times (PBS) the following morning and incubated for minimally 2 hours at room temperature with secondary antibody in PBS, followed by wash treatment with PBS. DAPI staining was performed (1mg/ml 1:5000) for 5 min, after which section were washed with PBS. Finally, sections were embedded with Fluorsave.
Primary antibodies that were used: Rabbit-α-Th (Pelfreeze, 1:1000), Chicken-α-GFP (Abcam, 1:1000). Secondary antibodies that were used: Goat-α-Rabbit Alexa 555 (1:1000), Goat-α-Chicken Alexa 488 (1:1000), all Invitrogen.
In situ hybridization
In situ hybridization was performed as described previously [24]. The Digoxigenin-labeled probe for Nurr1 was used as described [22]
Statistical analysis
Values are expressed as means ± standard error of the mean. Comparisons were made using, two-tailed Student's t-test. P<0.05 was considered significant, and indicated using an *, additionally ** = P<0.01; *** = P<0.001.
Results
Generation of double En1KO;Pitx3GFP/GFP animals
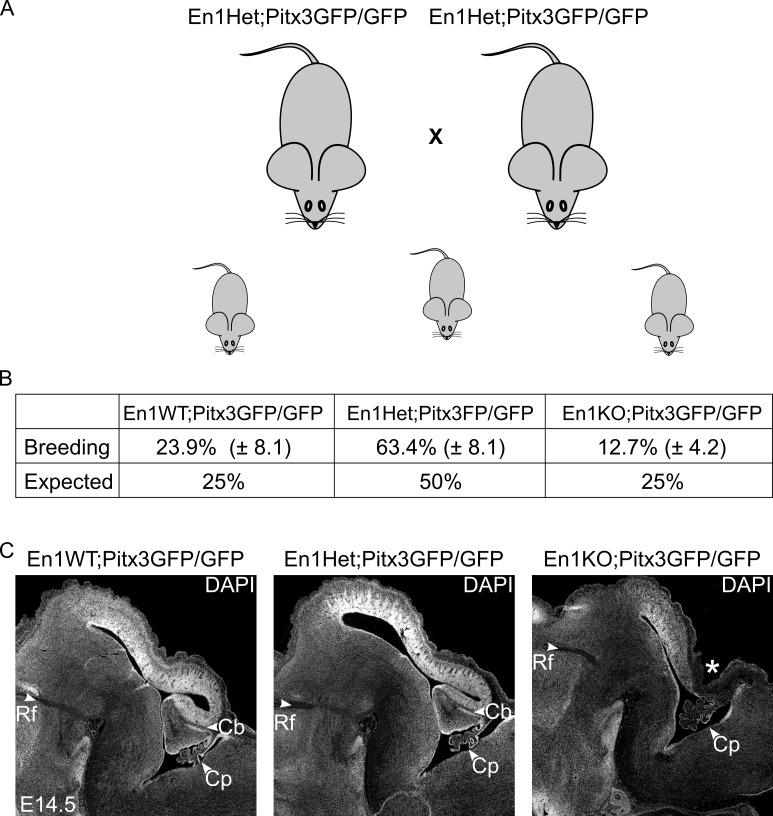
Our research group previously investigated single Pitx3KO and En1KO animals to better understand the role of Pitx3 and En1 during the development of mdDA neurons. We showed that in absence of Pitx3 the SNc is absent [31] and in absence of En1 we observed a dramatic loss of Th-expressing cells, that included mdDA neurons from both the SNc and VTA [13]. Moreover, we reported that Pitx3 and En1 heavily modulate each other’s expression levels (through transcriptional control, transcriptional complex composition and/or protein-protein interactions) [13]. To further improve our understanding of the regulatory relationship between Pitx3 and En1 during the development of mdDA neurons, we inter-crossed En1tm1Alj/+ animals with Pitx3gfp/gfp animals to create double En1tm1Alj/tm1Alj;Pitx3gfp/gfp (En1KO;Pitx3GFP/GFP) animals. First of all, in order to create the desired En1KO;Pitx3GFP/GFP genotype, we initially bred an 'intermediate genotype' En1tm1Alj/+;Pitx3gfp/gfp (Fig 1A). These animals were bred to generate litters that included En1+/+;Pitx3gfp/gfp (En1WT;Pitx3GFP/GFP), En1tm1Alj/+;Pitx3gfp/gfp (En1Het;Pitx3GFP/GFP) and En1tm1Alj/tm1Alj;Pitx3gfp/gfp (En1KO;Pitx3GFP/GFP). En1Het;Pitx3GFP/GFP animals were blind and smaller, due to the absence of Pitx3 as described previously [29,31]. As a consequence the minimum weight to conceive was reached later, i.e. after four months compared to 6–8 weeks in control C57BL/6J animals housed and bred in our animal facility (Table 1). Moreover, upon birth En1KO;Pitx3GFP/GFP animals were smaller and skinny, and displayed perinatal lethality and were therefore isolated at P0 at the latest. Furthermore, litter size was relatively small and the En1KO;Pitx3GFP/GFP genotype presented itself significantly less in litters than expected based on Mendelian distribution (Fig 1B). Due to these breeding constrains we were only able to obtain and examine one litter at P0, and four litters at E14.5 (n = 3 for FACS analysis, n = 1 for anatomical descriptions).
Fig 1. Breeding schedule and outcome of the generation of En1KO;Pitx3GFP/GFP animals.
(A) En1Het;Pitx3GFP/GFP animals were inter-crossed to generate a litter that includes three genotypes: En1WT;Pitx3GFP/GFP, En1Het;Pitx3GFP/GFP, and En1KO;Pitx3GFP/GFP. (B) The presence of double En1KO;Pitx3GFP/GFP animals occurred below (Mendelian) predicted chance level (12.7% versus 25%). (C) DAPI staining at E14.5 reveals that in En1WT;Pitx3GFP/GFP and En1Het;Pitx3GFP/GFP animals the cerebellar anlage is correctly developed (Cb), whilst it is absent in the double En1KO;Pitx3GFP/GFP (*). (Rf: retroflexus, Cb: cerebellum, Cp: choroid plexus).
Table 1. Breeding constrains in creating double En1KO;Pitx3GFP/GFP animals.
| En1Het;Pitx3GFP/GFP X En1Het;Pitx3GFP/GFP |
C57BL/6J (wild-type)§ | |
|---|---|---|
| Average age of pregnancy | 4.4 months (± 0.5) | 6–8 weeks |
| Average weight at conception | 22.3 grams (± 1.0) | 20 grams |
| Average litter size | 5.8 (± 0.7) pups | 8 pups |
§ Based on observations from our animal facility.
The earliest studies on En1-ablated mice reported perinatal lethality, due to cerebellar ablation [32], however our group and others reported that this phenomenon may be circumvented by back-crossing the original 129/Sv line to a C57BL/6J background [13,33]. To determine the influence of the mouse-model background in these double En1KO;Pitx3GFP/GFP animals, we first examined the cytoarchitecture of the mid- and hindbrain area (using a DAPI staining). At E14.5 the anlage of the cerebellum is clearly present in En1WT;Pitx3GFP/GFP and En1Het;Pitx3GFP/GFP animals (Cb, Fig 1C), whereas the anlage was absent in En1KO;Pitx3GFP/GFP animals (asterisk, Fig 1C). These morphological changes are highly similar to the original En1KO. Even though the En1tm1Alj/+;Pitx3gfp/gfp animals were back-crossed unto C57BL/6J animals this cerebellar ablation suggests that the current analysis of the double loss of En1 and Pitx3 is influenced by the genetic background effects as described for the original En1-mutation [32].
The cytoarchitecture of the developing mdDA area is affected in En1/Pitx3 double mutants
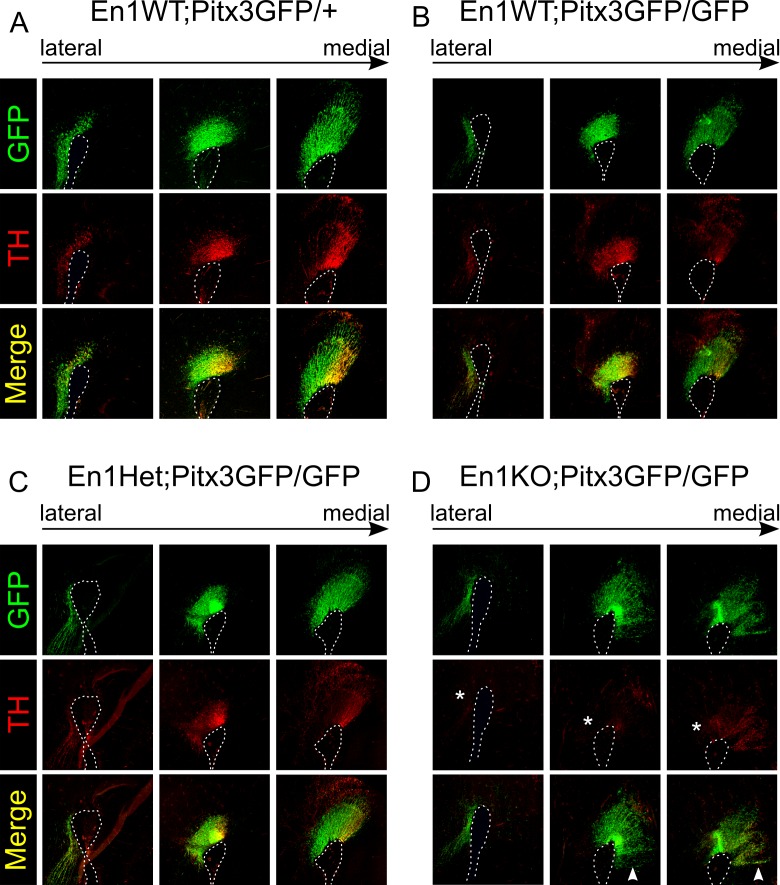
To examine the combined role of Pitx3 and En1 in the cytoarchitecture of the mdDA system, we analysed TH protein and GFP expression at E14.5 in En1WT;Pitx3GFP/GFP, En1Het;Pitx3GFP/GFP, En1KO;Pitx3GFP/GFP litter mates and in an En1WT;Pitx3GFP/+ controls from a different litter. The phenotypical changes that have been previously ascribed to the single En1KO or the single Pitx3KO mutants are visible in the double En1KO;Pitx3GFP/GFP animal at E14.5 (Fig 2). First of all, similar to both single knock-outs TH protein was strongly diminished in lateral mdDA regions (asterisk, Fig 2D). Noteworthy, in the En1KO;Pitx3GFP/GFP animals the loss of TH immunoreactivity is more severe, as TH is also lost in more medial sections (compared to all other genotypes, Fig 2). Second, in (para)medial sections the presence of ectopic mdDA neurons (eDA neurons) was evident, which was recently attributed to changes in the maintenance of the Isthmic Organizer, in absence of En1 [30]. Note that these eDA neurons are marked by both by TH and GFP expression (arrow head, Fig 2D). Finally, using GFP as a marker for mdDA neurons in these models, it appears that in the medial sections fewer mdDA neurons are present in absence of both Pitx3 and En1 (Fig 2D), compared to all other genotypes (Fig 2A–2C). Together, these data suggest that in absence of both Pitx3 and En1 the survival and/or generation of mdDA neurons is more severely affected, as are the programming defects in terms of number of mdDA neurons that contain TH protein.
Fig 2. Analysis of TH and GFP expression in multiple En1-/Pitx3-mutants at E14.5.
(A) Immunohistochemistry of GFP and TH in different sections from medial to lateral encompassing the mdDA neuronal pool in Pitx3GPF/+ animals. (B-D) Same setup as described for A for (B) En1WT;Pitx3GFP/GFP, (C) En1Het;Pitx3GFP/GFP, and (D) En1KO;Pitx3GFP/GFP animals (matching sections with A). (D) Asterisk indicate the loss of TH immunoreactivity in more medial sections. Arrowheads indicate the presence of ectopic mdDA neurons.
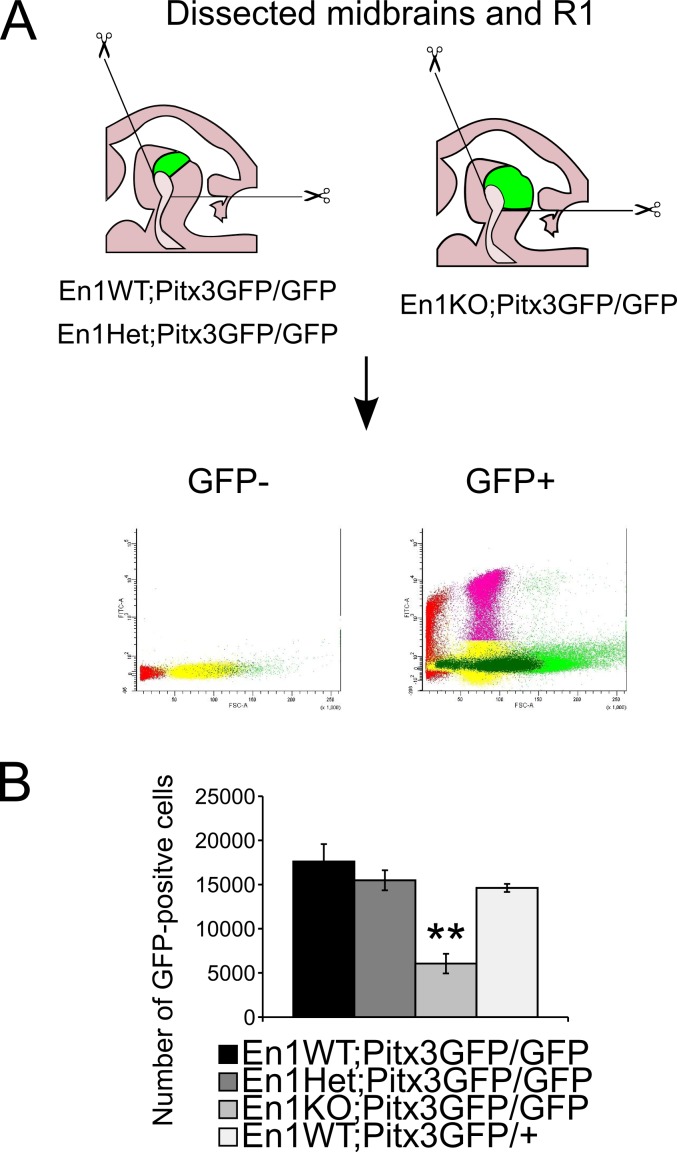
To get an objective measurement of the number of GFP-positive mdDA neurons that are still present in the midbrain of double En1KO;Pitx3GFP/GFP animals, we used the presence of GFP under the promoter of Pitx3 to selectively sort GFP-positive mdDA neurons [22,29]. Previous work from our group revealed the eDA neurons that are present in the rostral hindbrain of En1-ablated animals are indistinguishable from mdDA neurons [30], thus we dissected midbrains and rostral hindbrains of litter mates En1WT;Pitx3GFP/GFP, En1Het;Pitx3GFP/GFP, En1KO;Pitx3GFP/GFP and En1WT;Pitx3GFP/+ control animals from a different litter, at E14.5 (Fig 3A). This approach enabled us to quantify the number of GFP-positive mdDA neurons that are present in the four different genotypes. At E14.5, ~15000 GFP-positive mdDA neurons were sorted from control animals, and this number did not change significantly in En1WT;Pitx3GFP/GFP and En1Het;Pitx3GFP/GFP animals. However, in the absence of both En1 and Pitx3 this number decreased 3-fold to ~5000 GFP-positive mdDA neurons (P<0.01, n = 3/4, Fig 3B). These data substantiated our observations that in the double En1KO;Pitx3GFP/GFP animal fewer mdDA neurons are present at E14.5, compared to the other analysed genotypes.
Fig 3. Quantification of the number of GFP-positive neurons present in the midbrains of multiple En1-/Pitx3-mutants at E14.5.
(A) Schematic representation of the isolation of midbrain and R1, and subsequent FAC-sorting setup at E14.5 to be used for quantification of number of GFP-positive neurons. (B) At E14.5, ~15000 GFP-positive mdDA neurons were sorted from control, En1WT;Pitx3GFP/GFP and En1Het;Pitx3GFP/GFP animals. In contrast, only ~5000 GFP-positive mdDA neurons were present in the En1KO;Pitx3GFP/GFP midbrain/R1 (** = P<0.01, n = 3/4).
En1 and Pitx3 activate the dopaminergic program synchronously
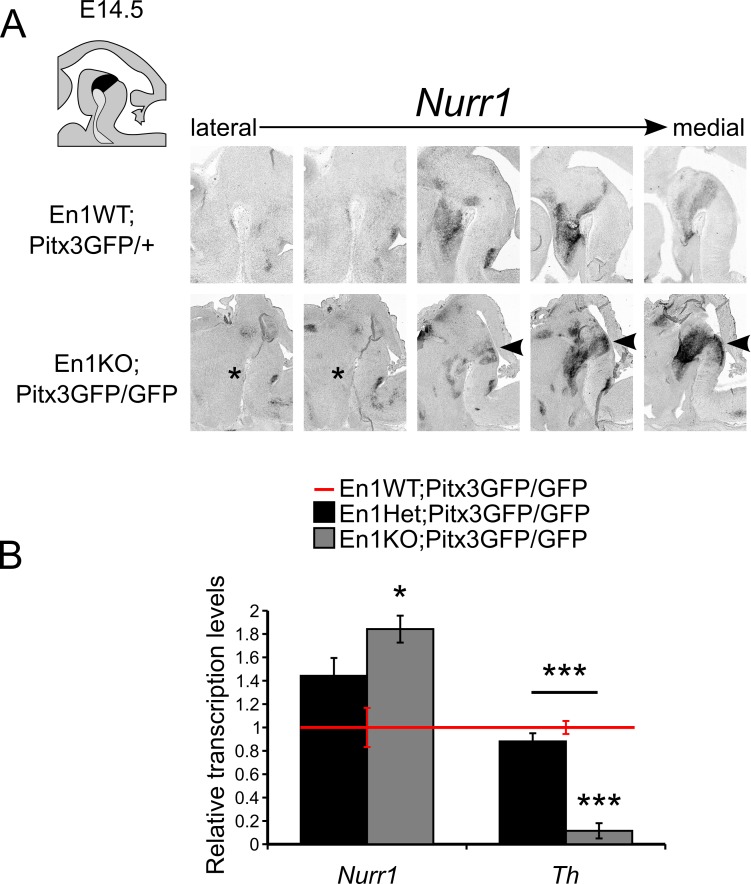
Both En1 and Pitx3 are pivotal players for the proper induction of the molecular dopaminergic profile, as illustrated by the loss of expression of Nurr1-targets in the single En1KO and the single Pitx3KO animal [13,31]. Thus, we aimed to determine which parts of the molecular dopaminergic machinery were still intact in the absence of both En1 and Pitx3. We used in situ hybridization to investigate the expression pattern of Nurr1 in En1WT;Pitx3GFP/+, and En1KO;Pitx3GFP/GFP animals (Fig 4). This analysis revealed regional alterations: in lateral sections of the double En1KO;Pitx3GFP/GFP a small area in the ventral diencephalon positive for Nurr1 transcript cannot be detected (Fig 4A, asterisk), whilst in (para)medial sections Nurr1 was present in a changed level and architecture in midbrain and ectopically extend caudally (arrowhead, Fig 4A). In order to further investigate the programming consequences of En1- and Pitx3-ablation we sorted GFP-positive neurons and subjected the RNA in those cells to qPCR analyses. Since the chosen breeding scheme did not allow for the presence of a control Pitx3GFP/+ litter mate (Fig 1), we elected to use the En1WT;Pitx3GFP/GFP genotype as a point of reference to analyze relative transcript levels, as genome wide expression analysis of En1WT;Pitx3GFP/GFP animals compared to control have already been published [27]. These analyses revealed that Nurr1 transcript levels were significantly increased in En1KO;Pitx3GFP/GFP mdDA neurons compared to En1WT;Pitx3GFP/GFP animals (Fig 4B, P<0.05, n = 3–4).
Fig 4. Nurr1-positive mdDA neurons are present in double En1KO;Pitx3GFP/GFP animals at E14.5.
(A) Schematic, sagittal section of the embryonic mouse brain, mdDA area is indicated in black. Lateral to medial sections of in situ hybridization experiments for Nurr1 at E14.5 in En1WT;Pitx3GFP/+ and En1KO;Pitx3GFP/GFP midbrains. Asterisk indicates diminished expression of Nurr1 in lateral sections, whilst in (para)medial sections Nurr1 is still present in the midbrain and is ectopically extended caudally (arrowheads). (B) Quantitative PCR on FAC-sorted mdDA neurons demonstrates elevated level of Nurr1 in the En1KO;Pitx3GFP/GFP midbrain, compared to En1WT;Pitx3GFP/GFP (* = P<0.05, n = 3/4). The expression of Th expression is significantly down-regulated in the En1KO;Pitx3GFP/GFP midbrain, compared to En1WT;Pitx3GFP/GFP (*** = P<0.01, n = 3/4).
Furthermore, we investigated the transcript level of Th, as we previously established that in both the single Pitx3KO and the single En1KO, Th transcript is significantly diminished [13,27,31]. We observed an even further drop in Th transcript expression in En1KO;Pitx3GFP/GFP mdDA neurons compared to both En1WT;Pitx3GFP/GFP and En1Het;Pitx3GFP/GFP mdDA neurons (Fig 4B, P<0.001, n = 3–4). This observation is in line with the loss of TH protein, identified in immunohistochemistry experiments at E14.5 (Fig 3). Together these data confirm that Pitx3 and En1 act in synchrony to induce the Th gene in mdDA neurons, independent of Nurr1.
En1 and Pitx3 program mdDA neurons to a specific rostral/caudal subtype
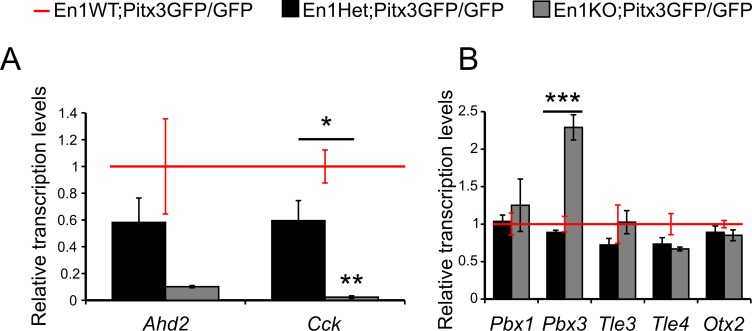
The mdDA neurons that make up the SNc are located in the rostrolateral midbrain during development (E14.5) and express Ahd2, while the mdDA neurons that will develop into the VTA express Cck and are located caudomedially [13,26]. Previous research revealed that the absence of either Pitx3 or En1 affects each subset differently; En1 initially promotes Pitx3 in the rostral subset, but is subsequently repressed by Pitx3. This was illustrated by an increase of En1 in the Pitx3KO, and the loss of Pitx3 in the rostral subset in the En1KO. It has been proposed that Pitx3 and En1 play an interrelated role in the programming of the rostral versus caudal subset. To refine our understanding of the regional-specific actions of En1 and Pitx3, we examined the expression levels of subset-specific markers Ahd2 and Cck in double En1KO;Pitx3GFP/GFP animals at stage E14.5 of development (Fig 5). In absence of both En1 and Pitx3 the expression of Ahd2 is not significantly decreased further relative to En1WT;Pitx3GFP/GFP mdDA neurons (Fig 5A, P>0.05). It is worth pointing out that in absence of solely Pitx3, the expression of Ahd2 is already severely affected [26], which is equally true for En1-null mice [13]. These data support the notion that En1 and Pitx3 both promote Ahd2 expression in unison: Pitx3 most likely in a direct manner (binding to the Ahd2-promotor [26]), while En1 could promote Ahd2 expression either directly or via its control on Pitx3 [13] or both.
Fig 5. Quantitative PCR analysis of mdDA neurons in double En1KO;Pitx3GFP/GFP animals at E14.5.
(A) Quantitative PCR demonstrates no further loss of Ahd2 expression in En1KO;Pitx3GFP/GFP animals compared to En1WT;Pitx3GFP/GFP (P>0.05, n = 3/4). Significant loss of Cck expression in both the En1Het;Pitx3GFP/GFP (P<0.05, n = 4) and En1KO;Pitx3GFP/GFP animals (P<0.01, n = 3/4), compared to En1WT;Pitx3GFP/GFP. (B) Quantitative PCR demonstrates no changes in Pbx1, Tle3, Tle4 and Otx2 expression in En1KO;Pitx3GFP/GFP animals compared to En1WT;Pitx3GFP/GFP (P>0.05, n = 3/4). Significantly increased expression of Pbx3 in En1KO;Pitx3GFP/GFP animals, compared to both the En1Het;Pitx3GFP/GFP and En1WT;Pitx3GFP/GFP animals (*** = P<0.01, n = 3/4).
In contrast, En1 and Pitx3 exert opposing effects on the caudal-subset programming, i.e. Cck expression. In absence of Pitx3, Cck is up-regulated [27] whereas Cck expression is lost in absence of En1 [13]. Thus, we queried whether the combined absence of En1 and Pitx3 would either restore Cck expression (through a loss of Pitx3-mediated repression) or inhibit its expression (due to the loss of En1-mediated activation). Indeed, double En1KO;Pitx3GFP/GFP animals display a significant loss of Cck compared to the single Pitx3KO (P<0.01, Fig 5A). Furthermore, the graded decline of Cck transcript expression with the loss of each En1 allele reveals its dose-dependent sensitivity to the (in)activation of En1, independent of Pitx3 (P<0.05, Fig 5A). This suggest that Cck is only dependent on its activation by En1.
In addition to Pitx3, En1 and Nurr1, other transcription factors have been shown to contribute to the development of (a subset of) mdDA neurons [34]. In order to better characterize the subset of mdDA neurons that is still present in the midbrain in absence of Pitx3 and En1, we elected to investigate the expression levels of certain relevant transcription factors. To start, we previously established clear expression of several members of the Tle/Groucho family, known interactors of En1 and Pitx3, within mdDA neurons. In addition, we revealed that the expression of Pbx1 and Tle3 is enriched in caudal mdDA neurons, whereas Pbx3 and Tle4 are rostrally enriched. Finally, we reported that the expression levels of Pbx1, Pbx3 and Tle3 are dependent on Pitx3 activity, [13]. Thus, we examined their relative expression in the absence of both Pitx3 and En1. Here, we report no changes in the expression levels of Pbx1, Tle3 and Tle4 between En1WT;Pitx3GFP/GFP and En1KO;Pitx3GFP/GFP (Fig 5B, P>0.05). Interestingly, the relative expression of Pbx3 is significantly increased in mdDA neurons of En1KO;Pitx3GFP/GFP, compared to both En1WT;Pitx3GFP/GFP and En1Het;Pitx3GFP/GFP (Fig 5B, P<0.001, n = 3–4). Since the expression of Pbx3 is diminished in the single Pitx3KO, its remarkable increase in the double En1KO;Pitx3GFP/GFP suggests that the absence of En1 has lifted an inhibition of En1 on Pbx3 expression.
Finally, we investigated the expression of Otx2, as previous research has determined that it plays an important role in the survival and programming of (a subset) of mdDA neurons [35,36]. We report no changes in the expression levels of Otx2 between En1WT;Pitx3GFP/GFP and En1KO;Pitx3GFP/GFP (Fig 5B, P>0.05). Thus, the remaining population of mdDA neurons in the En1KO;Pitx3GFP/GFP is positive for Otx2, a transcription factor that is known to contribute to the survival of mdDA neurons during adverse conditions [37] and mainly present in a caudal subset of mdDA neurons.
Loss of both En1 and Pitx3 affects survival of mdDA neurons upon P0
Single Pitx3KO animals are characterized by an embryonic loss of Ahd2 and the post-natal loss of the SNc, while in the En1KO both Ahd2 and Cck are lost embryonically, which is accompanied by a post-natal defect in both the SNc and VTA. We just established that the midbrain of double En1KO;Pitx3GFP/GFP animals contains ~60% less GFP-positive mdDA neurons (E14.5), and that these mdDA neurons do not express their characteristic rostral and caudal marks. In order to obtain a better understanding of the (combined) role of En1 and Pitx3 in survival of mdDA neurons, we examined the cytoarchitecture through GFP immunohistochemistry at the latest possible time-point (Fig 6).
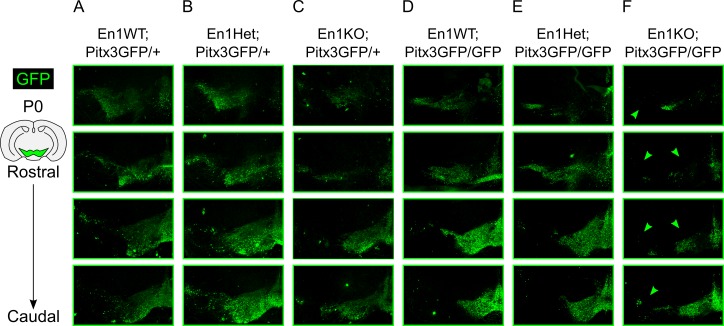
Fig 6. Qualitative analysis of GFP expression in multiple En1-/Pitx3-mutants at P0.
Schematic, coronal section of the adult mouse brain, mdDA area is indicated in green. (A) Immunohistochemistry of GFP in different sections from rostral to caudal encompassing the mdDA neuronal pool in Pitx3GPF/+ animals. (B-F) Same setup as described for A for (B) En1Het;Pitx3GFP/+, (C) En1KO;Pitx3GFP/+, (D) En1WT;Pitx3GFP/GFP, (E) En1Het;Pitx3GFP/GFP, and (F) En1KO;Pitx3GFP/GFP animals (matching sections with A). Arrowheads indicate the loss of GFP-positive mdDA neurons in the SNc and VTA region (n = 1).
Due to the described breeding difficulties we were only able to obtain one double Pitx3/En1-ablated mouse at P0, thus the immunohistochemistry of the GFP is a qualitative description of the cytoarchitecture, in which we included six different genotypes: En1WT;Pitx3GFP/+; En1Het;Pitx3GFP/+; En1KO;Pitx3GFP/+; En1WT;Pitx3GFP/GFP; En1Het;Pitx3GFP/GFP; En1KO;Pitx3GFP/GFP. First, the largest differences in cytoarchitecture of the mdDA area are observed when at least two alleles of one gene are ablated i.e. the En1KO;Pitx3GFP/+, the single Pitx3KO, the En1Het;Pitx3GFP/GFP, and finally the En1KO;Pitx3GFP/GFP (arrowheads, Fig 6C–6F). Second, the absence of Pitx3 mainly affects the SNc, as described in great detail before (Fig 6D; [29,31,38–40]. Third, in contrast, the absence of two alleles of En1 seemed to target the GFP-positive mdDA neurons of both SNc and VTA (Fig 6C, 6D and 6F), which has also been previously described (albeit at a later age)[13]. Finally, double En1KO;Pitx3GFP/GFP animals display severe defects in the entire mdDA area: The SNc is mostly lost, except for a small spot of GFP-positive cells, and the VTA is much smaller compared to any other genotype (arrowheads, Fig 6F). Although the data on the Pitx3/En1 double ablated animals is only present at an N = 1 we conclude that the data substantiate previous data, in the sense that En1 and Pitx3 act together in generating the mdDA neuronal pool and that a small portion might not not depend on both transcription factors for their generation and survival (until P0).
Discussion
Genetic background influences the phenotype of En1KO;Pitx3GFP/GFP animals
In the current manuscript we aimed to refine our understanding of the effects of Pitx3 and En1 activity on each other, and the development of mdDA neurons. Our group and others have previously investigated the single Pitx3KO [29,31,38–40] and the single En1KO [13,30] in great detail, and we elected to include that data in our study to allow us to draw comparisons between the single Pitx3KO, the single En1KO, and the double En1KO;Pitx3GFP/GFP. To start, upon birth En1KO;Pitx3GFP/GFP animals were smaller, and skinny, and displayed perinatal lethality. Furthermore, we observed that the cerebellar anlage was lost in the double En1KO;Pitx3GFP/GFP animal (Fig 1). This phenotype is identical to the En1-mutation in the 129/Sv mouse background [32]. Both Pitx3gfp/gfp animals and En1tm1Alj/+ animals were back-crossed onto a C57BL6/J background, however it takes several generations of back-crossing to completely neutralize the 129/Sv mouse background, and generate viable En1KO animals [13,30,33]. We thus hypothesize that the perinatal lethality is most likely the consequence of the presence of the 129/Sv mouse background. Consequently, we excluded viability as a criterion in our analyses in double En1KO;Pitx3GFP/GFP animals.
Absence of both En1 and Pitx3 severely affects mdDA neurons
We examined the cytoarchitecture of several Pitx3-/En1-mutants at E14.5 and P0, and quantified the number of GFP-positive mdDA neurons in the midbrain at E14.5. Both the single Pitx3KO and the single En1KO are characterized by diminished expression of Nurr1/Pitx3/En1-targets during embryonic development, but mdDA neuronal cell loss is not yet present at E14.5 [31,39]. Both the single En1KO and the Pitx3KO demonstrate normal expression of Nurr1 at E12.5 suggesting that the neurogenesis of early post-mitotic mdDA neurons occurs correctly [13]. When examining double En1KO;Pitx3GFP/GFP animals it is important to take into account that until E11.5 the development of mdDA neurons in the single En1KO and the double En1KO;Pitx3GFP/GFP animal are identical. Still, we demonstrate that the double loss of En1 and Pitx3 does not merely mimic the En1KO phenotype, but represents a more severe phenotype. This suggest that the combined loss of Pitx3 and En1 (and their subsequent targets) after E11.5 is causative to the significant cell loss observed at E14.5 in double En1KO;Pitx3GFP/GFP animals compared to controls and Pitx3KOs. Previous data from single En1KO- and Pitx3KO animals revealed that both transcription factors control the expression of a broad myriad of genes and other transcription factors [13,22,31,41]. After E11.5, the combined loss of any of such targets and their subsequent molecular pathways contribute to the phenotype of double En1KO;Pitx3GFP/GFP animals. Taken together, the diminished number of mdDA neurons in double En1KO;Pitx3GFP/GFP animals may find its origin in an absence of survival factors, or an absence of proper dopaminergic differentiation. In sum, both En1 and Pitx3 are important for the survival of developing mdDA neurons, and thus the size of the mdDA neuronal pool.
Does a compensatory mechanism of transcription factors contribute to the survival of (a subset of) mdDA neurons?
The importance of Nurr1 in the embryonic development of mdDA neurons has been clearly established for years [21,22,24]. The expression of Nurr1 is thought to be independent of both Pitx3 and En1, as expression levels and patterns of Nurr1 are unchanged in single Pitx3KO and single En1KO animals [13,22]. Still, we report here a significant increase in Nurr1 transcript levels in mdDA neurons of double En1KO;Pitx3GFP/GFP animals. This could suggest that through the double absence of Pitx3 and En1 a regulatory inhibition is lifted. Alternatively, this might suggest that via a still unknown mechanism, the expression of Nurr1 is boosted as a possible compensatory mechanism, to promote the survival and functioning of the remaining mdDA neurons.
Moreover, we previously demonstrated that the expression of Pbx3 is enriched in rostral embryonic mdDA neurons, adult neurons of the SNc and is dependent on activation by Pitx3 [13,24]. Here we report a significant increase in Pbx3 expression in mdDA neurons in the absence of both Pitx3 and En1 (Fig 5). Even though Pbx3 did not surface as a target of En1 activity in genome wide expression analysis on En1-ablated embryonic midbrains [13], these data could suggest that En1 represses the expression of Pbx3 during wild type embryonic development. This aligns with the hypothesis that En1 favours the programming of caudal mdDA neurons. Furthermore, other authors have speculated on a possible redundancy between Pbx1 and Pbx3 [42–44]. This notion was recently strengthened by an elaborate study which investigated several Pbx1/Pbx3-mutants, and revealed that a conditional Pbx1;Pbx3 knock-out resulted in more severe loss of TH-positive neurons than either the single Pbx1 or Pbx3 knock out mouse. In fact, Pbx3 levels were increased in the Pbx1 null-mouse [45]. In similar line of thought, the elevated levels of Pbx3 in double Pitx3/En1-ablated mdDA neurons that we report in the current study (Fig 5B) could also act as a compensatory mechanism, and contribute to the survival of the small group of remaining mdDA neurons.
Finally, we confirmed that the presence of Otx2 in the remaining mdDA neurons of the double En1KO;Pitx3GFP/GFP animal, is not different from the Otx2 expression in single Pitx3KO animals (Fig 5B). Furthermore, Otx2 is known marker of a subset of VTA neurons, and has been shown to promote survival of mdDA neurons in multiple adverse conditions (e.g. MPTP toxicity and En1-haplo-insufficiency) [35,37]. Thus, it might be possible that the combined presence of Nurr1, Pbx3 and Otx2 contribute to the (temporary) survival of a small subset of mdDA neurons in double En1KO;Pitx3GFP/GFP animals.
Relative levels of Pitx3 and En1 determine rostral-caudal identity and the size of the embryonic mdDA neuronal pool
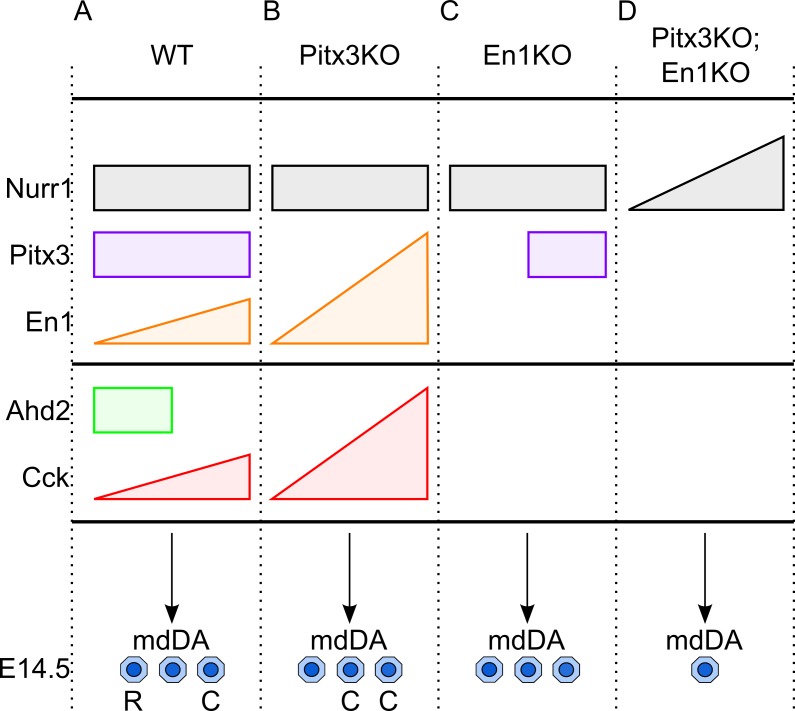
During wild-type development the transcriptional regulation of Nurr1, Pitx3 and En1 induce mdDA neurons. Two important subsets that can be identified at E14.5 within the total and divers pool of mdDA neurons are rostral (R) mdDA neurons that express Ahd2 and become SNc neurons, and caudal (C) mdDA neurons (Cck+) that develop into the VTA (Fig 7A). In Pitx3-deficient mice unaltered Nurr1 expression represents the normal initiation of mdDA progenitors, however Ahd2 expression is lost. Moreover, the inhibitory regulation of Pitx3 on En1 is released, and therefore En1 expression is significantly increased, resulting in the concomitant up-regulation of Cck expression [13,26]. These changes in the programming of mdDA neurons result in the absence of rostral-coded mdDA neurons, and an over-representation of caudally programmed mdDA neurons (Fig 7B). In En1-null mice, unchanged Nurr1 expression reveals the initiation of mdDA progenitors, though the expression of Pitx3 and Ahd2 is affected in the rostral midbrain, and the expression of Cck is lost as well [13]. Together these deficiencies result in the presence of mdDA neurons that are devoid of the rostral-caudal markers which might be a representation of the loss of the correct programming of the rostral and caudal subset (Fig 7C). Finally, in absence of Pitx3 and En1 mdDA progenitors are still initiated, revealed by the (elevated) presence of Nurr1, but significantly fewer GFP-positive mdDA neurons are present at E14.5. Furthermore, the expression of Ahd2 and Cck is completely lost (Fig 7D). In sum, the current study supports the hypothesis that Pitx3 primarily promotes the specification of mdDA neurons into a SNc neuron, whereas En1 drives mdDA neurons towards a VTA fate and may be higher-up in the hierarchy of the transcriptional programming [34]].
Fig 7. Schematic representation of roles of En1 and Pitx3 in the programming of the rostral-caudal identity of mdDA neurons.
(A) In wild-type midbrain Nurr1 initiates the development of mdDA differentiation, Pitx3 promotes Ahd2 expression and represses En1 in rostral midbrain, whilst En1 promotes Pitx3 and Cck expression. The mdDA neuronal pool includes rostral-coded and caudal-coded neurons. (B) In Pitx3-ablated animals Nurr1 initiates the development of mdDA differentiation, though Ahd2 expression is lost, and the inhibition of Pitx3 on En1 is lifted, thus En1 and subsequently Cck are up-regulated. The mdDA neuronal pool includes only caudal-coded neurons. (C) In En1-ablated animals Nurr1 initiates the differentiation of mdDA progenitors, though Cck expression is lost, and Pitx3 expression in the rostral midbrain is not initiated, thus Ahd2 expression is lost as well. The mdDA neuronal pool includes only non-coded neurons. (D) In double En1KO;Pitx3GFP/GFP animals elevated levels Nurr1 promotes the differentiation of mdDA progenitors, though Cck and Ahd2 are lost.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek grant no 865.09.002 to MPS. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999. August;122 (Pt 8):1437–48. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature. 1988. July 28;334(6180):345–8. doi: 10.1038/334345a0 [DOI] [PubMed] [Google Scholar]
- 3.Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, Isacson O. Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet. 2005. July 1;14(13):1709–25. doi: 10.1093/hmg/ddi178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene JG, Dingledine R, Greenamyre JT. Gene expression profiling of rat midbrain dopamine neurons: implications for selective vulnerability in parkinsonism. Neurobiol Dis. 2005. February;18(1):19–31. doi: 10.1016/j.nbd.2004.10.003 [DOI] [PubMed] [Google Scholar]
- 5.Grimm J, Mueller A, Hefti F, Rosenthal A. Molecular basis for catecholaminergic neuron diversity. Proc Natl Acad Sci USA. 2004. September 21;101(38):13891–6. doi: 10.1073/pnas.0405340101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brichta L, Greengard P. Molecular determinants of selective dopaminergic vulnerability in Parkinson’s disease: an update. Front Neuroanat. 2014;8:152 doi: 10.3389/fnana.2014.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakrabarty K, Von Oerthel L, Hellemons A, Clotman F, Espana A, Groot Koerkamp M, et al. Genome wide expression profiling of the mesodiencephalic region identifies novel factors involved in early and late dopaminergic development. Biol Open. 2012. August 15;1(8):693–704. doi: 10.1242/bio.20121230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoekstra EJ, von Oerthel L, van der Linden AJA, Smidt MP. Phox2b influences the development of a caudal dopaminergic subset. PLoS ONE. 2012;7(12):e52118 doi: 10.1371/journal.pone.0052118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoekstra EJ, von Oerthel L, van der Heide LP, Kouwenhoven WM, Veenvliet JV, Wever I, et al. Lmx1a encodes a rostral set of mesodiencephalic dopaminergic neurons marked by the Wnt/B-catenin signaling activator R-spondin 2. PLoS ONE. 2013;8(9):e74049 doi: 10.1371/journal.pone.0074049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smits SM, von Oerthel L, Hoekstra EJ, Burbach JPH, Smidt MP. Molecular marker differences relate to developmental position and subsets of mesodiencephalic dopaminergic neurons. PLoS ONE. 2013;8(10):e76037 doi: 10.1371/journal.pone.0076037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poulin J-F, Zou J, Drouin-Ouellet J, Kim K-YA, Cicchetti F, Awatramani RB. Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling. Cell Rep. 2014. November 6;9(3):930–43. doi: 10.1016/j.celrep.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.La Manno G, Gyllborg D, Codeluppi S, Nishimura K, Salto C, Zeisel A, et al. Molecular Diversity of Midbrain Development in Mouse, Human, and Stem Cells. Cell. 2016. October 6;167(2):566–580.e19. doi: 10.1016/j.cell.2016.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veenvliet JV, Alves Dos Santos MTM, Kouwenhoven WM, von Oerthel L, Lim JL, van der Linden AJA, et al. Specification of dopaminergic subsets involves interplay of En1 and Pitx3. Development. 2013. July 17; [DOI] [PubMed] [Google Scholar]
- 14.Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. 2011. April;3(4):385–428. doi: 10.3390/nu3040385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duester G, Mic FA, Molotkov A. Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chem Biol Interact. 2003. February 1;143–144:201–10. [DOI] [PubMed] [Google Scholar]
- 16.Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: implications for Parkinson’s disease pathogenesis. Brain Res. 2003. November 7;989(2):205–13. [DOI] [PubMed] [Google Scholar]
- 17.Liu G, Yu J, Ding J, Xie C, Sun L, Rudenko I, et al. Aldehyde dehydrogenase 1 defines and protects a nigrostriatal dopaminergic neuron subpopulation. J Clin Invest. 2014. July;124(7):3032–46. doi: 10.1172/JCI72176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotzinger S, Vaccarino FJ. Cholecystokinin receptor subtypes: role in the modulation of anxiety-related and reward-related behaviours in animal models. J Psychiatry Neurosci. 2003. May;28(3):171–81. [PMC free article] [PubMed] [Google Scholar]
- 19.Sugaya K, Takahashi M, Kubota K. Cholecystokinin protects cholinergic neurons against basal forebrain lesion. Jpn J Pharmacol. 1992. May;59(1):125–8. [DOI] [PubMed] [Google Scholar]
- 20.Reisi P, Ghaedamini AR, Golbidi M, Shabrang M, Arabpoor Z, Rashidi B. Effect of cholecystokinin on learning and memory, neuronal proliferation and apoptosis in the rat hippocampus. Adv Biomed Res. 2015;4:227 doi: 10.4103/2277-9175.166650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zetterström RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997. April 11;276(5310):248–50. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs FMJ, van Erp S, van der Linden AJA, von Oerthel L, Burbach JPH, Smidt MP. Pitx3 potentiates Nurr1 in dopamine neuron terminal differentiation through release of SMRT-mediated repression. Development. 2009. February;136(4):531–40. doi: 10.1242/dev.029769 [DOI] [PubMed] [Google Scholar]
- 23.Saucedo-Cardenas O, Quintana-Hau JD, Le WD, Smidt MP, Cox JJ, De Mayo F, et al. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci USA. 1998. March 31;95(7):4013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smits SM, Ponnio T, Conneely OM, Burbach JPH, Smidt MP. Involvement of Nurr1 in specifying the neurotransmitter identity of ventral midbrain dopaminergic neurons. Eur J Neurosci. 2003. October;18(7):1731–8. [DOI] [PubMed] [Google Scholar]
- 25.Smidt MP, van Schaick HS, Lanctôt C, Tremblay JJ, Cox JJ, van der Kleij AA, et al. A homeodomain gene Ptx3 has highly restricted brain expression in mesencephalic dopaminergic neurons. Proc Natl Acad Sci USA. 1997. November 25;94(24):13305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs FMJ, Smits SM, Noorlander CW, von Oerthel L, van der Linden AJA, Burbach JPH, et al. Retinoic acid counteracts developmental defects in the substantia nigra caused by Pitx3 deficiency. Development. 2007. July;134(14):2673–84. doi: 10.1242/dev.02865 [DOI] [PubMed] [Google Scholar]
- 27.Jacobs FMJ, Veenvliet JV, Almirza WH, Hoekstra EJ, von Oerthel L, van der Linden AJA, et al. Retinoic acid-dependent and -independent gene-regulatory pathways of Pitx3 in meso-diencephalic dopaminergic neurons. Development. 2011. December;138(23):5213–22. doi: 10.1242/dev.071704 [DOI] [PubMed] [Google Scholar]
- 28.Davis CA, Joyner AL. Expression patterns of the homeo box-containing genes En-1 and En-2 and the proto-oncogene int-1 diverge during mouse development. Genes Dev. 1988. December;2(12B):1736–44. [DOI] [PubMed] [Google Scholar]
- 29.Maxwell SL, Ho H-Y, Kuehner E, Zhao S, Li M. Pitx3 regulates tyrosine hydroxylase expression in the substantia nigra and identifies a subgroup of mesencephalic dopaminergic progenitor neurons during mouse development. Dev Biol. 2005. June 15;282(2):467–79. doi: 10.1016/j.ydbio.2005.03.028 [DOI] [PubMed] [Google Scholar]
- 30.Kouwenhoven WM, Veenvliet JV, van Hooft JA, van der Heide LP, Smidt MP. Engrailed 1 shapes the dopaminergic and serotonergic landscape through proper isthmic organizer maintenance and function. Biol Open. 2016. February 15; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smidt MP, Smits SM, Bouwmeester H, Hamers FPT, van der Linden AJA, Hellemons AJCGM, et al. Early developmental failure of substantia nigra dopamine neurons in mice lacking the homeodomain gene Pitx3. Development. 2004. March;131(5):1145–55. doi: 10.1242/dev.01022 [DOI] [PubMed] [Google Scholar]
- 32.Wurst W, Auerbach AB, Joyner AL. Multiple developmental defects in Engrailed-1 mutant mice: an early mid-hindbrain deletion and patterning defects in forelimbs and sternum. Development. 1994. July;120(7):2065–75. [DOI] [PubMed] [Google Scholar]
- 33.Bilovocky NA, Romito-DiGiacomo RR, Murcia CL, Maricich SM, Herrup K. Factors in the genetic background suppress the engrailed-1 cerebellar phenotype. J Neurosci. 2003. June 15;23(12):5105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veenvliet JV, Smidt MP. Molecular mechanisms of dopaminergic subset specification: fundamental aspects and clinical perspectives. Cell Mol Life Sci. 2014. July 27; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Salvio M, Di Giovannantonio LG, Acampora D, Prosperi R, Omodei D, Prakash N, et al. Otx2 controls neuron subtype identity in ventral tegmental area and antagonizes vulnerability to MPTP. Nat Neurosci. 2010. December;13(12):1481–8. doi: 10.1038/nn.2661 [DOI] [PubMed] [Google Scholar]
- 36.Puelles E, Annino A, Tuorto F, Usiello A, Acampora D, Czerny T, et al. Otx2 regulates the extent, identity and fate of neuronal progenitor domains in the ventral midbrain. Development. 2004. May;131(9):2037–48. doi: 10.1242/dev.01107 [DOI] [PubMed] [Google Scholar]
- 37.Di Giovannantonio LG, Di Salvio M, Acampora D, Prakash N, Wurst W, Simeone A. Otx2 selectively controls the neurogenesis of specific neuronal subtypes of the ventral tegmental area and compensates En1-dependent neuronal loss and MPTP vulnerability. Dev Biol. 2013. January 1;373(1):176–83. doi: 10.1016/j.ydbio.2012.10.022 [DOI] [PubMed] [Google Scholar]
- 38.Hwang D-Y, Ardayfio P, Kang UJ, Semina EV, Kim K-S. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Molecular Brain Research. 2003. June 10;114(2):123–31. [DOI] [PubMed] [Google Scholar]
- 39.van den Munckhof P, Luk KC, Ste-Marie L, Montgomery J, Blanchet PJ, Sadikot AF, et al. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development. 2003. June;130(11):2535–42. [DOI] [PubMed] [Google Scholar]
- 40.Nunes I, Tovmasian LT, Silva RM, Burke RE, Goff SP. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci USA. 2003. April 1;100(7):4245–50. doi: 10.1073/pnas.0230529100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng C, Aron L, Klein R, Li M, Wurst W, Prakash N, et al. Pitx3 is a critical mediator of GDNF-induced BDNF expression in nigrostriatal dopaminergic neurons. J Neurosci. 2011. September 7;31(36):12802–15. doi: 10.1523/JNEUROSCI.0898-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferretti E, Li B, Zewdu R, Wells V, Hebert JM, Karner C, et al. A conserved Pbx-Wnt-p63-Irf6 regulatory module controls face morphogenesis by promoting epithelial apoptosis. Dev Cell. 2011. October 18;21(4):627–41. doi: 10.1016/j.devcel.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selleri L, DiMartino J, van Deursen J, Brendolan A, Sanyal M, Boon E, et al. The TALE homeodomain protein Pbx2 is not essential for development and long-term survival. Mol Cell Biol. 2004. June;24(12):5324–31. doi: 10.1128/MCB.24.12.5324-5331.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sgadò P, Ferretti E, Grbec D, Bozzi Y, Simon HH. The atypical homeoprotein Pbx1a participates in the axonal pathfinding of mesencephalic dopaminergic neurons. Neural Dev. 2012. July 2;7:24 doi: 10.1186/1749-8104-7-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villaescusa JC, Li B, Toledo EM, Rivetti di Val Cervo P, Yang S, Stott SR, et al. A PBX1 transcriptional network controls dopaminergic neuron development and is impaired in Parkinson’s disease. EMBO J. 2016. September 15;35(18):1963–78. doi: 10.15252/embj.201593725 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.