Abstract
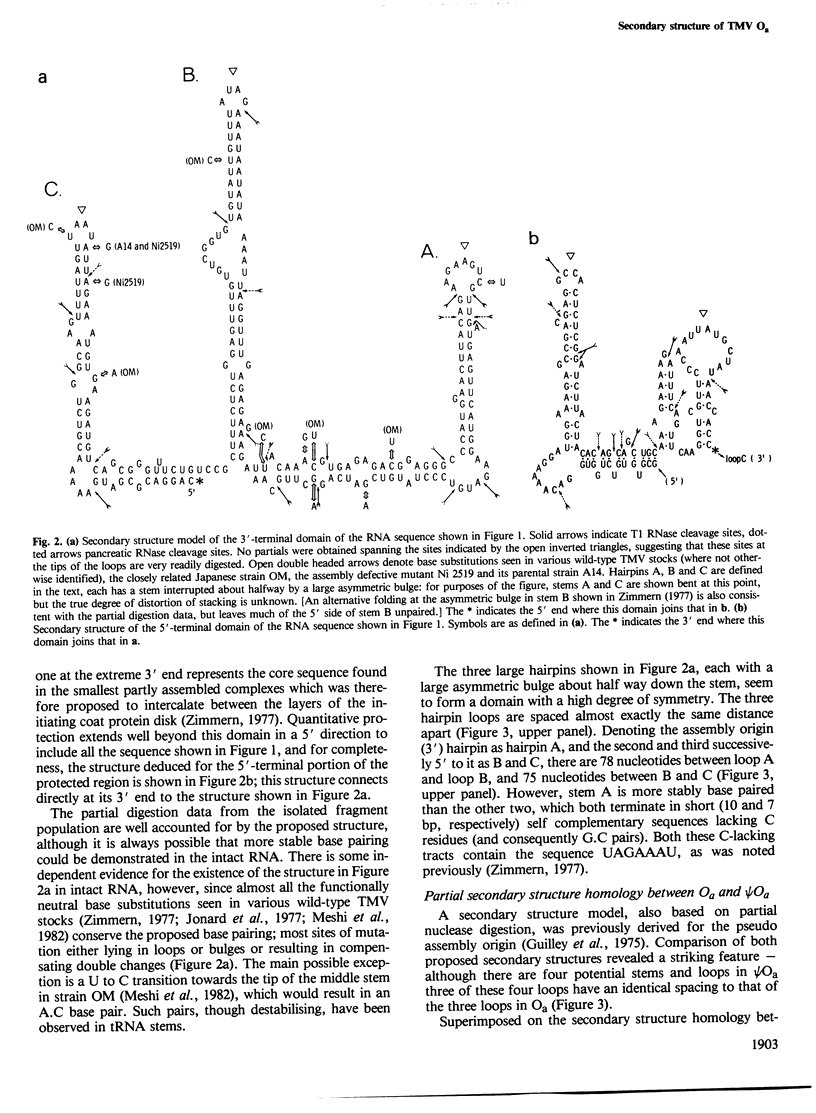
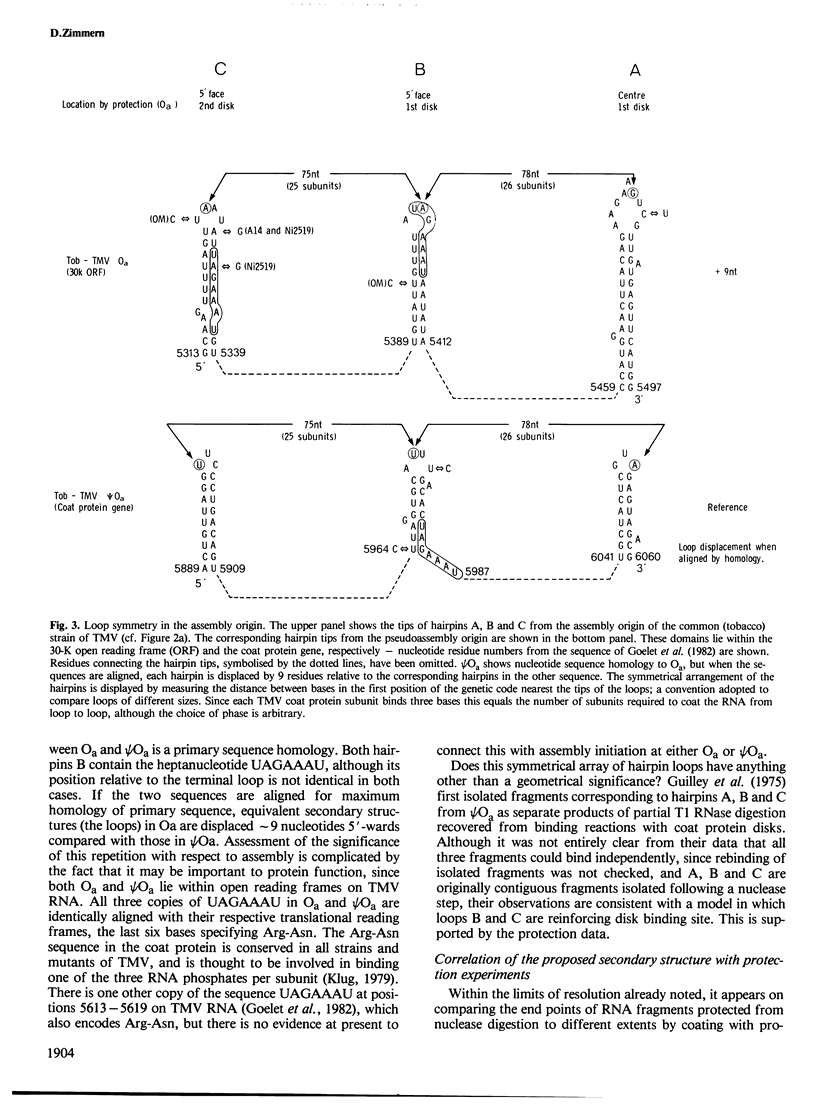
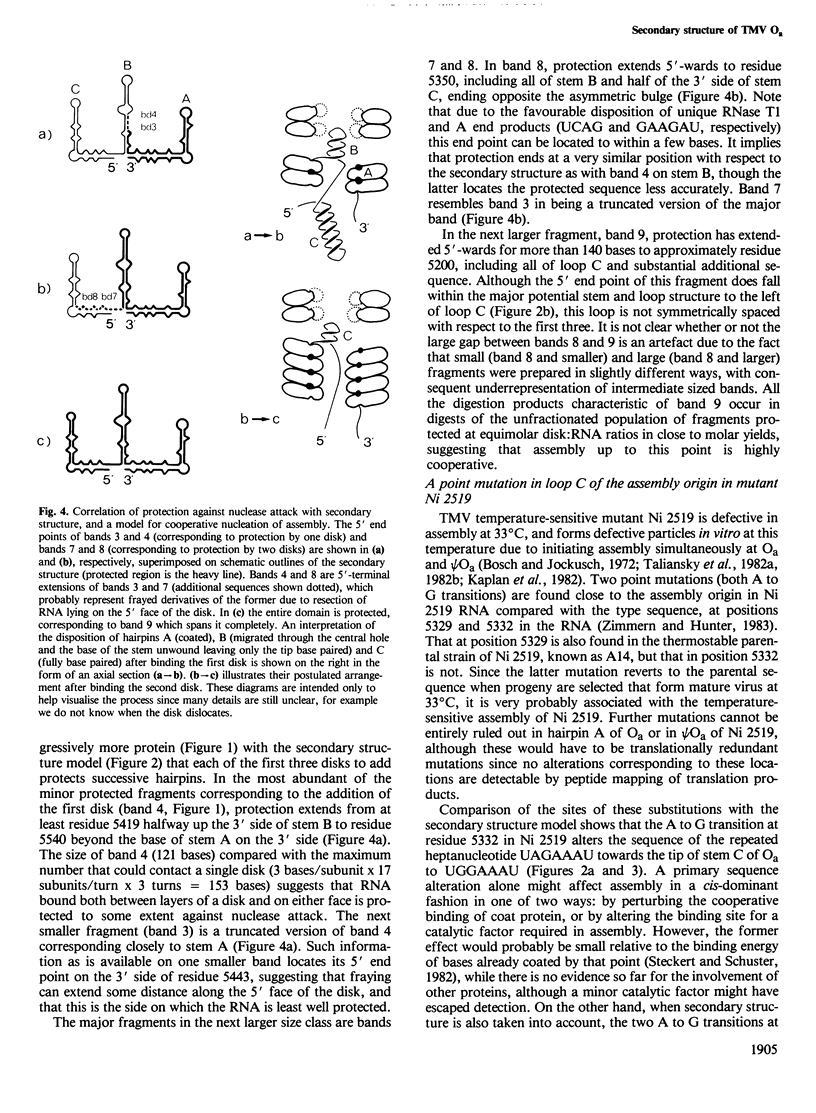
Recognition of the unique internal assembly origin on tobacco mosaic virus (TMV) RNA by the disk aggregate of the viral coat protein probably involves an extended region of the RNA (larger than that coated by a single disk) folded into a specific conformation. A secondary structure model is proposed for the RNA preferentially coated by limiting amounts of coat protein disks on the basis of partial nuclease digestion data. Part of this sequence can form three symmetrically spaced hairpins with marginally stable base paired sequences at the tips of the stems. The pattern of progressive protection of the RNA from nuclease attack during assembly suggests that these three hairpins are successively coated by the first three disks to add. The spacing of these hairpins is identical to that of three hairpins in the pseudo assembly origin (part of the coat protein gene homologous to the assembly origin). In Ni 2519, a TMV mutant whose assembly is defective at high temperature because it can no longer discriminate between the true and pseudo assembly origins, a point mutation has occurred near the tip of the third metastably base paired stem of the true assembly origin which would disrupt its structure and alter one copy of a repeated heptanucleotide. This suggests an important role for the ordered and cooperative recognition of successive loops in determining the specificity of assembly.
Keywords: assembly origin, protein-nucleic acid interactions, RNA secondary structure, temperature-sensitive RNA, tobacco mosaic virus
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosch F. X., Jockusch H. Temperature-sensitive mutants of TMV: behavior of a non-coat protein mutant in isolated tobacco cells. Mol Gen Genet. 1972;116(1):95–98. doi: 10.1007/BF00334265. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Lomonossoff G. P. Quantized incorporation of RNA during assembly of tobacco mosaic virus from protein disks. J Mol Biol. 1978 Dec 25;126(4):877–882. doi: 10.1016/0022-2836(78)90027-x. [DOI] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilley H., Jonard G., Richards K. E., Hirth L. Observations concerning the sequence of two additional specifically encapsidated RNA fragments originating from the tobacco-mosaic-virus coat-protein cistron. Eur J Biochem. 1975 May;54(1):145–153. doi: 10.1111/j.1432-1033.1975.tb04123.x. [DOI] [PubMed] [Google Scholar]
- Jonard G., Richards K. E., Guilley H., Hirth L. Sequence from the assembly nucleation region of TMV RNA. Cell. 1977 Jul;11(3):483–493. doi: 10.1016/0092-8674(77)90066-6. [DOI] [PubMed] [Google Scholar]
- Klug A. The assembly of tobacco mosaic virus: structure and specificity. Harvey Lect. 1980;74:141–172. [PubMed] [Google Scholar]
- Meshi T., Ohno T., Okada Y. Nucleotide sequence and its character of cistron coding for the 30 K protein of tobacco mosaic virus (OM strain). J Biochem. 1982 Apr;91(4):1441–1444. doi: 10.1093/oxfordjournals.jbchem.a133833. [DOI] [PubMed] [Google Scholar]
- Salser W. Globin mRNA sequences: analysis of base pairing and evolutionary implications. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):985–1002. doi: 10.1101/sqb.1978.042.01.099. [DOI] [PubMed] [Google Scholar]
- Shire S. J., Stegkert J. J., Schuster T. M. Mechanism of tobacco mosaic virus assembly: Incorporation of 4S and 20S protein at pH 7.0 and 20 degrees C. Proc Natl Acad Sci U S A. 1981 Jan;78(1):256–260. doi: 10.1073/pnas.78.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckert J. J., Schuster T. M. Sequence specificity of trinucleoside diphosphate binding to polymerized tobacco mosaic virus protein. Nature. 1982 Sep 2;299(5878):32–36. doi: 10.1038/299032a0. [DOI] [PubMed] [Google Scholar]
- Tyulkina L. G., Nazarova G. N., Kaftanova A. S., Ledneva R. K., Bogdanov A. A., Atabekov J. G. Reassembly of TMV 20-S protein disks with 3-S RNA fragments. Virology. 1975 Jan;63(1):15–29. doi: 10.1016/0042-6822(75)90366-9. [DOI] [PubMed] [Google Scholar]
- Zimmern D., Butler P. J. The isolation of tobacco mosaic virus RNA fragments containing the origin for viral assembly. Cell. 1977 Jul;11(3):455–462. doi: 10.1016/0092-8674(77)90064-2. [DOI] [PubMed] [Google Scholar]
- Zimmern D. The nucleotide sequence at the origin for assembly on tobacco mosaic virus RNA. Cell. 1977 Jul;11(3):463–482. doi: 10.1016/0092-8674(77)90065-4. [DOI] [PubMed] [Google Scholar]



