Abstract
Background & Aims
Chronic granulomatous disease (CGD) is an inherited disorder of the NADPH oxidase complex within phagocytic cells that predisposes people to bacterial and fungal infections. Approximately 40% of patients with CGD have gastrointestinal involvement. We aimed to characterize the endoscopic features of gastrointestinal CGD and define the role of endoscopy in patients.
Methods
We created a database of all patients with CGD seen at the National Institutes of Health (NIH) from 1990 through 2010. We identified patients who had an endoscopy, and collected information from those with CGD-associated inflammatory bowel disease (CGD-IBD). We analyzed clinical data (demographic information and symptoms), endoscopic data (indication, preparation quality, degree of inflammation, mucosal findings, and complications), and pathologic data.
Results
A total of 211 endoscopies (96 esophagogastroduodenoscopies, 82 colonoscopies. and 33 flexible sigmoidoscopies) were performed at the NIH on 78 patients with CGD. Esophageal, gastric, and duodenal inflammation were detected in 21%, 74%, and 37% of patients, respectively. Esophageal dysmotility and structural abnormalities were noted in 26%. Of the patients who had colonic CGD-IBD, 74% had skip lesions and 93% had ano-rectal disease. Enteric fistulae were found in 18% of patients; 73% of these were perianal. Colonic strictures were observed in 24% of patients; 80% were in the ano-rectal area.
Conclusions
Based on an analysis of clinical and endoscopic data from 78 patients, CGD-IBD is a distinct entity, primarily involving the anus and rectum, with skip lesions in the remaining bowel. Bowel strictures and fistulae are present in a significant number of patients. Upper gastrointestinal tract inflammatory disease is common, though typically not as severe as colonic disease. Upper and lower endoscopies are important in characterizing the gastrointestinal features of CGD.
Keywords: database analysis, GI, intestine, disease phenotype
Introduction
Chronic granulomatous disease (CGD) is a genetic disorder that affects a component of the NADPH oxidase (NOX) complex, originally described in 1954.1 The defect prevents the generation of reactive oxygen species (ROS) required for microbicidal activity in phagocytes, leading to recurrent bacterial and fungal infections.
CGD is typically diagnosed before the age of 5 (76%), however many patients are diagnosed in the second (10%) or third decade (4%) of life.2 CGD is diagnosed by testing for neutrophil superoxide production, and dihydrorhodamine-123 is commonly used.4 Genetic testing can identify molecular defects and is helpful in risk stratifying the patients. Increased recognition of CGD and early institution of prophylactic antibiotic and antifungal therapy has led to increased life expectancy into the fifth and sixth decades of life.2,3
Patients with CGD are known to have gastrointestinal involvement, presenting as inflammatory bowel disease (IBD). Prior reports have found GI involvement in 27% to 44% of patients with CGD.5,6 In some instances, the diagnosis of CGD has been made after the onset of gastrointestinal disease.7–9
Endoscopy is important in the diagnosis and management of gastrointestinal CGD. Previous reports define CGD-IBD as ulcerative colitis-like10–12 or Crohn’s disease-like6–8. We here describe the endoscopic features of GI disease in CGD and how it is distinguished from other colitides. We present our experience and define the role of endoscopy in chronic granulomatous disease.
Methods
Collection of data
A retrospective data analysis was performed on all CGD patients followed at the National Institutes of Health Clinical Center (NIH) from May 1990 to December 2010. For detailed methods, refer to supplemental material. During this period, 251 patients with CGD were evaluated, of whom 78 underwent a clinically indicated endoscopy. Patients who presented with diarrhea were ruled out for infectious colitis prior to inclusion into the endoscopy database.
Histologic Findings
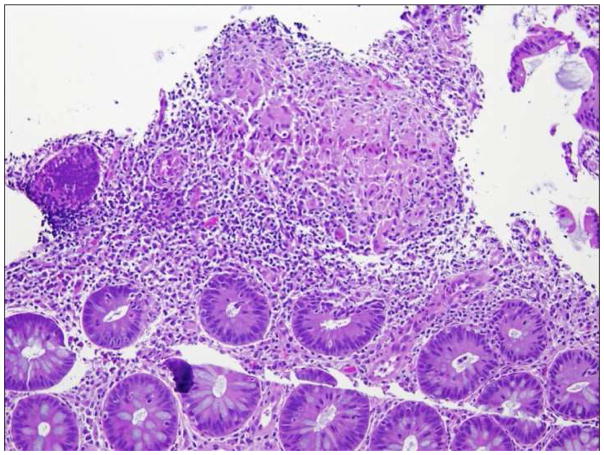
Endoscopic biopsies were taken to confirm CGD-IBD. CGD-IBD was characterized by inflammation in addition to pigmented macrophages and granulomas, which are common findings throughout the body in CGD. Acute colitis showed cryptitis/crypt abscesses (Figure 1). Chronic colitis was marked by crypt distortion and basal cell plasmacytosis. Granulomas ranged from poorly to well-formed with occasional giant cells14. Such changes were consistent with previously published literature5,6
Figure 1.
Acute colitis
For statistical analysis, refer to supplemental material.
Results
78 patients with CGD underwent a total of 211 endoscopies at the NIH. These included 96 EGDs in 57 patients, 82 colonoscopies in 52 patients, and 33 flexible sigmoidoscopies in 24 patients. A median of two (1–8) endoscopies were performed on each patient. Five patients underwent capsule endoscopy. There were 67 males and 11 females. The median age was 21.5 years (4–67 years) with 69% of patients >18 years of age. The majority of patients were Caucasian (Supplemental Table 2). 21 endoscopists performed or supervised these procedures, while 113 endoscopies were performed or supervised by a single endoscopist (TH).
The indication for endoscopy was guided by symptoms. The most common indication for an upper endoscopy was abdominal pain, for a colonoscopy was diarrhea and for a flexible sigmoidoscopy was a known history of CGD-IBD. Indications and basic findings for lower endoscopies can be found in Table 1. The bowel prep was poor in 15% of patients undergoing colonoscopy and in 3% undergoing flexible sigmoidoscopy.
Table 1.
Primary Indications and findings for lower endoscopy
| Inflammation† | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Indication | None | Mild | Moderate | Severe | Rectal involvement | Rectal structure | Rectal ulceration | Perianal disease | Anal structure |
| Diarreha (n=24) | 12.5% | 20.8% | 45.8% | 16.7% | 75.0% | 4.2% | 37.5% | 50.0% | 29.2% |
| Constipation (n=5) | 0.0% | 20.0% | 60.0% | 20.0% | 100.0% | 40.0% | 40.0% | 60.0% | 40.0% |
| Abdominal pain (n=22) | 13.6%# | 18.2% | 54.5% | 13.6% | 72.7% | 4.5% | 40.9% | 36.3% | 13.6% |
| Rectal pain (n=6) | 33.3% | 16.7% | 33.3% | 16.7% | 66.7% | 0.0% | 66.7% | 66.7% | 33.3% |
| Rectal bleeding (n=16) | 18.8% | 25.0% | 43.8% | 12.5% | 56.2% | 12.5% | 37.5% | 37.5% | 18.8% |
| Other indications: | |||||||||
| Protocol f/u (n=12) | |||||||||
| CRC Screening (n=6) | |||||||||
| Abnormal CT (n=2) | |||||||||
| IDA (n=3) | |||||||||
| Unknown (n=9) | |||||||||
Of those without colitis or perianal disease, 1 had mild atrophic gastritis, and the other did not have an EGO performed
Worst degree of inflammation noted on endoscopy
Endoscopic Findings
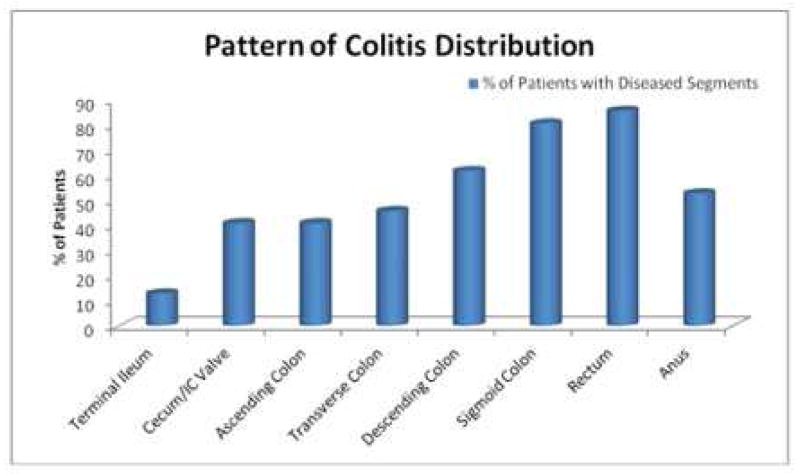
CGD-IBD was characterized by discontinuous areas of mucosal inflammation leading to “skip lesions”, which involved both the upper and lower gastrointestinal tracts. Colonic and gastric involvement were seen in similar (74%) numbers of patients, and disease was more severe in the colon. The frequency of disease decreased from the ano-rectum to the cecum (Figure 2).
Figure 2.
Pattern of colitis distribution
Colon
Of the patients who had a colonoscopy performed, 74% have findings suggestive of colitis and related complications. Of the 46 patients who have evidence of colitis, 8 (17%) had mild colitis, 16 (35%) had moderate colitis and 22 (48%) had severe colitis.
Ano-rectal involvement, in the form of anal erythema, fissures, ulcers, stenosis, and/or perianal fistulae, was a major feature of CGD-IBD. Ano-rectal disease was seen in 93% of patients who had IBD (Figure 3A, 3B, 3C, 3D).
Figure 3.
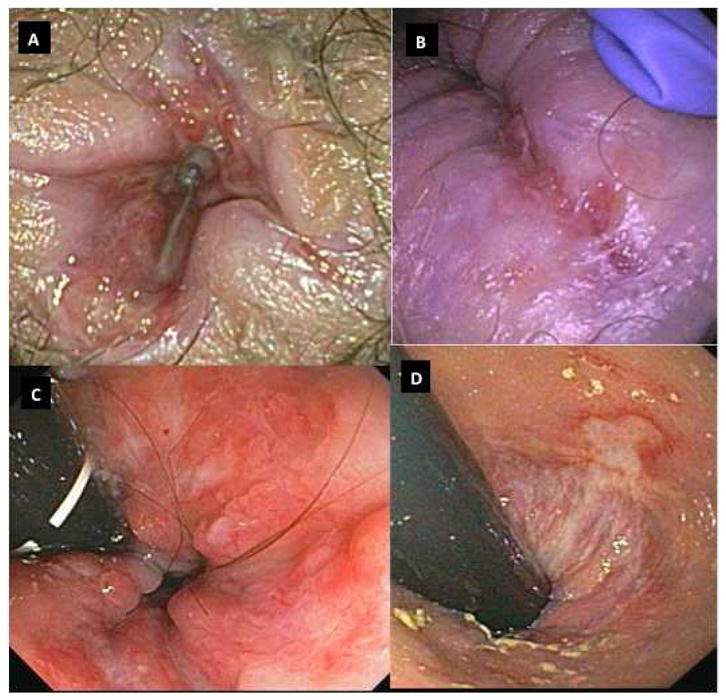
Figure 3A: Anal Ulcers
Figure 3B: Gluteal cleft fissure
Figure 3C: Ano-rectum with ulcers on retroflexion
Figure 3D: Rectal ulcer extending in to anal canal
Colonic strictures were seen in 15/62 (24%) patients. Of these strictures 12/15 (80%) were noted in the ano-rectal area, 2/15 (13%) in sigmoid colon and 1/15 (7%) in the ascending colon. Eight patients required anal stricture dilation. A mean of 2.25 (1–7) dilations were performed in these patients. One patient, who did not have control of luminal disease, required multiple anal dilations, whereas the other patients, whose luminal disease were controlled, maintained patency after a single adequate dilation.
Since CGD-IBD is a transmural process, enteric fistulae developed.6,7,15 These were seen in 11/62 (18%) patients, with 8/11 (73%) being in the perianal area, and then one each at the splenic flexure (9%), rectum (9%) and duodenum (to colon) (9%). Two patients had colo-vesicular fistulae noted clinically and on imaging but not on endoscopy.
None of the patients were noted to have dysplasia on non-protocolized biopsies. Eleven patients were noted to have polyps that were resected. These polyps were inflammatory in nature.
Ileum
The terminal ileum was examined in 30/52 (58%) patients who had colonoscopy and 24/46 (52%) with CGD-IBD. In those with evidence of IBD, only 4 had superficial ileal ulcers. Of these four patients, two had diverting ileostomies and the ulcers were noted near the stomas.
Esophagus
Of the patients who underwent upper endoscopy, 12/57 (21%) were noted to have esophageal inflammation, of whom 4/12 (33%) had esophageal ulcers. Biopsies of these ulcers were negative for fungal and viral etiologies. Furthermore, these biopsies showed evidence of chronic inflammation, similar to that found on biopsies of colonic ulcers. The spectrum of esophageal inflammation ranged from subtle mucosal changes with altered vascular pattern, to linear furrows, to superficial or deep linear ulcers along the length of the esophagus (Supplemental Figure 2A).
Suspected esophageal dysmotility, including esophageal spasm with tertiary contractions, was observed during the endoscopies in 15/57 (26%) of patients, however formal motility studies were not necessarily performed to confirm this finding. Abnormal esophageal contour was noted in 16/57 (28%) of patients, which ranged from fine esophageal rings to a tortuous esophagus giving the lumen a keyhole-like appearance (Supplemental Figure 2B). These abnormal structural changes had been previously well corroborated with Barium radiographs16,18. No obvious strictures or esophageal webs were noted in our patients. None of these patients had esophageal dilation performed at NIH. Only 19% with suspected esophageal dysmotility or structural changes were symptomatic. Esophageal dysmotility and structural changes were noted irrespective of the patient’s age, duration or severity of IBD.
Stomach
Gastric involvement was a common finding on endoscopy 42/57 (74%) with gastric ulcers and erosions noted in 8/42 (19%) of these patients. The stomach had a characteristic “salt-and-pepper” like appearing mucosa (Supplemental Figure 3). Other features of gastric inflammation included fine, lacy mucosal pattern, islands of discolored mucosa with pallor, and patchy erythema and erosions. The gastric ulcers were shallow, slit-like with heaped edges. These were primarily located in the antrum, though they were also seen in the gastric body and fundus. Although not recorded in the database, in our observations, a small percentage of patients had retained gastric food or bilious fluid suggestive of gastric dysmotility.
Duodenum
Duodenal involvement ranged from erythema, villous atrophy, dilated lacteals to erosions and ulcers, and were noted in 21/57 (37%) of patients undergoing endoscopy. Duodenal ulcers and erosions were seen in 6/21(29%) of patients. These patients were negative for H.pylori infection.
Complications during endoscopy
No procedure-related complications were noted. One patient developed fever within 6 hours of colonoscopy with anal dilation. The etiology of the fever was noted to be a hepatic abscesses, identified on imaging performed the same day.
Capsule Endoscopy
Capsule endoscopy was performed in 5 patients. Two patients had an incomplete study: one due to poor prep and the second had a retained capsule in the stomach, requiring endoscopic removal. One patient had delayed transit. Another patient had nonspecific changes of villous edema and patchy erythema. There were no ulcers, fistulae or strictures in the small bowel on capsule endoscopy.
Discussion
Our study is the first comprehensive description of the gastrointestinal findings in a large, contemporary cohort of CGD patients in the literature. An overview of the endoscopic findings is provided in Supplemental Figure 4. The pathology described here will be of interest to physicians caring for CGD patients, and as the subject of future research efforts.
Endoscopy was helpful in diagnosing and differentiating CGD-IBD from other causes of colitis, to identifying the extent of luminal disease, to determining the severity of disease and to monitoring the response to medical management.
The earliest endoscopic findings in CGD colitis included mucosal erythema, edema, abnormal vascular pattern, mucosal friability and contact bleeding. “Target-like lesions,” which have a clear elevated center with surrounding erythema, were characteristic of mild disease in setting of CGD. This might have been followed by the development of superficial erosions and aphthoid ulcers. Superficial erosions and ulcers coalesced to form irregular, serpiginous ulcers or rake-like ulcers, which tended to be more severe in the left colon than right colon. With increasing severity of inflammation, extensive, deep, and circumferential ulcerations covered with mucopurulent exudate formed, which extended through the wall of the intestine. Extensive, poorly controlled colitis led to frank purulent material in the lumen, a featureless colon with loss of haustral markings, decreased contractility and “lead pipe like” appearance. In the quiescent stage, the mucosa appeared relatively normal, except for neovascular changes and evidence of prior disease such as scars and pseudopolyps.
Colonoscopy with ileoscopy was the preferred endoscopic procedure in the initial evaluation of patients with suspected CGD-IBD. Colonoscopy allowed for direct mucosal visualization and biopsies, thereby facilitating the diagnosis and determination of colonic extent, activity, and severity of CGD colitis. Mucosal biopsies were useful both for confirming the diagnosis and defining the extent of CGD colitis, which helped to direct medical therapy.
After the initial colonoscopy had defined the extent and severity of disease, flexible sigmoidoscopy was sufficient for surveillance of patients who had rectal symptoms such as urgency and tenesmus and had known distal colonic disease. In patients with poor response to medical therapy, repeat endoscopic evaluation was necessary to help guide decisions regarding altering or escalating therapy.
Endoscopy also helped to direct decisions regarding whether or not to intensify medical therapy versus proceeding with a surgical diversion. Surgical diversion was performed in patients refractory to medical therapy, and in some cases, was used as a bridge to hematopoietic stem cell transplantation31. A general treatment algorithm can be found in Supplemental Figures 5 and 6.
GI tract dysplasia or malignancy was not observed yet in patients with CGD-IBD despite the presence of long standing colonic inflammation. Twenty-four patients in this cohort had pancolitis with greater than 10 years of disease. Only some of these patients had a systematic evaluation for dysplasia, but on multiple random biopsies that were obtained, dysplasia was not found. None of the patients were noted to have adenomas or premalignant lesions in the colon. In fact, only 1 patient since the cohort was established in 1978 was diagnosed with any malignancy. Obviously, this cohort was limited by a small study size, the patients were predominantly young, Caucasian males, and only two of the patients were older than 50 years.
However, studies have shown that ROS produced by activated leukocytes can overwhelm the tissue’s antioxidant defense, and ROS damage is thought to be involved in the early events of colon dysplasia and carcinogenesis28. In the case of chronic inflammation from CGD-IBD, the inability to produce ROS due to defective NOX enzymes might be protective against tumor carcinogenesis, which suggests that ROS play a key role in the malignant potential in IBD but not in CGD-IBD.
Small bowel disease was unusual in patients with CGD. Two of the four patients who were noted to have ileal ulcers had diverting loop ileostomies, which predisposed them to dermal flora that could have been pathogenic in this setting. We had not encountered small bowel strictures or fistulae except in one patient who had duodeno-colonic fistula in the presence of colonic disease. Since starting data collection, we encountered one patient with deep, cratered ulcers in the small bowel noted both on ileoscopy and capsule endoscopy. As the full spectrum of disease continues to unfold, it is recommended that the terminal ileum be evaluated at least at the time of the index colonoscopy to define the extent of disease. When clinical suspicion is high, the small bowel should be evaluated with capsule endoscopy to help direct therapy.
We have seen an equal incidence of upper tract disease as colitis, although less severe. Since gastric and duodenal involvement are common, we suggest routine upper endoscopic evaluation for initial staging. Esophageal structural changes and suspected dysmotility have been observed in a significant number of patients despite the absence of symptoms. These changes have been noted across all age groups, irrespective of disease duration. This information has been useful in educating patients in terms of symptoms and prognosis. Although endoscopy is not the ideal test to evaluate dysmotility, particular attention should be paid to structural abnormalities and dysmotility on endoscopy in a symptomatic patient, and follow-up motility studies may be warranted.
Dysphagia with radiographic abnormalities have been described in patients with CGD in case reports. 16–18 In these reports, the biopsies were noted to be normal or had non-specific inflammation. Symptoms responded well to antibiotics and steroids, suggesting an inflammatory component to the underlying process leading to structural abnormalities.17,18,20,21 Despite some of these prior reports suggesting the presence of esophageal strictures, in our experience, we have not yet encountered endoscopically treatable lesions.
When upper endoscopy was performed for symptoms of nausea and vomiting, there was no evidence of gastric outlet obstruction, unlike previous reports22,23. On our endoscopies, obstructive lesions such as ulcers, masses or pyloric stenosis, were not found. It may be that in our patients these obstructive symptoms were more likely to be due to gastric or small bowel dysmotility leading to functional obstruction, although formal motility studies would be necessary to confirm this hypothesis
Endoscopy has helped in characterizing CGD-IBD as a distinct entity. At times, an individual patient with CGD-IBD might be difficult to distinguish from Crohn’s disease. However, when CGD-IBD is evaluated broadly, then CGD-IBD has overlapping features of Crohn’s disease and ulcerative colitis but remains a distinct entity (Table 2). Furthermore, CGD-IBD patents have been observed to present consistently with colonic involvement that is worse distally, often with perianal disease and skip lesions. In contrast, Crohn’s disease patients might present with a wider variety of symptoms and features of gastrointestinal involvement. A small percentage of patients with CGD may remain undiagnosed until adulthood with CGD-IBD as their first CGD manifestation.7,8 Recognition of the endoscopic features of CGD-IBD that distinguish it from other colitides is helpful for diagnosis and management.
Table 2.
Comparison between CGD related IBD Crohn’s disease and Ulcerative Colitis
| Chronic Granulomatous Disease | Crohn’s Disease | Ulcerative colitis | |
|---|---|---|---|
| Upper tract GI disease | + | 2–20% | 20% (Clinical Significance unclear) |
| Skip Lesions | + | + | − |
| Perianal and intrabdominal abscesses | + | + | − |
| Fistulae | + | + | − |
| Rental Involvement | Almost always | Can spare the rectum | 95% |
| Terminal ileal Involvement | Seldom | Common | Not typically, but 20% of pancolitis with backwash ileitis |
| Dysmotility | +† | − | − |
| Granulomas | + (not Pathognomonic | + | − |
| Risk of neoplasia | Not Yet observed* | Present | Present |
| Extra-intestinal manifestations | Rare (Arthralgias, Acne, Pyoderma gangrenosum) | + | + |
Dysplastic lesions or cancer have not been identified on non-screening protocol biopsies despite the presence of disease for greater than 10 years
Dymotility is suspected based on observations during endoscopy
Distinguishing CGD-IBD from Crohn’s disease or ulcerative colitis is also important for treatment purposes because immunosuppression commonly used in patients with IBD is not without risk in patients with CGD.24 For example, TNF-blockade therapy can lead to life threatening infections in patients with CGD.25 Physicians should monitor for, and treat infections, especially among patients on immunosuppressive therapy. A multidisciplinary approach is essential to caring for these patients.
There are currently no guidelines on screening for CGD in patients with suspected Crohn’s disease who plan to start TNF-blockade therapy. One study detected no cases of CGD disease or carrier states after neutrophil function testing in 120 patients with diagnosed IBD, however the study was not adequately powered27. However, in patients whom CGD is suspected based on a history of recurrent infections, infections with rare organisms, or a family history of CGD, neutrophil function testing would be reasonable. Further studies would be necessary to determine the utility of screening for CGD in all or select patients with IBD.
There are several limitations to these data. First, this was a retrospective data analysis, and important events during the course of the illness might have been lost. Second, some of the index endoscopies were performed outside of the NIH and these patients were already on therapy when they were first evaluated at the NIH. This could have changed the endoscopic appearance, disease extent and severity. Despite this limitation however, we have consistently observed the same patterns of this disease as those who had their index endoscopies at our institution. Third, we cannot exclude the possibility that as the NIH is a tertiary referral center, we might be overestimating the severity and prevalence of CGD-IBD compared to patients seen in the community. Fourth, we had not routinely employed narrow band imaging to assess for disease severity. A prior report suggests benefit when subtle findings with white light endoscopy might be missed or milder than expected when compared to symptoms.19 Finally, the majority of the patients in this cohort had X-linked disease, or a mutation in the gp91 subunit of the NOX enzyme, and these data might not reflect the entire CGD population with other genotypes. In general, mutations in the gp91 subunit leads to lower ROS production which portends a worse overall prognosis with higher mortality3, but it remains to be seen if genotype clearly predicts colitis severity and response to treatment29.
Conclusion
Endoscopy plays an important role in the diagnosis and management of patients with chronic granulomatous disease and gastrointestinal symptoms. Dysmotility and structural abnormalities can be seen on endoscopy, which are under-recognized but important features of the disease. CGD-IBD is distinct from Crohn’s disease and ulcerative colitis though it has overlapping features, and it is a common manifestation of CGD. Future directions for research include characterizing the dysmotility in this cohort, response to therapy in the setting of varying severity and genotype, and understanding the reasons for the high incidence and particular pattern of involvement of IBD in patients with CGD.
Supplementary Material
Figure 1A: Mild Coliti
Figure 1B: Mild colitis
Figure 1C: Moderate Colitis
Figure 1D: Moderate Colitis
Figure 1E: Severe Colitis
Figure 1F: Severe Colitis
Figure 2A: Linear esophageal ulcers
Figure 2B: Tortuous esophagus with rings
Figure 3: Gastric “salt and pepper” like mucosa with slit-like ulcer
Supplemental Figure 4: Spectrum of GI disease in Chronic Granulomatous Disease CGD
Supplemental Table 1: Endoscopic Differentiation of Severity of Colitis*
Supplemental Table 2: Demographics
In general, mild colonic disease was treated with a 5-ASA agent such as mesalamine, with or without prednisone induction. Thiopurine agents including 6-MP and azathioprine were added to treat patients with moderate to severe colitis with prednisone induction. Topical 5-ASA therapy was frequently added for distal colonic disease, and antibiotics were started for perianal disease.
Refractory disease was treated with a variety of medications including methotrexate, thalidomide, and mycophenolate mofetil with varying success. Elemental diet had been considered for treatment, but no data is currently available regarding remission rates. Bone marrow transplant was also considered in eligible candidates. Surgery was reserved for patient who failed all medical therapy and were not eligible transplant candidates. Abdominal perineal resection was reserved for patient with fistulizing or structuring perianal disease.
Acknowledgments
Financial Support:
Funded by the intramural program in NIDDK, NIAID, NCI and the clinical center of the NIH.
Douglas B. Kuhns who is funded in whole or part by NCI contract no. HHSN261200800001E
Abbreviations
- CGD
chronic granulomatous disease
- IBD
inflammatory bowel disease
- GI
gastrointestinal
- NOX
NADPH oxidase
- ROS
reactive oxidative species
- NIH
National Institutes of Health, Bethesda, MD
Footnotes
Conflicts of Interest: No authors have conflicts to declare
Author involvement:
Sajneet K. Khangura, MD: Patient care, drafted manuscript, collated data, study design
Natasha Kamal, BA: Collated data, maintained database, study design
Nancy Ho, MD: Edited manuscript, maintained database, study design
Martha Quezado, MD: Evaluated pathology
Xiongce Zhao, PhD: Statistical analysis
Beatriz Marciano, MD: Maintained database
Jennifer Simpson, BA: Maintained database
Christa Zerbe, MD: Patient care, collated data, study design
Gulbu Uzel, MD: Patient care, collated data
Michael D. Yao, MD: Patient care
Suk See DeRavin, MD: Patient care
Colleen Hadigan, MD: Patient care
Douglas B. Kuhns, PhD: Genotype analysis, maintained database
John I. Gallin, MD: Patient care
Harry L. Malech, MD: Patient care, collated data
Steven M. Holland, MD: Patient care, maintained databased, study design
Theo Heller, MB, ChB: Patient care, maintained database, study design, drafted manuscript, study design and concept
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Janeway CA, Craig J, Davidson M, Downey W, et al. Hypergammaglobulinemia associated with severe recurrent and chronic nonspecific infection. American Journal of Diseases of Children. 1954;88:388–92. [Google Scholar]
- 2.Winkelstein JA, Marino MC, Johnston RB, Jr, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–69. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Kuhns DB, Alvord WG, Heller T, et al. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med. 2010;363:2600–10. doi: 10.1056/NEJMoa1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vowells SJ, Sekhsaria S, Malech HL, et al. Flow cytometric analysis of the granulocyte respiratory burst: a comparison study of fluorescent probes. J Immunol Methods. 1995;178:89–97. doi: 10.1016/0022-1759(94)00247-t. [DOI] [PubMed] [Google Scholar]
- 5.Marciano BE, Rosenzweig SD, Kleiner DE, et al. Gastrointestinal involvement inchronic granulomatous disease. Pediatrics. 2004;114:462–8. doi: 10.1542/peds.114.2.462. [DOI] [PubMed] [Google Scholar]
- 6.Marks DJ, Miyagi K, Rahman FZ, et al. Inflammatory bowel disease in CGD reproduces the clinicopathological features of Crohn’s disease. Am J Gastroenterol. 2009;104:117–24. doi: 10.1038/ajg.2008.72. [DOI] [PubMed] [Google Scholar]
- 7.Isaacs D, Wright VM, Shaw DG, et al. Chronic granulomatous disease mimicking Crohn’s disease. Journal of pediatric gastroenterology and nutrition. 1985;4:498–501. doi: 10.1097/00005176-198506000-00030. [DOI] [PubMed] [Google Scholar]
- 8.Huang JS, Noack D, Rae J, et al. Chronic granulomatous disease caused by a deficiency in p47(phox) mimicking Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2:690–5. doi: 10.1016/s1542-3565(04)00292-7. [DOI] [PubMed] [Google Scholar]
- 9.Ament ME, Ochs HD. Gastrointestinal manifestations of chronic granulomatous disease. The New England journal of medicine. 1973;288:382–7. doi: 10.1056/NEJM197302222880802. [DOI] [PubMed] [Google Scholar]
- 10.Sloan JM, Cameron CH, Maxwell RJ, et al. Colitis complicating chronic granulomatous disease. A clinicopathological case report. Gut. 1996;38:619–22. doi: 10.1136/gut.38.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villanacci V, Falchetti D, Liserre B, et al. Diversion of the fecal stream resolves ulcerative colitis complicating chronic granulomatous disease in an adult patient. J Clin Gastroenterol. 2007;41:491–3. doi: 10.1097/01.mcg.0000212638.44735.78. [DOI] [PubMed] [Google Scholar]
- 12.Fisher JE, Khan AR, Heitlinger L, et al. Chronic granulomatous disease of childhood with acute ulcerative colitis: a unique association. Pediatr Pathol. 1987;7:91–6. doi: 10.1080/15513818709177120. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. The New England journal of medicine. 1987;317:1625–9. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 14.Alimchandani M, Lai JP, Aung PP, et al. Gastrointestinal histopathology in chronic granulomatous disease: a study of 87 patients. Am J Surg Pathol. 2013;37:1365–72. doi: 10.1097/PAS.0b013e318297427d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopal L, Forbes J, Uzel G, et al. Gastrointestinal fistulae in chronic granulomatous disease. Am J Gastroenterol. 2009;104:2112–3. doi: 10.1038/ajg.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markowitz JF, Aronow E, Rausen AR, et al. Progressive esophageal dysfunction in chronic granulomatous disease. J Pediatr Gastroenterol Nutr. 1982;1:145–9. doi: 10.1097/00005176-198201010-00024. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Contreras J, Bastero R, Serrano C, et al. Oesophageal narrowing in chronic granulomatous disease. Eur J Radiol. 1998;27:149–52. doi: 10.1016/s0720-048x(97)00049-1. [DOI] [PubMed] [Google Scholar]
- 18.Golioto M, O’Connor JB. Esophageal dysmotility in an adult with chronic granulomatous disease. J Clin Gastroenterol. 2001;33:330–2. doi: 10.1097/00004836-200110000-00016. [DOI] [PubMed] [Google Scholar]
- 19.von Rosenvinge EC, O’Donnell TG, Holland SM, Heller T. Chronic granulomatous disease. Inflamm Bowel Dis. 2010;16:9. doi: 10.1002/ibd.20912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chin TW, Stiehm ER, Falloon J, Gallin JI. Corticosteroids in treatment of obstructive lesions of chronic granulomatous disease. The Journal of pediatrics. 1987;111:349–52. doi: 10.1016/s0022-3476(87)80452-3. [DOI] [PubMed] [Google Scholar]
- 21.Renner WR, Johnson JF, Lichtenstein JE, Kirks DR. Esophageal inflammation and stricture: complication of chronic granulomatous disease of childhood. Radiology. 1991;178:189–91. doi: 10.1148/radiology.178.1.1984302. [DOI] [PubMed] [Google Scholar]
- 22.Danziger RN, Goren AT, Becker J, et al. Outpatient management with oral corticosteroid therapy for obstructive conditions in chronic granulomatous disease. The Journal of pediatrics. 1993;122:303–5. doi: 10.1016/s0022-3476(06)80138-1. [DOI] [PubMed] [Google Scholar]
- 23.Dickerman JD, Colletti RB, Tampas JP. Gastric outlet obstruction in chronic granulomatous disease of childhood. American Journal of Diseases of Children. 1986;140:567–70. doi: 10.1001/archpedi.1986.02140200077032. [DOI] [PubMed] [Google Scholar]
- 24.Vinh DC, Freeman AF, Shea YR, et al. Mucormycosis in chronic granulomatous disease: association with iatrogenic immunosuppression. The Journal of allergy and clinical immunology. 2009;123:1411–3. doi: 10.1016/j.jaci.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uzel G, Orange JS, Poliak N, et al. Complications of tumor necrosis factor-alpha blockade in chronic granulomatous disease-related colitis. Clin Infect Dis. 2010;51:1429–34. doi: 10.1086/657308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez-Downing MM, Kamal N, Inchauste SM, et al. The Role of Surgery in the Management of Patients With Refractory Chronic Granulomatous Disease Colitis. Diseases of the Colon & Rectum. 2013;56(5):609–14. doi: 10.1097/DCR.0b013e3182781504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaggi P, Scherzer R, Knieper R, et al. Utility of Screening for Chrnoic Granulomatous Disease in Patients with Inflammatory Bowel Disease. Journal of Clinical Immunology. 2011;32(1):78–81. doi: 10.1007/s10875-011-9608-5. [DOI] [PubMed] [Google Scholar]
- 28.Guina T, Biasi F, Calfapietra S, et al. Inflammatory and Redox Reactions in Colorectal Carcinogenesis. Annals of the New York Academy of Sciences. 2015;1340:95–103. doi: 10.1111/nyas.12734. [DOI] [PubMed] [Google Scholar]
- 29.Magnani A, Brosselin P, Beaute J, et al. Inflammatory Manifestations in a Single-center Cohort of Patients with Chronic Granulomatous Disease. Journal of Allergy and Clinical Immunology. 2014;134(3):655–62. doi: 10.1016/j.jaci.2014.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1A: Mild Coliti
Figure 1B: Mild colitis
Figure 1C: Moderate Colitis
Figure 1D: Moderate Colitis
Figure 1E: Severe Colitis
Figure 1F: Severe Colitis
Figure 2A: Linear esophageal ulcers
Figure 2B: Tortuous esophagus with rings
Figure 3: Gastric “salt and pepper” like mucosa with slit-like ulcer
Supplemental Figure 4: Spectrum of GI disease in Chronic Granulomatous Disease CGD
Supplemental Table 1: Endoscopic Differentiation of Severity of Colitis*
Supplemental Table 2: Demographics
In general, mild colonic disease was treated with a 5-ASA agent such as mesalamine, with or without prednisone induction. Thiopurine agents including 6-MP and azathioprine were added to treat patients with moderate to severe colitis with prednisone induction. Topical 5-ASA therapy was frequently added for distal colonic disease, and antibiotics were started for perianal disease.
Refractory disease was treated with a variety of medications including methotrexate, thalidomide, and mycophenolate mofetil with varying success. Elemental diet had been considered for treatment, but no data is currently available regarding remission rates. Bone marrow transplant was also considered in eligible candidates. Surgery was reserved for patient who failed all medical therapy and were not eligible transplant candidates. Abdominal perineal resection was reserved for patient with fistulizing or structuring perianal disease.