Abstract
The major issue in coronary heart disease (CHD) diagnosis and management is that symptoms onset in an advanced state of disease. Despite the availability of several clinical risk scores, the prediction of cardiovascular events is lacking, and many patients at risk are not well stratified according to the canonical risk factors alone. Therefore, adequate risk assessment remains the most challenging issue. Recently, the integration of imaging data with biochemical markers in a radiogenomic framework has been proposed in many fields of medicine as well as in cardiology. Multimodal imaging and advanced processing techniques can provide both direct (e.g., remodeling index, calcium score, total plaque volume, plaque burden) and indirect (e.g., myocardial perfusion index, coronary flow reserve) imaging features of CHD. Furthermore, the identification of novel non-invasive biochemical markers, mainly focused on plasma and/or serum samples, has increased the specificity of findings, reflecting several pathophysiological pathways of atherosclerosis, the principal actor in CHD. In this context, a multifaced approach, derived from the strengths of all these modalities, appears promising for finer risk stratification and treatment strategies, facilitating the decision-making and clinical management of patients. This review underlines the role of different imaging modalities in the quantification of coronary atherosclerosis and describes novel blood-based markers that could improve diagnosis and have a better predictive value in CHD.
Keywords: Atherosclerosis, coronary heart disease, imaging, biomarkers
Introduction
Cardiovascular diseases (CVDs) are the primary cause of mortality worldwide with 17.3 million deaths per year, and an estimation of 23.6 million in 2030, placing it as a relevant issue for the public health system [1,2]. Coronary heart disease (CHD) is the largest contributor of CVDs and mortality rate is due in prevalence to atherosclerosis, a chronic inflammatory condition of the arterial wall. Unfortunately, myocardial infarction (MI) is still a first common manifestation of CHD and, in about 50% of patients, angina pectoris is the first symptom of the pathology [1]. For this reason, an accurate and prompt diagnosis in CHD patients could improve prognosis and/or quality of life and allow timely and adequate therapeutic treatments (percutaneous or surgical myocardial revascularization, pharmacological therapy). Furthermore, efforts should be focused on primary prevention or early detection of subjects suffering from coronary atherosclerosis, in order to implement therapeutic strategies, so reducing morbidity, health expenditure, and mortality [3-6]. The risk of clinical manifestations of CHD is currently estimated according to multifactorial integrated prediction models developed on the basis of population studies that have allowed to evaluate the likelihood of cardiac events, even though some of them have a poor predictive value [7-13]. Imaging techniques have deeply increased early detection of CHD, although the invasive approach restricts their feasibility mainly to symptomatic patients. Among them, for their higher spatial resolution, intravascular ultrasound (IVUS), X-ray angiography (XRA), and computed tomography coronary angiography (CTCA) provide a direct evaluation and quantification of coronary artery alteration, while cardiac magnetic resonance (CMR) and nuclear medicine techniques (single photon computed tomography (SPECT), and positron emission tomography (PET)) provide indirect information of CHD, estimating myocardial perfusion and metabolism abnormalities that are consequent to coronary artery disease [14]. In addition, serum/plasma biomarkers can be mini-invasively extracted also in asymptomatic patients in order to analyze at different levels (e.g., cellular, biochemical, epigenetic and/or transcriptional) atherosclerosis and CHD development.
This review underlines the role of different imaging modalities in the setting of coronary atherosclerosis and describes novel blood-based markers that could improve diagnosis and have a better predictive value in CHD.
Imaging markers for direct CHD diagnosis
Several imaging techniques have been developed and used extensively over the last years in order to exclude and/or detect CHD with the aim to guide optimal patient management.
IVUS employs a miniature ultrasound probe placed at the tip of a coronary catheter; the signals received according to the different acoustic impedance are reconstructed into a real-time tomographic gray-scale image [15]. In coronary arteries, two borders are well defined: the blood-intimal border and the external elastic membrane (EEM). Some morphologic features of atherosclerotic coronary plaques can be invasively assessed by this tool such as soft, fibrous, calcified, necrotic and lipid components. The reliability of ultrasound imaging in predicting the composition of atherosclerotic plaque components has been demonstrated in histological comparative studies [16]. Indeed, IVUS allows to clearly depict the vascular wall and accurately calculate the remodeling index.
Many clinical trials have disclosed significant improvements in patient outcomes and reduced complications [17,18]. Meanwhile, plenty of clinical pharmacological trials employs IVUS to demonstrate its beneficial effects because this technique offers great value for the precise quantification of atherosclerosis progression or regression [19].
Differently, XRA is a procedure that uses a special dye (contrast medium) and x-rays to selectively highlight the arterial lumen (lumenogram), allowing the identification of caliber alterations, like stenoses/occlusions or aneurysms, and, at the same time, to treat these conditions. This technique is a reliable reference standard for the estimation of the real coronary lumen and intracoronary pressure measurement for the detection of functionally relevant stenoses and fractional flow reserve (FFR) calculation. However, XRA is an invasive technique like IVUS, but unlike it, it is not convincing with regard to prognostic value [20]. Moreover, conversely to other imaging techniques for direct CHD diagnosis, XRA does not allow the evaluation of vascular wall, and CHD is largely not a disease of the lumen itself but an abnormality of the vessel wall [21].
CTCA has overcome several of previous technique drawbacks, allowing an accurate quantitative evaluation of the arterial lumen, vessel wall and the extension, severity and composition of the atherosclerotic plaque [14,22]. A first level of evidence that can be extracted by CTCA is represented by the detection of coronary calcium deposits and the assessment of lumen stenosis [23]. Calcium burden is usually quantified using the ‘Agatston score’, an aggregate score across entire vessel territories rather than across specific atherosclerotic plaques, and mainly related to risk stratification of asymptomatic patients [24], than of symptomatic ones [25]. The amount of calcium correlates roughly to the total amount of coronary atherosclerosis but the correlation with the degree of luminal narrowing is poor. Even with severe calcifications, luminal stenosis is not necessarily present and a ‘zero’ calcium score cannot be used to rule out coronary artery stenosis in symptomatic individuals, especially in young subjects and in individuals with acute symptoms [24,26]. This evidence can be described by the remodeling index, a CTCA parameter used to define the main grow direction of plaque toward the inner or outer layers of the vascular wall and not evaluable by XRA technique [27]. Moreover, atherosclerotic plaques are often composed by both calcific and non-calcific components (mixed plaque), a feature highly predictive for CHD complications and easily quantifiable (e.g., total plaque volume, calcific plaque volume, non-calcific plaque volume) by advanced CTCA scanners, like dual energy CT or spectral CT [28,29]. These state-of-art computed tomographies, thanks to improved spatial and temporal resolution, better tissue characterization, and lowering of dose and iodine load, have a wide range of applications also in patients with high or irregular heart rate, encompassing from myocardial perfusion, coronary blood flow velocity and pressure and non-invasive FFR assessment by the application of the computational fluid dynamics and mathematical models [30]. In conclusion, among the imaging techniques for direct CHD diagnosis, CTCA is the unique that allows a “simultaneous” analysis of the entire coronary tree at a low dose of contrast medium load, proposing this tool as suboptimal for early diagnosis and CHD risk assessment (Figure 1).
Figure 1.
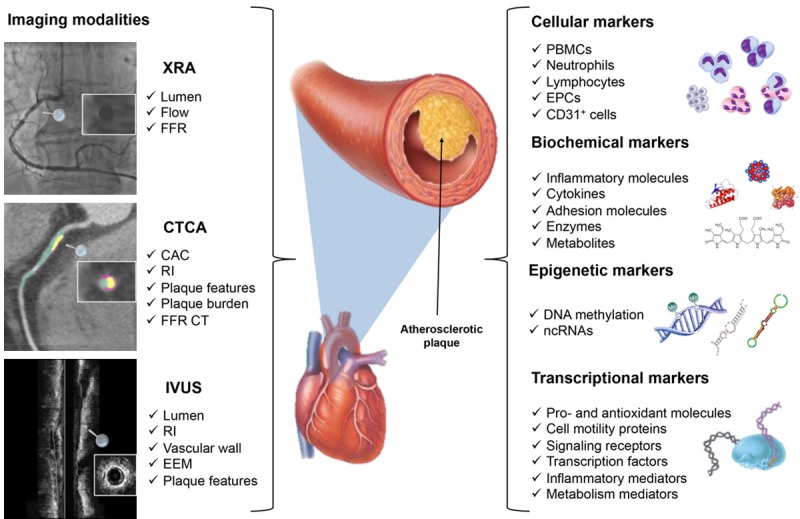
Integrated diagnostic approach in coronary heart disease. The figure shows the imaging modalities for direct assessment of coronaries and the diversity of circulating biomarkers that could be associated with the diagnosis, outcome prediction and risk assessment in coronary heart disease (CHD). The left panel represents X-ray angiography (XRA), coronary computed tomography angiography (CTCA) and intravascular ultrasound (IVUS) in coronary imaging and respective parameters. For XRA: luminal narrowing, blood flow and fractional flow reserve (FFR); for CTCA: coronary artery calcium (CAC), remodeling index (RI), plaque features, plaque burden and FFR CT; for IVUS: lumen, RI, vessel structure, external elastic membrane (EEM) and plaque features. The right panel depicts markers that can be analyzed in serum/plasma or whole blood and reflect coronary artery alterations: cellular: peripheral blood mononuclear cells (PBMCs), neutrophils, lymphocytes, endothelial progenitors cells (EPCs), CD31+ cells; biochemical markers: inflammatory pathways, cytokines, adhesion molecules, enzymes and metabolites; epigenetic markers: DNA methylation levels and differential expression of circulating non-coding RNAs (ncRNAs); and transcriptional markers: genes coding for pro-and antioxidant molecules, cell motility proteins, signaling receptors, transcription factors, inflammatory and metabolic mediators.
Imaging markers for indirect CHD diagnosis
CMR provides excellent soft tissue contrast, allowing functional, perfusional, morphological and anatomical evaluations, without exposing the patient to ionizing radiation, like both XRA and CTCA [14]. Recently, CMR has been applying also to direct anatomical coronary visualization, although it is still limited in the visualization of distal coronary segments due to inferior spatial resolution, compared to CTCA [31]. Several applications for the assessment and follow-up of patients with CHD include the estimation of myocardial viability thanks to T1 mapping, T2 mapping and the monitoring of left ventricle remodeling after acute myocardial infarct through diffusor tensor imaging besides first pass perfusion CMR and delayed contrast enhancement [32].
Nuclear medicine imaging by SPECT and PET, instead, provide, through the injection of a radiotracer, functional and perfusional analysis, the evaluation of ischemia and blood flow quantification [33,34]. Myocardial perfusion imaging triggered by ECG monitoring allows also consideration of wall motion and thickening; moreover, changes in blood flow are considered positive for suspected CHD. Cardiac PET is useful for perfusion imaging, functional evaluation and assessment of myocardial viability. For perfusion imaging, commonly used PET radiotracers are 82Rubidium, 13Nitrogen-ammonia and 15Oxygen-water. 18F-FDG is used for diagnosis of myocardial viability and it is considered the most sensitive modality for predicting left ventricular functional recovery post-coronary revascularization. In particular, the integration of perfusion and viability, detected by PET, allows the identification of myocardial stunning (normal perfusion but reduced metabolism) and myocardial hibernation (reduced perfusion but preserved metabolism) [35].
In vitro biomarkers for CHD diagnosis and risk prediction
Several studies focused on the introduction of multiplex biomarker assays analyzing different circulating molecules and their integration with current clinical scores in multivariable prediction model for a better diagnosis and risk stratification [36,37]. Concerning in vitro biomarkers, many molecules can be extracted from serum and/or plasma of asymptomatic subjects and CHD patients that could reflect at different levels (e.g., cellular, biochemical, epigenetic and/or transcriptional) atherosclerosis and coronary alterations (Figure 1).
Cellular markers
Circulating cells have been proposed as blood-derived biomarkers in CHD in the pathogenesis of CHD, secreting a wide plethora of specific biomarkers [38]. Several studies showed an increased number of monocytes in the blood of patients with atherosclerosis and in subjects with cardiovascular risk factors [38,39]. The multiple roles of monocytes in the atherogenic process are attributed to the existence of sub-populations characterized by the expression of different surface markers with consequent functional changes and response to stimuli as well as alterations in gene expression [40-42]. Circulating neutrophil count and neutrophil/lymphocyte ratio are also emerging markers of the presence and severity of CHD, correlating with plaque vulnerability [43,44]. In peripheral blood mononuclear cell (PBMCs) of CHD patients, were found a set of 190 genes differentially expressed compared to healthy controls [45]. Another heterogeneous class of cells involved in CVDs is represented by the endothelial progenitor cells (EPCs). Several studies have reported a variation of cell number and a functional impairment in patients with CHD strictly dependent on the presence of cardiovascular risk factors and associated with the severity of coronary lesions and clinical outcome [46-48]. Finally, Kim et al. performed a flow cytometry analysis of CD31(+) cells from the peripheral blood of CHD patients showing a significant correlation between CD31(+) cell count and the number of atherosclerotic vessels [49] (Table 1).
Table 1.
Cellular markers associated to CHD diagnosis and prognosis
| Biomarker | Source | Regulation | Clinical role | Ref. |
|---|---|---|---|---|
| CD14++CD16-CCR2+; CD14++CD16+CCR2+; CD14+CD16++CCR2- monocytes | PBMCs | Reduced expression of CD14 and CD14+CD16++CCR2- subpopulation in CHD | Diagnostic | [40] |
| CD4+CD28null T cells | PBMCs | CD4+CD28null population from RF and ACS groups had higher expression levels of cytotoxic molecules | Predictive for treatment | [41] |
| CD3+/CD31+ T cells | PBMCs | Increased levels in ACS patients | Predictive after PCI | [42] |
| Neutrophil/Lymphocyte ratio | Leucocytes | Increased N/L ratio associated with severity of CHD and plaque vulnerability | Diagnostic/Predictive of long term outcome | [43,44] |
| EPCs | PBMCs | Number reduction and functional impairment in CHD patients; cell count dependent on the number of RF; associated with the severity of coronary lesions and less sub-stent plaque burden | Diagnostic/Predictive of future CV events and PCI follow-up | [46-48] |
| CD31(+) cells | Blood | Increased CD31(+) cells in UA patients; significant correlations between cell count and the number of atherosclerotic coronaries | Diagnostic/Predictive of UA | [49] |
Abbreviations: CHD = Coronary heart disease; RF = risk factor; ACS = Acute coronary syndrome; PBMC = Peripheral blood mononuclear cell; PCI = Percutaneous coronary intervention; UA = Unstable angina.
Biochemical biomarkers
Inflammatory biomarkers appear to have an important prognostic value in patients with CVDs and may be useful in the diagnosis of apparently healthy subjects without known CHD who cannot be assessed with conventional risk factors. In the last years, several biomarkers have been identified, although not for everyone there are studies that show a possible correlation with instrumental imaging parameters.
In particular, the group of biomarkers of interest includes: transforming growth factor beta (TGF-β1) [50]; cellular adhesion molecules (CAMs) [51,52]; monocyte chemoattractant protein-1 (MCP-1) [53-55]; stromal cell-derived factor-1α (SDF-1α) [56-58]; lectin-like oxidized low density lipoprotein receptor 1 (LOX-1) [59-61]; pentraxin 3 (PTX3) [62-64]; bilirubin [65-67] and haemoglobin A1c (HbA1c) [68,69], whose serum and/or plasmatic levels were associated with the presence and severity of CHD.
In addition, among emerging biomarkers, we selected: serum amyloid A (SAA) proteins [70,71]; fibrinogen [72,73]; myeloperoxidase (MPO) [74-77]; paraoxonase-1 (PON1) [78]; matrix metalloproteinases (MMPs) [79,80]; proprotein convertase subtilisin/kexin type 9 (PCSK9) [81-83]; lipoprotein-associated phospholipase A2 (Lp-PLA2) [84,85]; retinol-binding protein-4 (RBP4) [86,87]; angiopoietin-like 4 (ANGPTL4) [88] and sphingolipids [89-91] that could be potential biomarkers for the assessment of CHD severity. Indeed, these emerging biochemical markers could potentially find a useful application for the diagnosis and prognosis assessment in CHD patients. Furthermore, several works focused on the introduction of multiplex biomarker assay analyzing different circulating molecules and their integration in new grading score for a better diagnosis and risk stratification [55,92-96] (Table 2).
Table 2.
Biochemical markers associated to CHD diagnosis and prognosis
| Biomarker | Source | Regulation | Clinical role | Ref. |
|---|---|---|---|---|
| TGF-β1 | Serum | High levels in AMI patients compared to SA and UA | Diagnostic/Risk stratification | [50] |
| sVCAM-1/sICAM-1 | Serum | Higher levels in UA patients | Predictive of ACS | [51,52] |
| MCP-1 | Plasma | Positive correlation with the extent of coronary atherosclerosis in UA patients detected by RXA; high concentrations in ACS patients | Diagnostic/Predictive of increased risk for death or AMI | [53-55] |
| SDF-1α | Plasma | Reduction in STEMI patients compared to SA or to NSTEMI; association with disease severity | Diagnostic/Predictive of negative outcome | [56-58] |
| LOX-1 | Serum | Increased concentrations in CHD patients in association with disease severity | Diagnostic/Predictive of increased risk of CHD | [59-61] |
| PTX3 | Plasma | Biomarker of plaque vulnerability | Diagnostic | [62-64] |
| Bilirubin | Serum | Lower levels in patients with critical stenosis and non-calcified/mixed plaques detected by CTCA | Diagnostic/Predictive of risk stratification, CHD onset and long term-mortality | [65-67,162] |
| HbA1c | Plasma | High levels associated to CAC and CHD severity in non diabetic patients | Diagnostic/Predictive | [68,69] |
| SAA | Plasma | Elevated levels in CHD patients | Predictive of disease risk and worse prognosis | [70,71] |
| MPO | Plasma | Marker of plaque instability and disease severity | Diagnostic | [74-77] |
| PON1 | Plasma | Reduced activity in CHD patients | Diagnostic/Predictive | [78] |
| MMP-9/MMP-8 | Plasma/Serum | Biomarkers of plaque instability | Diagnostic | [79,80] |
| PCSK9 | Serum | High concentrations are associated with CAC and disease severity | Diagnostic/Predictive of CV events | [81-83] |
| Lp-PLA2 | Plasma | High levels associated with CHD severity | Diagnostic/Predictive of CHD and mortality | [84,85] |
| RBP4 | Serum | High levels associated with coronary lesion severity in stable CHD and ACS | Diagnostic/Predictive of CV events | [86,87] |
| ANGPTL4 | Plasma | High levels in patients at risk | Predictive of CV event occurrence | [88] |
| Sphingolipids | Plasma | Positively related to CHD and subclinical atherosclerosis | Predictive of CV events | [89-91] |
| hs-TnT/hs-CRP | Serum | Associated with CHD burden and change in plaque composition detected by CCTA | Diagnostic | [154] |
| IL-10 | Serum | Reduced levels in patients with ACS | Diagnostic/Predictive of long-term adverse outcomes | [155,156] |
| IL-8 | Serum | High levels in patients in CHD patients | Predictive of long-term outcome | [157] |
| IL-6 | Plasma/Serum | High concentration in patients with multivessel atherosclerosis and calcified plaque assessed by CCTA | Diagnostic | [158] |
| Adiponectin | Serum | Low levels associated with lipid-rich, non-calcified plaques and multivessel CHD by CTCA | Diagnostic | [159-161] |
Abbreviations: ACS = Acute coronary syndrome; CAC = Coronary Artery Calcium; CHD = Coronary heart disease; CTCA = Coronary Computed Tomography Angiography; SA = stable angina; STEMI = ST-elevation myocardial infarction; NSTEMI = non-ST-elevation myocardial infarction; AMI = acute myocardial infarction; UA = unstable angina.
Epigenetic markers
Epigenetic modifications are involved in a variety of pathological conditions as well as in CVDs and atherosclerosis [4-6,97-101]. Epigenetic mechanisms include DNA methylation, histone modifications, and regulation by non-coding RNA (ncRNA) [102,103]. Several studies evaluated the methylation status of genomic DNA from blood cells in CHD and ACS patients and in subjects with cardiovascular risk factors, observing a different methylation pattern, as well as a methylation signature predictive of increased risk of cardiac events, ischemic heart disease, stroke, and mortality [104-108].
Furthermore altered specific DNA methylation has been reported in several promoters, particularly in genes related to inflammation [109,110], coagulation [111], hypertension [112], glucose [113,114] and lipid metabolism [115,116].
In addition, regulatory T (Treg) cells have also been shown to play a protective role in atherosclerosis avoiding plaque rupture. DNA demethylation of the transcription factor Forkhead box P3 (FOXP3) gene was found to be essential for the maintenance of the suppressive properties of this cellular subtype. Indeed, an increase of FOXP3 methylation was observed in cultures of PBMCs obtained from ACS patients compared to controls [117] (Table 3).
Table 3.
Epigenetic markers associated to CHD diagnosis and prognosis
| Biomarker | Source | Regulation | Clinical role | Ref. |
|---|---|---|---|---|
| Global DNA methylation | Lymphocytes | Hypermethylation in patients related to hyperhomocysteinemia | Diagnostic | [104] |
| LINE-1 | Leucocytes | Lower methylation in CHD patients | Diagnostic/Predictive of increased risk of acute events and mortality | [105] |
| Alu/Sat2 | Leucocytes | High methylation in CHD patients with cardiovascular risk factor | Diagnostic | [106] |
| Global DNA methylation | Leucocytes | Hypermethylated regions in patients with hyperhomocysteinemia | Diagnostic | [107] |
| Global DNA methylation | Leucocytes | Identification of 47 CpG islands associated with ACS | Diagnostic | [108] |
| PLA2G7 | Leucocytes | High promoter methylation in CHD patients | Diagnostic/Predictive of CHD risk gender and age specific | [109] |
| PTX3 | Leucocytes | Lower promoter methylation in CHD group | Diagnostic | [110] |
| Factor VII | PBMCs | Promoter hypomethylation in CHD patients | Diagnostic | [111] |
| HSD11B2 | PBMCs | Promoter methylation associated with hypertension | Diagnostic | [112] |
| INS/GNASAS | Leucocytes | Locus hypermethylation in AMI patients | Predictive of risk of AMI in women | [113] |
| GCK | Leucocytes | Hypomethylation in CHD patients compared to controls | Predictive of risk of CHD onset | [114] |
| ABCA1 | Leucocytes | Higher methylation in CHD patients associated with low HDL; association with aging and CHD in men | Diagnostic | [115,116] |
| FOXP3 | Regulatory T cells | Increased methylation in ACS patients | Diagnostic | [117] |
| Microarray | Plasma/Serum | Downregulation: miR-17-92 cluster, -126, -145, -155; Upregulation: miR-133, -208a associated with CHD severity | Diagnostic | [121-123] |
| Microarray | PBMCs | Upregulation of miR-134, -135a, -147, -198, -370 in UA compared to SA patients | Diagnostic/Predictive of acute events | [124] |
| Microarray | Plasma | Upregulation of miR-1, -126, and -483-5p in SA patients vs. controls; upregulation miR-1, -126, and -133a in UA patients vs. controls | Diagnostic | [125] |
| Microarray | Plasma | Overexpression of miR-106b/25 cluster, miR-17/92a cluster, miR-21/590-5p family, miR-126* and miR-451 in patients with vulnerable CHD | Diagnostic | [126] |
| mir-197/mir-223 | Serum | Elevated levels in CHD patients | Predictive of cardiovascular death | [127] |
| miR-31 | Serum | Higher levels in CHD patients with restenosis compared to patients without restenosis and in healthy controls | Diagnostic | [128] |
| miR-214 | Plasma | Circulating levels related to the severity of coronary stenosis | Diagnostic | [129,130] |
| Realtime PCR | Plasma | Upregulation of miR-122 and miR-370 in hyperlipidemic CHD patients; association of increased levels of miR-122 and miR-370 with disease severity | Diagnostic | [131] |
| Microarray | Plasma | Low levels of miR-145, miR-155 and let-7c levels in CHD patients compared to controls | Diagnostic | [132] |
| Realtime PCR | Plasma | High levels of miR-17-5p are associated with CHD severity | Diagnostic | [133] |
| Microarray | Platetets | Upregulation of miRNA340* and miRNA624 in patients with CHD | Diagnostic | [134] |
| Realtime PCR | Plasma/PBMCs | Downregulation of miR-155 and miR-146a/b during ACEI/ARB treatment | Predictive markers of CHD risk and treatment efficacy | [135,136] |
| EPCs | PBMCs | Upregulation of miR-221, miR-222 and miR-92a; increased EPC number and decreased miR-221/222 levels after atorvastatin therapy | Predictive markers of treatment efficacy | [137,138] |
Abbreviations: ACEI = Angiotensin converting-enzyme inhibitor; ARB = Angiotensin II receptor blocker; CHD = Coronary heart disease; PBMC = Peripheral blood mononuclear cells; AMI = acute myocardial infarction; SA = stable angina; UA = unstable angina; EPC = endothelial progenitor cell.
MicroRNAs (MiRNAs) are short noncoding single-strand RNAs with the function of inhibiting gene expression through mRNA degradation or translational repression [118]. MiRNAs are stable in several bodily fluids, so several studies focused on the identification of these molecules in plasma specimens as well as in blood cells with the aim to correlate their expression with CHD [119,120].
Circulating levels of specific endothelial cell, smooth muscle cell and inflammation associated miRNAs, were significantly reduced in patients with stable CHD, while cardiac-specific miRNAs were upregulated [121-123].
Several studies also showed the important role of miRNA signature in vulnerable CHD, particularly for their ability to discriminate patients with UA from those with SA, suggesting that these circulating molecules could be useful to identify patients at risk for ACS and predict the clinical outcome [124-127].
Serum levels of miR-31 were higher in CHD patients with in stent restenosis compared to patients without stent restenosis and miR-214 downregulation was linked to disease severity [128-130]. In addition, other studies also showed that miRNA alterations are associated with disease severity [131,132].
Furthermore, miR-17-5p overexpression was reported to be an independent factor associated with the severity of atherosclerosis [133].
Platelets play an important role, both during plaque rupture and in the formation of atherosclerotic plaque. Indeed, miRNA expression profiles of platelets from patients with premature CHD revealed an upregulation of miR-340* and miR-624* in patients compared to healthy controls [134].
The pattern of miRNA expression is also influenced by therapeutic treatments, suggesting important implications for patient management. In whole blood samples of CHD patients and controls, 11 miRNAs were significantly down-regulated in CHD group. Particularly, miR-155, which is known to target the AT1 receptor, was found to be associated with ACEI/ARB use [135]. Furthermore, miR-146a/b were higher in PBMCs of CHD patients but under angiotensin II receptor blocker inhibitors and statin treatments, their expression levels decreased [136]. Interestingly, also lipid lowering therapy with statins is able to influence miRNA expression in CHD [137,138] (Table 3).
Transcriptional markers
Genome-wide gene expression profiling is a promising strategy for the identification of novel disease biomarkers [139]. Several studies on gene expression profiling of blood cells identified a differential transcriptional signature in CHD patients and healthy subjects. Particularly, the major alterations were discovered in genes codifying for pro- and antioxidant molecules, cell motility proteins, signaling receptors, transcription factors, inflammatory molecules and mediators. Interestingly, the expression pattern was found to correlate with the severity of CHD and gene expression in vascular tissues, indicating a mirroring between circulating cells and changes in the atherosclerotic vessel wall [140-146]. Transcriptional deregulations were also found in genes involved in DNA repair, as reported in a recent study by Ahmadi el al. [147].
Gene expression changes may reflect not only the presence and activity of disease but also environmental modifier effects, as well as treatment response. Indeed, in a study by Taurino et al. 365 differentially expressed genes were found in patients with CHD vs. healthy controls (175 genes were upregulated, and 190 genes were downregulated). Furthermore, in a group of patients was analyzed whole-blood gene expression before and after a cardiac rehabilitation following surgical coronary revascularization. In patients underwent rehabilitation, were found 645 genes differentially expressed at the beginning and the end of the program, with 196 genes upregulated and 449 genes downregulated. The expression levels of genes involved in oxidative phosphorylation and mitochondrial dysfunction were higher in CHD patients compared to control subjects. Completion of the rehabilitation treatment was characterized by a downregulation of the genic signature of oxidative stress and mitochondrial impairment [148]. Furthermore, a whole genome expression analysis identified six genes with a differential expression in monocytes of patients versus controls; ABCA1, ABCG1, and RGS1 were down-regulated in patients, whereas ADRB2 and FOLR3 were upregulated compared to matched controls and this expression pattern was influenced by aspirin and statin therapy [149] (Table 4).
Table 4.
Transcriptional markers associated to CHD diagnosis and prognosis
| Biomarker | Source | Regulation | Clinical role | Ref. |
|---|---|---|---|---|
| Microrray | PBMCs | Upregulation of cytokine and EGR family genes; EGR1 levels able to discriminate ischemic and non-ischemic CHD patients | Diagnostic | [140] |
| Whole-genome microarray | PBMCs | Differential expression of 14 genes related to coronary stenosis | Diagnostic | [141] |
| Array | PBMCs | 160 differently expressed genes in patients; expression signature correlated with the severity of CHD and gene expression in vascular tissues | Diagnostic | [142] |
| Myocardin/GATA4/Nkx2.5 | PBMCs | Higher transcriptional levels in patients in relation to disease severity | Diagnostic | [143] |
| Nrf2 | PBMCs | Lower gene expression in patients than control subjects | Diagnostic | [144] |
| Homer1/IL-1β/TNF-α | Leucocytes | Higher mRNA levels in CHD patients compared to controls | Diagnostic | [145] |
| MT-COI | Monocytes | Low mRNA levels in patients at risk | Predictive of CV events | [146] |
| MSH2/XRCC1/ATM | PBMCs | Upregulation in diabetic CHD patients | Diagnostic | [147] |
| Microarray | Leucocytes | Downregulation of COX7C, ATP5I NDUFA1 and CASP3 | Predictive markers after cardiac rehabilitation | [148] |
| Microarray | Monocytes | Downregulation of ABCA1, ABCG1 and RGS1, upregulation of ADRB2 and FOLR3 | Diagnostic | [149] |
| GES score | Whole blood | GES score is associated with plaque volume and phenotype by IVUS and atherosclerotic plaque burden and stenosis by CTCA | Diagnostic | [152,153] |
Abbreviations: EGR = Early growth response; CHD = Coronary heart disease; PBMCs = Peripheral blood mononuclear cell.
Integration of biomarkers and direct imaging features in CHD
While several studies have tested circulating biomarkers in imaging-detected CHD (only recently by CTCA), some authors have tried to correlate imaging features to serum/plasma biomarkers, in order to increase diagnostic power and risk stratification of patients with coronary alterations.
In a multicenter study a gene expression score (GES) based on age, sex and the expression levels of 23 genes in peripheral blood cells was developed to assess the likelihood of CHD in non diabetic patients [150]. A subsequent study showed that GES could be a more accurate predictor of obstructive CHD compared to clinical estimation scores [151].
In the Atlanta study, Joshi et al. correlated the validated GES score and coronary plaque composition by IVUS with radiofrequency backscatter analysis (IVUS/VH). GES was significantly associated with plaque volume, necrotic core composition, and dense calcium. Data suggested that this composite gene expression score is not only predictive of obstructive coronary artery disease, as previously reported, but also predictive of larger atherosclerotic plaque burden with a more vulnerable phenotype [152]. GES was also significantly associated with plaque burden and luminal stenosis assessed by CTCA. Indeed, in a study on 610 patients, Voros et al. reported a significant association between GES score, plaque burden expressed by coronary artery calcium and stenosis severity reported as score index. Particularly, GES significantly correlated with maximum luminal stenosis and segment stenosis score index. A low score had a sensitivity of 0.90 and a high score a specificity of 0.87 for stenosis ≥ 70% [153].
C-reactive protein (CRP) is the most extensively studied systemic marker of inflammation. A recent study by Seifarth et al. has shown that plasmatic levels of high-sensitivity C-reactive protein (hs-CRP) and high-sensitivity troponin T (hs-TnT) are weakly associated with a significant increase in CHD burden and change in plaque composition detected by CTCA using a semiquantitative crosssection-based score in a 2-year follow-up [154].
Several studies showed also the involvement of interleukins in plaque instability and their possible predictive value of cardiovascular events in long-term outcome [155-157]. Indeed, in a recent study Harada K. et al. evaluated the association between inflammatory markers and coronary artery plaque parameters assessed by CTCA on 220 subjects with suspected CHD showing that circulating levels of hs-CRP and IL-6 were significantly higher in CHD patients. Particularly, plasma IL-6 concentration was significantly associated with 4-9 segment plaques compared to patients without or with 1-3 segments. On the other hand, plasma hs-CRP level was associated with the presence of calcified plaque, independently of traditional cardiovascular risk factors [158].
MCP-1 plays a key role in the recruitment of monocytes to sites of inflammation, promoting the initiation of the fatty streak, plaque instability, as well as remodeling after MI. A positive correlation between circulating MCP-1 levels and the extent of coronary atherosclerosis, expressed by an angiographic severity score, was found in patients with UA. These findings suggest that circulating MCP-1 concentration likely reflects the coronary wall damage [53].
Adiponectin is a protein hormone playing a protective role of vascular walls from atherosclerosis. Several studies showed a significant inverse correlation between multivessel CHD and lipid-rich non-calcified plaques assessed by CTCA and serum levels of adiponectin independently by other significant risk factors [159-161]. Although bilirubin has long been considered a waste product, it is currently recognized as an endogenous antioxidant molecule, involved in the attenuation of lipid peroxidation and playing an anti-atherogenic role. In a study on 1151 patients the association between total serum bilirubin levels and presence, severity and plaque composition evaluated by CTCA was determined. Data showed that subjects with primarily non-calcified plaques and mixed plaques had lower bilirubin levels compared to patients with calcified plaques and normal subjects. Furthermore, serum total bilirubin levels were found to be lower in patients with any coronary plaque [162].
Conclusions and future perspectives
The need to improve diagnosis and risk prediction has prompted the search for novel markers in cardiovascular medicine. Literature data suggest that CTCA could substantially reduce the number of invasive procedures, increasing the safety of patients, and allows a more precise planning of potential treatment options. Furthermore, strong evidence has also emerged on the usefulness of coronary calcium score assessed by CTCA. In association with imaging improvements, novel high-throughput platforms investigating proteomic, metabolomic, epigenomic, and transcriptomics profiles together with genome-wide association studies may generate “multimarker CHD scores” with a higher predictive power than the use of a single biomarker. Surrogate biomarkers of coronary atherosclerosis and advanced imaging techniques could represent important cornerstones to characterize sub-clinical and clinical atherosclerosis with a consequent facilitation in the decision-making and clinical management of patients.
Acknowledgements
This work was supported by “Giovani Ricercatori 2011-12 Grant” (project code GR-2011-02349436) and “Ricerca Corrente 2013-2015” (project code RRC-2015-2360454) from Italian Ministry of Health.
Disclosure of conflict of interest
None.
References
- 1.Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. The epidemic of the 20(th) century: coronary heart disease. Am J Med. 2014;127:807–812. doi: 10.1016/j.amjmed.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Wong ND. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol. 2014;11:276–289. doi: 10.1038/nrcardio.2014.26. [DOI] [PubMed] [Google Scholar]
- 3.Napoli C, Lerman LO, de Nigris F, Gossl M, Balestrieri ML, Lerman A. Rethinking primary prevention of atherosclerosis-related diseases. Circulation. 2006;114:2517–2527. doi: 10.1161/CIRCULATIONAHA.105.570358. [DOI] [PubMed] [Google Scholar]
- 4.Napoli C, Crudele V, Soricelli A, Al-Omran M, Vitale N, Infante T, Mancini FP. Primary prevention of atherosclerosis: a clinical challenge for the reversal of epigenetic mechanisms? Circulation. 2012;125:2363–2373. doi: 10.1161/CIRCULATIONAHA.111.085787. [DOI] [PubMed] [Google Scholar]
- 5.Napoli C, Grimaldi V, De Pascale MR, Sommese L, Infante T, Soricelli A. Novel epigeneticbased therapies useful in cardiovascular medicine. World J Cardiol. 2016;8:211–219. doi: 10.4330/wjc.v8.i2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiano C, Vietri MT, Grimaldi V, Picascia A, De Pascale MR, Napoli C. Epigenetic-related therapeutic challenges in cardiovascular disease. Trends Pharmacol Sci. 2015;36:226–235. doi: 10.1016/j.tips.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935–2939. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 9.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA GRACE Investigators. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month post discharge death in an international registry. JAMA. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 10.Alnasser SM, Huang W, Gore JM, Steg PG, Eagle KA, Anderson FA Jr, Fox KA, Gurfinkel E, Brieger D, Klein W, van de Werf F, Avezum Á, Montalescot G, Gulba DC, Budaj A, Lopez-Sendon J, Granger CB, Kennelly BM, Goldberg RJ, Fleming E, Goodman SG GRACE Investigators. Late consequences of acute coronary syndromes: global registry of acute coronary events (GRACE) follow-up. Am J Med. 2015;128:766–775. doi: 10.1016/j.amjmed.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Versteylen MO, Joosen IA, Shaw LJ, Narula J, Hofstra L. Comparison of Framingham, PROCAM, SCORE, and diamond forrester to predict coronary atherosclerosis and cardiovascular events. J Nucl Cardiol. 2011;18:904–911. doi: 10.1007/s12350-011-9425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Backus BE, Six AJ, Kelder JC, Bosschaert MA, Mast EG, Mosterd A, Veldkamp RF, Wardeh AJ, Tio R, Braam R, Monnink SH, van Tooren R, Mast TP, van den Akker F, Cramer MJ, Poldervaart JM, Hoes AW, Doevendans PA. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168:2153–2158. doi: 10.1016/j.ijcard.2013.01.255. [DOI] [PubMed] [Google Scholar]
- 13.Sørgaard M, Linde JJ, Kofoed KF, Kühl JT, Kelbæk H, Nielsen WB, Hove JD. Diagnostic value of the updated diamond and forrester score to predict coronary artery disease in patients with acute-onset chest pain. Cardiology. 2016;133:10–17. doi: 10.1159/000438980. [DOI] [PubMed] [Google Scholar]
- 14.Schuijf JD, Shaw LJ, Wijns W, Lamb HJ, Poldermans D, de Roos A, van der Wall EE, Bax JJ. Cardiac imaging in coronary artery disease: differing modalities. Heart. 2005;91:1110–1117. doi: 10.1136/hrt.2005.061408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CP, Honye J, Saito S. New modality for evaluating plaque characteristics of the culprit lesion in a patient with acute coronary syndrome and no reflow phenomenon. Int Heart J. 2010;51:207–210. doi: 10.1536/ihj.51.207. [DOI] [PubMed] [Google Scholar]
- 16.Schoenhagen P, Nissen S. Understanding coronary artery disease: tomographic imaging with intravascular ultrasound. Heart. 2002;88:91–96. doi: 10.1136/heart.88.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuzcu EM, Kapadia SR, Tutar E, Ziada KM, Hobbs RE, McCarthy PM, Young JB, Nissen SE. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001;103:2705–2710. doi: 10.1161/01.cir.103.22.2705. [DOI] [PubMed] [Google Scholar]
- 18.Mintz GS, Painter JA, Pichard AD, Kent KM, Satler LF, Popma JJ, Chuang YC, Bucher TA, Sokolowicz LE, Leon MB. Atherosclerosis in angiographically “normal” coronary artery reference segments: an intravascular ultrasound study with clinical correlations. J Am Coll Cardiol. 1995;25:1479–1485. doi: 10.1016/0735-1097(95)00088-l. [DOI] [PubMed] [Google Scholar]
- 19.Tuzcu EM, Hobbs RE, Rincon G, Bott-Silverman C, De Franco AC, Robinson K, McCarthy PM, Stewart RW, Guyer S, Nissen SE. Occult and frequent transmission of atherosclerotic coronary disease with cardiac transplantation: insights from intravascular ultrasound. Circulation. 1995;91:1706–1713. doi: 10.1161/01.cir.91.6.1706. [DOI] [PubMed] [Google Scholar]
- 20.Gorenoi V, Schönermark MP, Hagen A. CT coronary angiography vs. invasive coronary angiography in CHD. GMS Health Technol Assess. 2012;8:Doc02. doi: 10.3205/hta000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forte E, Aiello M, Inglese M, Infante T, Soricelli A, Tedeschi C, Salvatore M, Cavaliere C. Coronary artery aneurysms detected by computed tomography coronary angiography. Eur Heart J Cardiovasc Imaging. 2016 doi: 10.1093/ehjci/jew218. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Forte E, Inglese M, Infante T, Schiano C, Napoli C, Soricelli A, Salvatore M, Tedeschi C. Anomalous left main coronary artery detected by CT angiography. Surg Radiol Anat. 2016;38:987–990. doi: 10.1007/s00276-016-1634-9. [DOI] [PubMed] [Google Scholar]
- 23.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 24.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography); Society of Atherosclerosis Imaging and Prevention; Society of Cardiovascular Computed Tomography. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American college of cardiology foundation clinical expert consensus task force (ACCF/AHA writing committee to update the 2000 expert consensus document on electron beam computed tomography) developed in collaboration with the society of atherosclerosis imaging and prevention and the society of cardiovascular computed tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Marwan M, Ropers D, Pflederer T, Daniel WG, Achenbach S. Clinical characteristics of patients with obstructive coronary lesions in the absence of coronary calcification: an evaluation by coronary CT angiography. Heart. 2009;95:1056–1060. doi: 10.1136/hrt.2008.153353. [DOI] [PubMed] [Google Scholar]
- 26.Task Force Members; Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ ESC Committee for Practice Guidelines; Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S Document Reviewers. Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European society of cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 27.Achenbach S, Ropers D, Hoffmann U, MacNeill B, Baum U, Pohle K, Brady TJ, Pomerantsev E, Ludwig J, Flachskampf FA, Wicky S, Jang IK, Daniel WG. Assessment of coronary remodeling in stenotic and nonstenotic coronary atherosclerotic lesions by multidetector spiral computed tomography. J Am Coll Cardiol. 2004;43:842–847. doi: 10.1016/j.jacc.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 28.SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383–2391. doi: 10.1016/S0140-6736(15)60291-4. [DOI] [PubMed] [Google Scholar]
- 29.Danad I, Fayad ZA, Willemink MJ, Min JK. New applications of cardiac computed tomography: dual-energy, spectral, and molecular CT imaging. JACC Cardiovasc Imaging. 2015;8:710–723. doi: 10.1016/j.jcmg.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douglas PS, Pontone G, Hlatky MA, Patel MR, Norgaard BL, Byrne RA, Curzen N, Purcell I, Gutberlet M, Rioufol G, Hink U, Schuchlenz HW, Feuchtner G, Gilard M, Andreini D, Jensen JM, Hadamitzky M, Chiswell K, Cyr D, Wilk A, Wang F, Rogers C, De Bruyne B PLATFORM Investigators. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFR(CT): outcome and resource impacts study. Eur Heart J. 2015;36:3359–3367. doi: 10.1093/eurheartj/ehv444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doesch C, Papavassiliu T. Diagnosis and management of ischemic cardiomyopathy: role of cardiovascular magnetic resonance imaging. World J Cardiol. 2014;6:1166–1174. doi: 10.4330/wjc.v6.i11.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saeed M, Van TA, Krug R, Hetts SW, Wilson MW. Cardiac MR imaging: current status and future direction. Cardiovasc Diagn Ther. 2015;5:290–310. doi: 10.3978/j.issn.2223-3652.2015.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machac J. Cardiac positron emission tomography imaging. Semin Nucl Med. 2005;35:17–36. doi: 10.1053/j.semnuclmed.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Berman DS, Hachamovitch R, Shaw LJ, Friedman JD, Hayes SW, Thomson LE, Fieno DS, Germano G, Wong ND, Kang X, Rozanski A. Roles of nuclear cardiology, cardiac computed tomography, and cardiac magnetic resonance: noninvasive risk stratification and a conceptual framework for the selection of noninvasive imaging tests in patients with known or suspected coronary artery disease. J Nucl Med. 2006;47:1107–1118. [PubMed] [Google Scholar]
- 35.Ghosh N, Rimoldi OE, Beanlands RS, Camici PG. Assessment of myocardial ischaemia and viability: role of positron emission tomography. Eur Heart J. 2010;31:2984–2995. doi: 10.1093/eurheartj/ehq361. [DOI] [PubMed] [Google Scholar]
- 36.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, Harrell FE Jr, Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand SL, O’Donnell CJ, Smith SC Jr, Wilson PW American Heart Association Expert Panel on Subclinical Atherosclerotic Diseases and Emerging Risk Factors and the Stroke Council. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162:55–63. doi: 10.7326/M14-0697. [DOI] [PubMed] [Google Scholar]
- 38.Poitou C, Dalmas E, Renovato M, Benhamo V, Hajduch F, Abdennour M, Kahn JF, Veyrie N, Rizkalla S, Fridman WH, Sautès-Fridman C, Clément K, Cremer I. CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:2322–2330. doi: 10.1161/ATVBAHA.111.230979. [DOI] [PubMed] [Google Scholar]
- 39.Hristov M, Leyendecker T, Schuhmann C, von Hundelshausen P, Heussen N, Kehmeier E, Krötz F, Sohn HY, Klauss V, Weber C. Circulating monocyte subsets and cardiovascular risk factors in coronary artery disease. Thromb Haemost. 2010;104:412–414. doi: 10.1160/TH10-01-0069. [DOI] [PubMed] [Google Scholar]
- 40.Shantsila E, Tapp LD, Wrigley BJ, Pamukcu B, Apostolakis S, Montoro-García S, Lip GY. Monocyte subsets in coronary artery disease and their associations with markers of inflammation and fibrinolysis. Atherosclerosis. 2014;234:4–10. doi: 10.1016/j.atherosclerosis.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Téo FH, de Oliveira RT, Mamoni RL, Ferreira MC, Nadruz W Jr, Coelho OR, Fernandes J de L, Blotta MH. Characterization of CD4+CD28null T cells in patients with coronary artery disease and individuals with risk factors for atherosclerosis. Cell Immunol. 2013;281:11–19. doi: 10.1016/j.cellimm.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Kakizaki M, Nobori K, Watanabe H, Iino K, Ishida M, Ito H. Increased circulating CD3+/CD31+ T cells in patients with acute coronary syndrome. Heart Vessels. 2013;28:566–569. doi: 10.1007/s00380-012-0284-z. [DOI] [PubMed] [Google Scholar]
- 43.Arbel Y, Finkelstein A, Halkin A, Birati EY, Revivo M, Zuzut M, Shevach A, Berliner S, Herz I, Keren G, Banai S. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis. 2012;225:456–460. doi: 10.1016/j.atherosclerosis.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson L, Wieringa WG, Pundziute G, Gjerde M, Engvall J, Swahn E, Jonasson L. Neutrophil/Lymphocyte ratio is associated with non-calcified plaque burden in patients with coronary artery disease. PLoS One. 2014;9:e108183. doi: 10.1371/journal.pone.0108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoefer IE, Sels JW, Jukema JW, Bergheanu S, Biessen E, McClellan E, Daemen M, Doevendans P, de Groot P, Hillaert M, Horsman S, Ilhan M, Kuiper J, Pijls N, Redekop K, van der Spek P, Stubbs A, van de Veer E, Waltenberger J, van Zonneveld AJ, Pasterkamp G. Circulating cells as predictors of secondary manifestations of cardiovascular disease: design of the CIRCULATING CELLS study. Clin Res Cardiol. 2013;102:847–856. doi: 10.1007/s00392-013-0607-9. [DOI] [PubMed] [Google Scholar]
- 46.Berezin AE, Kremzer AA. Circulating endothelial progenitor cells as markers for severity of ischemic chronic heart failure. J Card Fail. 2014;20:438–447. doi: 10.1016/j.cardfail.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Padfield GJ, Tura-Ceide O, Freyer E, Barclay GR, Turner M, Newby DE, Mills NL. Endothelial progenitor cells, atheroma burden and clinical outcome in patients with coronary artery disease. Heart. 2013;99:791–798. doi: 10.1136/heartjnl-2012-302949. [DOI] [PubMed] [Google Scholar]
- 48.Otto S, Nitsche K, Jung C, Kryvanos A, Zhylka A, Heitkamp K, Gutiérrez-Chico JL, Goebel B, Schulze PC, Figulla HR, Poerner TC. Endothelial progenitor cells and plaque burden in stented coronary artery segments: an optical coherence tomography study six months after elective PCI. BMC Cardiovasc Disord. 2017;17:103. doi: 10.1186/s12872-017-0534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim MH, Guo L, Kim HS, Kim SW. Characteristics of circulating CD31(+) cells from patients with coronary artery disease. J Cell Mol Med. 2014;18:2321–2330. doi: 10.1111/jcmm.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen C, Lei W, Chen W, Zhong J, Gao X, Li B, Wang H, Huang C. Serum TGF-β1 and SMAD3 levels are closely associated with coronary artery disease. BMC Cardiovasc Disord. 2014;14:18. doi: 10.1186/1471-2261-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tretjakovs P, Jurka A, Bormane I, Mikelsone I, Elksne K, Krievina G, Reihmane D, Verbovenko J, Bahs G. Circulating adhesion molecules, matrix metalloproteinase-9, plasminogen activator inhibitor-1, and myeloperoxidase in coronary artery disease patients with stable and unstable angina. Clin Chim Acta. 2012;413:25–29. doi: 10.1016/j.cca.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Hulok A, Sciborski K, Marczak J, Bańkowski T, Poręba R, Negrusz-Kawecka M. Soluble cell adhesion molecules-does estimating sVCAM-1 and sICAM-1 concentration provide additional information about cardiovascular risk in patients with coronary artery disease? Adv Clin Exp Med. 2014;23:735–741. doi: 10.17219/acem/37232. [DOI] [PubMed] [Google Scholar]
- 53.Serrano-Martínez M, Palacios M, Lezaun R. Monocyte chemoattractant protein-1 concentration in coronary sinus blood and severity of coronary disease. Circulation. 2003;108:e75. doi: 10.1161/01.CIR.0000089100.20182.B7. [DOI] [PubMed] [Google Scholar]
- 54.de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP, Braunwald E. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690–695. doi: 10.1161/01.cir.0000049742.68848.99. [DOI] [PubMed] [Google Scholar]
- 55.Tajfard M, Latiff LA, Rahimi HR, Moohebati M, Hasanzadeh M, Emrani AS, Esmaeily H, Taghipour A, Mirhafez SR, Ferns GA, Mardan-Nik M, Mohammadzadeh E, Avan A, Hanachi P, Ghayour-Mobarhan M. Serum concentrations of MCP-1 and IL-6 in combination predict the presence of coronary artery disease and mortality in subjects undergoing coronary angiography. Mol Cell Biochem. 2017 doi: 10.1007/s11010-017-3054-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 56.Stellos K, Ruf M, Sopova K, Kilias A, Rahmann A, Stamatelopoulos K, Jorbenadze R, Geisler T, Gawaz M, Bigalke B. Plasma levels of stromal cell-derived factor-1 in patients with coronary artery disease: effect of clinical presentation and cardiovascular risk factors. Atherosclerosis. 2011;219:913–916. doi: 10.1016/j.atherosclerosis.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 57.Ghasemzadeh N, Hritani AW, De Staercke C, Eapen DJ, Veledar E, Al Kassem H, Khayata M, Zafari AM, Sperling L, Hooper C, Vaccarino V, Mavromatis K, Quyyumi AA. Plasma stromal cell-derived factor 1α/CXCL12 level predicts long-term adverse cardiovascular outcomes in patients with coronary artery disease. Atherosclerosis. 2015;238:113–118. doi: 10.1016/j.atherosclerosis.2014.10.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tavakolian Ferdousie V, Mohammadi M, Hassanshahi G, Khorramdelazad H, Khanamani Falahati-Pour S, Mirzaei M, Allah Tavakoli M, Kamiab Z, Ahmadi Z, Vazirinejad R, Shahrabadi E, Koniari I, Kounis NG, Esmaeili Nadimi A. Serum CXCL10 and CXCL12 chemokine levels are associated with the severity of coronary artery disease and coronary artery occlusion. Int J Cardiol. 2017;233:23–28. doi: 10.1016/j.ijcard.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 59.Lubrano V, Del Turco S, Nicolini G, Di Cecco P, Basta G. Circulating levels of lectin-like oxidized low-density lipoprotein receptor-1 are associated with inflammatory markers. Lipids. 2008;43:945–950. doi: 10.1007/s11745-008-3227-9. [DOI] [PubMed] [Google Scholar]
- 60.Inoue N, Okamura T, Kokubo Y, Fujita Y, Sato Y, Nakanishi M, Yanagida K, Kakino A, Iwamoto S, Watanabe M, Ogura S, Otsui K, Matsuda H, Uchida K, Yoshimoto R, Sawamura T. LOX index, a novel predictive biochemical marker for coronary heart disease and stroke. Clin Chem. 2010;56:550–558. doi: 10.1373/clinchem.2009.140707. [DOI] [PubMed] [Google Scholar]
- 61.Balın M, Celik A, Kobat MA. Circulating soluble lectin-like oxidized low density lipoprotein receptor-1 levels are associated with proximal/middle segment of the LAD lesions in patients with stable coronary artery disease. Clin Res Cardiol. 2012;101:247–253. doi: 10.1007/s00392-011-0386-0. [DOI] [PubMed] [Google Scholar]
- 62.Hudzik B, Danikiewicz A, Szkodzinski J, Polonski L, Zubelewicz-Szkodzinska B. Pentraxin-3 concentrations in stable coronary artery disease depend on the clinical presentation. Eur Cytokine Netw. 2014;25:41–45. doi: 10.1684/ecn.2014.0354. [DOI] [PubMed] [Google Scholar]
- 63.Soeki T, Niki T, Kusunose K, Bando S, Hirata Y, Tomita N, Yamaguchi K, Koshiba K, Yagi S, Taketani Y, Iwase T, Yamada H, Wakatsuki T, Akaike M, Sata M. Elevated concentrations of pentraxin 3 are associated with coronary plaque vulnerability. J Cardiol. 2011;58:151–157. doi: 10.1016/j.jjcc.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 64.Tazaki R, Tanigawa J, Fujisaka T, Shibata K, Takeda Y, Ishihara T, Hoshiga M, Hanafusa T, Ishizaka N. Plasma pentraxin3 level is associated with plaque vulnerability assessed by optical coherence tomography in patients with coronary artery disease. Int Heart J. 2016;57:18–24. doi: 10.1536/ihj.15-248. [DOI] [PubMed] [Google Scholar]
- 65.Song YS, Koo BK, Cho NH, Moon MK. Effect of low serum total bilirubin levels (≤0.32 mg/dl) on risk of coronary artery disease in patients with metabolic syndrome. Am J Cardiol. 2014;114:1695–1700. doi: 10.1016/j.amjcard.2014.08.043. [DOI] [PubMed] [Google Scholar]
- 66.Leem J, Koh EH, Jang JE, Woo CY, Oh JS, Lee MJ, Kang JW, Lim TH, Jung CH, Lee WJ, Park JY, Lee KU. Serum total bilirubin levels provide additive risk information over the framingham risk score for identifying asymptomatic diabetic patients at higher risk for coronary artery stenosis. Diabetes Metab J. 2015;39:414–423. doi: 10.4093/dmj.2015.39.5.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang FY, Peng Y, Huang BT, Yang Y, Pu XB, Chen SJ, Gui YY, Xia TL, Chen F, Liu RS, Zhu Y, Chen M. The correlation between serum total bilirubin and outcomes in patients with different subtypes of coronary artery disease. Clin Chim Acta. 2017;465:101–105. doi: 10.1016/j.cca.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 68.Garg N, Moorthy N, Kapoor A, Tewari S, Kumar S, Sinha A, Shrivastava A, Goel PK. Hemoglobin A(1c) in nondiabetic patients: an independent predictor of coronary artery disease and its severity. Mayo Clin Proc. 2014;89:908–916. doi: 10.1016/j.mayocp.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 69.Scicali R, Giral P, Gallo A, Di Pino A, Rabuazzo AM, Purrello F, Cluzel P, Redheuil A, Bruckert E, Rosenbaum D. HbA1c increase is associated with higher coronary and peripheral atherosclerotic burden in non diabetic patients. Atherosclerosis. 2016;255:102–108. doi: 10.1016/j.atherosclerosis.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 70.Filep JG, El Kebir D. Serum amyloid A as a marker and mediator of acute coronary syndromes. Future Cardiol. 2008;4:495–504. doi: 10.2217/14796678.4.5.495. [DOI] [PubMed] [Google Scholar]
- 71.Kosuge M, Ebina T, Ishikawa T, Hibi K, Tsukahara K, Okuda J, Iwahashi N, Ozaki H, Yano H, Kusama I, Nakati T, Umemura S, Kimura K. Serum amyloid A is a better predictor of clinical outcomes than C-reactive protein in non-STsegment elevation acute coronary syndromes. Circ J. 2007;71:186–190. doi: 10.1253/circj.71.186. [DOI] [PubMed] [Google Scholar]
- 72.Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, Butterworth AS, Amouyel P, Assmann G, Bakker SJ, Barr EL, Barrett-Connor E, Benjamin EJ, Björkelund C, Brenner H, Brunner E, Clarke R, Cooper JA, Cremer P, Cushman M, Dagenais GR, D’Agostino RB Sr, Dankner R, Davey-Smith G, Deeg D, Dekker JM, Engström G, Folsom AR, Fowkes FG, Gallacher J, Gaziano JM, Giampaoli S, Gillum RF, Hofman A, Howard BV, Ingelsson E, Iso H, Jørgensen T, Kiechl S, Kitamura A, Kiyohara Y, Koenig W, Kromhout D, Kuller LH, Lawlor DA, Meade TW, Nissinen A, Nordestgaard BG, Onat A, Panagiotakos DB, Psaty BM, Rodriguez B, Rosengren A, Salomaa V, Kauhanen J, Salonen JT, Shaffer JA, Shea S, Ford I, Stehouwer CD, Strandberg TE, Tipping RW, Tosetto A, Wassertheil-Smoller S, Wennberg P, Westendorp RG, Whincup PH, Wilhelmsen L, Woodward M, Lowe GD, Wareham NJ, Khaw KT, Sattar N, Packard CJ, Gudnason V, Ridker PM, Pepys MB, Thompson SG, Danesh J. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buljubasic N, Akkerhuis KM, Cheng JM, Oemrawsingh RM, Garcia-Garcia HM, de Boer SP, Regar E, van Geuns RM, Serruys PW, Boersma E, Kardys I. Fibrinogen in relation to degree and composition of coronary plaque on intravascular ultrasound in patients undergoing coronary angiography. Coron Artery Dis. 2017;28:23–32. doi: 10.1097/MCA.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 74.Schaub N, Reichlin T, Meune C, Twerenbold R, Haaf P, Hochholzer W, Niederhauser N, Bosshard P, Stelzig C, Freese M, Reiter M, Gea J, Buser A, Mebazaa A, Osswald S, Mueller C. Markers of plaque instability in the early diagnosis and risk stratification of acute myocardial infarction. Clin Chem. 2012;58:246–256. doi: 10.1373/clinchem.2011.172940. [DOI] [PubMed] [Google Scholar]
- 75.Yunoki K, Naruko T, Inaba M, Inoue T, Nakagawa M, Sugioka K, Ohsawa M, Iwasa Y, Komatsu R, Itoh A, Haze K, Yoshiyama M, Becker AE, Ueda M. Gender-specific correlation between plasma myeloperoxidase levels and serum high-density lipoprotein-associated paraoxonase-1 levels in patients with stable and unstable coronary artery disease. Atherosclerosis. 2013;231:308–314. doi: 10.1016/j.atherosclerosis.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 76.Baseri M, Heidari R, Mahaki B, Hajizadeh Y, Momenizadeh A, Sadeghi M. Myeloperoxidase levels predict angiographic severity of coronary artery disease in patients with chronic stable angina. Adv Biomed Res. 2014;3:139. doi: 10.4103/2277-9175.135155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hasanpour Z, Javanmard SH, Gharaaty M, Sadeghi M. Association between serum myeloperoxidase levels and coronary artery disease in patients without diabetes, hypertension, obesity, and hyperlipidemia. Adv Biomed Res. 2016;5:103. doi: 10.4103/2277-9175.183663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun T, Hu J, Yin Z, Xu Z, Zhang L, Fan L, Zhuo Y, Wang C. Low serum paraoxonase1 activity levels predict coronary artery disease severity. Oncotarget. 2017;8:19443–19454. doi: 10.18632/oncotarget.14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hou ZH, Lu B, Gao Y, Cao HL, Yu FF, Jing N, Chen X, Cong XF, Roy SK, Budoff MJ. Matrix metalloproteinase-9 (MMP-9) and myeloperoxidase (MPO) levels in patients with nonobstructive coronary artery disease detected by coronary computed tomographic angiography. Acad Radiol. 2013;20:25–31. doi: 10.1016/j.acra.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 80.Momiyama Y, Ohmori R, Tanaka N, Kato R, Taniguchi H, Adachi T, Nakamura H, Ohsuzu F. High plasma levels of matrix metalloproteinase-8 in patients with unstable angina. Atherosclerosis. 2010;209:206–210. doi: 10.1016/j.atherosclerosis.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 81.Li S, Zhang Y, Xu RX, Guo YL, Zhu CG, Wu NQ, Qing P, Liu G, Dong Q, Li JJ. Proprotein convertase subtilisin-kexin type 9 as a biomarker for the severity of coronary artery disease. Ann Med. 2015;47:386–393. doi: 10.3109/07853890.2015.1042908. [DOI] [PubMed] [Google Scholar]
- 82.Zhao X, Zhang HW, Li S, Zhang Y, Xu RX, Zhu CG, Wu NQ, Guo YL, Qing P, Li XL, Liu G, Dong Q, Sun J, Li JJ. Association between plasma proprotein convertase subtisilin/kexin type 9 concentration and coronary artery calcification. Ann Clin Biochem. 2017:4563217695351. doi: 10.1177/0004563217695351. [DOI] [PubMed] [Google Scholar]
- 83.Werner C, Hoffmann MM, Winkler K, Bohm M, Laufs U. Risk prediction with proprotein convertase subtilisin/kexin type 9 (PCSK9) in patients with stable coronary disease on statin treatment. Vascul Pharmacol. 2014;62:94–102. doi: 10.1016/j.vph.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 84.Cai A, Li G, Chen J, Li X, Li L, Zhou Y. Increased serum level of Lp-PLA2 is independently associated with the severity of coronary artery diseases: a cross-sectional study of Chinese population. BMC Cardiovasc Disord. 2015;15:14. doi: 10.1186/s12872-015-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lp-PLA(2) Studies Collaboration. Thompson A, Gao P, Orfei L, Watson S, Di Angelantonio E, Kaptoge S, Ballantyne C, Cannon CP, Criqui M, Cushman M, Hofman A, Packard C, Thompson SG, Collins R, Danesh J. Lipoproteinassociated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–1544. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lambadiari V, Kadoglou NP, Stasinos V, Maratou E, Antoniadis A, Kolokathis F, Parissis J, Hatziagelaki E, Iliodromitis EK, Dimitriadis G. Serum levels of retinol-binding protein-4 are associated with the presence and severity of coronary artery disease. Cardiovasc Diabetol. 2014;13:121. doi: 10.1186/s12933-014-0121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y, Wang D, Chen H, Xia M. Circulating retinol binding protein 4 is associated with coronary lesion severity of patients with coronary artery disease. Atherosclerosis. 2015;238:45–51. doi: 10.1016/j.atherosclerosis.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 88.Muendlein A, Saely CH, Leiherer A, Fraunberger P, Kinz E, Rein P, Vonbank A, Zanolin D, Malin C, Drexel H. Angiopoietin-like protein 4 significantly predicts future cardiovascular events in coronary patients. Atherosclerosis. 2014;237:632–638. doi: 10.1016/j.atherosclerosis.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 89.Yeboah J, McNamara C, Jiang XC, Tabas I, Herrington DM, Burke GL, Shea S. Association of plasma sphingomyelin levels and incident coronary heart disease events in an adult population: multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:628–633. doi: 10.1161/ATVBAHA.109.199281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nelson JC, Jiang XC, Tabas I, Tall A, Shea S. Plasma sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;163:903–912. doi: 10.1093/aje/kwj140. [DOI] [PubMed] [Google Scholar]
- 91.Othman A, Saely CH, Muendlein A, Vonbank A, Drexel H, von Eckardstein A, Hornemann T. Plasma C20-Sphingolipids predict cardiovascular events independently from conventional cardiovascular risk factors in patients undergoing coronary angiography. Atherosclerosis. 2015;240:216–221. doi: 10.1016/j.atherosclerosis.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 92.Onda T, Inoue K, Suwa S, Nishizaki Y, Kasai T, Kimura Y, Fukuda K, Okai I, Fujiwara Y, Matsuoka J, Sumiyoshi M, Daida H. Reevaluation of cardiac risk scores and multiple biomarkers for the prediction of first major cardiovascular events and death in the drug-eluting stent era. Int J Cardiol. 2016;219:180–185. doi: 10.1016/j.ijcard.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 93.Pereira EC, Bertolami MC, Faludi AA, Monte O, Xavier HT, Pereira TV, Abdalla DS. Predictive potential of twenty-two biochemical biomarkers for coronary artery disease in type 2 diabetes mellitus. Int J Endocrinol. 2015;2015:146816. doi: 10.1155/2015/146816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kleber ME, Goliasch G, Grammer TB, Pilz S, Tomaschitz A, Silbernagel G, Maurer G, März W, Niessner A. Evolving biomarkers improve prediction of long-term mortality in patients with stable coronary artery disease: the BIOVILCAD score. J Intern Med. 2014;276:184–194. doi: 10.1111/joim.12189. [DOI] [PubMed] [Google Scholar]
- 95.Sponder M, Fritzer-Szekeres M, Marculescu R, Litschauer B, Strametz-Juranek J. A new coronary artery disease grading system correlates with numerous routine parameters that were associated with atherosclerosis: a grading system for coronary artery disease severity. Vasc Health Risk Manag. 2014;10:641–647. doi: 10.2147/VHRM.S68919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eapen DJ, Manocha P, Patel RS, Hammadah M, Veledar E, Wassel C, Nanjundappa RA, Sikora S, Malayter D, Wilson PW, Sperling L, Quyyumi AA, Epstein SE. Aggregate risk score based on markers of inflammation, cell stress, and coagulation is an independent predictor of adverse cardiovascular outcomes. J Am Coll Cardiol. 2013;62:329–337. doi: 10.1016/j.jacc.2013.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Picascia A, Grimaldi V, Iannone C, Soricelli A, Napoli C. Innate and adaptive immune response in stroke: focus on epigenetic regulation. J Neuroimmunol. 2015;289:111–120. doi: 10.1016/j.jneuroim.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 98.Sommese L, Zullo A, Mancini FP, Fabbricini R, Soricelli A, Napoli C. Clinical relevance of epigenetics in the onset and management of type 2 diabetes mellitus. Epigenetics. 2017;12:401–415. doi: 10.1080/15592294.2016.1278097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grimaldi V, De Pascale MR, Zullo A, Soricelli A, Infante T, Mancini FP, Napoli C. Evidence of epigenetic tags in cardiac fibrosis. J Cardiol. 2017;69:401–408. doi: 10.1016/j.jjcc.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 100.Schleithoff C, Voelter-Mahlknecht S, Dahmke IN, Mahlknecht U. On the epigenetics of vascular regulation and disease. Clin Epigenetics. 2012;4:7. doi: 10.1186/1868-7083-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grimaldi V, Vietri MT, Schiano C, Picascia A, De Pascale MR, Fiorito C, Casamassimi A, Napoli C. Epigenetic reprogramming in atherosclerosis. Curr Atheroscler Rep. 2015;17:476. doi: 10.1007/s11883-014-0476-3. [DOI] [PubMed] [Google Scholar]
- 102.Udali S, Guarini P, Moruzzi S, Choi SW, Friso S. Cardiovascular epigenetics: from DNA methylation to microRNAs. Mol Aspects Med. 2013;34:883–901. doi: 10.1016/j.mam.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 103.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 104.Sharma P, Kumar J, Garg G, Kumar A, Patowary A, Karthikeyan G, Ramakrishnan L, Brahmachari V, Sengupta S. Detection of altered global DNA methylation in coronary artery disease patients. DNA Cell Biol. 2008;27:357–365. doi: 10.1089/dna.2007.0694. [DOI] [PubMed] [Google Scholar]
- 105.Baccarelli A, Wright R, Bollati V, Litonjua A, Zanobetti A, Tarantini L, Sparrow D, Vokonas P, Schwartz J. Ischemic heart disease and stroke in relation to blood DNA methylation. Epidemiology. 2010;21:819–828. doi: 10.1097/EDE.0b013e3181f20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim M, Long TI, Arakawa K, Wang R, Yu MC, Laird PW. DNA methylation as a biomarker for cardiovascular disease risk. PLoS One. 2010;5:e9692. doi: 10.1371/journal.pone.0009692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sharma P, Garg G, Kumar A, Mohammad F, Kumar SR, Tanwar VS, Sati S, Sharma A, Karthikeyan G, Brahmachari V, Sengupta S. Genome wide DNA methylation profiling for epigenetic alteration in coronary artery disease patients. Gene. 2014;541:31–40. doi: 10.1016/j.gene.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 108.Li J, Zhu X, Yu K, Jiang H, Zhang Y, Deng S, Cheng L, Liu X, Zhong J, Zhang X, He M, Chen W, Yuan J, Gao M, Bai Y, Han X, Liu B, Luo X, Mei W, He X, Sun S, Zhang L, Zeng H, Sun H, Liu C, Guo Y, Zhang B, Zhang Z, Huang J, Pan A, Yuan Y, Angileri F, Ming B, Zheng F, Zeng Q, Mao X, Peng Y, Mao Y, He P, Wang QK, Qi L, Hu FB, Liang L, Wu T. Genome-wide analysis of DNA methylation and acute coronary syndrome. Circ Res. 2017;120:1754–1767. doi: 10.1161/CIRCRESAHA.116.310324. [DOI] [PubMed] [Google Scholar]
- 109.Jiang D, Zheng D, Wang L, Huang Y, Liu H, Xu L, Liao Q, Liu P, Shi X, Wang Z, Sun L, Zhou Q, Li N, Xu L, Le Y, Ye M, Shao G, Duan S. Elevated PLA2G7 gene promoter methylation as a gender-specific marker of aging increases the risk of coronary heart disease in females. PLoS One. 2013;8:e59752. doi: 10.1371/journal.pone.0059752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guo TM, Huang LL, Liu K, Ke L, Luo ZJ, Li YQ, Chen XL, Cheng B. Pentraxin 3 (PTX3) promoter methylation associated with PTX3 plasma levels and neutrophil to lymphocyte ratio in coronary artery disease. J Geriatr Cardiol. 2016;13:712–717. doi: 10.11909/j.issn.1671-5411.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Friso S, Lotto V, Choi SW, Girelli D, Pinotti M, Guarini P, Udali S, Pattini P, Pizzolo F, Martinelli N, Corrocher R, Bernardi F, Olivieri O. Promoter methylation in coagulation F7 gene influences plasma FVII concentrations and relates to coronary artery disease. J Med Genet. 2012;49:192–199. doi: 10.1136/jmedgenet-2011-100195. [DOI] [PubMed] [Google Scholar]
- 112.Friso S, Pizzolo F, Choi SW, Guarini P, Castagna A, Ravagnani V, Carletto A, Pattini P, Corrocher R, Olivieri O. Epigenetic control of 11 betahydroxysteroid dehydrogenase 2 gene promoter is related to human hypertension. Atherosclerosis. 2008;199:323–327. doi: 10.1016/j.atherosclerosis.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 113.Talens RP, Jukema JW, Trompet S, Kremer D, Westendorp RG, Lumey LH, Sattar N, Putter H, Slagboom PE, Heijmans BT PROSPER Group. Hypermethylation at loci sensitive to the prenatal environment is associated with increased incidence of myocardial infarction. Int J Epidemiol. 2012;41:106–115. doi: 10.1093/ije/dyr153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu L, Zheng D, Wang L, Jiang D, Liu H, Xu L, Liao Q, Zhang L, Liu P, Shi X, Wang Z, Sun L, Zhou Q, Li N, Huang Y, Le Y, Ye M, Shao G, Duan S. GCK gene-body hypomethylation is associated with the risk of coronary heart disease. Biomed Res Int. 2014;2014:151723. doi: 10.1155/2014/151723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guay SP, Brisson D, Munger J, Lamarche B, Gaudet D, Bouchard L. ABCA1 gene promoter DNA methylation is associated with HDL particle profile and coronary artery disease in familial hypercholesterolemia. Epigenetics. 2012;7:464–472. doi: 10.4161/epi.19633. [DOI] [PubMed] [Google Scholar]
- 116.Guay SP, Légaré C, Houde AA, Mathieu P, Bossé Y, Bouchard L. Acetylsalicylic acid, aging and coronary artery disease are associated with ABCA1 DNA methylation in men. Clin Epigenetics. 2014;6:14. doi: 10.1186/1868-7083-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jia L, Zhu L, Wang JZ, Wang XJ, Chen JZ, Song L, Wu YJ, Sun K, Yuan ZY, Hui R. Methylation of FOXP3 in regulatory T cells is related to the severity of coronary artery disease. Atherosclerosis. 2013;228:346–352. doi: 10.1016/j.atherosclerosis.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 118.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Economou EK, Oikonomou E, Siasos G, Papageorgiou N, Tsalamandris S, Mourouzis K, Papaioanou S, Tousoulis D. The role of microRNAs in coronary artery disease: from pathophysiology to diagnosis and treatment. Atherosclerosis. 2015;241:624–633. doi: 10.1016/j.atherosclerosis.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 120.Bronze-da-Rocha E. MicroRNAs expression profiles in cardiovascular diseases. Biomed Res Int. 2014;2014:985408. doi: 10.1155/2014/985408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T, Müller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 122.Li HY, Zhao X, Liu YZ, Meng Z, Wang D, Yang F, Shi QW. Plasma MicroRNA-126-5p is associated with the complexity and severity of coronary artery disease in patients with stable angina pectoris. Cell Physiol Biochem. 2016;39:837–46. doi: 10.1159/000447794. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Y, Li HH, Yang R, Yang BJ, Gao ZY. Association between circulating microRNA-208a and severity of coronary heart disease. Scand J Clin Lab Invest. 2017:1–6. doi: 10.1080/00365513.2017.1328740. [DOI] [PubMed] [Google Scholar]
- 124.Hoekstra M, van der Lans CA, Halvorsen B, Gullestad L, Kuiper J, Aukrust P, van Berkel TJ, Biessen EA. The peripheral blood mononuclear cell microRNA signature of coronary artery disease. Biochem Biophys Res Commun. 2010;394:792–797. doi: 10.1016/j.bbrc.2010.03.075. [DOI] [PubMed] [Google Scholar]
- 125.D’Alessandra Y, Carena MC, Spazzafumo L, Martinelli F, Bassetti B, Devanna P, Rubino M, Marenzi G, Colombo GI, Achilli F, Maggiolini S, Capogrossi MC, Pompilio G. Diagnostic potential of plasmatic MicroRNA signatures in stable and unstable angina. PLoS One. 2013;8:e80345. doi: 10.1371/journal.pone.0080345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ren J, Zhang J, Xu N, Han G, Geng Q, Song J, Li S, Zhao J, Chen H. Signature of circulating microRNAs as potential biomarkers in vulnerable coronary artery disease. PLoS One. 2013;8:e80738. doi: 10.1371/journal.pone.0080738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schulte C, Molz S, Appelbaum S, Karakas M, Ojeda F, Lau DM, Hartmann T, Lackner KJ, Westermann D, Schnabel RB, Blankenberg S, Zeller T. miRNA-197 and miRNA-223 predict cardiovascular death in a cohort of patients with symptomatic coronary artery disease. PLoS One. 2015;10:e0145930. doi: 10.1371/journal.pone.0145930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang J, Yan CH, Li Y, Xu K, Tian XX, Peng CF, Tao J, Sun MY, Han YL. MicroRNA-31 controls phenotypic modulation of human vascular smooth muscle cells by regulating its target gene cellular repressor of E1A-stimulated genes. Exp Cell Res. 2013;319:1165–1175. doi: 10.1016/j.yexcr.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 129.Lu HQ, Liang C, He ZQ, Fan M, Wu ZG. Circulating miR-214 is associated with the severity of coronary artery disease. J Geriatr Cardiol. 2013;10:34–38. doi: 10.3969/j.issn.1671-5411.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jin Y, Yang CJ, Xu X, Cao JN, Feng QT, Yang J. MiR-214 regulates the pathogenesis of patients with coronary artery disease by targeting VEGF. Mol Cell Biochem. 2015;402:111–122. doi: 10.1007/s11010-014-2319-5. [DOI] [PubMed] [Google Scholar]
- 131.Gao W, He HW, Wang ZM, Zhao H, Lian XQ, Wang YS, Zhu J, Yan JJ, Zhang DG, Yang ZJ, Wang LS. Plasma levels of lipometabolism-related miR-122 and miR-370 are increased in patients with hyperlipidemia and associated with coronary artery disease. Lipids Health Dis. 2012;11:55. doi: 10.1186/1476-511X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Faccini J, Ruidavets JB, Cordelier P, Martins F, Maoret JJ, Bongard V, Ferrières J, Roncalli J, Elbaz M, Vindis C. Circulating miR-155, miR-145 and let-7c as diagnostic biomarkers of the coronary artery disease. Sci Rep. 2017;7:42916. doi: 10.1038/srep42916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen J, Xu L, Hu Q, Yang S, Zhang B, Jiang H. MiR-17-5p as circulating biomarkers for the severity of coronary atherosclerosis in coronary artery disease. Int J Cardiol. 2015;197:123–124. doi: 10.1016/j.ijcard.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 134.Sondermeijer BM, Bakker A, Halliani A, de Ronde MW, Marquart AA, Tijsen AJ, Mulders TA, Kok MG, Battjes S, Maiwald S, Sivapalaratnam S, Trip MD, Moerland PD, Meijers JC, Creemers EE, Pinto-Sietsma SJ. Platelets in patients with premature coronary artery disease exhibit upregulation of miRNA340* and miRNA624*. PLoS One. 2011;6:e25946. doi: 10.1371/journal.pone.0025946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weber M, Baker MB, Patel RS, Quyyumi AA, Bao G, Searles CD. MicroRNA expression profile in CAD patients and the impact of ACEI/ARB. Cardiol Res Pract. 2011;2011:532915. doi: 10.4061/2011/532915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takahashi Y, Satoh M, Minami Y, Tabuchi T, Itoh T, Nakamura M. Expression of miR-146a/b is associated with the toll-like receptor 4 signal in coronary artery disease: effect of renin-angiotensin system blockade and statins on miRNA-146a/b and Toll-like receptor 4 levels. Clin Sci (Lond) 2010;119:395–405. doi: 10.1042/CS20100003. [DOI] [PubMed] [Google Scholar]
- 137.Zhang Q, Kandic I, Kutryk MJ. Dysregulation of angiogenesis-related microRNAs in endothelial progenitor cells from patients with coronary artery disease. Biochem Biophys Res Commun. 2011;405:42–46. doi: 10.1016/j.bbrc.2010.12.119. [DOI] [PubMed] [Google Scholar]
- 138.Minami Y, Satoh M, Maesawa C, Takahashi Y, Tabuchi T, Itoh T, Nakamura M. Effect of atorvastatin on microRNA 221/222 expression in endothelial progenitor cells obtained from patients with coronary artery disease. Eur J Clin Invest. 2009;39:359–367. doi: 10.1111/j.1365-2362.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- 139.Siemelink MA, Zeller T. Biomarkers of coronary artery disease: the promise of the transcriptome. Curr Cardiol Rep. 2014;16:513. doi: 10.1007/s11886-014-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cappuzzello C, Napolitano M, Arcelli D, Melillo G, Melchionna R, Di Vito L, Carlini D, Silvestri L, Brugaletta S, Liuzzo G, Crea F, Capogrossi MC. Gene expression profiles in peripheral blood mononuclear cells of chronic heart failure patients. Physiol Genomics. 2009;38:233–240. doi: 10.1152/physiolgenomics.90364.2008. [DOI] [PubMed] [Google Scholar]
- 141.Wingrove JA, Daniels SE, Sehnert AJ, Tingley W, Elashoff MR, Rosenberg S, Buellesfeld L, Grube E, Newby LK, Ginsburg GS, Kraus WE. Correlation of peripheral-blood gene expression with the extent of coronary artery stenosis. Circ Cardiovasc Genet. 2008;1:31–38. doi: 10.1161/CIRCGENETICS.108.782730. [DOI] [PubMed] [Google Scholar]
- 142.Sinnaeve PR, Donahue MP, Grass P, Seo D, Vonderscher J, Chibout SD, Kraus WE, Sketch M Jr, Nelson C, Ginsburg GS, Goldschmidt-Clermont PJ, Granger CB. Gene expression patterns in peripheral blood correlate with the extent of coronary artery disease. PLoS One. 2009;4:e7037. doi: 10.1371/journal.pone.0007037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kontaraki JE, Kochiadakis GE, Marketou ME, Chlouverakis G, Igoumenidis NE, Saloustros IG, Vardas PE. Early cardiac gene transcript levels in peripheral blood mononuclear cells reflect severity in stable coronary artery disease. Hellenic J Cardiol. 2014;55:119–125. [PubMed] [Google Scholar]
- 144.Mozzini C, Fratta Pasini A, Garbin U, Stranieri C, Pasini A, Vallerio P, Cominacini L. Increased endoplasmic reticulum stress and Nrf2 repression in peripheral blood mononuclear cells of patients with stable coronary artery disease. Free Radic Biol Med. 2014;68:178–185. doi: 10.1016/j.freeradbiomed.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 145.Jing X, Chen SS, Jing W, Tan Q, Yu MX, Tu JC. Diagnostic potential of differentially expressed Homer1, IL-1β, and TNF-α in coronary artery disease. Int J Mol Sci. 2014;16:535–546. doi: 10.3390/ijms16010535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Holvoet P, Vanhaverbeke M, Bloch K, Baatsen P, Sinnaeve P, Janssens S. Low MT-CO1 in monocytes and microvesicles is associated with outcome in patients with coronary artery Disease. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.004207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ahmadi A, Behmanesh M, Boroumand MA, Tavallaei M. Up-regulation of MSH2, XRCC1 and ATM genes in patients with type 2 diabetes and coronary artery disease. Diabetes Res Clin Pract. 2015;109:500–506. doi: 10.1016/j.diabres.2015.05.049. [DOI] [PubMed] [Google Scholar]
- 148.Taurino C, Miller WH, McBride MW, McClure JD, Khanin R, Moreno MU, Dymott JA, Delles C, Dominiczak AF. Gene expression profiling in whole blood of patients with coronary artery disease. Clin Sci (Lond) 2010;119:335–343. doi: 10.1042/CS20100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sivapalaratnam S, Basart H, Watkins NA, Maiwald S, Rendon A, Krishnan U, Sondermeijer BM, Creemers EE, Pinto-Sietsma SJ, Hovingh K, Ouwehand WH, Kastelein JJ, Goodall AH, Trip MD. Monocyte gene expression signature of patients with early onset coronary artery disease. PLoS One. 2012;7:e32166. doi: 10.1371/journal.pone.0032166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thomas GS, Voros S, McPherson JA, Lansky AJ, Winn ME, Bateman TM, Elashoff MR, Lieu HD, Johnson AM, Daniels SE, Ladapo JA, Phelps CE, Douglas PS, Rosenberg S. A bloodbased gene expression test for obstructive coronary artery disease tested in symptomatic nondiabetic patients referred for myocardial perfusion imaging the COMPASS study. Circ Cardiovasc Genet. 2013;6:154–162. doi: 10.1161/CIRCGENETICS.112.964015. [DOI] [PubMed] [Google Scholar]
- 151.Rosenberg S, Elashoff MR, Lieu HD, Brown BO, Kraus WE, Schwartz RS, Voros S, Ellis SG, Waksman R, McPherson JA, Lansky AJ, Topol EJ PREDICT Investigators. Whole blood gene expression testing for coronary artery disease in nondiabetic patients: major adverse cardiovascular events and interventions in the PREDICT trial. J Cardiovasc Transl Res. 2012;5:366–374. doi: 10.1007/s12265-012-9353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Joshi PH, Rinehart S, Vazquez G, Qian Z, Sharma A, Anderson H, Murrieta L, Flockhart N, Karmpaliotis D, Kalynych A, Asztalos B, Elashoff MR, Blanchard J, Rosenberg S, Brown C 3rd, Voros S. A peripheral blood gene expression score is associated with plaque volume and phenotype by intravascular ultrasound with radiofrequency backscatter analysis: results from the ATLANTA study. Cardiovasc Diagn Ther. 2013;3:5–14. doi: 10.3978/j.issn.2223-3652.2013.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Voros S, Elashoff MR, Wingrove JA, Budoff MJ, Thomas GS, Rosenberg S. A peripheral blood gene expression score is associated with atherosclerotic plaque burden and stenosis by cardiovascular CT-angiography: results from the PREDICT and COMPASS studies. Atherosclerosis. 2014;233:284–290. doi: 10.1016/j.atherosclerosis.2013.12.045. [DOI] [PubMed] [Google Scholar]
- 154.Seifarth H, Schlett CL, Lehman SJ, Bamberg F, Donnelly P, Januzzi JL, Koenig W, Truong QA, Hoffmann U. Correlation of concentrations of high-sensitivity troponin T and high-sensitivity C-reactive protein with plaque progression as measured by CT coronary angiography. J Cardiovasc Comput Tomogr. 2014;8:452–458. doi: 10.1016/j.jcct.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Heeschen C, Dimmeler S, Hamm CW, Fichtlscherer S, Boersma E, Simoons ML, Zeiher AM CAPTURE Study Investigators. Serum level of the antiinflammatory cytokine interleukin-10 is an important prognostic determinant in patients with acute coronary syndromes. Circulation. 2003;107:2109–2114. doi: 10.1161/01.CIR.0000065232.57371.25. [DOI] [PubMed] [Google Scholar]
- 156.Cavusoglu E, Marmur JD, Hojjati MR, Chopra V, Butala M, Subnani R, Huda MS, Yanamadala S, Ruwende C, Eng C, Pinsky DJ. Plasma interleukin-10 levels and adverse outcomes in acute coronary syndrome. Am J Med. 2011;124:724–730. doi: 10.1016/j.amjmed.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 157.Inoue T, Komoda H, Nonaka M, Kameda M, Uchida T, Node K. Interleukin-8 as an independent predictor of long-term clinical outcome in patients with coronary artery disease. Int J Cardiol. 2008;124:319–325. doi: 10.1016/j.ijcard.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 158.Harada K, Amano T, Uetani T, Yoshida T, Kato B, Kato M, Marui N, Kumagai S, Ando H, Ishii H, Matsubara T, Murohara T. Association of inflammatory markers with the morphology and extent of coronary plaque as evaluated by 64-slice multidetector computed tomography in patients with stable coronary artery disease. Int J Cardiovasc Imaging. 2013;29:1149–1158. doi: 10.1007/s10554-013-0181-2. [DOI] [PubMed] [Google Scholar]
- 159.Bamberg F, Truong QA, Koenig W, Schlett CL, Nasir K, Butler J, Kurtz E, Nikolaou K, Hoffmann U, Januzzi JL Jr. Differential associations between blood biomarkers of inflammation, oxidation, and lipid metabolism with varying forms of coronary atherosclerotic plaque as quantified by coronary CT angiography. Int J Cardiovasc Imaging. 2012;28:183–192. doi: 10.1007/s10554-010-9773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kunita E, Yamamoto H, Kitagawa T, Ohashi N, Utsunomiya H, Oka T, Horiguchi J, Awai K, Kihara Y. Association between plasma highmolecular-weight adiponectin and coronary plaque characteristics assessed by computed tomography angiography in conditions of visceral adipose accumulation. Circ J. 2012;76:1687–96. doi: 10.1253/circj.cj-11-1442. [DOI] [PubMed] [Google Scholar]
- 161.Matsuda M, Tamura R, Kishida N, Segawa T, Kanno K, Nishimoto O, Nakamoto K, Nishiyama H, Kawamoto T. Predictive value of adiponectin in patients with multivessel coronary atherosclerosis detected on computed tomography angiography. J Atheroscler Thromb. 2013;20:767–776. doi: 10.5551/jat.18036. [DOI] [PubMed] [Google Scholar]
- 162.Canpolat U, Aytemir K, Yorgun H, Hazırolan T, Kaya EB, Şahiner L, Sunman H, Tokgözoğlu L, Kabakcı G, Oto A. Association of serum total bilirubin levels with the severity, extent and subtypes of coronary atherosclerotic plaques detected by coronary CT angiography. Int J Cardiovasc Imaging. 2013;29:1371–1379. doi: 10.1007/s10554-013-0209-7. [DOI] [PubMed] [Google Scholar]


