Abstract
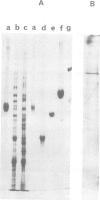
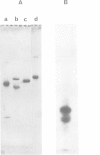
Assimilatory nitrate reductase has been purified with 55% recovery from a Neurospora crassa nmr-1 nit-6 mutant, using a modification of a published procedure. It possesses one heme per 240 000 g, and subunits of mol. wt. 68 000. Upon digestion with chymotrypsin, a heme-binding domain was isolated by gel filtration; its visible spectrum was highly similar to that of cytochrome b5. On SDS gels, the fraction showed two heme-containing bands of ˜10 000 and 12 5000 daltons; their amino acid composition was not very different, suggesting that they originated from the same region of the polypeptide chain. After S-carboxymethylation, the mixture of bands was submitted to cyanogen bromide cleavage, and the fragments were separated by h.p.l.c. The two largest fragments yielded an identical sequence upon automated degradation. This sequence (39 residues with some gaps) could be easily aligned with that of cytochrome b5 starting close to the N terminus. These results are discussed in terms of the possible quaternary structure of N. crassa nitrate reductase, whose heme-binding domain proves to be another member of the family of b5-like cytochromes.
Keywords: cytochrome b5, evolution, Neurospora crassa, nitrate reductase, structural domain
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Cohen H. J., Fridovich I. Hepatic sulfite oxidase. The nature and function of the heme prosthetic groups. J Biol Chem. 1971 Jan 25;246(2):367–373. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- De la Rosa M. A., Vega J. M., Zumft W. G. Composition and structure of assimilatory nitrate reductase from Ankistrodesmus braunii. J Biol Chem. 1981 Jun 10;256(11):5814–5819. [PubMed] [Google Scholar]
- Delepelaire P., Chua N. H. Lithium dodecyl sulfate/polyacrylamide gel electrophoresis of thylakoid membranes at 4 degrees C: Characterizations of two additional chlorophyll a-protein complexes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):111–115. doi: 10.1073/pnas.76.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas M., Finkelstein D., Butow R. A. Analysis of products of mitochondrial protein synthesis in yeast: genetic and biochemical aspects. Methods Enzymol. 1979;56:58–66. doi: 10.1016/0076-6879(79)56009-1. [DOI] [PubMed] [Google Scholar]
- Garrett R. H., Amy N. K. Nitrate assimilation in fungi. Adv Microb Physiol. 1978;18:1–65. doi: 10.1016/s0065-2911(08)60414-2. [DOI] [PubMed] [Google Scholar]
- Garrett R. H., Nason A. Further purification and properties of Neurospora nitrate reductase. J Biol Chem. 1969 Jun 10;244(11):2870–2882. [PubMed] [Google Scholar]
- Garrett R. H., Nason A. Involvement of a B-type cytochrome in the assimilatory nitrate reductase of Neurospora crassa. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1603–1610. doi: 10.1073/pnas.58.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais M., Tegoni M. Spontaneous dissociation of a cytochrome core and a biglobular flavoprotein after mild trypsinolysis of the bifunctional Saccharomyces cerevisiae flavocytochrome b2. Eur J Biochem. 1980 Oct;111(2):357–367. doi: 10.1111/j.1432-1033.1980.tb04949.x. [DOI] [PubMed] [Google Scholar]
- Giri L., Ramadoss C. S. Physical studies on assimilatory nitrate reductase from Chlorella vulgaris. J Biol Chem. 1979 Nov 25;254(22):11703–11712. [PubMed] [Google Scholar]
- Guerrero M. G., Gutierrez M. Purification and properties of the NAD(P)H:nitrate reductase of the yeast Rhodotorula glutinis. Biochim Biophys Acta. 1977 Jun 10;482(2):272–285. doi: 10.1016/0005-2744(77)90241-8. [DOI] [PubMed] [Google Scholar]
- Guiard B., Groudinsky O., Lederer F. Homology between bakers' yeast cytochrome b2 and liver microsomal cytochrome b5. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2539–2543. doi: 10.1073/pnas.71.6.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiard B., Lederer F. Amino acid sequence of the 'b5-like' heme-binding domain from chicken sulfite oxidase. Eur J Biochem. 1979 Oct 15;100(2):441–453. doi: 10.1111/j.1432-1033.1979.tb04187.x. [DOI] [PubMed] [Google Scholar]
- Guiard B., Lederer F., Jacq C. More similarity between bakers'yeast L-(+)-lactate dehydrogenase and liver microsomal cytochrome b5. Nature. 1975 May 29;255(5507):422–423. doi: 10.1038/255422a0. [DOI] [PubMed] [Google Scholar]
- Guiard B., Lederer F. The "b5-like" domain from chicken-liver sulfite oxidase: a new case of common ancestral origin with liver cytochrome b5 and bakers' yeast cytochrome b2 core. Eur J Biochem. 1977 Mar 15;74(1):181–190. doi: 10.1111/j.1432-1033.1977.tb11379.x. [DOI] [PubMed] [Google Scholar]
- Guiard B., Lederer F. The "cytochrome b5 fold": structure of a novel protein superfamily. J Mol Biol. 1979 Dec 15;135(3):639–650. doi: 10.1016/0022-2836(79)90169-4. [DOI] [PubMed] [Google Scholar]
- Howard W. D., Solomonson L. P. Quaternary structure of assimilatory NADH:nitrate reductase from Chlorella. J Biol Chem. 1982 Sep 10;257(17):10243–10250. [PubMed] [Google Scholar]
- Ito A. Hepatic sulfite oxidase identified as cytochrome b 5 -like pigment extractable from mitochondria by hypotonic treatment. J Biochem. 1971 Dec;70(6):1061–1064. doi: 10.1093/oxfordjournals.jbchem.a129716. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Hainline B. E., Rajagopalan K. V. Characterization of the molybdenum cofactor of sulfite oxidase, xanthine, oxidase, and nitrate reductase. Identification of a pteridine as a structural component. J Biol Chem. 1980 Mar 10;255(5):1783–1786. [PubMed] [Google Scholar]
- Johnson J. L., Rajagopalan K. V. Tryptic cleavage of rat liver sulfite oxidase. Isolation and characterization of molybdenum and heme domains. J Biol Chem. 1977 Mar 25;252(6):2017–2025. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lederer F., Ghrir R., Guiard B., Cortial S., Ito A. Two homologous cytochromes b5 in a single cell. Eur J Biochem. 1983 Apr 15;132(1):95–102. doi: 10.1111/j.1432-1033.1983.tb07330.x. [DOI] [PubMed] [Google Scholar]
- Mathews F. S., Levine M., Argos P. The structure of calf liver cytochrome b 5 at 2.8 A resolution. Nat New Biol. 1971 Sep 1;233(35):15–16. doi: 10.1038/newbio233015a0. [DOI] [PubMed] [Google Scholar]
- Mathews F. S., Levine M., Argos P. Three-dimensional Fourier synthesis of calf liver cytochrome b 5 at 2-8 A resolution. J Mol Biol. 1972 Mar 14;64(2):449–464. doi: 10.1016/0022-2836(72)90510-4. [DOI] [PubMed] [Google Scholar]
- Minagawa N., Yoshimoto A. Purification and characterization of the assimilatory NADPH-nitrate reductase of Aspergillus nidulans. J Biochem. 1982 Mar;91(3):761–774. doi: 10.1093/oxfordjournals.jbchem.a133763. [DOI] [PubMed] [Google Scholar]
- OZOLS J., STRITTMATTER P. THE INTERACTION OF PORPHYRINS AND METALLOPORHYRINS WITH APOCYTOCHROME BETA-5. J Biol Chem. 1964 Apr;239:1018–1023. [PubMed] [Google Scholar]
- Pajot P., Groudinsky O. Molecular weight and quaternary structure of yeast L-lactate dehydrogenase (cytochrome b2). 2. Revised heme extinction coefficients and minimal molecular weight. Eur J Biochem. 1970 Jan;12(1):158–164. doi: 10.1111/j.1432-1033.1970.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Pan S. S., Nason A. Purification and characterization of homogeneous assimilatory reduced nicotinamide adenine dinucleotide phosphate-nitrate reductase from Neurospora crassa. Biochim Biophys Acta. 1978 Apr 12;523(2):297–313. doi: 10.1016/0005-2744(78)90033-5. [DOI] [PubMed] [Google Scholar]
- Ryan L. D., Vestling C. S. Rapid purification of lactate dehydrogenase from rat liver and hepatoma: a new approach. Arch Biochem Biophys. 1974 Jan;160(1):279–284. doi: 10.1016/s0003-9861(74)80035-4. [DOI] [PubMed] [Google Scholar]
- Solomonson L. P. Purification of NADH-Nitrate Reductase by Affinity Chromatography. Plant Physiol. 1975 Dec;56(6):853–855. doi: 10.1104/pp.56.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner F. X., Downey R. J. Isoelectric focusing and two-dimensional analysis of purified nitrate reductase from Aspergillus nidulans. Biochim Biophys Acta. 1982 Sep 7;706(2):203–211. doi: 10.1016/0167-4838(82)90488-5. [DOI] [PubMed] [Google Scholar]
- Tomsett A. B., Dunn-Coleman N. S., Garrett R. H. The regulation of nitrate assimilation in Neurospora crassa: the isolation and genetic analysis of nmr-1 mutants. Mol Gen Genet. 1981;182(2):229–233. doi: 10.1007/BF00269662. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamaya T., Solomonson L. P., Oaks A. Action of Corn and Rice-inactivating Proteins on a Purified Nitrate Reductase from Chlorella vulgaris. Plant Physiol. 1980 Jan;65(1):146–150. doi: 10.1104/pp.65.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]