Abstract
Asthma is tightly related to the imbalance of Th1/Th2 cells, and Runx3 plays a pivotal role in the differentiation of T helper cells. The present study aimed to investigate dysregulated microRNAs that may target Runx3 in CD4+ T cells from asthmatic patients and reveal Runx3 function in Th1/Th2 balance regulation. We detected the levels of Th1- and Th2-related cytokines by ELISA and analyzed the differentiation marker gene of T helper cells by qRT-PCR. Results indicated that an imbalance of Th1/Th2 cells was present in our asthmatic subject. Runx3 expression was reduced in the CD4+ T cells from asthmatic patients. Overexpression of Runx3 could restore the Th1/Th2 balance. After performing microRNA microarray assay, we found a series of microRNAs that were considerably altered in the CD4+ T cells from asthmatic patients. Among these upregulated microRNAs, eight microRNAs that may target Runx3 were selected by bioinformatics prediction. Five microRNAs, namely miR-371, miR-138, miR-544, miR-145, and miR-214, were confirmed by qRT-PCR and selected as candidate microRNAs. Luciferase reporter assay showed that these five microRNAs could directly target the 3’-UTR of Runx3. However, only simultaneous inhibition of these five microRNAs could alter the expression of Runx3. Most importantly, only simultaneous inhibition could improve the Th1/Th2 balance. Thus, we suggest that miR-371, miR-138, miR-544, miR-145, and miR-214 can modulate the Th1/Th2 balance in asthma by regulating Runx3 in a combinatorial manner.
Keywords: Asthma, microRNA, Runx3, regulatory T cells
Introduction
Asthma is a common chronic immunoallergic disease characterized by reversible airway obstruction, airway hyper-responsiveness, mucus hypersecretion, and underlying airway inflammation [1,2]. The pathophysiologic mechanisms involved in the development of the allergy are intricate; in brief, inflammation has a prominent role in the pathophysiology of asthma [3,4]. Two major factors, host factors (for example, sex, genetics, innate immunity, and so on) and environmental exposures, may initiate the inflammatory process. Among host factors, there is increasingly more evidence showing that innate and adaptive immune responses are associated with both the development and regulation of inflammation in asthma [5,6]. In particular, the imbalance in T helper cell responses has been focused on in recent years. Thelper cells fall into two major group: Th1 and Th2. The cytokine profiles of Th1 cells includes IL-2 and interferon-γ (IFN-γ), which are involved in cellular defence mechanisms in response to infection [7]. Th2 cells produce a group of cytokines, namely IL-4, -5, -6, -9, -10, and -13, that mediate allergic inflammation [8]. Normally, Th1 cells tend to predominate in healthy subjects. However, Th1/Th2 imbalance is observed in asthma patients. Overactive Th2 response and overexpression of Th2 cytokines (accompanied with Th1 underexpression) causes a cascade of immune-activating events that result in increased IgE production, growth and differentiation of acidophilic granulocytes, and increased mucus secretion, thus inducing airway inflammation [9-11]. In Wang’s research, they found that salidroside treatment could reduce inflammation and edema in the airway of ovalbumin-induced asthma mice model by decreasing the IL-4 level and increasing the INF-γ level [12]. Huang and colleagues reported that astragaloside IV attenuated allergic inflammation in ovalbumin-induced asthma by regulating IFN-γ/IL-4 levels [13]. These studies demonstrate that regulation of Th1/Th2 might be a useful and novel therapeutic strategy for asthma.
Runx3 is one of the three mammalian Runt-domain transcription factors and encoded by the highly conserved and structurally similar Runx gene family. It is generally distributed in the peripheral blood and immune system such as in the bone marrow, spleen, thymus, spinal cord cells, B cells, and T cells [14,15]. Imbalances in Runx3 protein levels or functions are associated with various human diseases. Interestingly, Runx3 has crucial functions in T cell development in the thymus, and its involvement in Thelper cell differentiation was recently reported [16]. Runx3 has the dual action of enhancing IFN-γ and repressing IL-4 through cooperation with T-bet and plays an important role in the differentiation of T cells, affecting the balance of Th1/Th2 [17]. In addition, deletion of Runx3 in T cells resulted in the spontaneous development of asthma-like features (elevated IgE, IgA, and IgG1 with infiltration of lymphocytes and eosinophils in the lung tissue) in mice [18]. Because of its regulatory effect on the balance of Th1/Th2, we assumed that Runx3 could be a potential and useful target in the therapy of asthma.
MicroRNAs are short non-coding RNAs of 20-23 nucleotides that regulate target gene expression in a post-transcriptional manner [19]. In brief, microRNAs can bind to the 3’-untranslated region (3’-UTR) of target mRNAs, leading to their translational inhibition or degradation. Multiple studies have shown that microRNAs are subtle master controllers of gene expression and therefore play a crucial role in the pathogenesis of human diseases, especially in chronic multifactorial diseases such as asthma [20,21]. Bettina Levänen’s study demonstrated that 24 altered microRNAs including members of the let-7 and microRNA-200 families might be important in the inflammatory response leading to bronchial hyperresponsiveness in patients with asthma [22]. Lu’s group found that miR-22 is a master regulator of antigen presenting cell activation and thereby controls asthma and emphysema pathogenesis. Remarkably, the effects of microRNAs are cell and dose dependent for both the microRNAs and their target mRNAs [23]. In addition, the interaction between various microRNAs has been proven to target gene networks and influence disease outcomes. Thus, the functional role of multiple microRNAs in cooperating groups should be taken into account [24]. However, most of the previous studies only focused on individual microRNAs in asthma. In the present study, we hypothesized that multiple microRNAs might be dysregulated in asthmatic patients and involved in Th1/Th2 balance. We conducted an experiment to validate our hypothesis and explored the potential mechanism involved in Runx3 regulation.
Methods
Patients and ethics statement
Thirty asthmatic patients with a range of severity, including 20 males and 10 females with age of 37±7 years, were recruited from the Nanjing Drum Tower Hospital. Patients were diagnosed with asthma according to the history and lung function testing results (FEV1 reversible by greater than 12% and greater than 200 mL after bronchodilator or airway hyperresponsiveness caused by methacholine). These asthmatic patients were clinically stable and not undergoing an exacerbation. All patients are taking asthma medications regularly. 25 healthy subjects (14 males and 11 females, the age of 40±6 years) were selected as health control from a routine physical examination program in Nanjing Drum Tower Hospital. Nonsubject has a smoking history. All participants provided written informed consent. Ethical approval was given by the Medical Research Ethics Committee of Nanjing Drum Tower Hospital. Subjects’ characteristics are presented in Table 1.
Table 1.
Demographics of the subject
| Asthmatic patients (n=30) | Healthy controls (n=25) | P value | |
|---|---|---|---|
| Age (Mean ± SD, year) | 37±7 | 40±6 | >0.05 |
| Gender (Male/Female) | 20/10 | 14/11 | >0.05 |
| Duration of asthma (Mean ± SD, year) | 6±2 | N/A | |
| % predicted FEV1 (Mean ± SD) | 79.2±10.3 | 113.2±10.3 | <0.05 |
| BMI (kg/m2) | 28.5±7.6 | 24.4±7.1 | >0.05 |
| Serum IgE (kU/L) | 123.5±21.4 | 30.5±8.4 | <0.05 |
CD4+ T cells collection and culture
Venous peripheral blood samples collected from patients and healthy subjects were preserved with heparin. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation over Ficoll-Hypaque solution (Pharmacia, Uppsala, Sweden). Isolated PBMCs were washed twice with HEPES-buffered RPMI medium and resuspended in RPMI medium. And then PBMCs were incubated with anti-CD4+ monoclonal antibody-coated magnetic beads, the CD4+ T cells were isolated using a positive selection magnetic isolation system according to the manufacturer’s instruction. The purity of CD4+ T cells was accessed by flow cytometry. In our condition, the purity of CD4+ T cells could achieve more than 90%. Isolated CD4+ T cells were routinely maintained in human T cell culture medium (OpTmizerTM CTSTM T-Cell Expansion SFM, Gibco, Rockville, MD, USA).
Enzyme-linked immunosorbent assay (ELISA) for cytokines
The culture supernatant of CD4+ T cells was collected. The concentration of IL-4, IL-10, IFN-γ and IL-2 were measured by ELISA kits (R&D, Minneapolis, MN, USA). According to the manufacturer’s protocols, The OD value at 450 nm wavelength was detected using a BioTekMicroplate Reader System (BioTek, Winooski, VT, USA).
MicroRNA microarrays
To assessing the microRNA expression profiling in the CD4+ T cells from asthmatic patients and healthy controls, microRNA microarray assay was performed by a service provider (OBBiotech Co., China) using TaqManLow Density Arrays A and B cards set V2.0 (Applied Biosystems USA). TotalRNA samples were collected using TRIzol reagent (Thermo Fisher Scientific, USA). RNA samples were quality controlled by measuring the optical density at 260 and 280 nm. Megaplex RT reactions and pre-amplification reactions were run according to the manufacturer’s instruction. The pre-amplified product was loaded and the MicroRNA assay was performed. Relative microRNAs expression was calculated using the 2-ΔΔCT method. Small RNA U6 was used as areference gene. The data of microRNA microarray was analysed by the same service provider using Genespring GX version 11.0.1 (Agilent Technologies Inc, USA).
Quantitative real-time PCR (qRT-PCR)
Total RNA samples of CD4+ cells from asthmatic patients and healthy controls were extracted using TRIzol reagent (Thermo Fisher Scientific Inc., USA) in accordance with the manufacturer’s protocol. For validating the results of microRNA microarray assay and detecting the microRNA level change, cDNA was synthesised by a miScript II RT Kit (QIAGEN, USA) following the manufacturer’s instructions. qRT-PCR was performed using a miScript SYBR® Green PCR Kit (QIAGEN, USA) in an ABI7500 real-time PCR system (Applied Biosystems, USA). SNORD-48 was served as a reference gene. For detecting Runx3, t-bet and GATA-3 level changes, cDNA was synthesised by a PrimeScript® RT reagent Kit (Takara, China) and followed with amplified using SYBR® Premix Ex TaqTM II (Takara, China), GAPDH was used as reference gene. The level changes were calculated by 2-ΔΔCT method.
Bioinformatics analysis
To obtain the candidate microRNAs list which could be further researched in the present study, we used using TargetScan (http://www.targetscan.org/), Pictar (http://pictar.mdc-berlin.de/) and miRanda (http://www.microrna.org/microrna) to predict the candidate microRNAs which may target on Runx3 mRNA. Moreover, starBase (http://starbase.sysu.edu.cn/) was used to determine target-microRNA interaction in the database mentioned above.
Luciferase reporter assay
For confirmation the prediction results of bioinformatics analysis, a Luciferase reporter assay was performed using Promega Luciferase Assay System (Promega, USA). The wildtype (Wt) 3’-UTR sequence of RUXN3 containing candidate microRNAs (miR-371, miR-138, miR-544, miR-145 and miR-214) binding sites were amplified from human genomic DNA and cloned into PsiCHECK2 luciferase reporter vector, respectively (Promega, USA). Meanwhile, mutant (Mut) constructs were generated by mutating the seed region of the candidate microRNAs binding sites. Considering the potential challenging in transfection of primary T cells, we used a human T lymphoblast cell line (MOLT-4, purchased from American Type Culture Collection, USA) in Luciferase Assay instead of CD4+ T cells from subjects. MOLT-4 were seeded in 24-well plates and cultured in RPMI-1640 medium (Thermo Fisher Scientific, USA) with 10% fetal bovine serum (Thermo Fisher Scientific, USA) for 48 h. psiCHECK2-RUXN3-WT or psiCHECK2-RUXN3-Mut and different microRNA mimics were co-transfected into cells using Lipofectamine 2000 (Invitrogen, USA). The fluorescence signals were detected 48 h after transfection according to the manufacturer’s instruction, and relative fluorescence value (Luciferase/Renilla) was calculated.
Transfection
To knock-down the level miR-371, miR-138, miR-544, miR-145 and miR-214, relevant microRNA inhibitors and negative control were transfected in CD4+ T cells using Lipofectamine® RNAiMAX Reagent (Thermo Fisher Scientific, USA). The inhibitors were designed and provided by Shanghai GenePharmaCo, Ltd. To silencing the Runx3 expression, siRNA (GenePharmaCo, Ltd., China) and negative control (All star) were transfected in CD4+ T cells using Lipofectamine® RNAiMAX Reagent (Thermo Fisher Scientific, USA). For over-expression Runx3 in CD4+ T cells, an expression vector named pcDNA3.1-Runx3 was constructed and transfected into CD4+ T cells using Lipofectamine2000 (Thermo Fisher Scientific, USA). pcDNA3.1 empty plasmid was used as negative control. The transfection effect was confirmed after 48 h by qRT-PCR and western blot.
Western blotting
CD4+ T Cells were harvested and lysed in a RIPA lysis buffer (Beyotime, China). The protein samples’ concentrations were measured using a BCA kit (Beyotime, China). 50 μg protein sample was loaded in each lane and separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After transferred, NC Membranes were blocked with 5% non-fat milk, incubated with primary antibodies: Runx3 and GAPDH rabbit anti-human antibodies (Santa Cruz Biotechnology, USA). After washed with TBS-T buffer threetimes the membranes were incubated with the goat anti-rabbit secondary antibodies for 12 h at 4°C. The protein bands were visualised using an enhanced chemiluminescence reagent (Beyotime, China) and the intensity was quantified using Image J software 1.48 (National Institutes of Health, USA).
Flow cytometry
CD4+ T cells were collected and incubated with ionomycin, phorbolester and monensin for four h at 37°C.
To identify the Th1 and Th2 cells, specific fluorescein-labeled antibodies were used. The antibodies were FITC-labeled anti-CD4, FITC-labeled anti-CD4 and PE-labeled anti-IFN-γ (BD, San Diego, USA). The Flow cytometry was performed in a Guava easyCyteTM Cytometer system (Merck Millipore, Germany). The data was analysed by the software provided by Cytometer system. Th1 cells were identified as CD4+ IFN-γ+, and Th2 cells were identified as CD4+ IL4+.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 software (IBM, USA). The date was shown as Mean ± standard deviation (SD). To compared the difference between two groups or multi-groups, t-test and one-way ANOVA were performed, respectively. For correlation assay, Spearman’s correlation assay was used. A difference was considered significant at P<0.05.
Results
Th1/Th2 imbalance was observed in asthmatic patients
To investigate whether Th1/Th2 balance was dysregulated in asthmatic patients, we evaluated the level of Th1-related cytokines (IFN-γ, IL-2) and Th2-related cytokines (IL-4, IL-10) in the CD4+ T cells from patients and healthy controls by ELISA. As shown in Figure 1A, the levels of IL-2 and IFN-γ decreased, while the levels of IL-4 and IL-10 significantly increased in asthmatic patients, compared to the levels in healthy controls (P<0.05). We measured the expression levels of Th1-related transcription factor T-box expressed in T cells (T-bet) and Th2-related transcription factor GATA-binding protein 3 (GATA3), which also reflect the balance of Th1/Th2. The qRT-PCR results (Figure 1B) showed that the mRNA level of T-bet was lower in asthmatic patients, while that of GATA3 was higher in healthy controls (P<0.05). Moreover, the percentages of IFN-γ+ CD4+ T cells and IL-4+ CD4+ T cells were detected by flow cytometry (Figure 1C). As expected, a decreased percentage of IFN-γ+ CD4+ T cells and increased percentage of IL-4+ CD4+ T cells was observed in asthmatic patients, compared to that in healthy controls (P<0.05). This evidence suggested that there was a Th1/Th2 imbalance (Th2 hyperactivity and Th1 deficiency) in our asthmatic patients, which was consistent with the findings of other studies.
Figure 1.

Th1/Th2 balance related biomarkers level difference indicated a indicated an excessive activation of Th2 in asthmatic patients. A: The levels of Th1 and Th2 related cytokines were detected by ELISA, higher Th2 related cytokines levels including IL-4 and IL-10 were observed in asthmatic patients. B: t-bet and GATA3 mRNA levels were detected by qRT-PCR, Th2-related transcription factor-GATA3 was higher in asthmatic patients. C: The percentage of Th1/Th2 was measured by flow cytometry, a higher percentage of Th2 cells was found in asthmatic patients. *P<0.05 compared with healthy controls.
Decreased Runx3 levels in asthmatic patients and its role in Th1/Th2 balance
The expression levels of Runx3 in CD4+ T cells from asthmatic patients and healthy controls were detected by qRT-PCR and western blot, respectively. As shown in Figure 2A and 2B, the Runx3 level decreased in asthmatic patients compared with healthy controls, and the difference was significant (P<0.05). After transfecting the CD4+ T cells of asthmatic patients with pcDNA3.1-Runx3, the level of Th1-related cytokines increased, while that of Th2-related cytokines decreased (Figure 3A). Moreover, the levels of T-bet and GATA3 and the percentages of IFN-γ+ CD4+ T cells and IL-4+ CD4+ T cells also changed, which indicated that the Th1/Th2 balance improved (Figure 3B and 3C). This suggested that Runx3 plays a role in the differentiation of Th1 and Th2 cells.
Figure 2.
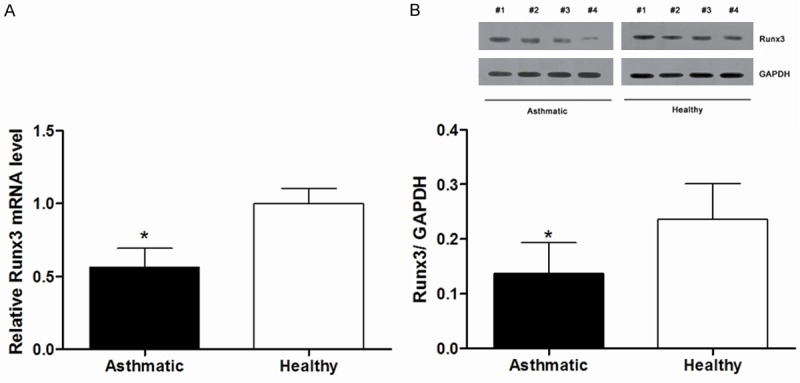
The Runx3 levels in CD4+ T cells decreased in asthmatic patients compared with healthy controls. A: Runx3 mRNA expression reduced in asthmatic patients. B: Runx3 protein expression was lower in asthmatic patients. The protein bands from 4 asthmatic patients and 4 healthy control (from #1 to #4). *P<0.05 compared with healthy controls.
Figure 3.
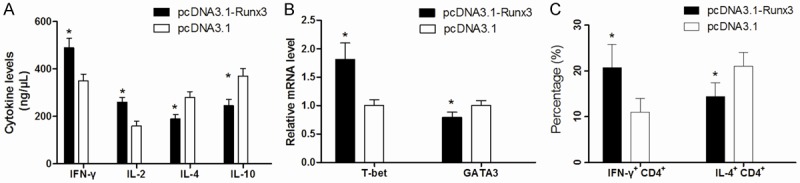
The Th1/Th2 balance in CD4+ T cells from asthmatic patients was restored by pcDNA3.1-Runx3 transfection. A: pcDNA3.1-Runx3 transfection reduced the levels of IL-4 and IL-10 in CD4+ T cells, meanwhile, it increased the levels of IFN-γ and IL-2. B: The mRNA level of T-bet and GATA3 were changed in pcDNA3.1-Runx3 transfected CD4+ T cells, Runx3 overexpression induced t-Bet but reduced GATA3 mRNA level. C: The percentage of Th1/Th2 was changed after pcDNA3.1-Runx3 transfection, overexpression of Runx3 could increase the percentage of Th1 in CD4+ T cells. *P<0.05 compared with pcDNA3.1 empty plasmid transfected cells.
Deregulated microRNA in CD4+ T cells from patients with asthma
To assess whether the microRNAs were deregulated in asthmatic CD4+ T cells, a microarray assay was performed. In total, 312 of the 754 arryed microRNAs could be detected in our samples. Among the 312 detectable microRNAs, 89 were significantly altered, including 37 upregulated microRNAs and 52 downregulated microRNAs (0.5< change fold <2.0). The changed microRNA profiling is showed in the heatmap (Figure 4A).
Figure 4.
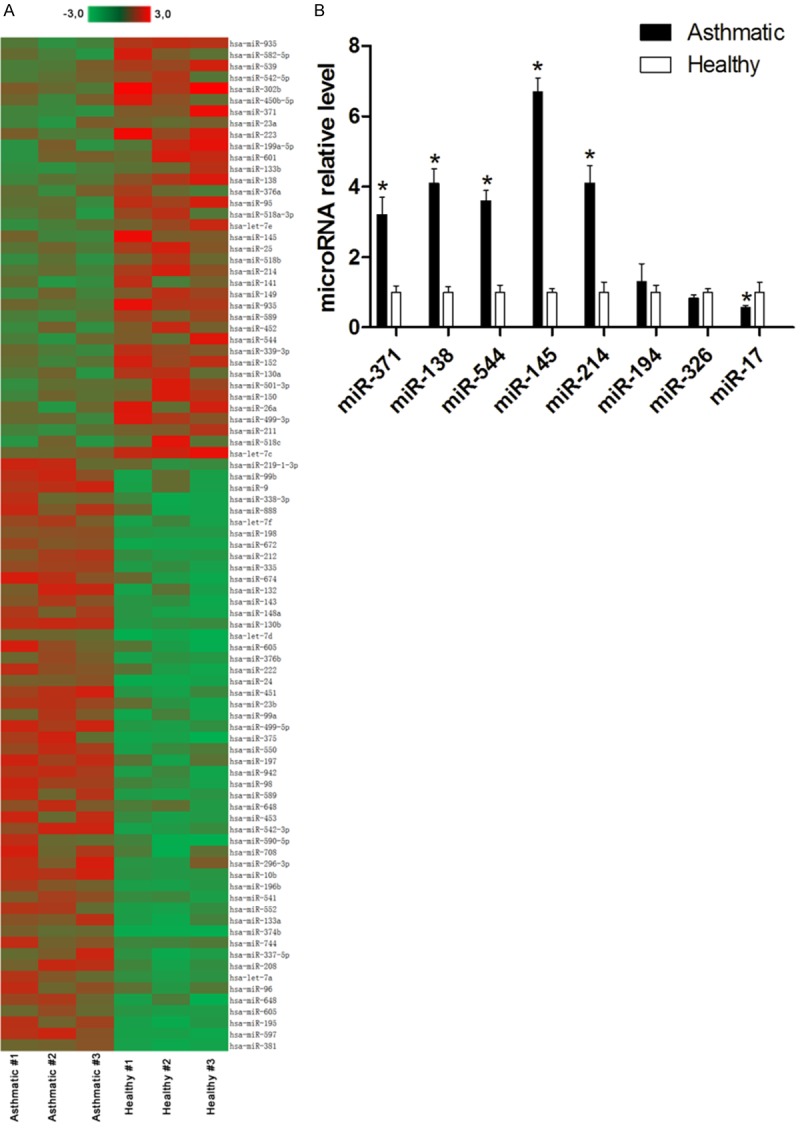
Dysregulated microRNA level changes in CD4+ T cells from asthmatic patients and healthy controls. A: Heatmap showed 89 microRNAs were significantly altered including 37 up-regulated microRNAs and 52 down-regulated microRNAs. B: 8 microRNAs which may target on Runx3 mRNA were confirmed by qRT-PCR, miR-371, miR-138, miR-544, miR-145 and miR-214 were increased in the CD4+ T cells from asthmatic patients. *P<0.05 compared with healthy controls.
miR-371, miR-138, miR-544, miR-145, and miR-214 potential group of microRNAs may target Runx3
In the present study, we focused on those microRNAs that may target Runx3. Among the 51 upregulated microRNAs, miR-371, miR-138, miR-544, miR-145, miR-214, miR-194, miR-326, and miR-17 may target Runx3 according to the bioinformatics analysis. These eight microRNAs were selected for further validation by qRT-PCR. As shown in Figure 4B, we only confirmed that miR-371, miR-138, miR-544, miR-145, and miR-214 levels were increased in the CD4+ T cells from asthmatic patients compared with healthy controls (P<0.05); however, miR-194, miR-326, and miR-17 showed no significant difference (P>0.05) or opposite changes. Hence, we selected miR-371, miR-138, miR-544, miR-145, and miR-214 as a candidate group of microRNAs for further experiments.
miR-371, miR-138, miR-544, miR-145, and miR-214 directly target the 3’-UTR of Runx3
To confirm whether Runx3 is a direct target of the candidate group of microRNAs, a renilla-luciferase reporter assay was performed. As shown in Figure 5, the relative luciferase activity in MOLT-4 significantly reduced after co-transfection with PsiCHECK2-Runx3 3’-UTR-Wt (WT) reporters and candidate miRs mimics. Co-transfection of PsiCHECK2-Runx3 3’-UTR-Mut (MUT) and miRs mimics had no effect on the relative luciferase activity. These results confirmed our bioinformatics prediction that miR-371, miR-138, miR-544, miR-145, and miR-214 directly target the 3’-UTR of Runx3.
Figure 5.
miR-371, miR-138, miR-544, miR-145 and miR-214 directly target on 3’-UTR of Runx3 mRNA. A: Binding sequence and the mutant binding sequence for each microRNA in the 3’-UTR of the RUNX3 mRNA; B-F: The relative luciferase activity in MOLT-4 significantly reduced after co-transfected with PsiCHECK2-Runx3 3’-UTR-Wt (WT) reporters and candidate miRs mimics. *P<0.05 compared with negative control.
Only simultaneous modulation of candidate microRNAs had expected the effect on the expression of Runx3
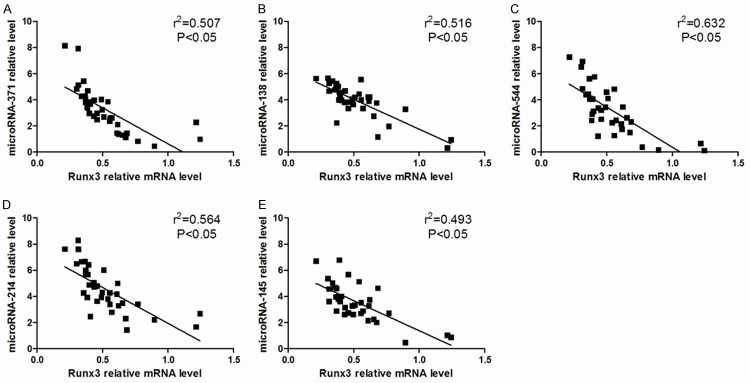
We found lower Runx3 expression with increased miR-371, miR-138, miR-544, miR-145, and miR-214 in asthmatic CD4+ T cells. Correlation analysis also demonstrated a negative correlation between these five microRNAs and Runx3 mRNA levels (r2=0.507, 0.516, 0.632, 0.564, and 0.493, P<0.05, Figure 6).
Figure 6.
Correlation between Runx3 mRNA level and candidate microRNAs levels (A-E) in CD4+ T cells from asthmatic patients, negative correlations were found.
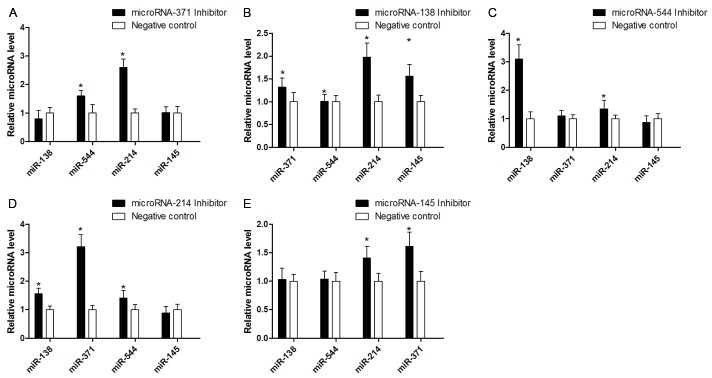
The bioinformatics analysis suggested that miR-371, miR-138, miR-544, miR-145, and miR-214 may target Runx3, and the same was also confirmed by luciferase reporter assay. Therefore, according to typical experience, we predicted that inhibition of transfection of the candidate microRNA could restore the level of Runx3. We thus analyzed the effects of individual inhibition of candidate microRNAs on the expression of Runx3. Interestingly, we found no effect on Runx3 mRNA and protein levels after individual miR modulation (Figure 7) in CD4+ T cells from asthmatic patients. We even found that miR-214 inhibitor decreased Runx3 levels further (P<0.05). However, when we transfected CD4+ T cells with a miR inhibitor mix against miR-371, miR-138, miR-544, miR-145, and miR-214, a significant increase in Runx3 was found.
Figure 7.

The Runx3 level changes after inhibition of candidates microRNAs. A: Individual inhibition of candidate microRNAs could not reduce the Runx3 mRNA level, but using mix-inhibitors could reduce the Runx3 mRNA level. *P<0.05 compared with negative control. B: Only simultaneous modulation of candidate microRNAs could reduce the Runx3 protein expression. *P<0.05 compared with mix inhibitor.
Effects of microRNA simultaneous silencing on Th1/Th2 balance
Our results so far indicated that inhibition of individual candidate microRNA levels did not have an effect on the Runx3 expression levels, which is in contrast to our preliminary analysis. Because Runx3 may play a role in the maintaining of Th1/Th2 balance, we also individually and simultaneously downregulated candidate microRNAs, and estimated their effect on Th1/Th2 balance. Similar to the effect on the expression of Runx3, we concluded that modulation of individual candidate microRNAs did not alter the Th1/Th2 imbalance in asthmatic patients (Figure 8). In contrast to individual regulation, we observed changed cytokine, t-bet, and GATA3 mRNA levels and improved percentage rate of Th1/Th2 after the transfection of miRs-Mix inhibitor. It indicated that only simultaneous knockdown of the group of miRs could restore the Th1/Th2 balance in asthmatic patients. Most importantly, we also conducted a rescue experiment. After transfecting Runx3 siRNA into the CD4+ T cells, simultaneous inhibition of candidate miRs could not change the Th1/Th2 balance anymore (Figure 8).
Figure 8.

The Th1/Th2 balance could be restored after simultaneous inhibition of candidates microRNAs. A-D: Only simultaneous modulation of candidate microRNAs could change the Th1 and Th2 related cytokines. Mix-inhibitor of candidate microRNAs transfection decreased the levels of IL-4 and IL-10. E, F: t-bet and GATA3 mRNA level changes after inhibition of candidates microRNAs, Mix-inhibitor of candidate microRNAs caused t-bet increasing but GATA3 decreasing. G, H: The percentage of Th1/Th2 was changed after using Mix-inhibitor of candidate microRNAs, the Th1 percentage increased. Meanwhile, the Th2 percentage decreased. *P<0.05 compared with negative control.
Interaction between miR-371, miR-138, miR-544, miR-145, and miR-214
To explain why the effects of microRNA knockdown were different when performed individually compared with simultaneous knockdown, we envisaged the hypothesis that the individual microRNAs might have an effect on rest of the microRNA members in the group. To test this hypothesis, we used qRT-PCR to determine the level changes in microRNA levels when individually transfected with a microRNA inhibitor. As shown in Figure 9, a complicated interaction between miR-371, miR-138, miR-544, miR-145, and miR-214 was found. In brief, only knockdown of one candidate microRNA could increase another microRNA’s levels; for example, which miR-371 was inhibited individually, the levels of miR-544 and miR-214 increased. The effect of individual microRNA inhibition could be eliminated by the interaction.
Figure 9.
The effect of single microRNA mimic transfection on changes of other candidate microRNAs’ level, knock-down one of candidate microRNAs could increase another microRNAs (A-E). *P<0.05 compared with negative control.
Discussion
It is well known that asthma is tightly related to the imbalance of Th1/Th2 cells and their characteristic cytokine profiles. In physiological conditions, there is a dynamic balance between immune responses of Th1 and Th2 cells, and when this balance is disturbed, asthma occurs [25,26]. In brief, Th2 cells mainly produce IL-4, IL-5, and IL-13, which increase the IgE levels in serum and recruit eosinophils, thus further inducing high air reactivity [27]. Th1 cells, which secrete IFN-γ and IL-12, can antagonize Th2-induced immune response and restrain the development of asthma [7,28]. We found that IL-2 and IFN-γ levels decreased, while the levels of IL-4 and IL-10 significantly increased in asthmatic patients, as detected by ELISA method; However, lower T-bet and higher GATA3 expression was observed. It indicated an imbalance of Th1/Th2 cells in our asthmatic subject. Restoring Th1/Th2 balance through improving Th1 cells and simultaneously inhibiting Th2 cells should be an effective therapy strategy for asthma. In fact, some evidencehas proven that regulating Th1/Th2 cytokine imbalance could attenuate asthma using rodent models. Guo et al. suggested that mangiferin, a natural C-glucosidexanthone, could exert anti-asthmatic effect through the modulation of Th1/Th2 cytokine imbalance and by inhibiting the STAT6 signalling pathway [29]. Wang and colleagues found that synthetic salidroside exhibited excellent anti-asthma effects through the regulation of TH1/Th2 balance in an OVA-induced mouse model [12]. However, these results were obtained depending on the administration of chemical agents. In the present study, we were more concerned with microRNAs rather than chemical agents. One reason is that microRNAs possess a unique characteristic, which is very attractive in terms of therapy for the disease, with a series of amazing benefits such as well-known sequences and easy to synthesize and flexible strategies for manipulation. Another reason is that some microRNAs play an indispensable role in asthma. For example, recent studies have found new evidence that upregulation of miR-155 was linked to the development of allergic asthma, and the mechanism was involved with the T helper cell immune response [30]. Huo et al. found that epithelial and plasma miR-181b-5p expression was decreased in asthma and contributed to eosinophilic airway inflammation by regulating proinflammatory cytokines [31]. Normally, microRNAs perform their functions by regulating relevant target genes. Besides investigating dysregulated microRNAs in asthma, it is also worth finding microRNA-related target gene and connecting them with Th1/th2 mediation.
Typically, the Th1/Th2 balance is mediated by transcription factors including GATA-3, STAT3, STAT5a, STAT6, c-Maf, notch, SOCS-3, and SOCS-5 through different functions [32]. For example, GATA-3 and STAT3 are important for the differentiation of Th2 cells; it has already been demonstrated that knockdown of GATA-3 and STAT3 could inhibit allergen-induced asthma as strategies to inhibit Th2 cell differentiation. SOCS-3 blocks Th1 cell development and is preferentially expressed in Th2 cells; in contrast, SOCS-5 is mainly expressed in Th1 cells and prevents Th2 cell from developing [33]. These factors are novel and attractive, but they were reported by previous studies frequently, whereas recently emerging evidence has linked Runx3 to Th1/Th2 balance and showed some microRNAs could regulate Th1/th2 balance via Runx3 regulation. Fu et al. suggested that inhibiting Runx3 expression regulates the balance of Th1/Th2 in psoriasis [34]. Naoe et al. demonstrated critical roles of Runx complexes in regulating immune responses; for instance, the deletion of Runx3 in T cells induced the spontaneous development of asthma-like features in targeted mice [35]. However, the effect of Runx3 on Th1/Th2 balance in asthmatic patients needs to be investigated. Our asthmatic subject showed decreased Runx3 levels, and when we overexpressed its expression by transfection, the balance of Th1/Th2 cells improved. This evidence showed that Runx3 played a role in the regulation of Th1/Th2 balance in asthma. As mentioned above, we focus on the microRNA changes and its function in asthma; thus, we tried to reveal the relationship between dysregulated miRs and Runx3. We found a series of microRNAs that were deregulated in CD4+ T cells in asthmatic patients using microRNA microarray assay. A group of significantly changed microRNAs, including miR-371, miR-138, miR-544, miR-145, and miR-21, that may target Runx3 were selected as candidate miRs. Among these five miRs, the target effects of miR-138 and miR-145 on Runx3 have been reported in previous studies. In psoriasis, insufficient miR-138 led to the overexpression of the target gene Runx3 and decreased the ratio of Th1/Th2 in CD4+ T cells [34]. However, no reports mentioned whether this interaction could exist in asthma. Fan’s study found that miR-145 could regulate the balance of Th1/Th2 by targeting Runx3 in asthma patients [36]. However, they only considered individual microRNAs. MicroRNAs may have a different effect depending on the expression of other microRNAs and their cross-talking. Thus, we considered a group of microRNAs. Using luciferase reporter assay, we found that all the five microRNAs could directly target the 3’-UTR of Runx3. Then we identified their effect on Runx3 expression and Th1/Th2 balance regulation. Interestingly, although the evidence of bioinformatics prediction and luciferase reporter assay strongly suggested that these candidate microRNAs could inhibit the expression of Runx3 and a negative correlation between Runx3 and candidate microRNAs was shown in patients, we did not observe an expected effect on Runx3 when we performed an individual miR modulation. After transfecting the CD4+ T cells with individual microRNA inhibitors, the Runx3 mRNA and protein levels were not restored. However, when we employed simultaneous inhibition of candidate microRNAs by transfecting miRs-inhibitor mix, the Runx3 mRNA and protein levels significantly increased. As similar as the effect on the Runx3 level, only microRNAs’ simultaneous silencing could correct the Th1/Th2 imbalance in asthmatic patients. However, knockdown expression of Runx3 could antagonize the effect induced by transfecting miRs-inhibitor mix. These results indicated that miR-371, miR-138, miR-544, miR-145, and miR-214 could regulate Th1/Th2 by targeting Runx3, but a combinatorial way is necessary. To explain why only simultaneous silencing of these five miRs could show influence on Runx3 and Th1/Th2 balance, we hypothesized that individual microRNAs might have a knock on effect on the other candidate microRNAs. As we expected, a complicated interaction between miR-371, miR-138, miR-544, miR-145, and miR-214 was found. In brief, knockdown of one candidate microRNA could increase the levels of other microRNAs. This interaction may eliminate the effects of individual microRNA inhibition. The limitation of the present study is that we only considered the interaction between microRNA level changes for explaining the contraction between individual and simultaneous microRNA inhibition; we did not analyze the potential network comprising candidate microRNAs. In addition to regulating Runx3, this microRNA network may exert its different effects on Th1/Th2 balance through other transcription factors. Thus, only simultaneous downregulation of these five microRNAs but not individual microRNAs may alter the different signalling pathways that lead to a change in the Runx3 and Th1/Th2 balance in an accumulation manner. This should be proven in our further study. Moreover, from our results, we suggest that prediction of in silico analysis of microRNA function should be confirmed prudently. Even with the evidence provided by the luciferase reporter assay, the interactions among different microRNAs should be considered especially in vivo.
In conclusion, we demonstrated a novel result: In the asthmatic patient, miR-371, miR-138, miR-544, miR-145, and miR-214 could regulate Th1/Th2 levels by targeting Runx3 in a combinatorial manner. These microRNAs have potential to be therapeutic targets in asthma. In the clinical practice, simultaneous regulation of several target microRNAs may increase the curative effect compared to that of individual target strategy.
Acknowledgements
This study was supported by the research funding from the department of human resources and social security of Jiangsu province (2012-ws-006) and the research funding for young researcher from the department of health of Nanjing city.
Disclosure of conflict of interest
None.
References
- 1.Tsilogianni Z, Ntontsi P, Papaioannou AI, Bakakos P, Loukides S. Biomarkers guided treatment strategies in adult patients with asthma: ready for the clinical field? Arch Immunol Ther Exp (Warsz) 2017;65:1–9. doi: 10.1007/s00005-016-0407-9. [DOI] [PubMed] [Google Scholar]
- 2.Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:215–20. doi: 10.1097/MOO.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 3.Chen YL, Chiang BL. Targeting TSLP with shRNA alleviates airway inflammation and decreases epithelial CCL17 in a murine model of asthma. Mol Ther Nucleic Acids. 2016;5:e316. doi: 10.1038/mtna.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sudini K, Diette GB, Breysse PN, McCormack MC, Bull D, Biswal S, Zhai S, Brereton N, Peng RD, Matsui EC. A randomized controlled trial of the effect of broccoli sprouts on antioxidant gene expression and airway inflammation in asthmatics. J Allergy Clin Immunol Pract. 2016;4:932–40. doi: 10.1016/j.jaip.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang Z, Zhu L. Update on the role of alternatively activated macrophages in asthma. J Asthma Allergy. 2016;9:101–7. doi: 10.2147/JAA.S104508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KS, Yu J, Shim D, Choi H, Jang MY, Kim KR, Choi JH, Cho SH. Local immune responses in children and adults with allergic and nonallergic rhinitis. PLoS One. 2016;11:e0156979. doi: 10.1371/journal.pone.0156979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang KL, Liu SY, Chou CC, Lee YH, Cheng TJ. The effect of size-segregated ambient particulate matter on Th1/Th2-like immune responses in mice. PLoS One. 2017;12:e0173158. doi: 10.1371/journal.pone.0173158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diao M, Min J, Guo F, Zhang CL. Effects of salbutamol aerosol combined with magnesium sulfate on T-lymphocyte subgroup and Th1/Th2 cytokines of pediatric asthma. Exp Ther Med. 2017;13:117–120. doi: 10.3892/etm.2016.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar A, Das S, Agrawal A, Mukhopadhyay I, Ghosh B. Genetic association of key Th1/Th2 pathway candidate genes, IRF2, IL6, IFNGR2, STAT4 and IL4RA, with atopic asthma in the Indian population. J Hum Genet. 2015;60:443–8. doi: 10.1038/jhg.2015.45. [DOI] [PubMed] [Google Scholar]
- 10.Tang F, Wang F, An L, Wang X. Upregulation of Tim-3 on CD4(+) T cells is associated with Th1/Th2 imbalance in patients with allergic asthma. Int J Clin Exp Med. 2015;8:3809–16. [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang T, Yang Z, Yang S, Du J, Wang S. Immunoregulatory effects of paeoniflorin exerts anti-asthmatic effects via modulation of the Th1/Th2 equilibrium. Inflammation. 2015;38:2017–25. doi: 10.1007/s10753-015-0182-5. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Jin RG, Xiao L, Wang QJ, Yan TH. Anti-asthma effects of synthetic salidroside through regulation of Th1/Th2 balance. Chin J Nat Med. 2014;12:500–4. doi: 10.1016/S1875-5364(14)60078-9. [DOI] [PubMed] [Google Scholar]
- 13.Huang X, Tang L, Wang F, Song G. Astragaloside IV attenuates allergic inflammation by regulation Th1/Th2 cytokine and enhancement CD4(+)CD25(+)Foxp3 T cells in ovalbumin-induced asthma. Immunobiology. 2014;219:565–71. doi: 10.1016/j.imbio.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Lotem J, Levanon D, Negreanu V, Bauer O, Hantisteanu S, Dicken J, Groner Y. Runx3 at the interface of immunity, inflammation and cancer. Biochim Biophys Acta. 2015;1855:131–43. doi: 10.1016/j.bbcan.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Voon DC, Hor YT, Ito Y. The RUNX complex: reaching beyond haematopoiesis into immunity. Immunology. 2015;146:523–36. doi: 10.1111/imm.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Gao J, Su Z, Dai X, Li Y, Liu Y, Chen J, Tong J, Zhang Y, Wu C, Zheng D, Wang S, Xu H. Downregulation of Hlx closely related to the decreased expressions of T-bet and Runx3 in patients with gastric cancer may be associated with a pathological event leading to the imbalance of Th1/Th2. Clin Dev Immunol. 2012;2012:949821. doi: 10.1155/2012/949821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SH, Jeong HM, Choi JM, Cho YC, Kim TS, Lee KY, Kang BY. Runx3 inhibits IL-4 production in T cells via physical interaction with NFAT. Biochem Biophys Res Commun. 2009;381:214–7. doi: 10.1016/j.bbrc.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Klunker S, Chong MM, Mantel PY, Palomares O, Bassin C, Ziegler M, Ruckert B, Meiler F, Akdis M, Littman DR, Akdis CA. Transcription factors RUNX1 and RUNX3 in the induction and suppressive function of Foxp3+ inducible regulatory T cells. J Exp Med. 2009;206:2701–15. doi: 10.1084/jem.20090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alipoor SD, Adcock IM, Garssen J, Mortaz E, Varahram M, Mirsaeidi M, Velayati A. The roles of miRNAs as potential biomarkers in lung diseases. Eur J Pharmacol. 2016;791:395–404. doi: 10.1016/j.ejphar.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pattarayan D, Thimmulappa RK, Ravikumar V, Rajasekaran S. Diagnostic potential of extracellular MicroRNA in respiratory diseases. Clin Rev Allergy Immunol. 2016 doi: 10.1007/s12016-016-8589-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Zhou H, Li J, Gao P, Wang Q, Zhang J. miR-155: a novel target in allergic asthma. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levanen B, Bhakta NR, Torregrosa Paredes P, Barbeau R, Hiltbrunner S, Pollack JL, Skold CM, Svartengren M, Grunewald J, Gabrielsson S, Eklund A, Larsson BM, Woodruff PG, Erle DJ, Wheelock AM. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J Allergy Clin Immunol. 2013;131:894–903. doi: 10.1016/j.jaci.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu W, You R, Yuan X, Yang T, Samuel EL, Marcano DC, Sikkema WK, Tour JM, Rodriguez A, Kheradmand F, Corry DB. The microRNA miR-22 inhibits the histone deacetylase HDAC4 to promote T(H)17 cell-dependent emphysema. Nat Immunol. 2015;16:1185–94. doi: 10.1038/ni.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panganiban RP, Wang Y, Howrylak J, Chinchilli VM, Craig TJ, August A, Ishmael FT. Circulating microRNAs as biomarkers in patients with allergic rhinitis and asthma. J Allergy Clin Immunol. 2016;137:1423–32. doi: 10.1016/j.jaci.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 25.Li JG, Du YM, Yan ZD, Yan J, Zhuansun YX, Chen R, Zhang W, Feng SL, Ran PX. CD80 and CD86 knockdown in dendritic cells regulates Th1/Th2 cytokine production in asthmatic mice. Exp Ther Med. 2016;11:878–884. doi: 10.3892/etm.2016.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mlcek J, Jurikova T, Skrovankova S, Sochor J. Quercetin and its anti-allergic immune response. Molecules. 2016:21. doi: 10.3390/molecules21050623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wise SK, Laury AM, Katz EH, Den Beste KA, Parkos CA, Nusrat A. Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. Int Forum Allergy Rhinol. 2017;4:361–370. doi: 10.1002/alr.21298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews NC, Pfeffer PE, Mann EH, Kelly FJ, Corrigan CJ, Hawrylowicz CM, Lee TH. Urban particulate matter-activated human dendritic cells induce the expansion of potent inflammatory Th1, Th2, and Th17 effector cells. Am J Respir Cell Mol Biol. 2016;54:250–62. doi: 10.1165/rcmb.2015-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo HW, Yun CX, Hou GH, Du J, Huang X, Lu Y, Keller ET, Zhang J, Deng JG. Mangiferin attenuates TH1/TH2 cytokine imbalance in an ovalbumin-induced asthmatic mouse model. PLoS One. 2014;9:e100394. doi: 10.1371/journal.pone.0100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plank MW, Maltby S, Tay HL, Stewart J, Eyers F, Hansbro PM, Foster PS. MicroRNA expression is altered in an ovalbumin-induced asthma model and targeting miR-155 with antagomirs reveals cellular specificity. PLoS One. 2015;10:e0144810. doi: 10.1371/journal.pone.0144810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huo X, Zhang K, Yi L, Mo Y, Liang Y, Zhao J, Zhang Z, Xu Y, Zhen G. Decreased epithelial and plasma miR-181b-5p expression associates with airway eosinophilic inflammation in asthma. Clin Exp Allergy. 2016;46:1281–90. doi: 10.1111/cea.12754. [DOI] [PubMed] [Google Scholar]
- 32.Wang HY, Ding JB, Halmurat U, Hou M, Xue ZQ, Zhu M, Tian SG, Ma XM. [The effect of Uygur medicine Hyssopus officinalis L on expression of T-bet, GATA-3 and STAT-3 mRNA in lung tissue of asthma rats] . Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2011;27:876–9. [PubMed] [Google Scholar]
- 33.Evans CM, Jenner RG. Transcription factor interplay in T helper cell differentiation. Brief Funct Genomics. 2013;12:499–511. doi: 10.1093/bfgp/elt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu D, Yu W, Li M, Wang H, Liu D, Song X, Li Z, Tian Z. MicroRNA-138 regulates the balance of Th1/Th2 via targeting RUNX3 in psoriasis. Immunol Lett. 2015;166:55–62. doi: 10.1016/j.imlet.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Naoe Y, Setoguchi R, Akiyama K, Muroi S, Kuroda M, Hatam F, Littman DR, Taniuchi I. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to the Il4 silencer. J Exp Med. 2007;204:1749–55. doi: 10.1084/jem.20062456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan L, Wang X, Fan L, Chen Q, Zhang H, Pan H, Xu A, Wang H, Yu Y. MicroRNA-145 influences the balance of Th1/Th2 via regulating RUNX3 in asthma patients. Exp Lung Res. 2016;42:417–424. doi: 10.1080/01902148.2016.1256452. [DOI] [PubMed] [Google Scholar]