Abstract
Purpose: The aims were two-fold: first, to examine the expression of Yes-activated protein (YAP), a key Hippo pathway regulator, in clinical thyroid papillary carcinoma samples and to correlate this with clinicopathological parameters; second, to explore the role of YAP in regulating cell growth and division in vitro. Methods and results: YAP expression was determined by immunohistochemistry of clinical thyroid papillary carcinoma tissue microarrays and expression was correlated with clinicopathological parameters. YAP expression positively correlated with TNM stage and lymph node metastasis. The effect of YAP gene silencing by siRNA on BCPAP and KI cell migration, invasion, apoptosis, cell cycle progression (including expression of the cell cycle regulators, p21, p27, c-Myc, and Foxo3a1), and the expression of autophagy markers (Belcin1, LC3-I, LC3-II, Atg12, Atg16L1, and Atg5) were examined. YAP gene silencing decreased cell proliferation, migration, and invasion. In contrast, there was no effect on cell apoptosis, but cells arrested at G0/G1, and this was accompanied by down-regulation of c-Myc and Foxo3a and up-regulation of the cell cycle proteins, p21 and p27. The autophagy marker LC3-I was expressed at slightly higher levels than LC3-II; YAP silencing decreased both LC3-1 and LC3-II protein expression, resulting in an increase in the LC3-II/LC3-I ratio, this process was accompanied by decreases in Beclin1 and Atg5-Atg12-Atg16 complex expression. Conclusions: In papillary thyroid cancer YAP protein expression is positively correlated with the extent of TNM stage and positive lymph node metastasis. In thyroid cancer cell lines YAP appears to be important in stimulating cell proliferation while inhibiting autophagy.
Keywords: YAP, BCPAP, KI, immunohistochemistry, proliferation, cell cycle arrest, apoptosis, autophagy
Introduction
The incidence of thyroid cancer (TC) has increased dramatically over the past few decades [1,2]. Papillary thyroid carcinoma (PTC) is the most common malignant thyroid neoplasm, accounting for 80% of all thyroid cancers, and its incidence has also increased strikingly over this period [3].
The Hippo pathway was first discovered through genetic screening in Drosophila and was found to be an evolutionarily conserved pathway that is responsible for controlling organ size primarily through controlling cell proliferation, apoptosis, stem-cell self-renewal, and tumorigenesis [4-6]. It consists of a kinase cascade, the activation of which results in the down-regulation of a variety of key regulators, and these key regulators are involved in cell contact inhibition, transmembrane receptor signalling as well as other processes that have yet to be defined. YAP and transcriptional coactivator with PDZ-binding motif (TAZ) are the most critical Hippo pathway effectors [4]; both these proteins are transcriptional activators that regulate the expression of multiple target genes in the Hippo pathway. Unphosphorylated YAP/TAZ was the key for Hippo pathway kinases transferring into the nucleus, therefore, when YAP/TAZ is in the “off” state, Hippo pathway kinases effectively entered the nucleus and further activated proliferation, epithelial-mesenchymal transition and transcription and so on [4,7].
YAP primarily regulates the expression of genes in concert with transcription factors of the TEAD/TEF family. Numerous studies have reported that expression of the YAP protein is altered in different malignant tumours including liver cancer, breast cancer, and gastric cancer [4,8-10]. However, expression of the YAP protein in papillary thyroid carcinoma, which is also epithelially derived, has not been reported to date. Here, we focused on the relationship between the YAP protein and high-risk characteristics in patients with papillary thyroid cancer, and through the use of siRNA mediated gene silencing, we investigated the role of YAP in proliferation, migration, invasion, cell cycle, apoptosis and autophagy of the papillary thyroid cancer cell lines, BCPAP and KI.
Materials and methods
Tissue microarray analysis
Three tissue microarrays containing a total of 88 papillary thyroid cancer tissue specimens were purchased from the National Engineering Center for BioChips (Shanghai, China). All specimens and clinical information were obtained from Shanghai Zhongshan Hospital. A YAP specific antibody (#4912; Cell Signaling Technology, Danvers, MA, USA) was used at a dilution of 1:50 to evaluate YAP expression in these tissue microarrays by immunohistochemical (IHC) staining. All immunohistochemical staining was evaluated by two experienced pathologists who were blinded to the clinical information. Both, the % of cells staining positive for YAP as well as the staining intensity were evaluated in the cytoplasm and graded according to either the percentage of YAP-positive cells using a scale from 0 to 4 (0-5%, 6-25%, 26-50%, 51-75%, and 76-100% respectively) or the intensity of YAP staining using a scale from 0 to 3. The final YAP expression score (score 0,1) was calculated from the summation of those two grades (total grade 0-1, 2-3, and 4-5, 6-7, separately) (Figure 1). The slides were scanned using a ScanScope slide scanner (Aperio, Vista, CA, USA), and images of representative areas were acquired using Image Scope software (Aperio) and analysed using Illustrator (Adobe).
Figure 1.
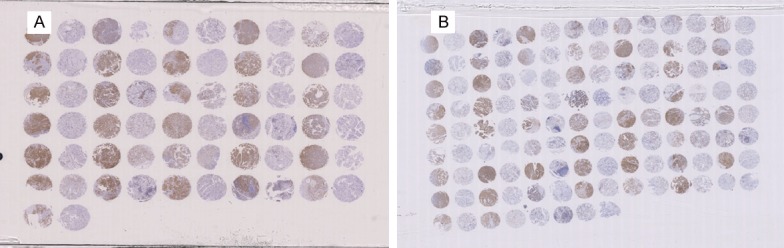
Tissue microarray (TMA) of YAP in PTC tumor samples. A. TMA 1; B. TMA 2.
Cell culture and treatment
The human PTC-derived cell lines, BCPAP and KI, were obtained from the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RPMI-1640 medium (Gibco-Life Technologies, Grand Island, NY, USA) supplemented with 10% foetal bovine serum (FBS; Gibco-Life Technologies) and 1% penicillin/streptomycin (Beyotime Institute of Biotechnology, Nanjing, Jiangsu, China) in the presence of 5% CO2 at 37°C.
Small interfering RNA (siRNA) and transfection
BCPAP and KI cells (3 × 105 cells/well) were seeded onto a six-well plate containing 1 mL Opti-MEM (Gibco) 24 h before transfection. Briefly, 75 pg YAP siRNA or a scrambled siRNA control in 10 m L of Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) were added and gently mixed. The cells were then incubated for 48 h before further assay.
Transwell migration and invasion assays
The upper transwell chamber (8 µm pore size; Corning Inc., Union City, CA, USA), coated with ECM gel (Sigma-Aldrich; invasion assay) or without ECM gel (migration assay) was covered with 6 × 104 cells in 200 µL of media containing 0.1% BSA. The lower chamber was then filled with 200 µL RPMI-1640 medium containing 30% FBS (HyClone Laboratories). The cells were then cultured at 37°C and 5% CO2 for 22 h, prior to being fixed with 70% ethanol and stained with 0.1% crystal violet. The cells were counted in multiple random fields using an Olympus IX71 fluorescence microscope (Olympus, Janpan).
Cell proliferation assay
Cell proliferation was assessed using the fluorescent cell staining dye carboxyfluorescein succinimidyl ester (CFSE) according to the manufacturer’s instructions. Briefly, cells were collected and washed twice with PBS, and then resuspended in 1 mL PBS; 5 μL CFSE stock solution was then added to the cell suspension and mixed gently, and the suspension was then incubated at room temperature in the dark for 5 min. Pure GIBGOserum (200 μL) was then added to the cell suspension to terminate the reaction, and the mixture was then incubated on ice in the dark for 10 min. Following this, the mixture was centrifuged at 1,000 g for 5 min, the supernatant discarded, and the cells were washed twice with cold PBS. Cells were counted, and then incubated in the dark in a 5% CO2 incubator at 37°C. Following this, the cells were collected and washed twice with PBS and were then analysed by flow cytometry using a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) over a period of one hour. The experiment was repeated three times.
Flow cytometric analysis for apoptosis
To assess apoptosis, an Annexin V-FITV/PI apoptosis kit (Nanjing KeyGen Biotech, Co., Ltd., Nanjing, China) was used according to the manufacturer’s instructions. Briefly, the cells (1-5 × 105) were isolated by digestion with EDTA-free trypsin (Beyotime Institute of Biotechnology). The cells were then centrifuged at 1,200 g for 5 min, and then washed three times with cold PBS. The cells were then resuspended in 500 µL binding buffer containing 5 µL Annexin V-FITC and 5 µL propidium iodide (PI). Apoptotic cells were detected by flow cytometry using a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) over an hour. The experiment was repeated three times.
Cell cycle assay
PI staining was performed according to the to the manufacturer’s instructions. Briefly, cells were collected and were washed once with cold PBS, and then resuspended in 75% ethanol chilled to -20°C. Cells were counted and the cell concentration was adjusted to 2 × 106 cell/tube; cells were then fixed by incubation at -20°C for 1-24 h. Following this, the cells were centrifuged at 2,000 g for 5 min, and then washed twice with cold PBS. The washed cells were re-suspended in 200 μL PBS and RNase was added to a final concentration of 100 μg/mL. Following mixing the cells were then incubated at 37°C for 30 min. Finally, 235 μL PBS and 60 μL PI (50 μg/mL final concentration) were added to the cells and they were then incubated at 37°C for 30 min in the dark. Stained cells were detected by flow cytometry using a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) over an hour. The experiment was repeated three times.
Western blot analysis
BCPAP and KI cells, which had been treated as appropriate, were collected at 60-80% confluence. Protein extraction was performed using an assay kit (Cell Signaling Technology, America), and the total protein concentration was examined using the BCA Protein Assay kit (Beyotime Institute of Biotechnology). For western blot analysis, SDS-PAGE (Beyotime Institute of Biotechnology) was used to separate equal amounts (30 µg) of total protein, and the proteins were transferred onto PVDF membranes (Millipore, Billerica, MA, USA) using transfer buffer (200 mM glycine, 40 mM Tris and 20% methanol) at 240 mA for 30-90 min, depending on the molecular weight of the proteins to be detected. Membranes were blocked using 5% non-fat milk (Cell Signaling Technology) in 0.1% TBST (Cell Signaling Technology) for 1 h at 37°C and then incubated with the primary antibody overnight at 4°C. After washing three times in 0.1% TBST, 5 min each time, the membranes were incubated in horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody (Cell Signaling Technology) at a dilution of 1:3,000 for 1 h, 37°C. The membranes were then then washed three times in 0.1% TBST, 5 min each time. The ECL Chemiluminescent Substrate Reagent kit (Cell Signaling Technology) was added to the membranes to visualize the immune-stained proteins. Quantification of the band intensities was determined using Image Lab software (Bio-Rad Laboratories, Hercules, CA, USA). GAPDH was used as an internal protein loading standard.
Statistical analysis
Results were obtained from three repeat experiments are expressed as the means ± standard deviation (SD), and analysed using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). The Student’s t-test was used to determine the differences between the groups. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
Association between Yap protein expression and patient clinicopathological characteristics in papillary thyroid cancer
Clinical details associated with the patient tissue samples as well as the immunohistochemical analysis of YAP expression are shown in Figures 1 and 2, respectively. The association between Yap protein expression levels and patient clinicopathological characteristics are shown in Table 1. After excluding samples that could not be read, a total of 88 papillary thyroid cancer samples were included for analysis. As shown in Table 1, of the 88 patients with PTC, 73 (82.9%) cases were scored positive for YAP expression and the remaining 15 (17.1%) scored negative for YAP expression. YAP expression was positively correlated with TNM stage and lymph node metastasis (LNM) (P=0.030 and 0.044 respectively). However, there was no significant correlation between YAP expression and age, sex, tumour size, or tumour location (all P>0.05).
Figure 2.
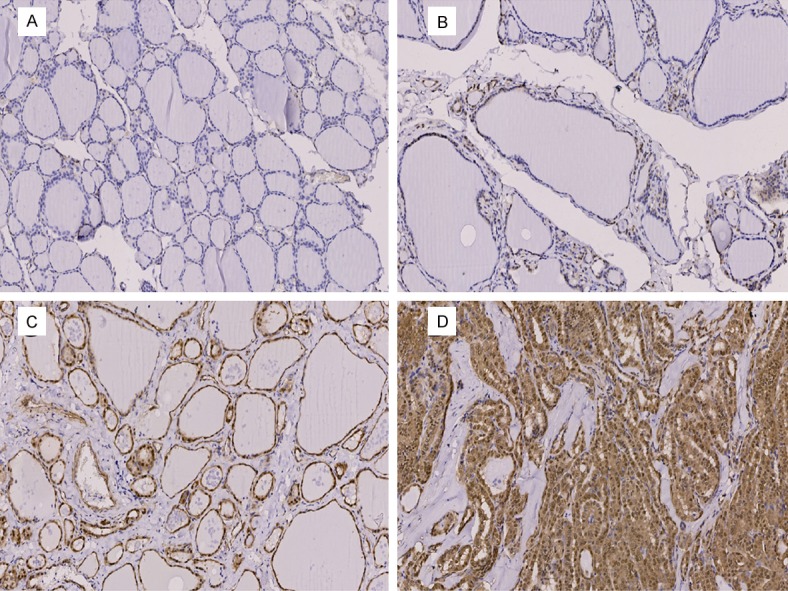
IHC staining of YAP in PTC tumor samples. A. Negative; B. Weakly Positive; C. Moderate Positive; D. Positive.
Table 1.
Association between the Yap protein and high-risk characteristics in patients with papillary thyroid cancer
| Characteristics | YAP- | YAP+ | P |
|---|---|---|---|
| Sex | 0.389 | ||
| Male | 7 | 25 | |
| Female | 8 | 48 | |
| Age (years) | 1.0 | ||
| <45 | 7 | 34 | |
| ≥45 | 8 | 39 | |
| Tumor size | 0.403 | ||
| ≤2 cm | 6 | 39 | |
| >2 cm | 9 | 34 | |
| TNM Stage | 0.030 | ||
| I | 8 | 28 | |
| II | 5 | 8 | |
| III | 2 | 31 | |
| IV | 0 | 6 | |
| Location | 1.0 | ||
| Unilateral | 13 | 64 | |
| Bilateral | 2 | 9 | |
| LNM | 0.044 | ||
| Negative | 12 | 35 | |
| Positive | 3 | 38 |
LNM: Lymph node metastasis.
A YAP targeted siRNA inhibits proliferation of BCPAP and KI
In order to clarify the role of YAP in BCPAP and KI cell proliferation, we used siRNA to silence the expression of YAP in BCPAP and KI cells. As shown in Figure 3A and 3B, an siRNA targeted to YAP significantly reduced YAP expression in both BCPAP and KI cells. BCPAP cell proliferation as assessed by CFSE labelling is shown in Figure 3C. The proliferation index (PI) in YAP siRNA-treated cells (PI=138.30±14.54) was significantly reduced compared with control siRNA-treated cells (PI=363.37±4.417). These data demonstrate that silencing of YAP expression inhibits cell proliferation in thyroid cancer BCAP and KI cells.
Figure 3.
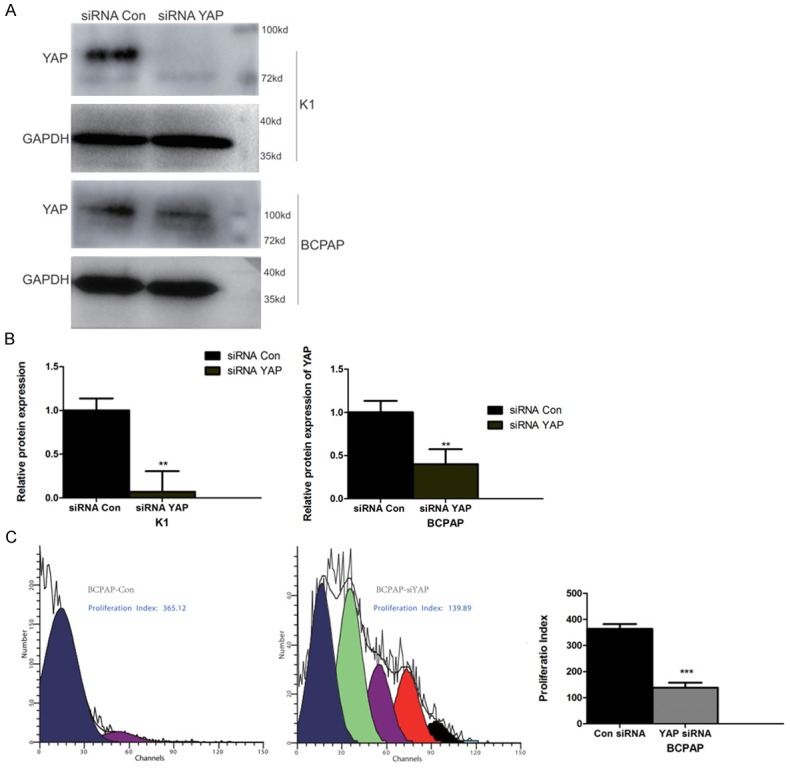
A YAP targeted siRNA suppresses YAP expression in KI and BCPAP cells and reduces proliferation in BCPAP cells. (A) Western blot of YAP protein in siRNA Con- and siRNA YAP-treated KI and BCPAP cells. GAPDH is presented as a loading control. (B) Quantification of YAP knockdown shown in (A). (C) Proliferation of BCPAP cells treated with siRNA Con or siRNA YAP. The proliferation index quantification is shown in the far right-hand graph. (**, *** represent P<0.01 and P<0.0001 in the t-test, compared with siRNA Con).
A YAP targeted siRNA inhibits the migration and invasion of BCPAP and KI cells
In order to clarify the role of YAP in BCPAP and KI cell migration and invasion, we used a transwell assay to assess cell migration and invasion after YAP knockout using siRNA (siRNA YAP). As shown in Figure 4, compared to the control group (siRNA Con), the migration and invasion capacities of BCPAP and KI cells in the siRNA YAP treated cells were inhibited significantly (*P<0.05, n=3 independent experiments).
Figure 4.
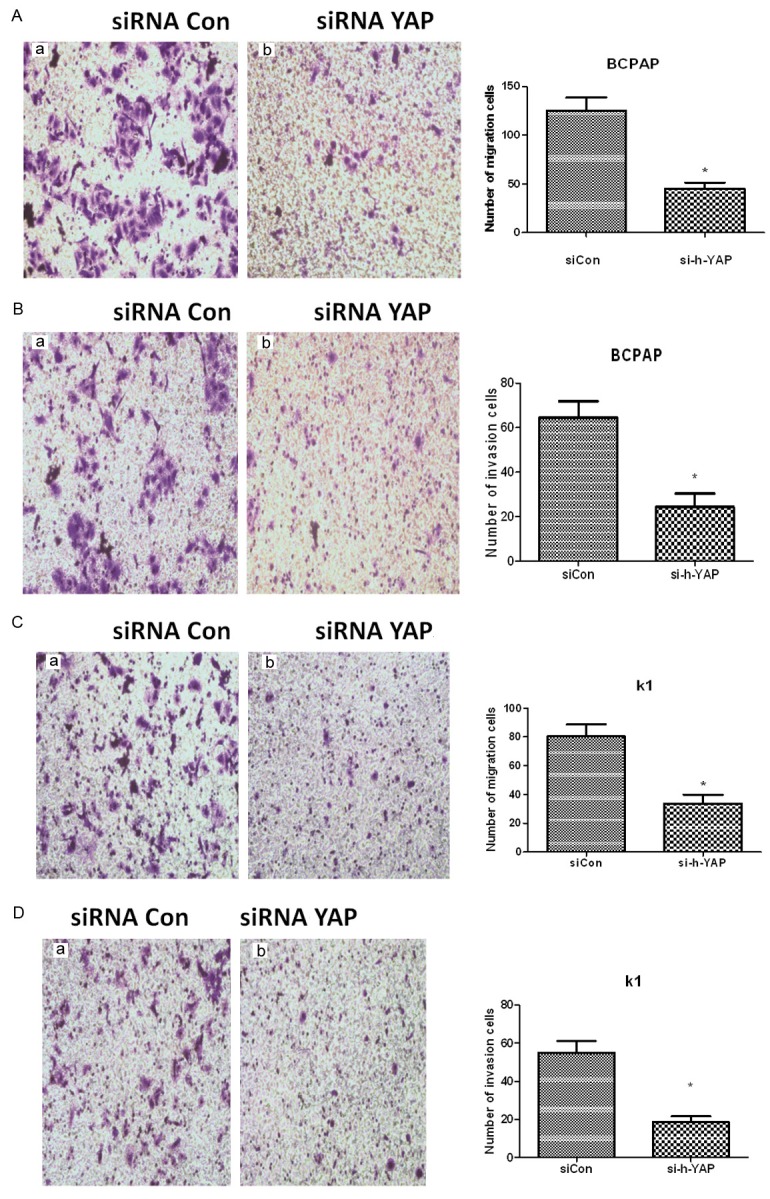
A. YAP knockout inhibits migration of BCPAP cells. BCPAP cells were treated with siRNA Con or siRNA YAP and cell migration assessed. The right hand graph is a quantification of BCPAP migration (*represents P<0.05 compared with siRNA Con, n=3 independent experiments). B. YAP knockout inhibits invasion of BCPAP cells. BCPAP cells were treated with siRNA Con or siRNA YAP and cell invasion ability assessed. The right hand graph is a quantification of BCPAP invasion (*represents P<0.05 compared with siRNA Con, n=3 independent experiments). C. YAP knockout inhibits migration of KI cells. KI cells were treated with siRNA Con or siRNA YAP and cell migration assessed. The right hand graph is a quantification of KI migration (*represents P<0.05 compared with siRNA Con, n=3 independent experiments). D. YAP knockout inhibits invasion of KI cells. KI cells were treated with siRNA Con or siRNA YAP and cell invasion ability assessed. The right hand graph is a quantification of KI invasion (*represents P<0.05 compared with siRNA Con, n=3 independent experiments).
A YAP targeted siRNA causes cell cycle arrest in BCPAP and KI cells
In order to clarify the role of YAP on cell cycle control in BCPAP and KI cells we used propidium iodide staining and cell sorting to quantitate the % of cells in G0/G1, S, and G2/M phases in siRNA Con or siRNA YAP treated cells. The results of this study showed that, compared to siRNA Con treated cells, the proportion of BCPAP and KI cells in the S and G2/M phases cells decreased whereas the proportion of cells in G0/G1 phase increased, indicating that siRNA YAP induced thyroid cancer BCPAP and KI cells to arrest at the G0/G1 phase (Figure 5A, 5B).
Figure 5.
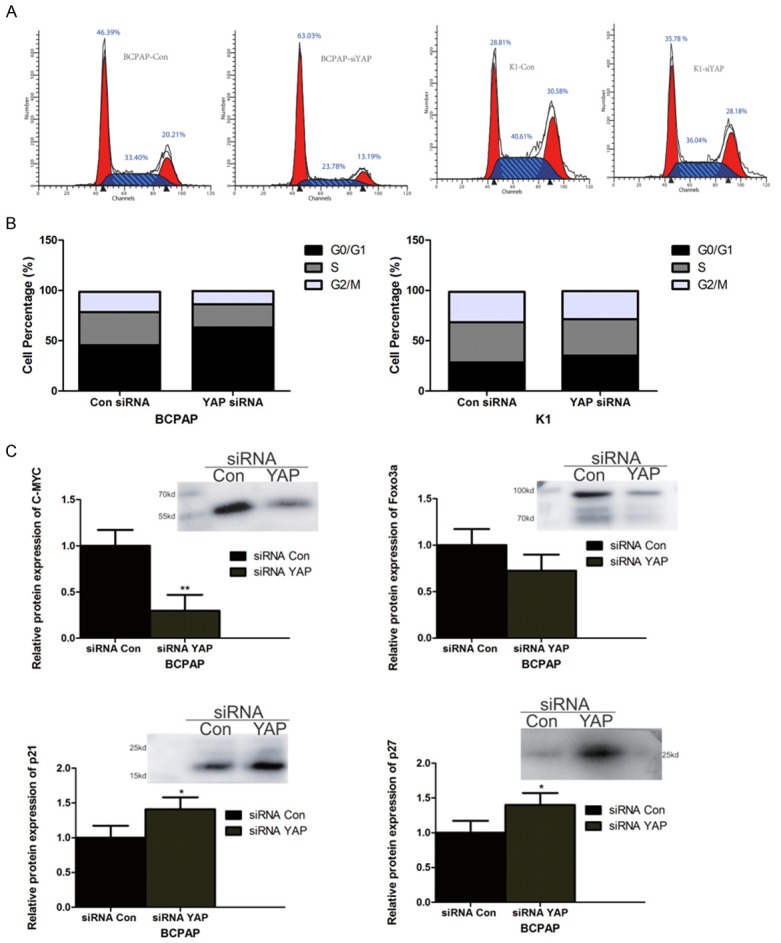
YAP knockout causes BCPAP and KI cells to arrest at the G0/G1 phase and decreases expression of c-Myc and Foxo3a expression while increasing p21 and p27 expression in BCPAP cells. A. Cell sorting of propidium iodide stained BCPAP or KI cells treated with siRNA Con or siRNA YAP. B. Quantification of the proportion of BCPAP and KI cells in G0/G1, S, and G2/M phases. C. Western blots of c-Myc, Foxo3a, p21 and p27 in siRNA Con and siRNA treated BCPAP cells. The graphs represent quantification of the respective protein signal from the Western blots. (*, ** represent P<0.05 and P<0.01 respectively compared with siRNA Con, n=3 independent experiments).
The cell cycle inhibitory protein p27 can arrest cells in the G1 phase by inhibiting cyclin E-CDK2 and cyclin A-CDK2 complexes, indicating that it plays a role in cell differentiation. p21 is also a cell cycle inhibitory protein that is related to stress response and DNA damage and repair. Both of these proteins are target genes for the transcription factors Foxo3a and c-Myc and they are found to be down-regulated in a variety of tumours. As shown in Figure 5C, after suppression of YAP expression in BCPAP cells, the expression levels of Foxo3a and c-Myc, which are closely related to transcriptional control of the cell cycle were both down-regulated. In contrast, the expression of both p21 and p27, which were both low in siRNA Con-treated BCPAP cells, were both significantly increased by YAP silencing.
A YAP targeted siRNA did not affect cell apoptosis in BCPAP and KI cells
As shown in Figure 6, siRNA YAP did not have any significant effect on apoptosis in BCPAP and KI cells, indicating that the apoptotic pathway is not significant in the inhibition of BCPAP and KI cell proliferation observed when YAP is silenced in these cells.
Figure 6.
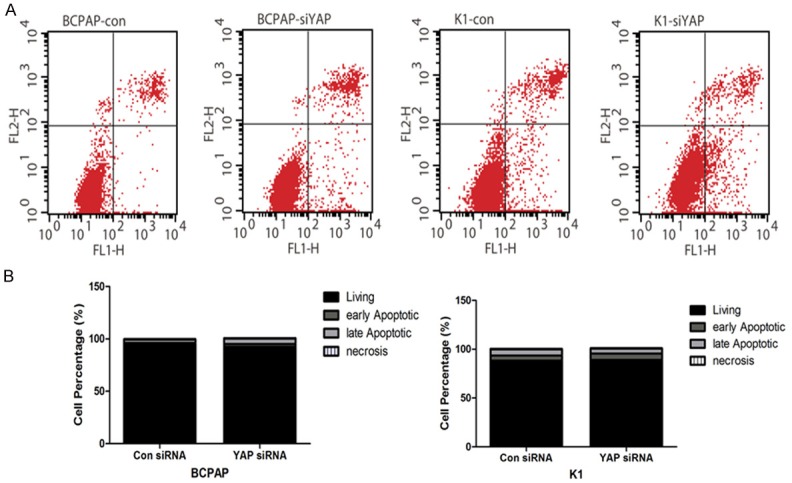
siRNA YAP does not affect cell apoptosis in BCPAP and KI cells. A. FACS sorting of BCPAP and KI cells treated with either siRNA Con or siRNA YAP. B. Quantification of living, early apoptotic, late apoptotic, or necrotic cells BCPAP or KI cells.
A YAP targeted siRNA effect on cell autophagy of BCPAP and KI
Recent studies have shown that, in addition to necrosis and apoptosis, autophagy is a third form of cell death; compared to type I programmed cell death (apoptosis), autophagy is believed to be a type II programmed cell death. One of the indicators of autophagy is lipidation of LC3, which occurs when the soluble LC3 protein (referred to as LC3-I) becomes engulfed by the autophagosome and then is converted into LC3-II by conjugation to phosphatidylethanolamine. We used western blotting to detect the expression levels of these two forms of LC-3 in BCPAP and KI cells. As shown in Figure 7A, the expression levels of LC3-I were slightly higher than LC3-II in siRNA Con-treated BCPAP and KI cells. Both LC3-1 and LC3-II expression levels were inhibited after silencing of YAP by siRNA, which is particularly evident for LC3-I, resulting in a big increase in the LC3-II/LC3-I ratio (Figure 7B, 7C). These data indicate siRNA YAP induces autophagy in both BCPAP and KI cells.
Figure 7.
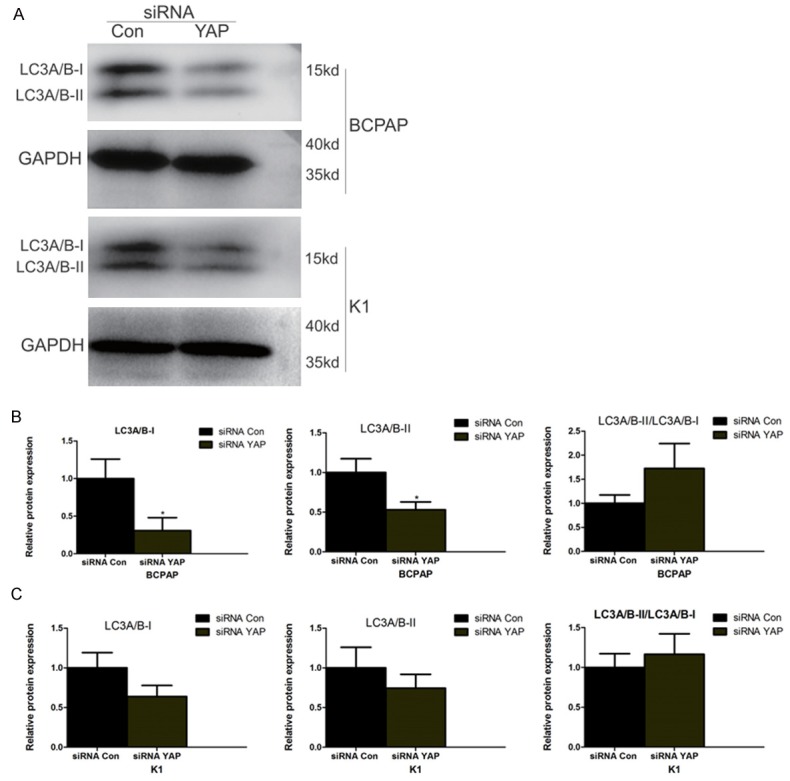
siRNA YAP increases cell autophagy in BCPAP and KI cells. A. Western blots of BCPAP or KI cells treated with siRNA Con or siRNA YAP showing the different forms of LC3A/B namely LC3A/B-1 (non-lipidated) and LC3A/B-II (lipidated). GAPDH was used to normalize protein loading. B and C. Quantification of LC3A/B-I and LC3A/B-II from Western blots and calculation of the LC3A/B-II/LC3A/B-I ratio.
Autophagy is regulated by a variety of cell regulatory factors to maintain its homeostasis, including the autophagy related protein Beclin1, and the autophagy nucleation and extension related Atg5-Atg12-Atg16 complex. The levels of these proteins were detected by western blotting in BCPAP and KI cells after siRNA Con or siRNA YAP treatment. As shown in Figure 8, siRNA YAP treatment significantly reduced expression of Beclin-1 protein as well as the levels of Atg5, Atg12, and Atg16L1 in BCPAP cells. These data confirm that YAP is involved in regulating autophagy in BCPAP and KI cells.
Figure 8.
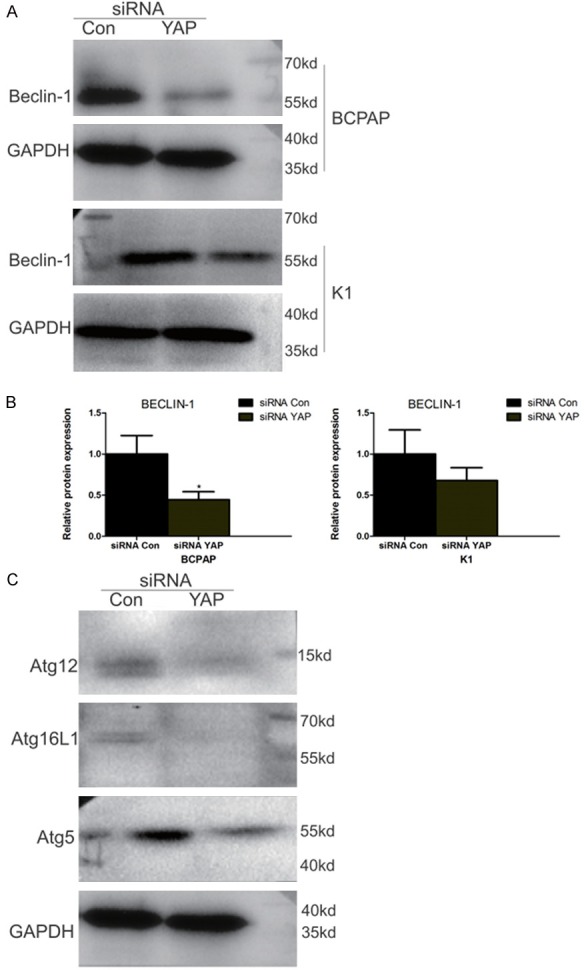
siRNA YAP significantly decreases levels of cell autophagy related proteins in BCPAP cells. (A) Western blots of BCPAP or KI cells treated with siRNA Con or siRNA YAP showing the expression of Beclin-1. GAPDH was used to normalize protein loading. (B) Quantification of Beclin-1 levels in (A). (C) Western blots of BCPAP treated with siRNA Con or siRNA YAP showing the expression of Atg12, Atg16L, and Atg5. GAPDH was used to normalize protein loading.
Discussion
The biological effects of the Hippo pathway are mainly related to the preservation of tissue and organ size and the pathway appears to operate by regulating cell proliferation, cell apoptosis, cell contact inhibition and tumour development, amongst other processes [11-13]. The YAP protein is a key downstream component of the Hippo pathway mediating many of its biological effects through control of transcription. In human malignancies, YAP has been found to function as a proto-oncogene. If the Hippo pathway is inhibited, YAP/TAZ, with the aid of Zona occludens-2 protein, translocate to the nucleus and together with DNA testing and transcription factors (TFs) promote cell proliferation as well as the transcription of anti-apoptotic genes [14]. Therefore, the Hippo pathway kinase cluster must effectively elicit YAP/TAZ phosphorylation in order to prevent their transfer to the nucleus, thereby avoiding activation in the nucleus of genes that promote cell and inhibit apoptosis.
Abnormal protein expression of YAP has been found in many human malignancies. Interestingly, YAP activity plays an important role in both epithelial cell tumours (e.g. breast, liver, and gastrointestinal tract) and stromal tumours (e.g. soft tissue sarcoma). Alizee et al. have shown that the YAP protein, acting as a positive effector of the Hippo pathway, regulated the proliferation of human schwannoma cells and may indeed serve as a potential therapeutic target [15]. Nan et al. also showed that the YAP can directly regulate the P53 promoter thereby increasing P53 expression and that during chemotherapy to treat liver cancer, the YAP protein can induce hepatoma cells apoptosis by regulating P53 to achieve a tumour suppression effect [16]. Yuan et al. have also reported that the YAP protein inhibited proliferation of breast cancer as a tumour suppressor by regulating P73 [17]. Taken together the current data shows an inconsistent picture with the YAP protein being both proliferative and anti-proliferative.
In the nucleus, YAP interacts with transcription factors of the TEAD/TEF family to regulate the transcription of its target genes and thus regulate tumour cell proliferation, epithelial-mesenchymal transition, and metastasis. In our tissue microarray analysis of papillary thyroid carcinoma, we found a significant positive association between YAP protein expression and TNM clinical stage as well as positive lymph node metastasis.
Based on the in vitro result presented here, silencing of YAP can significantly inhibit cell proliferation, migration, invasion, and induce G0/ G1 phase arrest, as well as inducing autophagy in two papillary thyroid cancer cell lines namely BCPAP and KI cells. These affects are therefore consistent with our tissue microarray analysis of human papillary thyroid carcinoma. Serrano et al. have reported that dasatinib (a broad-spectrum tyrosine kinase inhibitor) can inhibit activation of MST1 and LATS, both of which are core kinases in the Hippo pathway, resulting in inactivation of YAP/TAZ-mediated transcription to therapy cancer. Therefore, an inhibitor of YAP could also provide a new direction for potentially treating papillary thyroid cancer.
The limitations of our study include the fact that no studies have yet been conducted in papillary thyroid cancer animal models to confirm the in vitro findings. Other limitations include the failure to demonstrate the specific mechanism by which YAP affects the processes described here and whether it affects the mechanism tolerance of iodine 131intake in papillary thyroid carcinoma and so on.
Conclusion
Our study shows that YAP expression is closely correlated with clinicopathological factors in papillary thyroid cancer, and that silencing YAP in vitro in BCPAP and KI cell lines causes inhibition of proliferation, migration, invasion, inhibition of resistance to stalling in the G0/G1 phase, and up-regulation of p21 and p27. YAP also affects c-Myc and Foxo3a (cycle regulatory transcription factor) expression, both of which are known to participate in thyroid cancer cell cycle regulation. Moreover, YAP appears to regulate autophagy in BCPAP and KI cell lines since inhibition of the expression of YAP can promote cell autophagy.
Acknowledgements
Our results have been presented at the 86th Annual Meeting of the American Thyroid Association on Sep 23, 2016.
Disclosure of conflict of interest
None.
References
- 1.Colonna M, Uhry Z, Guizard AV, Delafosse P, Schvartz C, Belot A, Grosclaude P. Recent trends in incidence, geographical distribution, and survival of papillary thyroid cancer in France. Cancer Epidemiol. 2015;39:511–518. doi: 10.1016/j.canep.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 4.Ye S, Eisinger-Mathason TS. Targeting the Hippo pathway: Clinical implications and therapeutics. Pharmacol Res. 2016;103:270–278. doi: 10.1016/j.phrs.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos A, Camargo FD. The Hippo signaling pathway and stem cell biology. Trends Cell Biol. 2012;22:339–346. doi: 10.1016/j.tcb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren F, Zhang L, Jiang J. Hippo signaling regulates Yorkie nuclear localization and activity through 14-3-3 dependent and independent mechanisms. Dev Biol. 2010;337:303–312. doi: 10.1016/j.ydbio.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Wu Y, Wang H, Zhang Y, Mei L, Fang X, Zhang X, Zhang F, Chen H, Liu Y, Jiang Y, Sun S, Zheng Y, Li N, Huang L. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci U S A. 2014;111:E89–98. doi: 10.1073/pnas.1319190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, Lowe SW, Poon RT, Luk JM. Yesassociated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song M, Cheong JH, Kim H, Noh SH, Kim H. Nuclear expression of Yes-associated protein 1 correlates with poor prognosis in intestinal type gastric cancer. Anticancer Res. 2012;32:3827–3834. [PubMed] [Google Scholar]
- 11.Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008;20:638–646. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Da CL, Xin Y, Zhao J, Luo XD. Significance and relationship between Yes-associated protein and survivin expression in gastric carcinoma and precancerous lesions. World J Gastroenterol. 2009;15:4055–4061. doi: 10.3748/wjg.15.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu AM, Xu Z, Luk JM. An update on targeting Hippo-YAP signaling in liver cancer. Expert Opin Ther Targets. 2012;16:243–247. doi: 10.1517/14728222.2012.662958. [DOI] [PubMed] [Google Scholar]
- 15.Boin A, Couvelard A, Couderc C, Brito I, Filipescu D, Kalamarides M, Bedossa P, De Koning L, Danelsky C, Dubois T, Hupe P, Louvard D, Lallemand D. Proteomic screening identifies a YAP-driven signaling network linked to tumor cell proliferation in human schwannomas. Neuro Oncol. 2014;16:1196–1209. doi: 10.1093/neuonc/nou020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai N, Zhang C, Liang N, Zhang Z, Chang A, Yin J, Li Z, Luo N, Tan X, Luo N, Luo Y, Xiang R, Li X, Reisfeld RA, Stupack D, Lv D, Liu C. Yesassociated protein (YAP) increases chemosensitivity of hepatocellular carcinoma cells by modulation of p53. Cancer Biol Ther. 2013;14:511–520. doi: 10.4161/cbt.24345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan M, Tomlinson V, Lara R, Holliday D, Chelala C, Harada T, Gangeswaran R, Manson-Bishop C, Smith P, Danovi SA, Pardo O, Crook T, Mein CA, Lemoine NR, Jones LJ, Basu S. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15:1752–1759. doi: 10.1038/cdd.2008.108. [DOI] [PubMed] [Google Scholar]


