Abstract
Mitochondrial dysfunction plays an important role in the pathogenesis of diaphragm weakness during sepsis. Recently, hydrogen sulfide (H2S), a gaseous transmitter endogenously generated by cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (3-MST), is found to improve mitochondrial function. The present study aimed to examine whether H2S synthases are expressed in the diaphragm, and investigated the effect of H2S donor in sepsis-induced diaphragm weakness and its relationship with mitochondrial function. Immunohistochemical staining of the rat diaphragm revealed that positive immunoreactivity for CBS, CSE as well as 3-MST was predominately localized to muscle cells. Using a cecal ligation and puncture (CLP)-induced sepsis model, it was found that CBS and CSE, but not 3-MST, was significantly down-regulated in the diaphragm at 24 h post-CLP compared with sham group. To determine the effect of H2S on sepsis-induced diaphragm weakness, H2S donor NaHS was intraperitoneally administered 30 min after CLP operation. NaHS at a dose of 50 μmol/kg significantly decreased the mortality in septic rats. CLP markedly reduced diaphragm-specific force generation (force/cross-sectional area and maximal titanic force), which was improved by NaHS treatment. In addition, CLP caused mitochondrial damage in the diaphragm tissues as evidenced by increased mitochondrial superoxide production, decreased mitochondrial membrane potential and ATP production, as well as mitochondrial ultrastructural abnormalities, which was also attenuated by NaHS treatment. These findings indicate that H2S donor may prevent sepsis-induced diaphragm weakness by preservation of mitochondrial function, suggesting that modulation of H2S levels may be considered as a potential therapeutic approach for diaphragm dysfunction during sepsis.
Keywords: Hydrogen sulfide, diaphragm weakness, sepsis, mitochondria
Introduction
It has been well established that sepsis is a major risk factor for the development of ventilatory muscle dysfunction in critically ill patients [1,2]. Decreased ventilatory muscle contraction, in turn, contributes to respiratory failure and prolonged mechanical ventilation in critically ill patients with septic shock [3]. The diaphragm is recognized as the primary muscle of respiration that contributes 60-80% of respiratory forces [4]. Severe dysfunction of the diaphragm, consisting of decreased maximal force production as well as an increased susceptibility to fatigue, has been documented in several experimental models of sepsis, such as after endotoxin administration [5], cecal ligation and puncture (CLP)-induced bacterial peritonitis [6], and pneumonia [7]. The diaphragm stands out from most skeletal muscles because of its high mitochondrial volume density (2- to 5-fold greater than most limb muscles) and its constant activity [8]. Increasing evidence indicates that mitochondrial dysfunction may play an important role in producing the biochemical abnormalities that are characteristic of sepsis-associated diaphragm weakness [9]. Thus, agent exhibiting mitochondria-protective effects may contribute to ameliorating sepsis-associated diaphragm dysfunction.
Hydrogen sulfide (H2S) has recently been suggested to be “the third endogenous gaseous signaling transmitter” in mammalian tissues [10,11]. Endogenous H2S is generated by cystathionine-b-synthase (CBS), cystathionine-c-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (3-MST). Previous studies have demonstrated that impaired endogenous H2S production is involved in the pathophysiological processes in sepsis-associated multiple organ dysfunction [12,13]. On the other hand, H2S treatment has been shown to exert protective effects via improving mitochondrial function in cardiovascular and nervous system [14]. Our previous studies have also demonstrated that H2S donor protected against endotoxin-induced adrenal insufficiency through improving mitochondrial function [15]. To date, there is no study examining the roles of H2S in regulating the mitochondrial function and contractility of diaphragm muscle in particular, during sepsis.
Thus, in the present study, we first examined the localization of H2S synthases in rat diaphragm muscle, and determined the level of H2S synthases in rat diaphragm muscles obtained from control and septic rats. Then, we evaluated the effects of H2S treatment in sepsis-induced diaphragm weakness and its relationship with mitochondrial function.
Materials and methods
Ethics statement
All experiments were performed in accordance with the animal experimental guidelines issued by the Animal Care and Use Committee at Shanghai Jiaotong University School of Medicine. This study was approved by the Ethical Committee of Experimental Animals of Xinhua Hospital (protocol approval number 2012-1023).
CLP-induced sepsis
Male Sprague-Dawley rats (180-200 g in weight) were obtained from Shanghai SLAC Laboratory Animal Co. (Shanghai, China) and housed at controlled room temperature with free access to food and water under a natural day/night cycle. All animals were acclimatized for 1 week before the experiment in our laboratory. All animals underwent either sham abdominal surgery or cecal ligation puncture as previously described [6]. Briefly, rats were anesthetized with ketamine (80 mg/kg, i.p.). The abdomen was shaved, wiped with 10% povidoneiodine solution, and a 2-cm ventral midline abdominal incision was made. The cecum was exteriorized, ligated with a sterile 3-0 silk suture at the middle of cecum, and punctured once with an 18-gauge needle. The cecum was squeezed to assure expression of a small amount of fecal material and was returned to the abdominal cavity. The incision was closed and kept clean by povidoneiodine solution. The sham-operated rats underwent laparotomy, and the cecum was manipulated without cecal ligation or puncture.
Experimental groups
For the first set of experiments, rats were subjected to sham operation or CLP. Twenty-four hours later, the diaphragm tissues were collected for determining the expression of H2S synthases.
For the second set of experiments, five groups of animals were studied: (1) Sham, sham-operated rats given saline (0.5 ml i.p.); (2) NaHS, sham-operated rats given H2S donor NaHS (50 μmol/kg in 0.5 ml saline i.p.); (3) CLP, rats subjected to CLP and given saline (0.5 ml i.p.); (4) CLP+NaHS (14 μmol/kg), rats subjected to CLP and given 14 μmol/kg NaHS (in 0.5 ml saline i.p.); (5) CLP+NaHS (50 μmol/kg), rats subjected to CLP and given 50 μmol/kg NaHS (in 0.5 ml saline i.p.). Either saline or NaHS was administered 30 min after sham or CLP operation. The mortality rate in each group was observed until 7 days after the surgery.
For the third set of experiments, four groups of animals were studied: (1) Sham, sham-operated rats given saline (0.5 ml i.p.); (2) NaHS, sham-operated rats given H2S donor NaHS (50 μmol/kg in 0.5 ml saline i.p.); (3) CLP, rats subjected to CLP and given saline (0.5 ml i.p.); (4) CLP+NaHS, rats subjected to CLP and given 50 μmol/kg NaHS (in 0.5 ml saline i.p.). Either saline or NaHS was administered 30 min after sham or CLP operation. Twenty-four hours later, the diaphragm tissue samples were collected for the measurements of muscle contraction and biomarkers of mitochondrial function.
Immunohistochemistry
Paraffin sections (5 μm) were rehydrated and microwaved in citric acid buffer to retrieve antigens. After inhibition of endogenous peroxidases with 3% H2O2, sections were incubated with antibodies against CBS (sc-46830, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA), CSE (sc-131905, 1:100), or 3-MST (sc-374326, 1:100) overnight at 4°C. The bound antibodies were detected with the biotin streptavidin-peroxidase system (Santa Cruz). To confirm the specificity of primary antibody, preabsorption of the primary antibody with a 10-fold excess of the blocking peptides sc-46830P, sc-131905P, sc-374326P (Santa Cruz) was performed as a negative control.
Western blot analysis
Rat diaphragm tissues were homogenized in cold RIPA buffer (Beyotime, Jiangsu, China). Equal amounts of approximately 30 μg of protein samples were separated by 10% SDS-PAGE and subsequently transferred to nitrocellulose membranes (Millipore Corp, Bedford, MA). After blockage in 5% skim milk powder in 0.1% Tris-buffered saline/Tween 20 (TBST) for 1.5 h at room temperature, membranes were immunostained using an antibody against CBS, CSE, or 3-MST overnight at 4°C at a dilution of 1:500. After washed with TBST, the membranes were incubated with a secondary horseradish peroxidase-conjugated antibody for 1 h at room temperature. Immunoreactive proteins were visualized using the enhanced chemiluminescence Western blotting detection system (Santa Cruz). The chemiluminiscent signal from the membranes was quantified by a GeneGnome HR scanner using GeneTools software (SynGene). To control sampling errors, the ratio of band intensities to GAPDH was obtained to quantify the relative protein expression level.
In vitro diaphragm contractile measurements
The diaphragm muscle was excised rapidly from the mid-costal region, and a diaphragm muscle strip (-5 mm wide), with intact fibers inserted at the ribs and central tendon, was used for isometric contractile measurements as previously described [16]. Briefly, the diaphragm muscle strip was suspended vertically in a 37°C tissue bath containing Krebs solution: 137 mM NaCl, 4 mM KCl, 1 mM KH2PO4, 2 mM CaCl2, 1 mM MgCl2, 12 mM NaHCO3, and 6.5 mM glucose. The solution was aerated continuously with a gas mixture of 95% O2 and 5% CO2, and a pH of 7.40 was maintained. The rib-end of the muscle was tied to a rigid supporter, and the central muscle tendon was connected to an isometric force transducer mounted on a micrometer. Two silver stimulating electrodes were placed parallel to the muscle strip. After equilibration for 15 min, isometric contractions were recorded at the optimal muscle length (L0) at which maximal isometric tetanic force was observed. Stimuli were applied using rectangular pulse duration of 0.2 ms and train duration of 250 ms, respectively. To ensure supramaximal stimulation, the strips were stimulated at 20% above voltage to obtain maximal forces. The signal was amplified and recorded using a data acquisition system (MPA 2000; Alcott Biotech, Shanghai, China). Maximal tetanic tension was produced by a supramaximal, 250-ms stimulus train (160 HZ). To measure the force-frequency response, each strip was stimulated with a 250-ms train at 10, 20, 40, 60, 80, 100, and 120 HZ, with at least a 2-min interval between each stimulus train. Following completion of all measurements, forces were normalized for muscle cross-sectional areas (CSA), which were estimated using the following formula: CSA (cm2) = muscle mass (g)/[L0 (cm) × muscle density (g/cm3)], where 1.056 g/cm3 represented muscle density.
Isolation of mitochondria
Mitochondrial isolation was performed using Mitochondria Fractionation Kit (Beyotime Biotech) as previously described [15]. Rat diaphragm tissues were quickly removed and placed in chilled (4°C) isolation media (0.25 M sucrose, 10 mM Tris-HCl buffer, pH 7.4, 1 mM EDTA, and 250 µg BSA/ml). The tissues were minced and washed with the isolation media, and 10% (w/v) homogenates were prepared. Nuclei and cell debris were sedimented by centrifugation at 600 g for 10 min at 4°C and discarded. The supernatant was subjected to centrifugation at 11,000 g for 10 min at 4°C. The resulting mitochondrial pellets were suspended in the mitochondrial storage fluid.
Detection of mitochondrial superoxide production
MitoSOX (Molecular Probes) is a cell-permeable probe that accumulates specifically in mitochondria and becomes fluorescent after oxidation by superoxide [15]. MitoSOX was dissolved in DMSO immediately before use, and then applied to isolated mitochondria at a final concentration of 5 μM. After incubation under dark condition for 30 min at 37°C, red fluorescence was read at 485 nm excitation and 590 nm emission using a SynergyTM fluorescence plate reader (Bio-Tek Instruments).
Assessment of mitochondrial membrane potential loss
Mitochondrial membrane potential was detected with fluorescent probe JC-1 (Sigma-Aldrich), which exists predominantly in monomeric form in cells with depolarized mitochondria and displays fluoresced green [17]. Cells with polarized mitochondria predominantly contain JC-1 in aggregate form and show fluoresced red. Loading was done by incubating isolated mitochondria with 2 μM JC-1 for 15 min under dark condition at 37°C. After staining, the red fluorescent signals were excited at 530 nm and detected at 630 nm, and the green fluorescence was excited at 488 nm and detected at 530 nm using a SynergyTM fluorescence plate reader. Mitochondrial damage was assessed by examining mitochondrial membrane depolarization, which was indicated by the ratio of red and green fluorescences.
Measurement of ATP concentration
Samples of isolated mitochondria were homogenized in a protein extraction solution (Pierce). The supernatant after centrifugation at 10,000 g for 10 min was subject to determination of ATP concentration, using an ATP bioluminescence assay (Beyotime). Light emitted from a luciferase-mediated reaction was measured by a tube luminometer (Tecan).
Electron microscopy
Fresh rat diaphragm tissues were fixed in a 4% solution of paraformaldehyde for 24 h. Then, tissues were postfixed with osmium tetraoxide, dehydrated in a graded ethanol series, and embedded in epoxy resin. Samples were sectioned (50 nm), counterstained with uranyl acetate and lead citrate. Finally, the ultrastructure of diaphragm muscle fibers and mitochondria were observed with a Hitachi H-800 Transmission Electron Microscope (Hitachi).
Statistical analysis
Data were expressed as means ± SEM. Statistical significance in experiments comparing only two groups was determined by two-tailed Student’s t-test. The significance of the difference in mean values among more than two groups was evaluated by one-way analysis of variance (ANOVA) followed by a Tukey post hoc test. Comparisons between groups for force frequency measurements were made by a two-way ANOVA followed by Tukey test. To determine statistical significance between survival curves, Kaplan-Meier test was used. All statistical analyses were done with SPSS 19.0. A p-value of less than 0.05 was considered significant.
Results
H2S synthesize enzymes are expressed in rat diaphragm
Immunohistochemical staining in rat diaphragm sections revealed that positive immunoreactivity for CBS, CSE as well as 3-MST was predominately localized to muscle cells of the rat diaphragm (Figure 1A, 1C, 1E). Immunoreactivity was abolished when the antibody was pre-absorbed with excess peptide, thereby confirming the specificity of the antibodies (Figure 1B, 1D, 1F). Western Blot analysis detected single protein bands of approximately 61 kDa, 43 kDa and 33 kDa corresponding to CBS, CSE and 3-MST in rat diaphragm tissues, respectively (Figure 1G).
Figure 1.
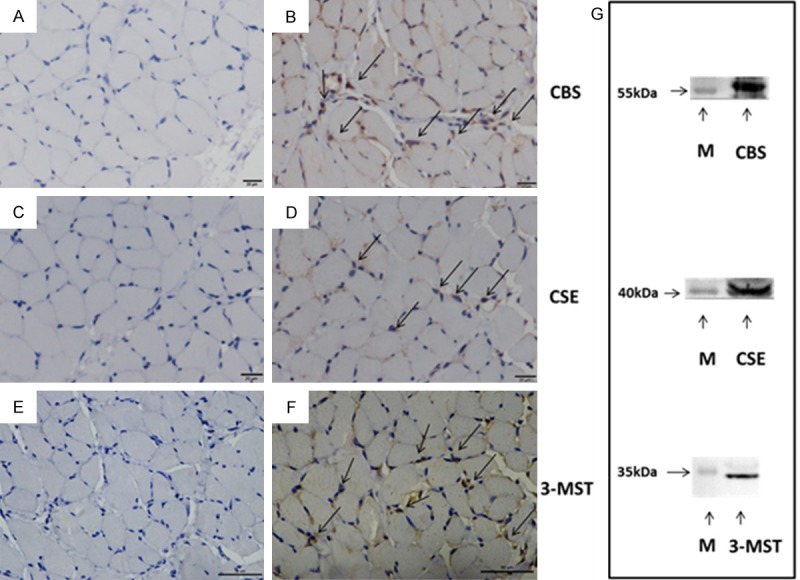
Expression of H2S synthetic enzymes in rat diaphragm. (A, C, E) The negative control of immunohistochemistry, preabsorption of the CBS (A), CSE (C) or 3-MST (E) primary antibody was performed with a 10-fold excess of the blocking peptide, respectively. (B, D, F) Representative sections for positive staining of CBS, CSE and 3-MST in rat diaphragm, respectively. The positive staining was localized to diaphragm muscle cells as indicted by arrows. Scale bars correspond to 20 μm in (A-D), and 50 μm in (E, F), respectively. (G) Western blot analysis of CBS, CSE and 3-MST in rat diaphragm. Positions of molecular weight markers (M) in kDa are shown on the left.
Expression of CBS and CSE, but not 3-MST, are down-regulated in rat diaphragm during sepsis
We next evaluated the protein levels of H2S synthases in the diaphragm of septic rats. Sepsis was induced by performing CLP, a widely used animal model of polymicrobial sepsis that closely resembles sepsis in humans [18]. As shown in Figure 2, it was found that the protein expressions of CBS and CSE were markedly decreased in the diaphragm at 24 h post-CLP compared with sham; however, CLP had no significant effect on 3-MST expression in the diaphragm.
Figure 2.
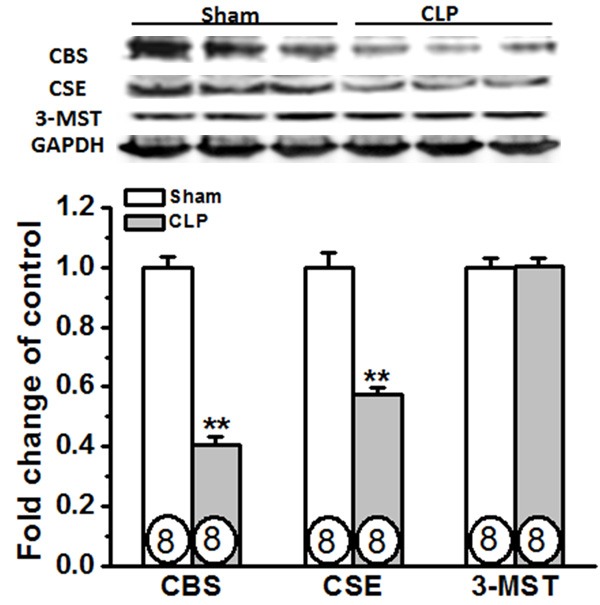
Expression of CBS and CSE, but not 3-MST, are down-regulated in rat diaphragm during sepsis. Rats were subjected to sham operation or CLP. Twenty-four hours later, the diaphragm tissues were collected for determining the expression of H2S synthases. Circles inside bars denoted n per group. Data are expressed as mean ± SEM. **P<0.01 vs Sham.
H2S donor NaHS decreases the mortality in septic rats
Previous in vivo studies assessing the impact of exogenous H2S on sepsis-induced mortality have reported conflicting results, revealing both a protective and a deleterious effect of H2S [12,13]. In the present study, H2S donor NaHS (14 μmol/kg or 50 μmol/kg, i.p.) was administered 30 min after sham or CLP operation. We first carried out a mortality study, and monitored survival for 7 days. Remarkably, we found that 45% (9 of 20) of the septic rats treated with 50 μmol/kg NaHS survived compared with 10% (2 of 20) of untreated septic mice (P<0.05, Figure 3). However, NaHS at a lower dose of 14 μmol/kg had no significant improvement on the survival rate of septic rats. Thus, adopting the similar dosage and delivery of H2S used in the study by Ahmad et al [12], we confirmed a significant increase in the survival of septic rats treated with exogenous H2S donor.
Figure 3.
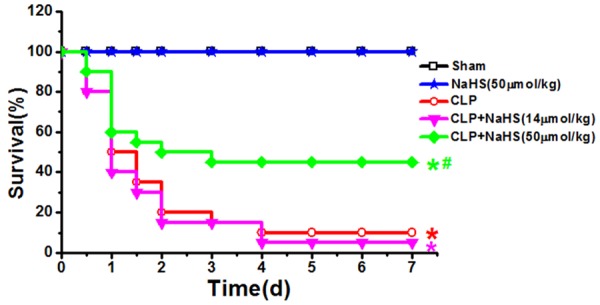
Effect of NaHS on survival rate after CLP. Rats were subjected to sham operation or CLP. Either saline or NaHS at the indicated doses was administered 30 min after sham or CLP operation. The mortality rate in each group was observed until 7 days after the surgery. *P<0.05 vs Sham; #P<0.05 vs CLP.
H2S donor NaHS improves diaphragmatic contractility in septic rats
To determine the effect of exogenous H2S upon contractile function, diaphragms were excised and electrically stimulated in vitro. Figure 4A showed that diaphragms obtained from septic rats elicited a substantial decline in diaphragmatic force generation with mean force averaging 53, 51, 46, 45, 48, 49, and 51% of those generated in response to 10, 20, 40, 60, 80, 100, and 120 Hz of stimulation in diaphragms obtained from sham rats. Administration of NaHS (50 μmol/kg) improved diaphragmatic contractility in septic rats as evidenced by profoundly enhanced diaphragmatic force (Figure 4). NaHS per se had no effect on the diaphragmatic force generation. Figure 4B also showed that the maximal tetanic force was significantly higher in the NaHS treatment group than that in the CLP group.
Figure 4.
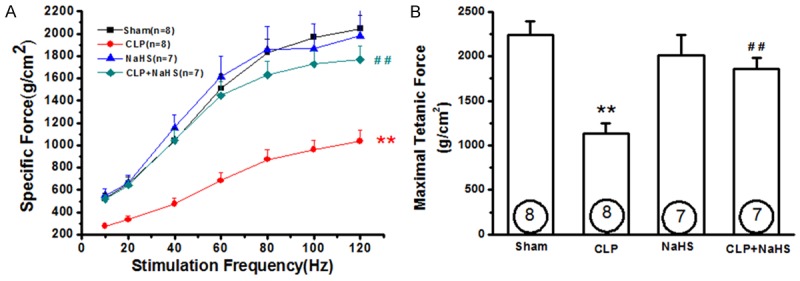
NaHS treatment improves diaphragmatic contractility in septic rats. Rats were subjected to sham operation or CLP. Either saline or NaHS (50 μmol/kg) was administered 30 min after sham or CLP operation. Twenty-four hours later, the diaphragm strips were collected for measurements of diaphragm contractility. A. Force-frequency curves of the diaphragm muscles. B. Diaphragm maximal tetanic force was measured at 160 HZ at the beginning of the protocol. Data are expressed as mean ± SEM. **P<0.01 vs Sham. ##P<0.01 vs CLP.
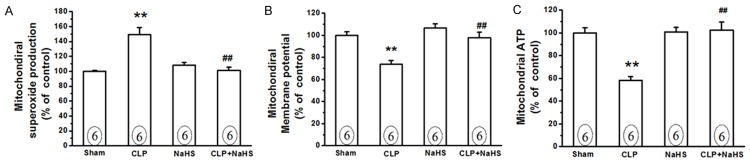
H2S donor NaHS alleviates mitochondrial damage in the diaphragm of septic rats
Previous studies have shown that mitochondrial damage contributes to diaphragmatic weakness during sepsis [9,19]. In the present study, we found that mitochondrial superoxide production was significantly increased (Figure 5A), whereas mitochondrial membrane potential and ATP production were decreased in the diaphragms obtained from septic rats compared with those from sham rats (Figure 5B and 5C), thus confirming a significant mitochondrial oxidative damage in the diaphragm of septic rats. We then examined the effect of exogenous H2S on mitochondrial function in the diaphragm. It was found that NaHS (50 μmol/kg) treatment significantly decreased the mitochondrial superoxide production (Figure 5A, P<0.01 vs CLP group), and reversed decreased mitochondrial membrane potential and ATP production (Figure 5B and 5C, P<0.01 vs CLP group) in the diaphragms of septic rats. NaHS per se had no impact on mitochondrial function in the diaphragm.
Figure 5.
NaHS treatment attenuates mitochondrial damage in the diaphragm of septic rats. Rats were subjected to sham operation or CLP. Either saline or NaHS (50 μmol/kg) was administered 30 min after sham or CLP operation. Twenty-four hours later, the diaphragm tissues were collected for isolation of mitochondria, which were used for measurements of mitochondrial superoxide production (A), membrane potential loss (B), and ATP production (C) as described in “Material and Methods”. Data are expressed as mean ± SEM. **P<0.01 vs Sham. ##P<0.01 vs CLP.
We then assessed ultrastructural changes in the diaphragm using electron microscope analysis. As shown in Figure 6, diaphragm tissue samples obtained from sham-operated rats exhibited intact Z-band and normal mitochondria with dense internal vesicular cristae. In contrast, evidence for Z-band disintegration was found in diaphragm tissue samples obtained from septic rat. In addition, the mitochondria in diaphragm tissues of septic rats exhibited cristae deformation and a significant alteration of the matrix structure. These ultrastructural changes of the diaphragm during sepsis were ameliorated by NaHS (50 μmol/kg) treatment.
Figure 6.
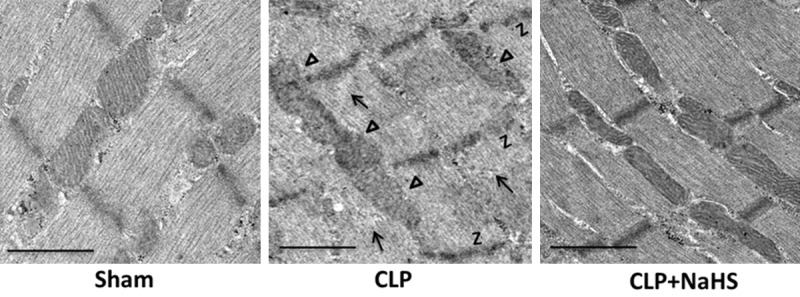
NaHS treatment attenuates ultrastructural abnormalities in the diaphragm of septic rats. Rats were subjected to sham operation or CLP. Either saline or NaHS (50 μmol/kg) was administered 30 min after sham or CLP operation. Twenty-four hours later, the diaphragm tissues were collected for electron microscopy examination. Arrows indicated Z-band disintegration, and triangles indicated mitochondrial morphological abnormalities in the diaphragm obtained from septic rats. Original magnification, ×5000. Scale bars correspond to 10 μm.
Discussion
The present study is the first to show that CBS and CSE, two main synthases of H2S in diaphragm muscles, are markedly down-regulated during sepsis. Moreover, exogenous H2S prevents diaphragm weakness by preservation of mitochondrial function, and meanwhile significantly improves the survival in CLP-induced sepsis.
Species specific differences have been reported with regard to expression of H2S synthases in skeletal muscles. It is found that human skeletal muscles express significant amounts of CBS and CSE, whereas mouse skeletal muscles express non-detectable levels of these enzymes [20,21]. By using immunohistochemistry technique, Du et al [22] demonstrate a detectable, but low expression of CBS and CSE in rat hind-limb skeletal muscle samples. In addition, 3-MST is located in rat hind-limb muscles at a comparable level with that observed in the liver and kidney. These findings was confirmed by our immunohistochemistry results showing the relatively low levels of CBS and CSE, as well as high levels of 3-MST in diaphragm muscle samples.
Severe inflammation has been found to cause dysregulation of H2S synthases in a variety of tissues including lung, liver, kidney, heart and adrenal glands [12,13,15]. For example, liver CBS expression and cardiac CSE expression are significantly up-regulated during CLP-induced sepsis in rodents [12,13]. Upregulation of CBS and CSE is also found in liver and kidney tissues obtained from rodent endotoxemia models [23,24]. In contrast, our previous study indicates that protein levels of CBS and CSE are markedly decreased in adrenal glands during endotoxemia [15]. A novel finding of this study is the demonstration that experimental sepsis leads to significant down-regulation of CBS and CSE protein expression in diaphragm muscles. Taken together, these results suggest that systemic inflammatory challenge causes dysregulation of endogenous H2S synthases in a tissue-specific manner. In addition, we have previous found that MG132, a proteasome inhibitor, completely reversed the inhibitory effect of endotoxin on CBS and CSE expression, thus suggesting a post-transcriptional, proteasome-dependent regulation of CBS and CSE expression by endotoxin in adrenocortical cells [15]. Activation of proteasome proteolytic pathway has been implicated in the development of diaphragm dysfunction during endotoxemia and CLP-induced sepsis [25,26]. Thus, it is of interest to know whether proteasome proteolytic pathway contributes to the down-regulation of CBS and CSE expression in diaphragm muscles during severe inflammation, which merits further investigation.
The effects of H2S on smooth muscle contractility, either inhibitory or stimulatory, have been well documented in a variety of organs throughout the body such as blood vessels [27], bladder [28], gastrointestinal tract [29], trachea [30], oviduct [31], and uterus [32]. However, few studies have examined the effect of H2S on striated muscle contractility. It has been found that NaHS treatment attenuates isoproterenol-augmented contraction in the electrically-stimulated rat ventricular myocytes in vitro [33]. Using an Akt2-knockout murine model of insulin resistance, Hu et al [34] demonstrate that NaHS treatment significantly alleviates Akt2 deficiency-induced cardiac contractile dysfunction in vivo. More recently, Shiina et al [35] report the inhibitory effect of H2S on the motility of esophageal striated muscle in rats. In the present study, we provided the first direct evidence that intraperitoneal administration of NaHS 30 min after the onset of sepsis could exert significant protection against sepsis-induced contractile dysfunction in rat diaphragm muscle.
A number of mechanisms have been implicated in the pathogenesis of diaphragmatic weakness in established sepsis [36,37]. Since high mitochondrial volume density is one of the most prominent ultrastructural features of the diaphragm muscle [8], recent interest has focused on the contribution of mitochondrial dysfunction to severe inflammation-associated diaphragmatic weakness. Previous studies have shown that either sepsis or endotoxemia can induce mitochondrial dysfunction in the diaphragm, as evidenced by increase in mitochondria ROS production, downregulation of diaphragm electron transport chain, and derangements in mitochondrial bioenergetics [38-40]. On the other hand, H2S is known to be a direct scavenger of ROS [10,11]. Moreover, H2S has been found to exert cytoprotection by attenuating mitochondrial ROS production, increasing ATP synthesis as well as inhibiting mitochondrial membrane depolarization in a variety of tissues including cardiovascular, nervous system and adrenal glands [14,15,41]. Consistent with above studies, the present study showed that CLP-induced sepsis led to mitochondrial oxidative injury in diaphragm muscles, which could be reversed by H2S donor NaHS. These data suggest that the protective effect of H2S against sepsis-induced diaphragm weakness may be, at least in part, related to the preservation of mitochondrial function.
Previous studies have shown conflicting results about the effects of exogenous H2S on experimental sepsis. Studies from Bathia’s group show that an i.v. injection of NaHS at a dose of 10 mg/kg significantly aggravated sepsis-associated systemic inflammation and organ injury in mice [13]. In contrast, a beneficial effect has been observed by Cunha’s group [42] and Ferlito et al [43] when H2S is administered s.c. at a dose of 100 µmol/kg (about 5.6 mg/kg) in murine models of CLP-induced sepsis. More recently, Szabo’s group investigate the effect of various doses of NaHS (1, 3 or 6 mg/kg) on sepsis-induced mortality in a rat model of CLP-induced sepsis [12]. Their results indicate that only treatment at the 3 mg/kg NaHS dose level (administered i.p. every 6 hours over a 24-hour period) significantly improved the survival rate in CLP-induced sepsis. However, neither 1 mg/kg nor 6 mg/kg NaHS treatment affects CLP-induced mortality. In addition, they also find that NaHS treatment at a single dose of 3 mg/kg significantly attenuates the sepsis-induced multiple organ dysfunction and systemic inflammation. In line with the findings of Szabo’s group [12], our study found that i.p. administration of NaHS at a dose of 50 µmol/kg (about 2.8 mg/kg), but not a lower dose of 14 µmol/kg (about 0.78 mg/kg), protected rats from sepsis-induced mortality.
In summary, we report that the suppression of CBS and CSE expression is associated with diaphragm weakness during cecal ligation puncture-induced sepsis. Additionally, exogenous H2S prevents diaphragm weakness by preservation of mitochondrial function, and meanwhile significantly improves the survival in CLP-induced sepsis. Notably, Li et al [44,45] recently demonstrate that H2S can protect the medullary respiratory centers against hypoxia-induced injury, thus antagonizing the inhibitory effect of hypoxia on rhythmic discharge of the diaphragm in rats. It has been well recognized that sepsis is a major cause of hypoxemia among critically ill patients [46]. Thus, we cannot exclude the possibility that the beneficial effect of H2S on respiratory centers may also contribute to the H2S-mediated protection against sepsis-induced mortality. Taken together, these findings suggest that H2S donor might be important agents for treatment of respiratory failure in patients with severe sepsis.
Acknowledgements
We acknowledge the extensive technical assistance provided by Chang-Nan Wang (Second Military Medical University, China). H. Zhang was supported by Shanghai Municipal Commission of Health and Family Planning (20154Y0099). Y. Liu, X. Zhu and L. Jiang were supported by National Natural Science Foundation of China (No. 31671213, 31371164, 31271270, 81672266, 81571929, 81272144).
Disclosure of conflict of interest
None.
References
- 1.Jung B, Nougaret S, Conseil M, Coisel Y, Futier E, Chanques G, Molinari N, Lacampagne A, Matecki S, Jaber S. Sepsis is associated with a preferential diaphragmatic atrophy: a critically ill patient study using tridimensional computed tomography. Anesthesiology. 2014;120:1182–1191. doi: 10.1097/ALN.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 2.Lanone S, Mebazaa A, Heymes C, Henin D, Poderoso JJ, Panis Y, Zedda C, Billiar T, Payen D, Aubier M, Boczkowski J. Muscular contractile failure in septic patients: role of the inducible nitric oxide synthase pathway. Am J Respir Crit Care Med. 2000;162:2308–2315. doi: 10.1164/ajrccm.162.6.2001097. [DOI] [PubMed] [Google Scholar]
- 3.Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003;68:10–48. doi: 10.1164/rccm.2206020. [DOI] [PubMed] [Google Scholar]
- 4.Wilson TA, Legrand A, Gevenois PA, De Troyer A. Respiratory effects of the external and internal intercostal muscles in humans. J Physiol. 2001;530:319–330. doi: 10.1111/j.1469-7793.2001.0319l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Supinski GS, Callahan LA. Calpain activation contributes to endotoxin induced diaphragmatic dysfunction. Am J Respir Cell Mol Biol. 2010;42:80–87. doi: 10.1165/rcmb.2008-0275OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Supinski GS, Wang W, Callahan LA. Caspase and calpain activation both contribute to sepsis-induced diaphragmatic weakness. J Appl Physiol. 2009;107:1389–1396. doi: 10.1152/japplphysiol.00341.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demoule A, Divangahi M, Yahiaoui L, Danialou G, Gvozdic D, Petrof BJ. Chemokine receptor and ligand upregulation in the diaphragm during endotoxemia and Pseudomonas lung infection. Mediators Inflamm. 2009;2009:860565. doi: 10.1155/2009/860565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoppeler H, Mathieu O, Krauer R, Claassen H, Armstrong RB, Weibel ER. Design of the mammalian respiratory system. VI Distribution of mitochondria and capillaries in various muscles. Respir Physiol. 1981;44:87–111. doi: 10.1016/0034-5687(81)90078-5. [DOI] [PubMed] [Google Scholar]
- 9.Callahan LA, Supinski GS. Diaphragm and cardiac mitochondrial creatine kinases are impaired in sepsis. J Appl Physiol. 2007;102:44–53. doi: 10.1152/japplphysiol.01204.2005. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia M. Hydrogen sulfide as a vasodilator. IUBMB Life. 2005;57:603–606. doi: 10.1080/15216540500217875. [DOI] [PubMed] [Google Scholar]
- 11.Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal. 2010;12:1111–1123. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad A, Druzhyna N, Szabo C. Delayed treatment with sodium hydrosulfide improves regional blood flow and alleviates cecal ligation and puncture (CLP)-induced septic shock. Shock. 2016;46:183–193. doi: 10.1097/SHK.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Zhi L, Moore PK, Bhatia M. Role of hydrogen sulfide in cecal ligation and puncture-induced sepsis in the mouse. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1193–1201. doi: 10.1152/ajplung.00489.2005. [DOI] [PubMed] [Google Scholar]
- 14.Fu M, Zhang W, Wu L, Yang G, Li H, Wang R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc Natl Acad Sci U S A. 2012;109:2943–2948. doi: 10.1073/pnas.1115634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang CN, Liu YJ, Duan GL, Zhao W, Li XH, Zhu XY, Ni X. CBS and CSE are critical for maintenance of mitochondrial function and glucocorticoid production in adrenal cortex. Antioxid Redox Signal. 2014;21:2192–2207. doi: 10.1089/ars.2013.5682. [DOI] [PubMed] [Google Scholar]
- 16.Yang M, Wang H, Han G, Chen L, Huang L, Jiang J, Li S. Phrenic nerve stimulation protects against mechanical ventilation-induced diaphragm dysfunction in rats. Muscle Nerve. 2013;48:958–962. doi: 10.1002/mus.23850. [DOI] [PubMed] [Google Scholar]
- 17.Murugan RS, Priyadarsini RV, Ramalingam K, Hara Y, Karunagaran D, Nagini S. Intrinsic apoptosis and NF-κB signaling are potential molecular targets for chemoprevention by black tea polyphenols in HepG2 cells in vitro and in a rat hepatocarcinogenesis model in vivo. Food Chem Toxicol. 2010;48:3281–3287. doi: 10.1016/j.fct.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW 3rd, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24:52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 19.Callahan LA, Supinski GS. Sepsis induces diaphragm electron transport chain dysfunction and protein depletion. Am J Resp Crit Care Med. 2005;172:861–868. doi: 10.1164/rccm.200410-1344OC. [DOI] [PubMed] [Google Scholar]
- 20.Chen NC, Yang F, Capecci LM, Gu Z, Schafer AI, Durante W, Yang XF, Wang H. Regulation of homocysteine metabolism and methylation in human and mouse tissues. FASEB J. 2010;24:2804–2817. doi: 10.1096/fj.09-143651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veeranki S, Tyagi SC. Role of hydrogen sulfide in skeletal muscle biology and metabolism. Nitric Oxide. 2015;46:66–71. doi: 10.1016/j.niox.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du JT, Li W, Yang JY, Tang CS, Li Q, Jin HF. Hydrogen sulfide is endogenously generated in rat skeletal muscle and exerts a protective effect against oxidative stress. Chin Med J (Engl) 2013;126:930–936. [PubMed] [Google Scholar]
- 23.Collin M, Anuar FB, Murch O, Bhatia M, Moore PK, Thiemermann C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. Br J Pharmacol. 2005;146:498–505. doi: 10.1038/sj.bjp.0706367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 25.Calder PC. A novel effect of eicosapentaenoic acid: improved diaphragm strength in endotoxemia. Crit Care. 2010;14:143. doi: 10.1186/cc8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Supinski GS, Vanags J, Callahan LA. Effect of proteasome inhibitors on endotoxin-induced diaphragm dysfunction. Am J Physiol Lung Cell Mol Physiol. 2009;296:L994–L1001. doi: 10.1152/ajplung.90404.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang G, Wang R. H2S and blood vessels: an overview. Handb Exp Pharmacol. 2015;230:85–110. doi: 10.1007/978-3-319-18144-8_4. [DOI] [PubMed] [Google Scholar]
- 28.Fernandes VS, Xin W, Petkov GV. Novel mechanism of hydrogen sulfide-induced guinea pig urinary bladder smooth muscle contraction: role of BK channels and cholinergic neurotransmission. Am J Physiol Cell Physiol. 2015;309:C107–116. doi: 10.1152/ajpcell.00021.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallego D, Clavé P, Donovan J, Rahmati R, Grundy D, Jiménez M, Beyak MJ. The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogastroenterol Motil. 2008;20:1306–1316. doi: 10.1111/j.1365-2982.2008.01201.x. [DOI] [PubMed] [Google Scholar]
- 30.Kubo S, Doe I, Kurokawa Y, Kawabata A. Hydrogen sulfide causes relaxation in mouse bronchial smooth muscle. J Pharmacol Sci. 2007;104:392–396. doi: 10.1254/jphs.sc0070199. [DOI] [PubMed] [Google Scholar]
- 31.Ning N, Zhu J, Du Y, Gao X, Liu C, Li J. Dysregulation of hydrogen sulphide metabolism impairs oviductal transport of embryos. Nat Commun. 2014;5:4107. doi: 10.1038/ncomms5107. [DOI] [PubMed] [Google Scholar]
- 32.Hu R, Lu J, You X, Zhu X, Hui N, Ni X. Hydrogen sulfide inhibits the spontaneous and oxytocininduced contractility of human pregnant myometrium. Gynecol Endocrinol. 2011;27:900–904. doi: 10.3109/09513590.2010.551563. [DOI] [PubMed] [Google Scholar]
- 33.Yong QC, Pan TT, Hu LF, Bian JS. Negative regulation of beta-adrenergic function by hydrogen sulphide in the rat hearts. J Mol Cell Cardiol. 2008;44:701–710. doi: 10.1016/j.yjmcc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Hu N, Dong M, Ren J. Hydrogen sulfide alleviates cardiac contractile dysfunction in an Akt2-knockout murine model of insulin resistance: role of mitochondrial injury and apoptosis. Am J Physiol Regul Integr Comp Physiol. 2014;306:R761–771. doi: 10.1152/ajpregu.00327.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiina T, Shima T, Horii K, Naitou K, Nakamori H, Sano Y, Shimizu Y. Inhibitory action of hydrogen sulfide on esophageal striated muscle motility in rats. Eur J Pharmacol. 2016;771:123–129. doi: 10.1016/j.ejphar.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Berger D, Bloechlinger S, von Haehling S, Doehner W, Takala J, Z’Graggen WJ, Schefold JC. Dysfunction of respiratory muscles in critically ill patients on the intensive care unit. J Cachexia Sarcopenia Muscle. 2016;7:403–412. doi: 10.1002/jcsm.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanone S, Taillé C, Boczkowski J, Aubier M. Diaphragmatic fatigue during sepsis and septic shock. Intensive Care Med. 2005;31:1611–1617. doi: 10.1007/s00134-005-2748-4. [DOI] [PubMed] [Google Scholar]
- 38.Peruchi BB, Petronilho F, Rojas HA, Constantino L, Mina F, Vuolo F, Cardoso MR, Gonçalves CL, Rezin GT, Streck EL, Dal-Pizzol F. Skeletal muscle electron transport chain dysfunction after sepsis in rats. J Surg Res. 2011;167:e333–338. doi: 10.1016/j.jss.2010.11.893. [DOI] [PubMed] [Google Scholar]
- 39.Vanasco V, Cimolai MC, Evelson P, Alvarez S. The oxidative stress and the mitochondrial dysfunction caused by endotoxemia are prevented by alpha-lipoic acid. Free Radic Res. 2008;42:815–823. doi: 10.1080/10715760802438709. [DOI] [PubMed] [Google Scholar]
- 40.Zolfaghari PS, Carré JE, Parker N, Curtin NA, Duchen MR, Singer M. Skeletal muscle dysfunction is associated with derangements in mitochondrial bioenergetics (but not UCP3) in a rodent model of sepsis. Am J Physiol Endocrinol Metab. 2015;308:E713–725. doi: 10.1152/ajpendo.00562.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang CN, Duan GL, Liu YJ, Yu Q, Tang XL, Zhao W, Li XH, Zhu XY, Ni X. Overproduction of nitric oxide by endothelial cells and macrophages contributes to mitochondrial oxidative stress in adrenocortical cells and adrenal insufficiency during endotoxemia. Free Radic Biol Med. 2015;83:31–40. doi: 10.1016/j.freeradbiomed.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 42.Spiller F, Orrico MI, Nascimento DC, Czaikoski PG, Souto FO, Alves-Filho JC, Freitas A, Carlos D, Montenegro MF, Neto AF, Ferreira SH, Rossi MA, Hothersall JS, Assreuy J, Cunha FQ. Hydrogen sulfide improves neutrophil migration and survival in sepsis via K+ATP channel activation. Am J Respir Crit Care Med. 2010;182:360–368. doi: 10.1164/rccm.200907-1145OC. [DOI] [PubMed] [Google Scholar]
- 43.Ferlito M, Wang Q, Fulton WB, Colombani PM, Marchionni L, Fox-Talbot K, Paolocci N, Steenbergen C. Hydrogen sulfide [corrected] increases survival during sepsis: protective effect of CHOP inhibition. J Immunol. 2014;192:1806–1814. doi: 10.4049/jimmunol.1300835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Chen L, Hou X, Zhou H, Zheng Y. Hydrogen sulfide attenuates hypoxia-induced respiratory suppression in anesthetized adult rats. Respir Physiol Neurobiol. 2016;220:1–9. doi: 10.1016/j.resp.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Li H, Hou X, Ding Y, Nie L, Zhou H, Nie Z, Tang Y, Chen L, Zheng Y. Effects of H2S on the central regulation of respiration in adult rats. Neuroreport. 2014;25:358–366. doi: 10.1097/WNR.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 46.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R Surviving sepsis campaign guidelines committee including the pediatric subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]