Abstract
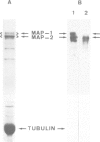
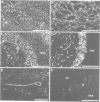
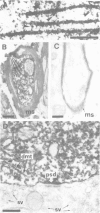
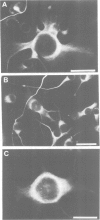
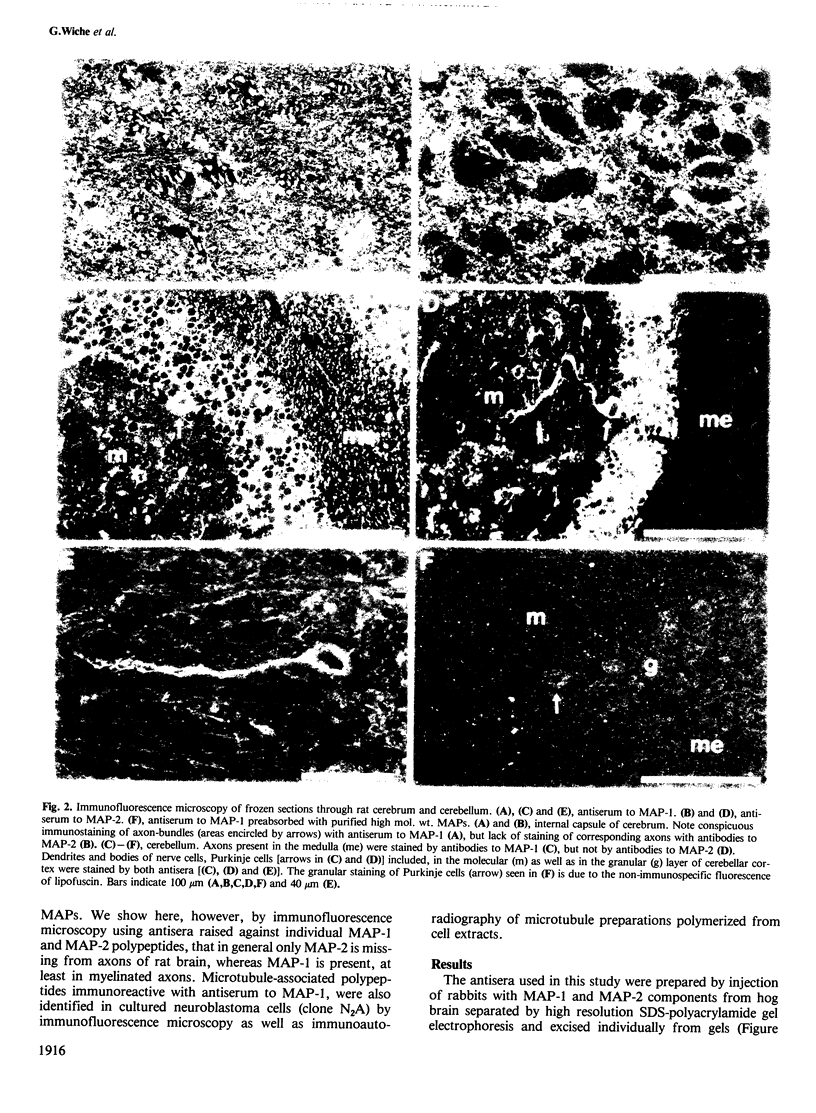
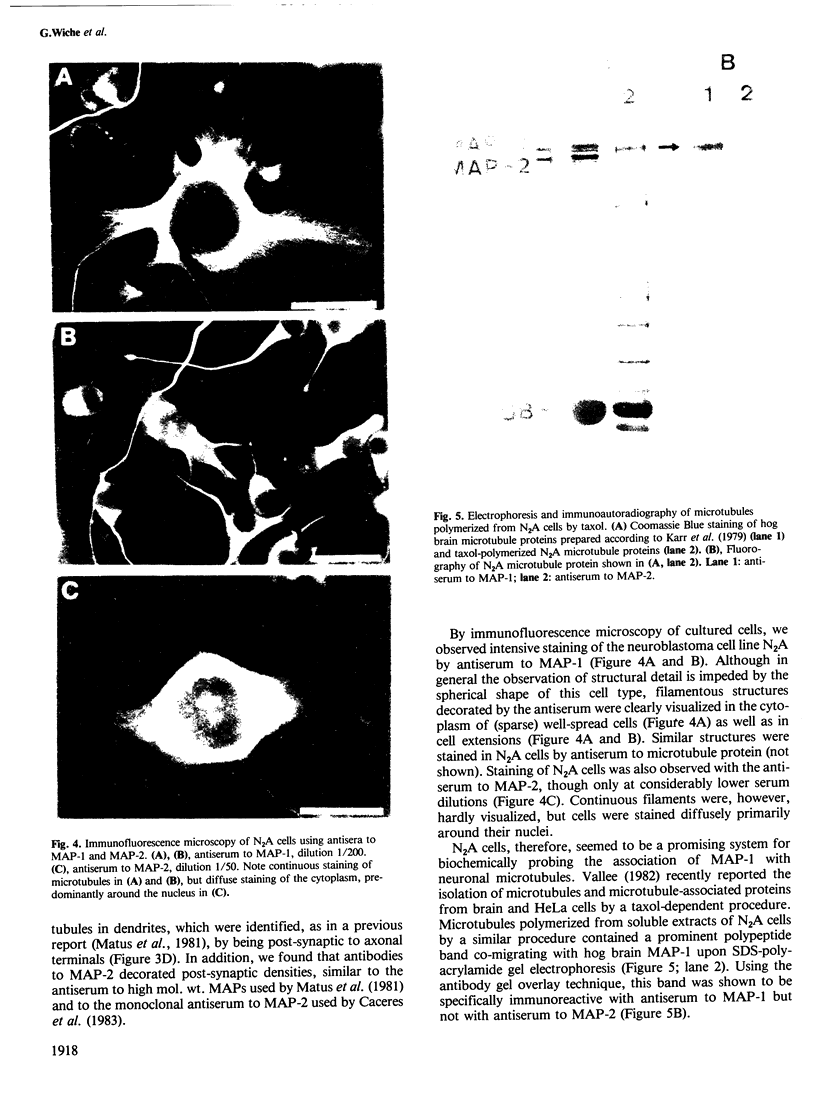
To study the individual location of the microtubule proteins MAP-1 and MAP-2 in neuronal tissues and cells, antisera to electrophoretically purified MAP-1 and MAP-2 components were raised in rabbits. When frozen sections through rat brain were examined by immunofluorescence microscopy the antibodies to MAP-1 strongly stained a variety of nerve cells including dendrites and myelinated axons in the cerebrum and cerebellum. Antibodies to MAP-2 showed similar staining patterns, except that myelinated axons were unstained. These results were confirmed by immunoelectron microscopy of frozen sections through cerebellum using the peroxidase technique. Thereby, the association of MAP-1 with microtubules was also clearly demonstrated. When cultured mouse neuroblastoma N2A cells were examined by immunofluorescence microscopy the antiserum to MAP-1 brightly stained filamentous structures resembling microtubules, whereas relatively weak and diffuse staining of the cytoplasm was observed with the antiserum to MAP-2. In agreement with the immunolocalization, MAP-1, but not MAP-2, was found as a prominent component of microtubules proteins polymerized in vitro by taxol from soluble N2A cell extracts. Together these results indicate that neuronal microtubules are preferentially associated with distinct high mol. wt. polypeptides. Therefore, they support the concept that different complements of associated proteins determine distinct functions of microtubules.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos L. A. Arrangement of high molecular weight associated proteins on purified mammalian brain microtubules. J Cell Biol. 1977 Mar;72(3):642–654. doi: 10.1083/jcb.72.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt R., Matus A. Initial phase of dendrite growth: evidence for the involvement of high molecular weight microtubule-associated proteins (HMWP) before the appearance of tubulin. J Cell Biol. 1982 Feb;92(2):589–593. doi: 10.1083/jcb.92.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibring T., Baxandall J., Denslow S., Walker B. Heterogeneity of the alpha subunit of tubulin and the variability of tubulin within a single organism. J Cell Biol. 1976 May;69(2):301–312. doi: 10.1083/jcb.69.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K. Changes in cellular glycoproteins after transformation: identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4457–4461. doi: 10.1073/pnas.73.12.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A., Payne M. R., Binder L. I., Steward O. Immunocytochemical localization of actin and microtubule-associated protein MAP2 in dendritic spines. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1738–1742. doi: 10.1073/pnas.80.6.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Hwo S. Y., Kirschner M. W. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol. 1977 Oct 25;116(2):207–225. doi: 10.1016/0022-2836(77)90213-3. [DOI] [PubMed] [Google Scholar]
- Connolly J. A., Kalnins V. I., Cleveland D. W., Kirschner M. W. Intracellular localization of the high molecular weight microtubule accessory protein by indirect immunofluorescence. J Cell Biol. 1978 Mar;76(3):781–786. doi: 10.1083/jcb.76.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentler W. L., Granett S., Rosenbaum J. L. Ultrastructural localization of the high molecular weight proteins associated with in vitro-assembled brain microtubules. J Cell Biol. 1975 Apr;65(1):237–241. doi: 10.1083/jcb.65.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Denk H., Schmid E., Osborn M., Weber K. Ultrastructural, biochemical, and immunologic characterization of Mallory bodies in livers of griseofulvin-treated mice. Fimbriated rods of filaments containing prekeratin-like polypeptides. Lab Invest. 1979 Feb;40(2):207–220. [PubMed] [Google Scholar]
- Franke W. W., Heid H. W., Grund C., Winter S., Freudenstein C., Schmid E., Jarasch E. D., Keenan T. W. Antibodies to the major insoluble milk fat globule membrane-associated protein: specific location in apical regions of lactating epithelial cells. J Cell Biol. 1981 Jun;89(3):485–494. doi: 10.1083/jcb.89.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I., Littauer U. Z. Tubulin microheterogeneity increases with rat brain maturation. Nature. 1978 Nov 23;276(5686):411–413. doi: 10.1038/276411a0. [DOI] [PubMed] [Google Scholar]
- Gozes I., Sweadner K. J. Multiple tubulin forms are expressed by a single neurone. Nature. 1981 Dec 3;294(5840):477–480. doi: 10.1038/294477a0. [DOI] [PubMed] [Google Scholar]
- Izant J. G., McIntosh J. R. Microtubule-associated proteins: a monoclonal antibody to MAP2 binds to differentiated neurons. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4741–4745. doi: 10.1073/pnas.77.8.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Weatherbee J. A., McIntosh J. R. A microtubule-associated protein in the mitotic spindle and the interphase nucleus. Nature. 1982 Jan 21;295(5846):248–250. doi: 10.1038/295248a0. [DOI] [PubMed] [Google Scholar]
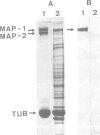
- Karr T. L., White H. D., Purich D. L. Characterization of brain microtubule proteins prepared by selective removal of mitochondrial and synaptosomal components. J Biol Chem. 1979 Jul 10;254(13):6107–6111. [PubMed] [Google Scholar]
- Kim H., Binder L. I., Rosenbaum J. L. The periodic association of MAP2 with brain microtubules in vitro. J Cell Biol. 1979 Feb;80(2):266–276. doi: 10.1083/jcb.80.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. A., Rodionov V. I., Bershadsky A. D., Gelfand V. I., Rosenblat V. A. High molecular weight protein MAP 2 promoting microtubule assembly in vitro is associated with microtubules in cells. Cell Biol Int Rep. 1980 Nov;4(11):1017–1024. doi: 10.1016/0309-1651(80)90174-5. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S. A., Rodionov V. I., Gelfand V. I., Rosenblat V. A. Microtubule-associated protein MAP1 promotes microtubule assembly in vitro. FEBS Lett. 1981 Dec 7;135(2):241–244. doi: 10.1016/0014-5793(81)80791-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mareck A., Fellous A., Francon J., Nunez J. Changes in composition and activity of microtubule-associated proteins during brain development. Nature. 1980 Mar 27;284(5754):353–355. doi: 10.1038/284353a0. [DOI] [PubMed] [Google Scholar]
- Matus A. I., NG M., Jones D. H. Immunohistochemical localization of neurofilament antigen in rat cerebellum. J Neurocytol. 1979 Aug;8(4):513–525. doi: 10.1007/BF01214806. [DOI] [PubMed] [Google Scholar]
- Matus A., Bernhardt R., Hugh-Jones T. High molecular weight microtubule-associated proteins are preferentially associated with dendritic microtubules in brain. Proc Natl Acad Sci U S A. 1981 May;78(5):3010–3014. doi: 10.1073/pnas.78.5.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P., Walter U., Theurkauf W. E., Vallee R. B., De Camilli P. Frozen tissue sections as an experimental system to reveal specific binding sites for the regulatory subunit of type II cAMP-dependent protein kinase in neurons. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5562–5566. doi: 10.1073/pnas.79.18.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Borisy G. G. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherline P., Schiavone K. Immunofluorescence localization of proteins of high molecular weight along intracellular microtubules. Science. 1977 Dec 9;198(4321):1038–1040. doi: 10.1126/science.337490. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Tack B. F., Dean J., Eilat D., Lorenz P. E., Schechter A. N. Tritium labeling of proteins to high specific radioactivity by reduction methylation. J Biol Chem. 1980 Sep 25;255(18):8842–8847. [PubMed] [Google Scholar]
- Vallee R. B. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J Cell Biol. 1982 Feb;92(2):435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiche G., Baker M. A. Cytoplasmic network arrays demonstrated by immunolocalization using antibodies to a high molecular weight protein present in cytoskeletal preparations from cultured cells. Exp Cell Res. 1982 Mar;138(1):15–29. doi: 10.1016/0014-4827(82)90086-6. [DOI] [PubMed] [Google Scholar]