Abstract
Objective: This study aims to examine the effects of low intensity pulsed ultrasound (LIPUS) on leukopenia induced by cyclophosphamide in a rabbit model. Methods: The leukopenia model in New Zealand rabbit was established by injecting cyclophosphamide into the ear vein. Forty leukopenia model rabbits were randomly allocated to control group (n = 20) and LIPUS group (n = 20). LIPUS group underwent 20 minutes of daily ultrasound treatment at femoral metaphysis for 7 days while control group received sham treatment. Diarrhea rate, mortality and blood cell count were calculated. IgA, IgG and IgM levels were measured by ELISA. Flow cytometry was used to detect CD44, CD49d, and PU.1. HE staining was performed to analyze bone marrow hyperplasia and changes of skin and muscle. Results: LIPUS treatment significantly promoted the proliferation of bone marrow nucleated cells, increased the number of WBC, IgA, IgG and IgM in the peripheral blood, and reduced the diarrhea rate and mortality. The irradiated skin and muscle tissues showed no obvious damages. LIPUS treatment promoted the migration of hematopoietic cells to peripheral blood by decreasing the expression of CD49d and CD44 on the surface of CD34 positive cells. It also promoted the differentiation of hematopoietic stem cells into granulocytes and lymphocytes by decreasing the expression of PU.1. Conclusion: LIPUS can be used as a safe and effective clinical treatment for cyclophosphamide induced leukopenia.
Keywords: Low intensity pulsed ultrasound, leukopenia, immunoglobulin, PU.1, adhesion molecules
Introduction
In year 2012, about 14.1 million new cases of cancer occurred globally and caused about 8.2 million deaths or 14.6% of human deaths [1]. Chemotherapy, as one of the most common treatments for malignant tumors, has been rapidly developed [2,3]. However, many chemotherapy drugs such as busulfan, cyclophosphamide, nitrosourea, and mitomycin C have side effects. These drugs cause myelosuppression, particularly suppression in granulocyte-macrophage cells, and lead to leukopenia [4,5].
Chemotherapy-induced leukopenia is mainly due to the lack of specificity in anti-tumor drugs. When killing tumor cells, they also cause serious damage on normal cells, for instance, the bone marrow hematopoietic cells [6]. The white blood cell (WBC) count usually declines first, because these cells have the shortest life span in the blood. Blood cell growth factors are clinically used to boost WBC, most commonly, the granulocyte colony stimulating factor (G-CSF). G-CSF can promote granulocyte proliferation, differentiation and functional activation, and it is widely used in treatment of neutropenia induced by chemotherapy or bone marrow transplantation and for peripheral blood progenitor cell mobilization [7,8]. However, the disadvantages of G-CSF are obvious. For example, the WBC count is prone to relapse. Adverse effects of G-CSF such as muscle pain, bone pain, joint pain, fatigue, fever, gastrointestinal reactions are common [9,10]. G-CSF is expensive and the economic burden to the patient family is great. Additionally, G-CSF may stimulate the proliferation of multipotent hematopoietic stem cells, progenitor cells and immature cells [11]. Therefore, it is of great significance to development safe, effective, and affordable methods to treat chemotherapy-induced leukopenia.
LIPUS is approved by the US FDA in 1994 and is now commonly used for fracture healing. Studies suggest that LIPUS can promote the osteoblast proliferation and osteogenic differentiation [12-14]. Besides, LIPUS can also promote the cell proliferation of the articular cartilage, intervertebral disc, and ligament, and, the secretion of related factors, thus accelerating the recovery process [15-18]. The mechanism is assumed to be through its promotive effect on the proliferation and differentiation of bone marrow mesenchymal stem cells (BMSC) [19,20]. In addition, BMSC is believed to have an impact on hematopoietic stem cell (HSC) proliferation, migration and pluripotency [21], and can differentiate into adipose cells, osteoblasts, fibroblasts and other bone marrow stromal cells [22], which are involved in hematopoiesis micro-environment. Previous experiments have shown that direct LIPUS stimulation on femoral metaphysis promoted the proliferation of bone marrow-derived nucleated cells and bone marrow hyperplasia was evident [23]. However, the effect of LIPUS on chemotherapy-induced leukopenia is unclear.
In this study, the leucopenia model was induced by cyclophosphamide in rabbits. The aim of this study is to investigate whether LIPUS treatment can relieve chemotherapy-induced myelosuppression, restore hematopoietic function, and then serve as an safe, effective and economical therapy for leukopenia.
Materials and methods
Animals
Forty New Zealand white rabbits (weight 2.0-2.5 Kg) were obtained from the Experimental Animal Center of Chongqing Medical University (production license No. SCXY 2012-0001). All experimental animals were kept under standard conditions (humidity 45 ± 5%, temperature 23 ± 5°C, light time 12 h/d). The experimental protocol was approved by the Ethics Committee of Chongqing Medical University. All experiments were performed in accordance with Guide for the Care and Use of Laboratory Animals issued by the Ministry of Science and Technology.
Model establishment
The leukopenia model in New Zealand rabbit was established by injecting cyclophosphamide (Shanxi Pude Pharmaceutical Co., Ltd., China) into the ear vein as described by Luo [24]. Briefly, 50 mg/kg cyclophosphamide injected daily for four consecutive days. The rabbit general conditions including eating, drinking, activity, mental state, diarrhea, and mortality were daily monitored from the first day of cyclophosphamide (-4) to 14 days after the model establishment.
LIPUS treatment
The leukopenia model rabbits were randomized into two groups: control group (n = 20) and LIPUS group (n = 20). After model establishment, the LIPUS group underwent 20 minutes of daily ultrasound treatment (0.2 W/cm2, 0.6 MHz) at femoral metaphysis started from day 1 and continued for seven days. The control group received sham treatment.
Sampling
Blood (0.5 ml) was collected from ear artery of each rabbit before model establishment and on days 0, 2, 5, 7, 11 and 14 after model establishment. Bone marrow (1 ml) was taken from distal femoral of each rabbit before model establishment and on days 0 and 7 of model establishment. Skin and muscle tissues of distal femoral at the irradiated area were collected on day 7.
Blood cells counting
WBC, neutrophil, lymphocyte and monocyte in blood were detected using an automatic blood cell count analyzer (Bowlinman Sunshine Co., Ltd, Beijing, China). The 95% confidence interval was used to calculate the upper and lower limits of normal values for WBC, neutrophil, lymphocyte, and monocyte in the blood.
IgA, IgG and IgM detection by ELISA
The serum was isolated from blood by centrifugation at 3000 r/min for 15 min. The detection of IgA, IgG and IgM was performed using ELISA kits (Hushang, Shanghai, China) according to the manufacturer’s instructions. The upper and lower limits of normal values for IgA, IgG and IgM were calculated by the 95% confidence interval.
Flow cytometry
Mononuclear cells were isolated from bone marrow by density gradient centrifugation. The cells were labeled with antibodies of PU.1, CD34, CD44, and CD49d (Novus Biologicals, USA). Flow cytometry (BD Bioscience, CA, USA) was used to detect levels of PU.1, CD34, CD44, and CD49d on bone marrow mononuclear cells.
HE staining
Tissues of bone marrow, skin and muscle tissue were fixed in 4% paraformaldehyde. HE staining was performed according to routine procedure. Briefly, samples were dehydrated in a graded series of ethanol solutions, cleared in xylene, embedded in paraffin, cut into sections and stained with hematoxylin and eosin and observed under a microscope.
Statistical analysis
All the data were expressed as mean ± SD. SPSS 22.0 software (IBM, US) was used for statistical analysis. Chi-square test was used to analyze the difference in diarrhea rate and mortality. Multivariate analysis of variance was used to analyze the difference in the results of blood cell count and immunoglobulin level. One-way analysis of variance and paired t test were used to analyze the difference in the results of PU.1, CD44 and CD49d. A P-value less than 0.05 was considered statistically significant.
Results
General conditions of rabbits
After injection of cyclophosphamide, New Zealand rabbits generally began to develop symptoms of lack of activity, unresponsive, decreased intake of food and water, diarrhea. The general conditions of rabbits were improved after LIPUS treatment. In LIPUS group, 11 rabbits had diarrhea and 2 rabbits died, with diarrhea rate of 55% and death rate of 10%. In control group, 17 rabbits had diarrhea and 14 rabbits died, with diarrhea rate of 85% and death rate of 70%. Both diarrhea and mortality rate were significantly lower in the LIPUS group than those in the control group (both P < 0.05), as shown in Figure 1.
Figure 1.

The diarrhea rate and mortality rate of New Zealand rabbits, comparing between the LIPUS group and the control group. *indicates P < 0.05.
Peripheral blood cell counting
To determine the effect of LIPUS treatment on blood cell count, blood routine test was performed. The normal ranges of blood cell count are summarized in Table 1. As shown in Figure 2A and 2B, WBC count and neutrophil count increased first and then decreased in both groups, with peak value on day 2. WBC count and neutrophil count in the LIPUS group maintained to be above the lower limit of normal value during the observation period, while the control group did not reach the normal range. The WBC of the LIPUS group was significantly higher than that of the control group on days 2, 7, 11, and 14 (P < 0.05). The neutrophil count of the LIPUS group was significantly higher than that of the control group on days 2 and 14 (P < 0.05). The lmphocyte count in the LIPUS group peaked on day 5 while that in the control group fluctuated below the lower limit of normal value (Figure 2C). LIPUS group had significantly higher lmphocyte count than control group on day 7 (P < 0.05). In contrast, no significant difference in was observed monocyte count between the two groups (Figure 2D). This data indicates that LIPUS can significantly increase the number of peripheral blood WBC, especially neutrophil and lymphocyte, thereby enhancing the body’s anti-infection ability.
Table 1.
The normal range reference of blood cell count
| Item | WBC (109/L) | Neutrophil (109/L) | Lymphocyte (109/L) | Monocyte (109/L) |
|---|---|---|---|---|
| Upper limit | 12.19 | 5.99 | 6.55 | 0.64 |
| Lower limit | 8.78 | 3.37 | 3.81 | 0.51 |
Figure 2.
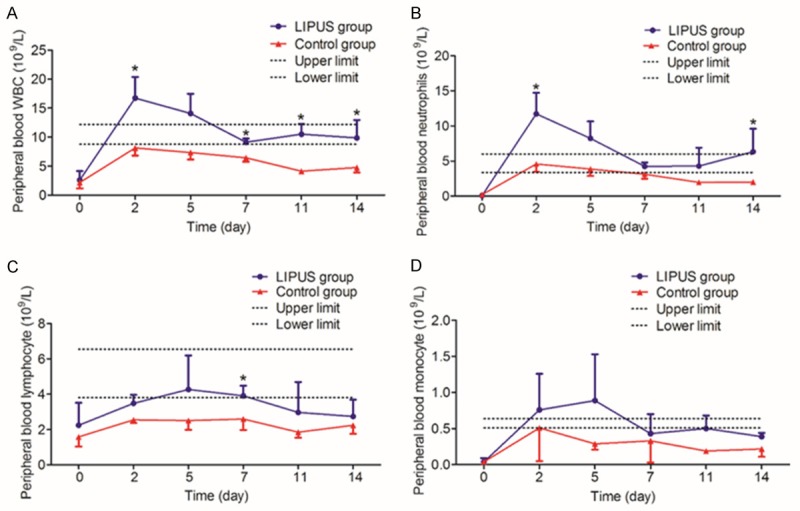
The number of peripheral WBCs (A), neutrophils (B), lymphocytes (C) and monocytes (D) in the LIPUS group and the control group on days 0, 2, 5, 7, 11 and 14 after modeling. Compared with control group, *P < 0.05.
Serum immunoglobulin levels
To investigate the effect of LIPUS on serum IgA, IgG and IgM levels, ELISA was performed. The normal ranges are listed in Table 2. Serum levels of IgA (Figure 3A), IgG (Figure 3B) and IgM (Figure 3C) in the LIPUS group were significantly higher than those in the control group on days 11 and 14; differences of IgA and IgM were also significant on day 7 (P < 0.05). And at the end of the observation period, the levels of immunoglobulin in the LIPUS group were higher than the upper limit of normal range, while those in the control group were around or below the lower limit of the normal range. The results show that LIPUS promotes secretion of IgA, IgG and IgM levels and enhances humoral immunity.
Table 2.
The immunoglobulin normal range reference
| Item | IgA (μg/ml) | IgG (mg/ml) | IgM (μg/ml) |
|---|---|---|---|
| Upper limit | 93.36 | 1.28 | 84.73 |
| Lower limit | 73.19 | 0.88 | 62.86 |
Figure 3.

The levels of IgA (A), IgG (B) and IgM (C) in the peripheral blood of the LIPUS group and control group were detected by ELISA on days 0, 2, 5, 7, 11 and 14 after modeling. Compared with control group, *P < 0.05.
Bone marrow tissues
To analyze the effect of LIPUS on bone marrow, HE staining was performed. The bone marrow hematopoietic tissue was rich and cell proliferation was active in normal rabbits (Figure 4A). After modeling, the normal structure of bone marrow tissue in New Zealand rabbits were destroyed, with decreased hematopoietic tissues and increased adipose tissues (Figure 4B). After LIPUS treatment, hematopoietic tissues increased and bone marrow hyperplasia was evident (Figure 4C). There was an increase in bone marrow hematopoietic tissue in the control group, but not as evident as in the LIPUS group (Figure 4D). The results suggest LIPUS treatment can promote the proliferation of bone marrow nucleated cells, thereby increasing the number of peripheral WBCs.
Figure 4.

HE staining results of bone marrow. The bone marrow was collected from each rabbit on day 0 and 7, Magnification × 100. The arrows indicate the hematopoietic tissue. A. Bone marrow from rabbit before modeling. B. Bone marrow of model rabbit on day 0. C. Bone marrow from LIPUS group on day 7; D. Bone marrow from sham treated control rabbit on day 7.
Flow cytometry detection
To investigate whether LIPUS can promote the migration and differentiation of hematopoietic cells into peripheral blood, we detected the expression of adhesion molecules CD44 and CD49d on bone marrow CD34+ cells and the expression of PU.1 on bone marrow mononuclear cells (Figure 5).
Figure 5.

CD44, CD49d, PU.1 expressions in LIPUS group and control group. The comparing time points were days-4 (before modeling), 0 and 7. (A, D, G) Were the summaries, and (B), (C), (E), (F), (H), (I) were the comparisons within each group. *P < 0.05.
CD44 expression
The expression of CD44 on the surface of bone marrow CD34+ cells in the leukopenia rabbit was compared on days-4 (before modeling), 0 and 7 (Figure 5A). The expression of CD44 peaked on day 0 and then decreased to a level around the level before modeling on day 7 (P < 0.05). The CD44 expression changes at different time points within group were shown in Figure 5B and 5C. The results suggest that LIPUS treatment reduces the expression of CD44.
CD49d expression
The expression of CD49d on the surface of bone marrow CD34+ cells in the leukopenia rabbit was compared on days-4 (before modeling), 0 and 7 (Figure 5D). The expression of CD49d showed a comparable trend with CD44 expression and peaked on day 0, then decreased on day 7 to a level around the level before modeling (P < 0.05). The CD49d expression changes at different time points within group were shown in Figure 5E and 5F. The difference of CD49d expression between LIPUS group and control group was statistically significant on day 7 (P < 0.05). The results suggest that LIPUS treatment reduces the expression of CD49d.
Pu.1 expression
The expression of PU.1 on the bone marrow mononuclear cells in the leukopenia rabbit was compared on days-4 (before modeling), 0 and 7 (Figure 5G). The PU.1 expressed decreased from day-4 to day 0, and continued to decrease on day 7. These decreases within group were of statistical significance (Figure 5H and 5I). On day 7, the PU.1 expression in LIPUS treatment was lower than that in the control group (Figure 5G). The results suggest that LIPUS can not only promote the migration of HSC to the peripheral blood but also can affect the differentiation of HSC.
Safety evaluation of LIPUS treatment
The skin and muscle tissues that received the LIPUS irradiation were used to prepare the specimens for safety evaluation of LIPUS treatment. Compared with control group, no obvious damages were observed in the skin surface of LIPUS group after seven consecutive days of irradiation (Figure 6A and 6B). HE staining was conducted to analyzed histological changes. As shown in Figure 6C and 6D, the skin tissues of both control group and LIPUS group were intact under light microscope. Cell shapes were normal. The epidermis and dermis were normal. Similarly, the muscle fiber of both control group and LIPUS group was clear without obvious inflammatory infiltration (Figure 6E and 6F). The results suggested that LIPUS treatment causes no obvious injuries to the skin and muscle tissues, and thus it is a safe treatment.
Figure 6.

General condition of skin and HE staining results of skin and muscle tissue. A. Skin surfaces of control rabbit on day 7. B. Skin surfaces of LIPUS treated rabbit on day 7. The skin and muscle tissue was collected from each rabbit on day 7 and analyzed with HE staining. Magnification × 100. C and D. Representative HE results of skin tissues of control and LIPUS group, respectively, on day 7. E and F. Representative HE results of muscle tissues of control and LIPUS group, respectively, on day 7.
Discussion
Leukopenia refers to the peripheral blood WBC absolute count below 4.0 × 109/L. Tumor chemotherapy or radiotherapy, viral infection, drugs and certain immune diseases can cause WBC reduction [25-30]. Leukopenia is particularly common in the course of chemotherapy [31], and it is the clinical reason for frequent or secondary infections, resulting in non-optimal treatment efficacy. Preventing and managing bone marrow suppression after chemotherapy is key to promote bone marrow hematopoietic recovery, increase peripheral blood WBC, reduce the risk of infection, and improve the clinical efficacy.
In recent years, in addition to promoting fracture, the widespread use of LIPUS has gradually extended to articular cartilage regeneration [32], soft tissue repair [33], anti-infection and inflammation [34], nerve regeneration [35], and blood vessel thrombolysis [36]. LIPUS can produce a variety of biological reactions and their synergies-increased cytoplasmic motility, changes in membrane potential and changes in enzymes-to promote cell proliferation and extracellular matrix secretion [37,38]. Our previous studies have shown that LIPUS can promote the proliferation of live rabbit bone marrow-derived nucleated cells [23] and in vitro cultured BMSCs [19,20]. Bone marrow-derived nucleated cells are the source of peripheral blood cells. BMSCs can differentiate into a variety of bone marrow stromal cells that support hematopoietic function, and play a key role in the proliferation, differentiation, migration and pluripotency of HSC [39-41]. Both of them can contribute to the recovery of bone marrow hematopoietic function. While leukopenia can be caused by myelosuppression, so we investigated the effect of LIPUS on cyclophosphamide-induced leukopenia.
The results showed that LIPUS effectively reduced diarrhea rate and mortality in the model rabbits. We further measured the number of peripheral blood WBCs. WBC is a group of blood cells, including neutrophil, lymphocyte and monocyte, and they can effectively kill the invasion bacteria, reduce the incidence of infection, thereby reducing diarrhea rate and mortality. In this study, WBC began to rise after LIPUS treatment, peaked at 2 days, and remained within the normal range after treatment withdrawal. This trend in WBC was consistent with the time course of diarrhea and death in New Zealand rabbits, and we therefore believe that LIPUS reduces the diarrhea rate and mortality by increasing the number of WBCs and enhancing immunity in New Zealand rabbits. To further understand the immune status of New Zealand rabbits, we detected the peripheral blood immunoglobulin levels of IgA, IgG, and IgM. The results showed that LIPUS effectively increased the peripheral blood IgA, IgG, and IgM levels, suggesting LIPUS can prevent infection through enhancing humoral immunity.
Kronenwett et al [42] postulate that adhesion effects of hematopoietic stem/progenitor cells with bone marrow stromal microenvironment may determine their colonization or migration from bone marrow. Studies have shown that G-CSF can down-regulate the expression of adhesion molecules on the hematopoietic stem/progenitor cells surfaces [43,44]. After G-CSF mobilization, expressions of CD49d, c-kit and LFA-3, CD621, CD31, CD44 and LFA-1 on CD34+ cells in peripheral blood are lower than those in the bone marrow, especially the expression of CD49d [45,46]. Our results revealed that LIPS had similar effects as G-CSF. Levels of adhesion molecules on those cells were lower after LIPUS treatment. In addition, LIPS down-regulated the expression of PU.1 in bone marrow mononuclear cells in our study. PU.1 mainly is expressed in hematopoietic cells, including CD34+ cells, macrophages, B lymphocytes, neutrophils, mast cells and primitive red blood cells [47]. A high level of PU.1 induces megakaryocyte differentiation while a low level of PU.1 induces granulocyte differentiation [48]. In addition, when PU.1 level is low, it can directly induce production of cytokine IL-7 and its receptor alpha to promote B cell and T cell proliferation and maturation [49]. Our results are in line with these studies, which showed an increase of peripheral blood neutrophils and lymphocytes when PU.1 expression in bone marrow decreased. These results suggest that LIPUS may promote the differentiation of HSC to WBC by down-regulating the expression of PU.1 and may promote the migration of HSC to peripheral blood by down-regulating the expression of adhesion molecules on HSC.
Conclusion
In summary, we demonstrate here that LIPUS can increase the number of peripheral WBCs and reduce diarrhea rate and mortality of New Zealand rabbits with cyclophosphamide induced leukopenia. We suggest that LIPUS may serve as a safe, effective and economical treatment for chemotherapy-induced leukopenia in the future.
Acknowledgements
This study was supported by the National Basic Research Program of China (No. 2011CB707900), the Chinese National Science Foundation (No. 81127901, 31000435, 11574039, 31571453 and 11274404), the National Instrumentation Program (No. 2013YQ03062906), Chongqing Research Program of Basic Research and Frontier Technology (No. cstc2016jcyjA0599) and Scientific and Technological Research Program of Chongqing Municipal Education Commission (No. KJ130329).
Disclosure of conflict of interest
None.
Authors’ contribution
B. L. performed the experiments, collected the data and prepared the manuscript; Y. L. performed part of the experiments; D. L. promoted figures quality and collected data; W.Z. collected experimental data; Y. Z., R. H., J. L., and Yong W. assisted with the experiment; Yan W. designed the experiment; W. C. conceived and supervised the experiment.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chabner BA, Roberts TG Jr. Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 3.Galmarini D, Galmarini CM, Galmarini FC. Galmarini. Cancer chemotherapy: a critical analysis of its 60 years of history. Crit Rev Oncol Hematol. 2012;84:181–199. doi: 10.1016/j.critrevonc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Barreto JN, McCullough KB, Ice LL, Smith JA. Antineoplastic agents and the associated myelosuppressive effects: a review. J Pharm Pract. 2014;27:440–446. doi: 10.1177/0897190014546108. [DOI] [PubMed] [Google Scholar]
- 5.Ozkan K, Turkkan E, Ender K, Mutlu D, Murat A, Nalan B, Abdulmecit Y, Osman M. 5-Fluorouracil, epirubicin and cisplatin in the treatment of metastatic gastric carcinoma: a retrospective analysis of 68 patients. Indian J Cancer. 2005;42:85–88. doi: 10.4103/0019-509x.16697. [DOI] [PubMed] [Google Scholar]
- 6.Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100:228–237. doi: 10.1002/cncr.11882. [DOI] [PubMed] [Google Scholar]
- 7.Anderlini P, Przepiorka D, Champlin R, Körbling M. Biologic and clinical effects of granulocte colony-stimulating factor in normal individuals. Blood. 1996;88:2819–2825. [PubMed] [Google Scholar]
- 8.Welte K, Gabrilove J, Bronchud MH, Platzer E, Morstyn G. Filgrastim (r-metHuG-CSF): the first 10 years. Blood. 1996;88:1907–1929. [PubMed] [Google Scholar]
- 9.Chen SH, Yang SH, Chu SC, Su YC, Chang CY, Chiu YW, Kao RH, Li DK, Yang KL, Wang TF. The role of donor characteristics and post-granulocyte colony-stimulating factor white blood cell counts in predicting the adverse events and yields of stem cell mobilization. Int J Hematol. 2011;93:652–659. doi: 10.1007/s12185-011-0844-5. [DOI] [PubMed] [Google Scholar]
- 10.Beaupain B, Leblanc T, Reman O, Hermine O, Vannier JP, Suarez F, Lutz P, Bordigoni P, Jourdain A, Schoenvald M, Ouachee M, François S, Kohser F, Jardin F, Devouassoux G, Bertrand Y, Nove-Josserand R, Donadieu J French SCN Registry, Service d’Hémato Oncologie Pédiatrique. Is pegfilgrastim safe and effective in congenital neutropenia? Ananalysis of the French severe chronic neutropenia registry. Pediatr Blood Cancer. 2009;53:1068–1073. doi: 10.1002/pbc.22147. [DOI] [PubMed] [Google Scholar]
- 11.White SA, Shaw JA, Sutherland DE. Pancreas transplantation. Lancet. 2009;373:1808–1817. doi: 10.1016/S0140-6736(09)60609-7. [DOI] [PubMed] [Google Scholar]
- 12.Vezeridis PS, Semeins CM, Chen Q, Klein-Nulend J. Osteocytes subjected to pulsating fluid flow regulate osteoblast proliferation and differentiation. Biochem Biophys Res Commun. 2006;348:1082–1088. doi: 10.1016/j.bbrc.2006.07.146. [DOI] [PubMed] [Google Scholar]
- 13.Uddin SM, Qin YX. Enhancement of osteogenic differentiation and proliferation in human mesenchymal stem cells by a modified low intensity ultrasound stimulation under simulated microgravity. PLoS One. 2013;8:e73914. doi: 10.1371/journal.pone.0073914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fung CH, Cheung WH, Pounder NM, Harrison A, Leung KS. Osteocytes exposed to far field of therapeutic ultrasound promotes osteogenic cellular activities in pre-osteoblasts through soluble factors. Ultrasonics. 2014;54:1358–1365. doi: 10.1016/j.ultras.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Korstjens CM, van der Rijt RH, Albers GH, Semeins CM, Klein-Nulend J. Low-intensity pulsed ultrasound affects human articular chondrocytes in vitro. Med Biol Eng Comput. 2008;46:1263–1270. doi: 10.1007/s11517-008-0409-9. [DOI] [PubMed] [Google Scholar]
- 16.Iwashina T, Mochida J, Miyazaki T, Watanabe T, Iwabuchi S, Ando K, Hotta T, Sakai D. Low-intensity pulsed ultrasound stimulates cell proliferation and proteoglycan production in rabbit intervertebral disc cells cultured in alginate. Biomaterials. 2006;27:354–361. doi: 10.1016/j.biomaterials.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 17.DU D, Chen S, Yi G, Wang P, Tang Y, Zheng L, Chen J. Low-intensity pulsed ultrasound promotes extracellular matrix synthesis of human osteoarthritis chondrocytes. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016;32:1536–1540. [PubMed] [Google Scholar]
- 18.Jia L, Chen J, Wang Y, Zhang Y, Chen W. Focused low-intensity pulsed ultrasound affects extracellular matrix degradation via decreasing chondrocyteapoptosis and inflammatory mediators in a surgically induced osteoarthritic rabbit model. Ultrasound Med Biol. 2016;42:208–219. doi: 10.1016/j.ultrasmedbio.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 19.He R, Zhou W, Zhang Y, Hu S, Yu H, Luo Y, Liu B, Ran J, Wu J, Wang Y, Chen W. Combination of low-intensity pulsed ultrasound and C3H10-T1/2 cells promotes bone-defect healing. Int Orthop. 2015;39:2181–2819. doi: 10.1007/s00264-015-2898-0. [DOI] [PubMed] [Google Scholar]
- 20.Yu HS, Chen WZ, Wang Y, Ding XY, Liu Z, Luo YP. Study on proliferation effects of low intensity pulsed ultrasound on bone mesenchymal stem cells in vitro. Journal of China Medical University. 2011;40:971–974. [Google Scholar]
- 21.Jing D, Fonseca AV, Alakel N, Fierro FA, Muller K, Bornhauser M, Ehninger G, Corbeil D, Ordemann R. Hematopoietic stem cells in co-culture with mesenchymal stromal cells-modeling the niche compartments in vitro. Haematologica. 2010;95:542–550. doi: 10.3324/haematol.2009.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fani N, Ziadlou R, Shahhoseini M, Baghaban Eslaminejad M. Comparative epigenetic influence of autologous versus fetal bovine serum on mesenchymal stem cells through in vitro osteogenic and adipogenic differentiation. Exp Cell Res. 2016;344:176–182. doi: 10.1016/j.yexcr.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Yu HS. Study of low-intensity pulsed ultrasound promoting proliferation of bone marrow-derived cells. Chongqing Medical University. 2012 [Google Scholar]
- 24.Luo YP, Wang Y, Wang W, Yu HS, Zhang CY, Chen WZ. Establishment of New Zealand white rabbit model of cyclophosphamide-induced leucopenia leucopenia. Laser J. 2013;34:77–78. [Google Scholar]
- 25.Zhou W, Ding Q, Liang X, He Z, Zha X, Liu X, Wang S. The risk of amenorrhea is related to chemotherapy-induced leukopenia in breast cancer patients receiving epirubicin and taxane based chemotherapy. PLoS One. 2012;7:e37249. doi: 10.1371/journal.pone.0037249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguilar-Ponce JL, Granados-García M, Cruz López JC, Maldonado-Magos F, Alvarez-Avitia MA, Arrieta O, González-Ramírez I, Lara-Cruz G, Martinez-Juárez I, Medina-Santillan R, Castillo-Hernández C, De la Garza-Salazar J. Alternating chemotherapy: gemcitabine and cisplatin with concurrent radiotherapy for treatment of advanced head and neck cancer. Oral Oncol. 2013;49:249–254. doi: 10.1016/j.oraloncology.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Huang CS, Liu L, Liu J, Chen Z, Guo J, Li CZ, Zhou DG, Wang ZH. Association of chemotherapy-induced leukopenia with treatment outcomes in advanced non-small-cell lung cancer cases receiving the NP regimen. Asian Pac J Cancer Prev. 2012;13:4481–4485. doi: 10.7314/apjcp.2012.13.9.4481. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi K, Ohtsu F, Yano R, Sakakibara J, Goto N. Risk factors and subjective symptoms of drug-induced leukopenia. Yakugaku Zasshi. 2011;131:139–152. doi: 10.1248/yakushi.131.139. [DOI] [PubMed] [Google Scholar]
- 29.Hooper C, Slocombe R, Day R, Crawford S. Leukopenia associated with abalone viral ganglioneuritis. Aust Vet J. 2012;90:24–28. doi: 10.1111/j.1751-0813.2011.00877.x. [DOI] [PubMed] [Google Scholar]
- 30.Shafi MS, Sheikh NI, Rizvi F, Afzal M, Manzoor S. Treatment induced leukopenia in hepatitis C and Role of G-CSF in its management. J Coll Physicians Surg Pak. 2009;19:346–349. [PubMed] [Google Scholar]
- 31.Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C European Organisation for Research and Treatment of Cancer. 2010 update of EORTC guidelines for the use of granulocytecolony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Jia XL, Chen WZ, Zhou K, Wang ZB. Effects of low-intensity pulsed ultrasound in repairing injured articular cartilage. Chin J Traumatol. 2005;8:175. [PubMed] [Google Scholar]
- 33.Lu H, Liu F, Chen H, Chen C, Qu J, Xu D, Zhang T, Zhou J, Hu J. The effect of low-intensity pulsed ultrasound on bone-tendon junction healing: initiating after inflammation stage. J Orthop Res. 2016;34:1697–1706. doi: 10.1002/jor.23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang PF, Li D, Zhang SM, Wu Q, Tang J, Huang LK, Liu W, Xu XD, Chen SR. Efficacy of ultrasound in the treatment of osteoarthritis of the knee. Orthop Surg. 2011;3:181–187. doi: 10.1111/j.1757-7861.2011.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mourad PD, Lazar DA, Curra FP, Mohr BC, Andrus KC, Avellino AM, McNutt LD, Crum LA, Kliot M. Ultrasound accelerates functional recovery after peripheral nerve damage. Neurosurgery. 2001;48:1136–1141. doi: 10.1097/00006123-200105000-00035. [DOI] [PubMed] [Google Scholar]
- 36.Nesser HJ, Karia DH, Tkalec W. Therapeutic ultrasound in cardiology. Herz. 2002;27:269–278. doi: 10.1007/s00059-002-2362-y. [DOI] [PubMed] [Google Scholar]
- 37.Parvizi J, Parpura V, Greenleaf JF, Bolander ME. Calcium signaling is required for ultrasound stimulated aggrecan synthesis by rat chondrocytes. Orthop Res. 2002;20:51–57. doi: 10.1016/S0736-0266(01)00069-9. [DOI] [PubMed] [Google Scholar]
- 38.Saini V, Yadav S, McCormick S. Low-intensity pulsed ultrasound modulates shear stress induced PGHS-2 expression and PGE2 synthesis in MLO-Y4 osteocyte-like cells. Ann Biomed Eng. 2011;39:378–393. doi: 10.1007/s10439-010-0156-6. [DOI] [PubMed] [Google Scholar]
- 39.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 40.Devme SM, Hoffman R. Role of mesenchymal stem cells in hematopoietic stem cell transplantation. Curr Opin Hematol. 2000;7:358–363. doi: 10.1097/00062752-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Jing D, Fonseca AV, Alakel N, Fierro FA, Muller K, Bornhauser M, Ehninger G, Corbeil D, Ordemann R. Hematopoietic stem cells in co-culture with mesenchymal stromal cells-modeling the niche compartments in vitro. Haematologica. 2010;95:542–550. doi: 10.3324/haematol.2009.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kronenwett R, Martin S, Haas R. The role of cytokines and adhesion molecules for mobilization of peripheral blood stem cells. Stem Cells. 2000;18:320–330. doi: 10.1634/stemcells.18-5-320. [DOI] [PubMed] [Google Scholar]
- 43.Kröger N, Zeller W, Hassan HT, Dierlamm J, Zander AR. Difference between expression of adhesion molecules on CD34+ cells from bone marrow and G-CSF-stimulated peripheral blood. Stem Cells. 1998;16:49–53. doi: 10.1002/stem.160049. [DOI] [PubMed] [Google Scholar]
- 44.Lichterfeld M, Martin S, Burkly L, Haas R, Kronenwett R. Mobilization of CD34+ haematopoietic stem cells is associated with a functional inactivation of the integrin very late antigen 4. Br J Haematol. 2000;110:71–81. doi: 10.1046/j.1365-2141.2000.02130.x. [DOI] [PubMed] [Google Scholar]
- 45.Yano T, Katayama Y, Sunami K, Ishimaru F, Shinagawa K, Ikeda K, Omoto E, Niiya K, Harada M. Granulocyte colony-stimulating factor and lineage-independent modulation of VLA-4 expression on circulating CD34+ cells. Int J Hematol. 2000;71:328–333. [PubMed] [Google Scholar]
- 46.Kikuta T, Shimazaki C, Ashihara E, Sudo Y, Hirai H, Sumikuma T, Yamagata N, Inaba T, Fujita N, Kina T, Nakagawa M. Mobilization of hematopoietic primitive and committed progenitor cells into blood in mice by anti-vascular adhesion molecule-1 antibody alone or in combination with granulocyte colony-stimulating factor. Exp Hematol. 2000;28:311–317. doi: 10.1016/s0301-472x(99)00151-4. [DOI] [PubMed] [Google Scholar]
- 47.Zhang P, Zhang X, Iwama A, Yu C, Smith KA, Mueller BU, Narravula S, Torbett BE, Orkin SH, Tenen DG. PU. 1 inhibits GATA-1 function and erythroid differentiation by blocking GATA-1 DNA binding. Blood. 2000;96:2641–2648. [PubMed] [Google Scholar]
- 48.Dahl R, Simon MC. The importance of PU. 1 concentration in hematopoietic lineage commitment and maturation. Blood Cells Mol Dis. 2003;31:229–233. doi: 10.1016/s1079-9796(03)00152-9. [DOI] [PubMed] [Google Scholar]
- 49.Warren LA, Rothenberg EV. Regulatory coding of lymphoid lineage choice by hematopoietic transcription factors. Curr Opin Immunol. 2003;15:166–175. doi: 10.1016/s0952-7915(03)00011-6. [DOI] [PubMed] [Google Scholar]


