Abstract
Long non-coding RNAs (lncRNAs) act critical roles in many biological processes, including cell proliferation, apoptosis, development, invasion and migration. LncRNA maternally expressed gene 3 (MEG3) is found to be downregulated in several tumors; however, its role in the atherosclerosis is still unknown. In the present study, we demonstrated that MEG3 expression level was downregulated in the coronary artery disease (CAD) tissues compared to in the control tissues. We also showed that TNF-α enhanced EC cell proliferation. In addition, the expression of MEG3 was increased in EC after treated with TNF-α. Overexpression of MEG3 suppressed EC cell proliferation and inhibited the expression of cyclin D1, ki-67 and PCNA. Elevated expression of MEG3 suppressed the type I collagen, type V collagen and proteoglycan expression. In addition, we showed that elevated expression of MEG3 suppressed the miR-21 expression in the EC and promoted the expression of RhoB and PTEN, which were the direct target genes of miR-21. We demonstrated that miR-21 expression level was upregulated in the CAD tissues compared to in the control tissues. Moreover, miR-21 expression was reversely correlated with MEG3 expression in the CAD tissues. Overexpression of MEG3 suppressed EC cell proliferation and type I collagen, type V collagen and proteoglycan expression through inhibiting miR-21 expression. These results suggested that MEG3 played a critical role in regulating EC proliferation and type I collagen, type V collagen and proteoglycan expression partly through suppressing miR-21 expression.
Keywords: Atherosclerosis, LncRNAs, MEG3, miR-21
Introduction
Atherosclerosis is one of the most common chronic inflammatory disease which fibrous and lipids elements are deposited in the arterial wall [1-4]. Atherosclerosis is the most common cause of cardiovascular diseases including stroke and coronary heart disease [5-8]. Reendothelialization is an important step for vascular healing which depends on endothelial cells proliferation and migration [9-12]. Therefore, activation of endothelial cell viability and proliferation is important for promoting endothelial healing and improving the vascular function.
Long noncoding RNAs (lncRNAs) are longer than 200 nucleotides with no protein coding capacity or limited protein coding potential [13-17]. Recently, lncRNAs is identified to play important roles in several biological processes such as cell proliferation, differentiation, migration, invasion and development [18-20]. Increasing evidences have demonstrated that a lot of lncRNAs are deregulated in a variety of tumors including gastric cancer, bladder cancer, breast cancer, hepatocellular carcinoma and colorectal cancer [21-25]. Recently, a novel long noncoding, Maternally Expressed Gene 3 (MEG3) has been identified to be upregulated and plays as a tumor suppressor gene in a lot of tumors [25-28]. However, the role of MEG3 in the function of endothelial cells remains unknown.
In this study, we found that the MEG3 expression level was downregulated in the coronary artery disease (CAD) tissues compared to in the control tissues. Overexpression of MEG3 suppressed EC cell proliferation and inhibited the expression of cyclin D1, ki-67 and PCNA. Elevated expression of MEG3 suppressed the expression of type I collagen, type V collagen and proteoglycan.
Materials and methods
Cell line and cell culture and transfection
The human CAD tissues and normal arterial tissues (control tissues) were collected from our department. The ethics committee of The First Affiliated Hospital, School of Medicine, Zhejiang University has approved this research, and all subjects signed the informed consent forms. Human endothelial cell from umbilical vein (HU-VECs) was purchased from Cell Application (Santiago, USA) and kept in the EBM-2 medium supplemented with penicillin/streptomycin and FBS. MEG3 and control vector were purchased from the Gene Pharma (Shanghai, China). Cell transfection was performed by using Lipofectamine 2000 (Invitrogen, USA) following to the manufacturer’s information.
RNA extraction and real-time PCR
Total RNA from cell was extracted using the TRIzol Reagent (Invitrogen, CA) according to manufacturer’s information. Quantitative RT-PCR was used to detect the expression of MEG3 and GAPDH using the standard protocols on the ABI 7500 Real-Time PCR Detection system. The primers of genes were list: primer: MEG3, Forward, 5’-CCTTCCATGCTGAGCTGCT-3’, reverse primer: 5’-TGTTGGTGGGATCCAGGAAA-3’. GAPDH, Forward: 5’-GACTCATGACCACAGTCCATGC-3’, Reverse: 5’-AGAGGCAGGGATGATGTTCTG-3’.
Cell proliferation
Cell proliferation was performed using the MTT analysis as previously described. Cell was seeded in the 96-well plate and the 100 μl MTT was added to the culture medium. After incubated for 4 hours at 37°C, the absorbance of cell was determined at 570 nm using the microplate reader (Cell Application, CA, USA).
Statistical analysis
Statistical assay was performed by using SPSS 17.0 (SPSS, Chicago, USA). Significant difference among two or more than two groups was assessed by Student’s t-test or one-way analysis of variance. P<0.05 was considered to be the statistically significant.
Result
MEG3 expression was downregulated in CAD tissues and the proliferating EC
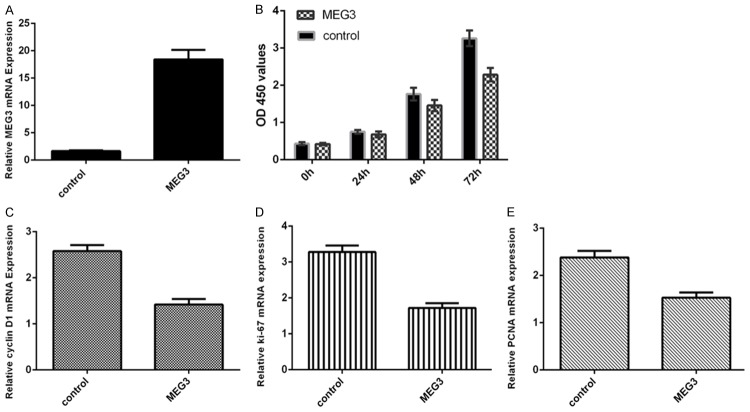
We firstly determined the MEG3 expression in CAD tissues. The expression of MEG3 in CAD tissues and normal arterial tissues (control tissues) was shown in the Figure 1A by using the qRT-PCR. MEG3 expression was downregulated in CAD tissues compared to in the control tissues (Figure 1B). TNF-α (50 ng/ml) enhanced EC cell proliferation (Figure 1C). The expression of MEG3 was decreased in the EC after treated with TNF-α (Figure 1D).
Figure 1.
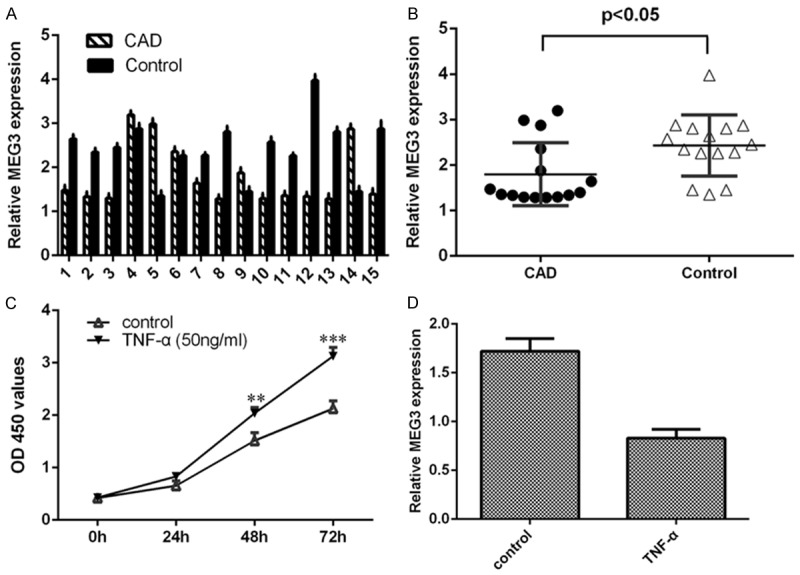
MEG3 expression was downregulated in CAD tissues and the proliferating EC. A. The expression of MEG3 in CAD tissues and normal arterial tissues was measured by qRT-PCR. B. MEG3 expression was downregulated in CAD tissues compared to in the control tissues. C. TNF-α (50 ng/ml) enhanced EC cell proliferation by using CCK-8 analysis. D. The expression of MEG3 was measured by qRT-PCR. **P<0.01 and ***P<0.001.
Overexpression of MEG3 suppressed the EC cell proliferation
We confirmed that the expression of MEG3 was significantly upregulated in EC treated with pcDNA-MEG3 (Figure 2A). MMT assay showed that overexpression of MEG3 inhibited EC cell proliferation (Figure 2B). Elevated expression of MEG3 decreased the expression of cyclin D1 (Figure 2C), ki-67 (Figure 2D) and PCNA (Figure 2E) by using the qRT-PCR.
Figure 2.
Overexpression of MEG3 suppressed the EC cell proliferation. A. The expression of MEG3 was in the EC treated with pcDNA-MEG3 ws determined by qRT-PCR. B. Overexpression of MEG3 inhibited EC cell proliferation. C. Elevated expression of MEG3 suppressed the expression of cyclin D1. D. Overexpression of MEG3 decreased the ki-67 expression. E. Elevated expression of MEG3 suppressed the expression of PCNA.
Elevated expression of MEG3 suppressed matrix gene expression in EC
Elevated expression of MEG3 inhibited the expression of type I collagen (Figure 3A). In addition, overexpression of MEG3 decreased the expression of type V collagen (Figure 3B). Moreover, ectopic expression of MEG3 suppressed proteoglycan expression (Figure 3C).
Figure 3.
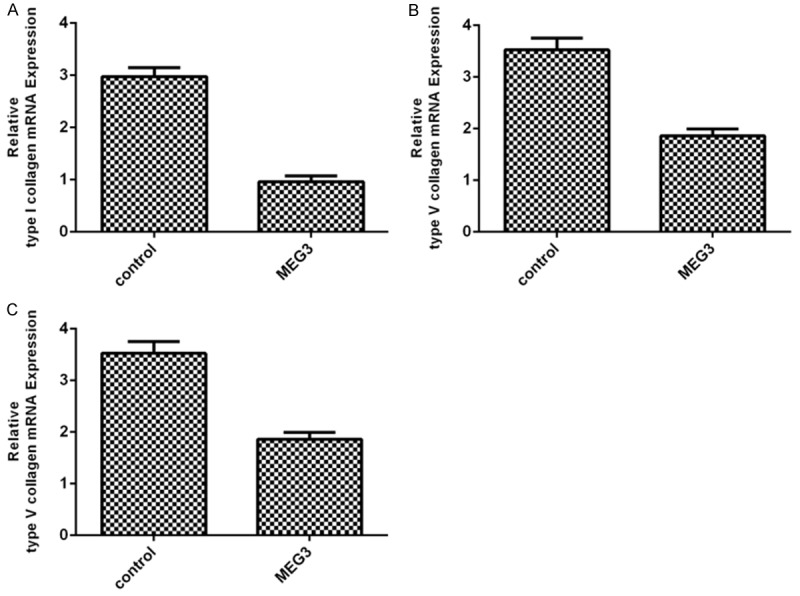
Elevated expression of MEG3 suppressed matrix gene expression in EC. A. Overexpression of MEG3 inhibited the expression of type I collagen. B. Overexpression of MEG3 decreased the expression of type V collagen. C. Overexpression of MEG3 suppressed the expression of proteoglycan.
Elevated expression of MEG3 decreased miR-21 expression in EC
Elevated expression of MEG3 suppressed miR-21 expression in EC (Figure 4A). We also confirmed the expression of miR-21 was decreased in EC using the PCR analysis (Figure 4B). Overexpression of MEG3 promoted the expression of RhoB (Figure 4C) and PTEN (Figure 4D), which were the direct target genes of miR-21.
Figure 4.
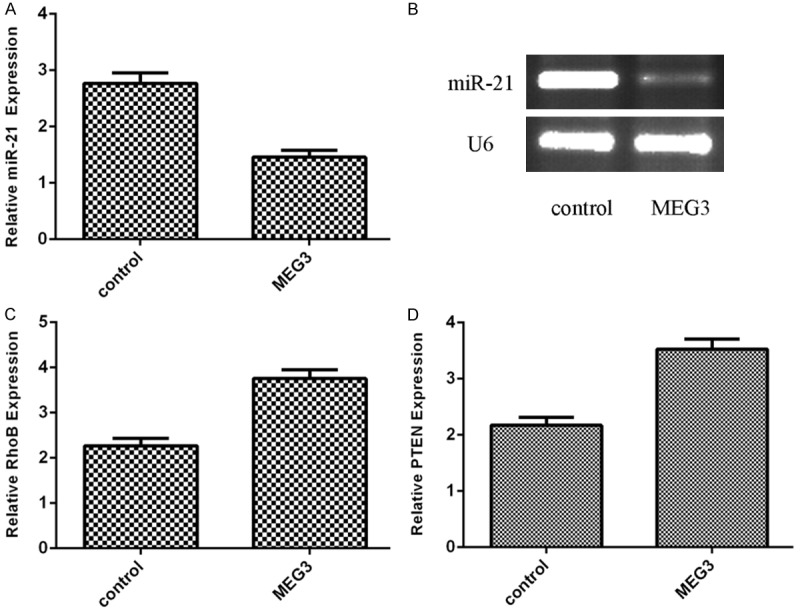
Elevated expression of MEG3 decreased miR-21 expression in EC. A. The miR-21 expression in EC was determined by qRT-PCR. B. The expression of miR-21 was downregulated in EC using the PCR analysis. C. Overexpression of MEG3 promoted the expression of RhoB in EC. D. Overexpression of MEG3 promoted the expression of PTEN in EC.
MiR-21 expression was reversely correlated with MEG3 expression in CAD tissues
The expression of miR-21 in the CAD tissues and control tissues was shown in the Figure 5A by using the qRT-PCR. MiR-21 expression level was upregulated in CAD tissues compared to in the control tissues (Figure 5B). Interestingly, miR-21 expression level was reversely correlated with MEG3 expression in CAD tissues (Figure 5C).
Figure 5.
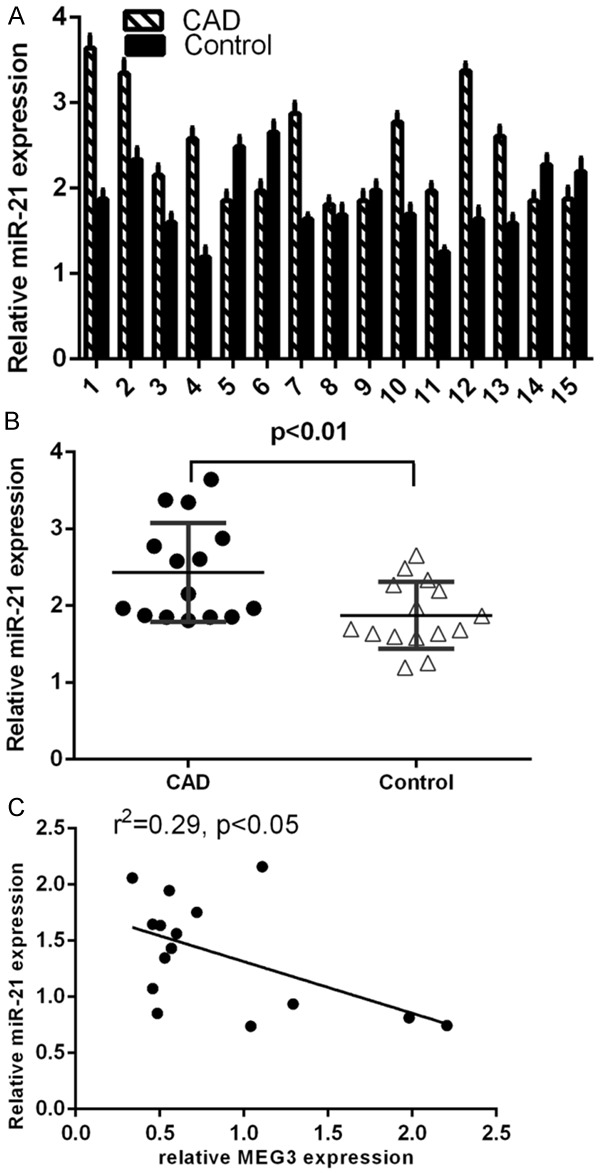
MiR-21 expression was reversely correlated with MEG3 expression in CAD tissues. A. The expression of miR-21 in the CAD tissues and control tissues was measured by using the qRT-PCR. B. MiR-21 expression level was upregulated in CAD tissues compared to in the control tissues. C. The miR-21 expression level was reversely correlated with MEG3 expression in CAD tissues.
Overexpression of MEG3 suppressed EC cell proliferation through regulating the miR-21 expression
We transfected the miR-21 mimic or scramble mimic to the MEG3-induced EC. We confirmed that overexpression of miR-21 promoted the MEG3-overexpressing EC cell proliferation (Figure 6A). Elevated expression of miR-21 increased the expression of cyclin D1 (Figure 6B), ki-67 (Figure 6C) and PCNA (Figure 6D) in the MEG3-overexpressing EC cell. Elevated expression of miR-21 enhanced the expression of type I collagen in the MEG3-overexpressing EC cell (Figure 6E). In addition, overexpression of miR-21 increased the expression of type V collagen in the MEG3-overexpressing EC cell (Figure 6F). Moreover, ectopic expression of miR-21 promoted proteoglycan expression (Figure 6G).
Figure 6.
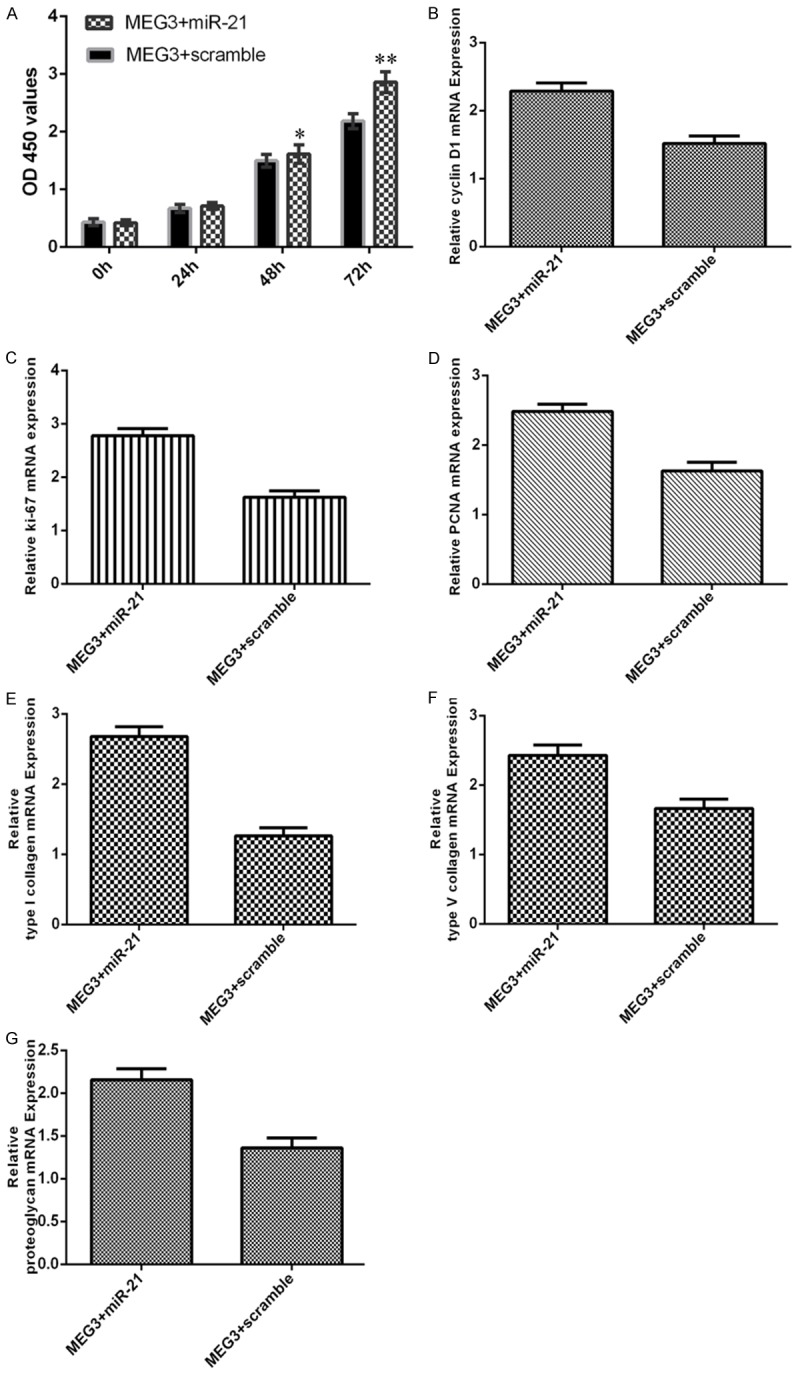
Overexpression of MEG3 suppressed EC cell proliferation through regulating the miR-21 expression. A. Overexpression of miR-21 promoted the MEG3-overexpressing EC cell proliferation. B. Elevated expression of miR-21 increased the expression of cyclin D1 in the MEG3-overexpressing EC. C. Overexpression of miR-21 increased the expression of ki-67 in the MEG3-overexpressing EC. D. Overexpression of miR-21 increased the expression of PCNA in the MEG3-overexpressing EC. E. The type I collagen expression in the MEG3-overexpressing EC cell was measured by qRT-PCR. F. Overexpression of miR-21 increased the expression of type V collagen in the MEG3-overexpressing EC. G. The proteoglycan expression in the MEG3-overexpressing EC cell was measured by qRT-PCR. *P<0.05 and **P<0.01.
Discussion
In the present study, we demonstrated that MEG3 expression level was downregulated in CAD tissues compared to in the control tissues. We also showed that TNF-α enhanced EC cell proliferation and the expression of MEG3 was increased in the EC treated with the TNF-α. Overexpression of MEG3 suppressed EC cell proliferation and inhibited the cyclin D1, ki-67 and PCNA expression. Elevated expression of MEG3 suppressed the expression of type I collagen, type V collagen and proteoglycan. In addition, we showed that elevated expression of MEG3 suppressed miR-21 expression in the EC and promoted the expression of RhoB and PTEN, which were the direct target genes of miR-21. We demonstrated that miR-21 expression level was upregulated in CAD tissues compared to in the control tissues. Moreover, miR-21 expression was reversely correlated with MEG3 expression in CAD tissues. Overexpression of MEG3 suppressed EC cell proliferation and the expression of type I collagen, type V collagen and proteoglycan through inhibiting miR-21 expression. These results suggested that MEG3 played a critical role in regulating EC proliferation and type I collagen, type V collagen and proteoglycan expression partly through suppressing miR-21 expression.
MEG3 was located at the chromosome 14q32, where allelic loss was commonly implicated in nasopharyngeal carcinoma [29]. Chak et al [29] demonstrated that MEG3 expression was downregulated in the nasopharyngeal carcinoma tissues and overexpression of MEG3 suppressed the nasopharyngeal carcinoma colony formation, cell proliferation, and inducted cell cycle arrest. Guo et al [28] investigated that the expression of MEG3 was decreased in the endometrial carcinoma tissues and elevated expression of MEG3 suppressed the endometrial carcinoma cell proliferation through inhibiting the Notch signaling pathway. Sun et al [25] showed that MEG3 expression was downregulated in the breast cancer tissues and suppressed the breast cancer cell proliferation, invasion and migration through regulating the p53 activity. Moreover, Zhang et al [26] demonstrated that the expression of MEG3 was decreased in the cervical cancer tissues and was associated with tumor size, FIGO stages, HR-HPV infection, lymphatic metastasis and miR-21 expression. Overexpression of MEG3 increased the cervical cancer cell apoptosis and suppressed the cervical cancer cell proliferation by regulating miR-21 expression. In line with this, we demonstrated that the MEG3 expression was downregulated in the CAD tissues compared to in the control tissues. We also showed that TNF-α can promoted the EC cell proliferation and the expression of MEG3 was increased in the EC after treated with the TNF-α. Overexpression of MEG3 inhibited the EC cell proliferation and suppressed the expression of cyclin D1, ki-67 and PCNA. Overexpression of MEG3 suppressed the expression of type I collagen, type V collagen and proteoglycan.
Previous studies demonstrated that miR-21 played an important role in the biological function of EC [30-33]. Yang et al [30] showed that miR-21 inhibited PTEN expression and bound directly with 3’-UTR of PTEN. The miR-21 expression was increased in the cardiac infarct model. MiR-21 acted as a protective role in the endothelial injury by regulating the PTEN/VEGF pathway after the acute myocardial infarction. Zuo et al [34] demonstrated that overexpression of miR-21 inhibited endothelial progenitor cell growth through promoting the TGF-β pathway by downregulation of WW domain-containing protein 1 (WWP1). Zeng et al [35] demonstrated that the circulating miR-21 expression was upregulated in the endothelial progenitor cell from the diabetes patients. Deregulated expression of miR-21 promoted the high glucose-induced the endothelial cytotoxicity. Overexpression of miR-21 protected endothelial cell through suppressing the DAXX expression. In our study, we demonstrated that overexpression of MEG3 inhibited miR-21 expression in the EC and promoted the expression of RhoB and PTEN, which were the direct target genes of miR-21. We demonstrated that miR-21 expression level was upregulated in the CAD tissues compared to in the control tissues. Moreover, miR-21 expression was reversely correlated with the MEG3 expression in CAD tissues. Overexpression of MEG3 suppressed the EC cell proliferation and type I collagen, type V collagen and proteoglycan expression through inhibiting miR-21 expression.
In conclusion, we demonstrated that MEG3 expression level was downregulated in the CAD tissues compared to in the control tissues. Overexpression of MEG3 suppressed the EC cell proliferation and decreased the type I collagen, type V collagen and proteoglycan expression partly through regulating miR-21 expression. These results suggested that MEG3 played a critical role in regulating EC proliferation and type I collagen, type V collagen and proteoglycan expression partly through suppressing miR-21 expression.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81200233/81200232), Natural Science Foundation of Zhejiang Province, China (LY16H020004/LY17H020011), and Zhejiang Medicine and Health Science and Technology Program (2014KYA080).
Disclosure of conflict of interest
None.
References
- 1.Yu X, Li Z. MicroRNAs regulate vascular smooth muscle cell functions in atherosclerosis (review) Int J Mol Med. 2014;34:923–933. doi: 10.3892/ijmm.2014.1853. [DOI] [PubMed] [Google Scholar]
- 2.Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z, Sharpless NE. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010;6:e1001233. doi: 10.1371/journal.pgen.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marquart TJ, Wu J, Lusis AJ, Baldan A. AntimiR-33 therapy does not alter the progression of atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2013;33:455–458. doi: 10.1161/ATVBAHA.112.300639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue Y, Wei Z, Ding H, Wang Q, Zhou Z, Zheng S, Zhang Y, Hou D, Liu Y, Zen K, Zhang CY, Li J, Wang D, Jiang X. MicroRNA-19b/221/222 induces endothelial cell dysfunction via suppression of PGC-1alpha in the progression of atherosclerosis. Atherosclerosis. 2015;241:671–681. doi: 10.1016/j.atherosclerosis.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 5.Loyer X, Potteaux S, Vion AC, Guerin CL, Boulkroun S, Rautou PE, Ramkhelawon B, Esposito B, Dalloz M, Paul JL, Julia PL, Maccario J, Boulanger CM, Mallat Z, Tedgui A. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ Res. 2014;114:434–43. doi: 10.1161/CIRCRESAHA.114.302213. [DOI] [PubMed] [Google Scholar]
- 6.Zhang CF, Kang K, Li XM, Xie BD. MicroRNA-136 promotes vascular muscle cell proliferation through the ERK1/2 pathway by targeting PPP2R2A in atherosclerosis. Curr Vasc Pharmacol. 2015;13:405–412. doi: 10.2174/1570161112666141118094612. [DOI] [PubMed] [Google Scholar]
- 7.Liu G, Li Y, Gao XG. microRNA-181a is upregulated in human atherosclerosis plaques and involves in the oxidative stress-induced endothelial cell dysfunction through direct targeting Bcl-2. Eur Rev Med Pharmacol Sci. 2016;20:3092–3100. [PubMed] [Google Scholar]
- 8.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, Fernandez-Hernando C, Fisher EA, Moore KJ. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Z, Han Y, Liu J, Jiang F, Hu H, Wang Y, Liu Q, Gong Y, Li X. MiR-135b-5p and MiR-499a-3p promote cell proliferation and migration in atherosclerosis by directly targeting MEF2C. Sci Rep. 2015;5:12276. doi: 10.1038/srep12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Qin W, Zhang L, Wu X, Du N, Hu Y, Li X, Shen N, Xiao D, Zhang H, Li Z, Yang H, Gao F, Du Z, Xu C, Yang B. MicroRNA-26a prevents endothelial cell apoptosis by directly targeting TRPC6 in the setting of atherosclerosis. Sci Rep. 2015;5:9401. doi: 10.1038/srep09401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araldi E, Suarez Y. MicroRNAs as regulators of endothelial cell functions in cardiometabolic diseases. Biochim Biophys Acta. 2016;1861:2094–2103. doi: 10.1016/j.bbalip.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang T, Tian F, Wang J, Jing J, Zhou SS, Chen YD. Endothelial cell autophagy in atherosclerosis is regulated by mir-30-mediated translational control of ATG6. Cell Physiol Biochem. 2015;37:1369–1378. doi: 10.1159/000430402. [DOI] [PubMed] [Google Scholar]
- 13.Qiu GZ, Tian W, Fu HT, Li CP, Liu B. Long noncoding RNA-MEG3 is involved in diabetes mellitus-related microvascular dysfunction. Biochem Biophys Res Commun. 2016;471:135–141. doi: 10.1016/j.bbrc.2016.01.164. [DOI] [PubMed] [Google Scholar]
- 14.Jiang W, Zhang D, Xu B, Wu Z, Liu S, Zhang L, Tian Y, Han X, Tian D. Long non-coding RNA BANCR promotes proliferation and migration of lung carcinoma via MAPK pathways. Biomed Pharmacother. 2015;69:90–95. doi: 10.1016/j.biopha.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Su W, Xie W, Shang Q, Su B. The long noncoding RNA MEG3 is downregulated and inversely associated with VEGF levels in osteoarthritis. Biomed Res Int. 2015;2015:356893. doi: 10.1155/2015/356893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naemura M, Murasaki C, Inoue Y, Okamoto H, Kotake Y. Long noncoding RNA ANRIL regulates proliferation of non-small cell lung cancer and cervical cancer cells. Anticancer Res. 2015;35:5377–5382. [PubMed] [Google Scholar]
- 17.Aguilo F, Di Cecilia S, Walsh MJ. Long noncoding RNA ANRIL and polycomb in human cancers and cardiovascular disease. Curr Top Microbiol Immunol. 2016;394:29–39. doi: 10.1007/82_2015_455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin L, Gu ZT, Chen WH, Cao KJ. Increased expression of the long non-coding RNA ANRIL promotes lung cancer cell metastasis and correlates with poor prognosis. Diagn Pathol. 2015;10:14. doi: 10.1186/s13000-015-0247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He X, Tan X, Wang X, Jin H, Liu L, Ma L, Yu H, Fan Z. C-Myc-activated long noncoding RNA CCAT1 promotes colon cancer cell proliferation and invasion. Tumour Biol. 2014;35:12181–12188. doi: 10.1007/s13277-014-2526-4. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Ding L, Wang L, Zhao Y, Sun Z, Karnes RJ, Zhang J, Huang H. LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget. 2015;6:41045–55. doi: 10.18632/oncotarget.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Su L, Chen X, Li P, Cai Q, Yu B, Liu B, Wu W, Zhu Z. MALAT1 promotes cell proliferation in gastric cancer by recruiting SF2/ASF. Biomed Pharmacother. 2014;68:557–564. doi: 10.1016/j.biopha.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Konishi H, Ichikawa D, Yamamoto Y, Arita T, Shoda K, Hiramoto H, Hamada J, Itoh H, Fujita Y, Komatsu S, Shiozaki A, Ikoma H, Ochiai T, Otsuji E. Plasma MALAT1 level is associated with liver damage and predicts development of hepatocellular carcinoma. Cancer Sci. 2015 doi: 10.1111/cas.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi P, Xu MD, Ni SJ, Huang D, Wei P, Tan C, Zhou XY, Du X. Low expression of LOC285194 is associated with poor prognosis in colorectal cancer. J Transl Med. 2013;11:122. doi: 10.1186/1479-5876-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang XF, Liu T, Li Y, Li S. Overexpression of long non-coding RNA CCAT1 is a novel biomarker of poor prognosis in patients with breast cancer. Int J Clin Exp Pathol. 2015;8:9440–9445. [PMC free article] [PubMed] [Google Scholar]
- 25.Sun L, Li Y, Yang B. Downregulated long non-coding RNA MEG3 in breast cancer regulates proliferation, migration and invasion by depending on p53’s transcriptional activity. Biochem Biophys Res Commun. 2016;478:323–329. doi: 10.1016/j.bbrc.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Yao T, Wang Y, Yu J, Liu Y, Lin Z. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21. Cancer Biol Ther. 2016;17:104–113. doi: 10.1080/15384047.2015.1108496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang NQ, Luo XJ, Zhang J, Wang GM, Guo JM. Crosstalk between Meg3 and miR-1297 regulates growth of testicular germ cell tumor through PTEN/PI3K/AKT pathway. Am J Transl Res. 2016;8:1091–1099. [PMC free article] [PubMed] [Google Scholar]
- 28.Guo Q, Qian Z, Yan D, Li L, Huang L. LncRNA-MEG3 inhibits cell proliferation of endometrial carcinoma by repressing Notch signaling. Biomed Pharmacother. 2016;82:589–594. doi: 10.1016/j.biopha.2016.02.049. [DOI] [PubMed] [Google Scholar]
- 29.Chak WP, Lung RW, Tong JH, Chan SY, Lun SW, Tsao SW, Lo KW, To KF. Downregulation of long non-coding RNA MEG3 in nasopharyngeal carcinoma. Mol Carcinog. 2017;56:1041–1054. doi: 10.1002/mc.22569. [DOI] [PubMed] [Google Scholar]
- 30.Yang F, Liu W, Yan X, Zhou H, Zhang H, Liu J, Yu M, Zhu X, Ma K. Effects of mir-21 on cardiac microvascular endothelial cells after acute myocardial infarction in rats: role of phosphatase and tensin homolog (PTEN)/vascular endothelial growth factor (VEGF) signal pathway. Med Sci Monit. 2016;22:3562–3575. doi: 10.12659/MSM.897773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ge X, Huang S, Gao H, Han Z, Chen F, Zhang S, Wang Z, Kang C, Jiang R, Yue S, Lei P, Zhang J. miR-21-5p alleviates leakage of injured brain microvascular endothelial barrier in vitro through suppressing inflammation and apoptosis. Brain Res. 2016;1650:31–40. doi: 10.1016/j.brainres.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Kuang DB, Zhou JP, Yu LY, Zeng WJ, Xiao J, Zhu GZ, Zhang ZL, Chen XP. DDAH1-V3 transcript might act as miR-21 sponge to maintain balance of DDAH1-V1 in cultured HUVECs. Nitric Oxide. 2016;60:59–68. doi: 10.1016/j.niox.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Wahlang B, Petriello MC, Perkins JT, Shen S, Hennig B. Polychlorinated biphenyl exposure alters the expression profile of microRNAs associated with vascular diseases. Toxicol In Vitro. 2016;35:180–187. doi: 10.1016/j.tiv.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuo K, Li M, Zhang X, Lu C, Wang S, Zhi K, He B. MiR-21 suppresses endothelial progenitor cell proliferation by activating the TGFbeta signaling pathway via downregulation of WWP1. Int J Clin Exp Pathol. 2015;8:414–422. [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng J, Xiong Y, Li G, Liu M, He T, Tang Y, Chen Y, Cai L, Jiang R, Tao J. MiR-21 is overexpressed in response to high glucose and protects endothelial cells from apoptosis. Exp Clin Endocrinol Diabetes. 2013;121:425–430. doi: 10.1055/s-0033-1345169. [DOI] [PubMed] [Google Scholar]