Abstract
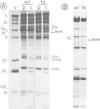
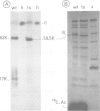
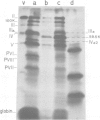
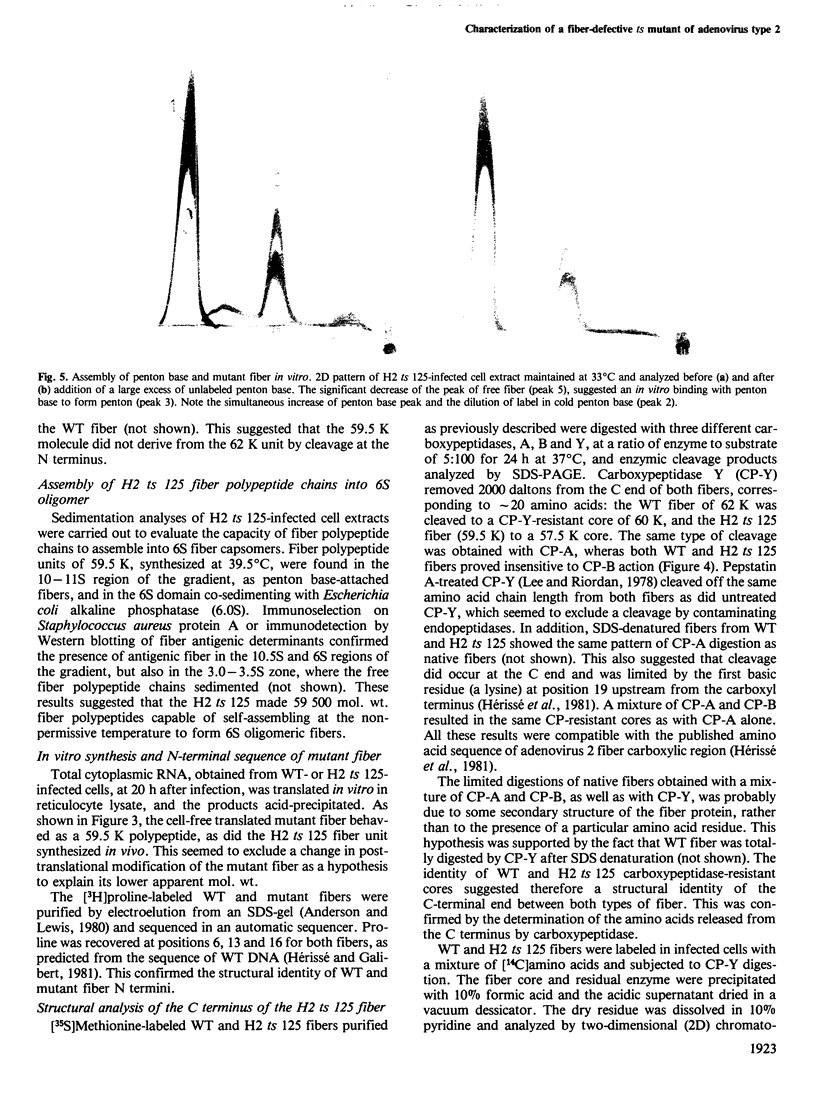
The adenovirus type 2 fiber mutant H2 ts 125 synthesized an unstable, temperature-sensitive fiber polypeptide with an apparent mol. wt. smaller by 2500 than the wild-type (62 K). The polypeptide of 59.5 K was found to be stable at the permissive temperature (33 degrees C). H2 ts 125 fiber synthesized in reticulocyte lysates had the same apparent mol. wt. of 59.5 K as the mutant fiber produced in vivo. Neither structural nor functional differences between wild-type and mutant fibers were detected in the N-terminal and C-terminal sequences, excluding the occurrence of a new initiation or termination codon. Restriction analysis of H2 ts 125 DNA also ruled out the hypothesis of a deletion mutant. The 59.5 K mutant fiber unit was normally glycosyated, N-acetylated, assembled into 6S oligomeric fiber and incorporated into virions. DNA sequencing of the H2 ts 125 fiber gene revealed two point mutations at nucleotides 3970 (C*TT leads to T*TT) and 4958 (GC*T leads to GT*T), corresponding to two amino acid changes at positions 105 and 434, respectively. The 105 mutation consisted of a conservative change Leu leads to Phe; the 434 interchange was Ala leads to Val, usually considered as nonconservative. The possibility of a donor site for splicing created by the mutation at codon GTT was eliminated on the basis of S1 nuclease analysis data. All these results suggested that either one or both mutations concerned highly organized domain(s) of the fiber polypeptide chain, resulting in aberrant mobility in SDS-polyacrylamide gels and temperature-sensitivity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Lewis J. B. Amino-terminal sequence of adenovirus type 2 proteins: hexon, fiber, component IX, and early protein 1B-15K. Virology. 1980 Jul 15;104(1):27–41. doi: 10.1016/0042-6822(80)90363-3. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Lewis J. B., Atkins J. F., Gesteland R. F. Cell-free synthesis of adenovirus 2 proteins programmed by fractionated messenger RNA: a comparison of polypeptide products and messenger RNA lengths. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2756–2760. doi: 10.1073/pnas.71.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boudin M. L., Boulanger P. Antibody-triggered dissociation of adenovirus penton capsomer. Virology. 1981 Sep;113(2):781–786. doi: 10.1016/0042-6822(81)90208-7. [DOI] [PubMed] [Google Scholar]
- Boudin M. L., Boulanger P. Assembly of adenovirus penton base and fiber. Virology. 1982 Jan 30;116(2):589–604. doi: 10.1016/0042-6822(82)90151-9. [DOI] [PubMed] [Google Scholar]
- Boudin M. L., Moncany M., D'Halluin J. C., Boulanger P. A. Isolation and characterization of adenovirus type 2 vertex capsomer (penton base). Virology. 1979 Jan 15;92(1):125–138. doi: 10.1016/0042-6822(79)90219-8. [DOI] [PubMed] [Google Scholar]
- Boulanger P. A., Puvion F. Large-scale preparation of soluble adenovirus hexon, penton and fiber antigens in highly purified form. Eur J Biochem. 1973 Nov 1;39(1):37–42. doi: 10.1111/j.1432-1033.1973.tb03100.x. [DOI] [PubMed] [Google Scholar]
- D'Halluin J. C., Cousin C., Boulanger P. Physical mapping of adenovirus type 2 temperature-sensitive mutations by restriction endonuclease analysis of interserotypic recombinants. J Virol. 1982 Feb;41(2):401–413. doi: 10.1128/jvi.41.2.401-413.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Halluin J. C., Martin G. R., Torpier G., Boulanger P. A. Adenovirus type 2 assembly analyzed by reversible cross-linking of labile intermediates. J Virol. 1978 May;26(2):357–363. doi: 10.1128/jvi.26.2.357-363.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Halluin J. C., Milleville M., Boulanger P. A. Restriction maps of human adenovirus types 2, 5 and 3 for BclI, ClaI, pvul and SphI endonucleases. Gene. 1983 Jan-Feb;21(1-2):165–169. doi: 10.1016/0378-1119(83)90158-0. [DOI] [PubMed] [Google Scholar]
- D'Halluin J. C., Milleville M., Martin G. R., Boulanger P. Morphogenesis of human adenovirus type 2 studied with fiber- and fiber and penton base-defective temperature-sensitive mutants. J Virol. 1980 Jan;33(1):88–99. doi: 10.1128/jvi.33.1.88-99.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedor M. J., Daniell E. Acetylation of histone-like proteins of adenovirus type 5. J Virol. 1980 Sep;35(3):637–643. doi: 10.1128/jvi.35.3.637-643.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hérissé J., Galibert F. Nucleotide sequence of the EcoRI E fragment of adenovirus 2 genome. Nucleic Acids Res. 1981 Mar 11;9(5):1229–1240. doi: 10.1093/nar/9.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H., Ohlsson H., Philipson L. An acetylated N-terminus of adenovirus type 2 hexon protein. Biochem Biophys Res Commun. 1974 Jan 23;56(2):304–310. doi: 10.1016/0006-291x(74)90842-0. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. M., Riordan J. F. Does carboxypeptidase Y have intrinsic endopeptidase activity? Biochem Biophys Res Commun. 1978 Dec 14;85(3):1135–1142. doi: 10.1016/0006-291x(78)90660-5. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Warocquier R., Boulanger P. A. Quantitation of adenovirus soluble antigens by crossed immunoelectrophoresis: application to serological characterization of mutants. Intervirology. 1975;5(3-4):162–172. doi: 10.1159/000149893. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Warocquier R., Cousin C., D'Halluin J. C., Boulanger P. A. Isolation and phenotypic characterization of human adenovirus type 2 temperature-sensitive mutants. J Gen Virol. 1978 Nov;41(2):303–314. doi: 10.1099/0022-1317-41-2-303. [DOI] [PubMed] [Google Scholar]
- Miller J. S., Ricciardi R. P., Roberts B. E., Paterson B. M., Mathews M. B. Arrangement of messenger RNAs and protein coding sequences in the major late transcription unit of adenovirus 2. J Mol Biol. 1980 Oct 5;142(4):455–488. doi: 10.1016/0022-2836(80)90258-2. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Jansson M., Philipson L. Synthesis and genomic site for an adenovirus type 2 early glycoprotein. J Mol Biol. 1980 Feb 5;136(4):375–394. doi: 10.1016/0022-2836(80)90396-4. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Höglund S. Sructural proteins of adenoviruses. 3. Purification and characterization of the adenovirus type 2 penton antigen. Virology. 1969 Sep;39(1):90–106. doi: 10.1016/0042-6822(69)90351-1. [DOI] [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunquist B., Pettersson U., Thelander L., Philipson L. Structural proteins of adenoviruses. IX. Molecular weight and subunit composition of adenovirus type 2 fiber. Virology. 1973 Jan;51(1):252–256. doi: 10.1016/0042-6822(73)90389-9. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer L. F., Ginsberg H. S. Synthesis, transport, and morphogenesis of type adenovirus capsid proteins. J Virol. 1970 Mar;5(3):338–352. doi: 10.1128/jvi.5.3.338-352.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm J. M., Ginsberg H. S. Synthesis in vitro of type 5 adenovirus capsid proteins. J Virol. 1972 Jun;9(6):973–980. doi: 10.1128/jvi.9.6.973-980.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]