Abstract
The serotonin (5-HT) transporter (SERT) is an integral membrane protein that functions to reuptake 5-HT released into the synapse following neurotransmission. This role serves an important regulatory mechanism in neuronal homeostasis. Previous studies have demonstrated that several clinically important antimalarial compounds inhibit serotonin (5-hydroxytryptamine, 5-HT) reuptake. In this study, we examined the details of antimalarial inhibition of 5-HT transport in both Drosophila (dSERT) and human SERT (hSERT) using electrophysiologic, biochemical and computational approaches. We found that the cinchona alkaloids quinidine and cinchonine, which have identical stereochemistry about carbons 8 and 9, exhibited the greatest inhibition of dSERT and hSERT transporter function whereas quinine and cinchonidine, enantiomers of quinidine and cinchonine, respectively, were weaker inhibitors of dSERT and hSERT. Furthermore, SERT mutations known to decrease the binding affinity of many antidepressants affected the cinchona alkaloids in a stereo-specific manner where the similar inhibitory profiles for quinine and cinchonidine (8S,9R) were distinct from quinidine and cinchonine (8R,9S). Small molecule docking studies with hSERT homology models predict that quinine and cinchonidine bind to the central 5-HT binding site (S1) whereas quinidine and cinchonine bind to the S2 site.
Taken together, the data presented here support binding of cinchona alkaloids to two different sites on SERT defined by their stereochemistry which implies separate modes of transporter inhibition. Notably, the most potent antimalarial inhibitors of SERT appear to preferentially bind to the S2 site. Our findings provide important insight related to how this class of drugs can modulate the serotonergic system as well as identify compounds that may discriminate between the S1 and S2 binding sites and serve as lead compounds for novel SERT inhibitors.
Keywords: antimalarial agents, serotonin, serotonin transporter, antagonist, structure-function
1. INTRODUCTION
1.1 The serotonin transporter
The serotonin transporter (SERT) is a member of the solute carrier 6 superfamily (SLC6) and functions in the nervous system to remove serotonin (5-HT) from the synaptic cleft following neurotransmitter release. In mammals, SERT is found in serotonergic neurons, platelets and in the gastrointestinal tract (Bröer and Gether, 2012; Kristensen et al., 2011; Pramod et al., 2013). SERT is the target of a number of important therapeutic drugs such as the selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs), and drugs of abuse such as 3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”), and cocaine (Fuller and Wong, 1990; Ramamoorthy et al., 1993; Rudnick and Wall, 1992). Molecular cloning of SERT (Blakely et al., 1991; Chang et al., 1996; Chen et al., 1998; Corey et al., 1994; Demchyshyn et al., 1994; Hoffman et al., 1991; Ramamoorthy et al., 1993) led to significant advancements in our understanding of how antagonists affect SERT function. Studies such as site-directed mutagenesis (SDM), substituted cysteine accessibility method (SCAM) and antagonist analog studies (Andersen et al., 2009; Bröer and Gether, 2012; Chen et al., 1997; Henry et al., 2006; Kristensen et al., 2011; Pramod et al., 2013) have pointed toward a single high-affinity binding site which was found to correspond to the location of the substrate and competitive inhibitor binding site from high-resolution crystal structures of the bacterial SLC6 protein, LeuT (Singh et al., 2008; Yamashita et al., 2005). On the other hand, recent co-crystals of LeuT with SSRIs (Zhou et al., 2009) and TCAs (Singh et al., 2007; Zhou et al., 2007) have suggested an alternative, non-competitive site for binding of antagonists to monoamine transporters and as a result the molecular mechanism of SERT inhibition remains unclear.
In addition to transport of 5-HT, SERT which is Na+, Cl− and K+-dependent, exhibits several current conducting states including: (1) a voltage-dependent, 5-HT-elicited current (Beckman and Quick, 2001; Corey et al., 1994; Galli et al., 1997; Lin et al., 1996; Mager et al., 1994), (2) a voltage sensitive transient current (3) a leak current (Galli et al., 1997; Mager et al., 1994; Petersen and DeFelice, 1999) and (4) a channel-like current (Galli et al., 1997; Petersen and DeFelice, 1999).
1.2 Antimalarial compounds modulate the serotonergic system
Similar to the better-known SERT antagonists (i.e. SSRIs and TCAs), several antimalarial compounds have been reported to bind and block 5-HT uptake by SERT. Early studies showed that the antimalarials quinidine, quinacrine, chloraquine and quinine could inhibit 5-HT accumulation by platelets (Bridges and Baldini, 1966; Davis and Wilson, 1969). Subsequently, quinine and quinidine were found to inhibit 5-HT uptake by hippocampal synaptosomes and compete with the binding of the SSRI [3H]paroxetine to hippocampal membranes (Clement et al., 1998). Recently, it was shown that the antimalarial, methylene blue, inhibits 5-HT-induced currents and 5-HT uptake in cultured EM4 (HEK-293) cells transfected with hSERT (Oz et al., 2012). However, as with classical SERT antagonists, the mechanism by which antimalarials inhibit SERT reuptake of 5-HT remains unclear and, in fact, both competitive or non-competitive modes of inhibition have been reported (Clement et al., 1998; Davis and Wilson, 1969).
Interestingly, in addition to blocking 5-HT reuptake through SERT, quinidine and quinine stimulate Ca2+-dependent release of intracellular stores of monoamines reportedly through activation of K+ channels (Clement et al., 1998). Quinidine activation of K+ channels has been linked to augmenting antidepressant activity in mouse behavioral studies (Guo et al., 1995) indicating that antimalarials can modulate the serotonergic system through the independent mechanisms of reuptake blockade and elevated vesicular release of 5-HT.
In this study, we look at structural and functional aspects of several antimalarial compounds to inhibit the serotonin transporter (SERT) function to identify structural features of the antimalarial compounds that are important for their antagonism of SERTs.
2. Materials and Methods
2.1 Reagents
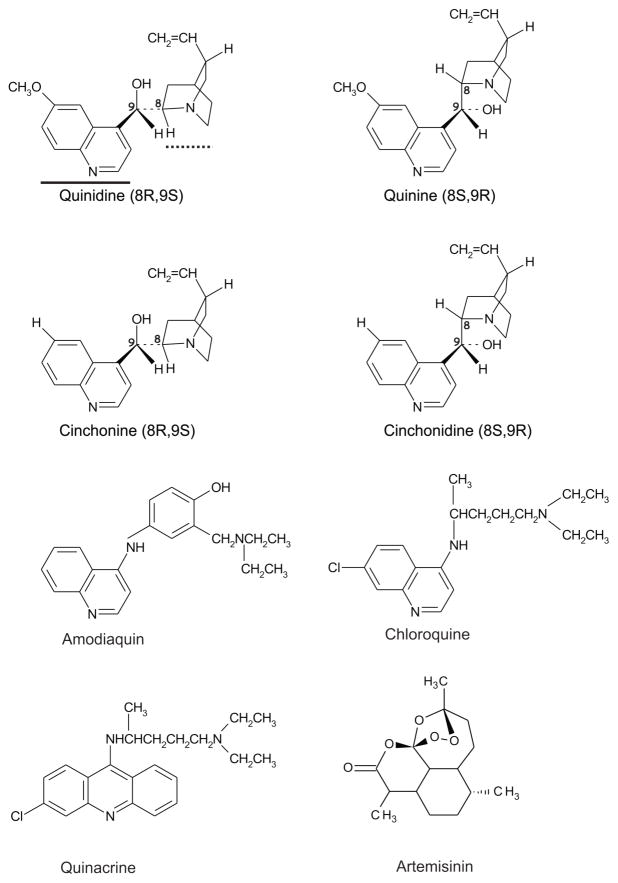
[3H]5-HT binoxalate and [3H]5-HT creatine sulfate (specific activity of 27 Ci/mmol) were purchased from New England Nuclear or Perkin Elmer Serotonin hydrochloride and quinidine sulfate were of the highest grade and purchased from RBI/Sigma (St. Louis, MO). Amodiaquine dihydrochloride, cinchonine hydrochloride, cinchonidine hydrochloride, chloroquine diphosphate, quinacrine dihydrochloride, artemisinin and quinine hemisulfate were of the highest grade and purchased from Sigma (St. Louis, MO). Their 2D structures are given in Figure 1.
Figure 1. Structures of antimalarial compounds.
Quinidine, quinine, cinchonine and cinchonidine are cinchona alkaloids. The thick solid line under quinidine denotes the quinoline ring while the thick dotted line corresponds to the quinuclidine ring. Note the configuration of these rings about carbons 8 and 9 where (quinidine and quinine) and (cinchonine and cinchonidine) are enantiomers. Amodiaquin and chloroquine also possess the quinoline ring but lack stereochemistry. Quinacrine is related to chloroquine but contains an additional ring. Artemisinin is structurally unrelated to the other antimalarials. Two-dimensional representations of the antimalarial compounds were rendered using ISIS Draw 2.3 (MDL Information Systems, Inc., San Leandro, CA).
2.2 Xenopus oocyte preparation, cRNA synthesis, and injection
Xenopus oocytes were prepared and maintained as previously described (Quick and Lester, 1994). Briefly, oocytes were defolliculated in collagenase A and maintained at 18°C in ND96 (in mM: 96 NaCl, 2 KCl, 1 MgCl2, 5 HEPES, 1.8 CaCl2) supplemented with 2.5 mM Na+ pyruvate, 50 μg/ml gentamycin, 10 U/ml penicillin, 10 μg/ml streptomycin, and 5% horse serum. The medium was changed daily.
dSERT cRNA was synthesized using Nhe I-linearized pGEMHE-dSERT as the template for in vitro transcription from the T7 promoter using mMessage mMachine (Ambion, Austin, TX). Individual oocytes were injected with 50 ng dSERT-HE cRNA. Experiments were carried out between 2–4 days post-injection.
2.3 Transient Expression of hSERT in HeLa Cells
HeLa cells were maintained in a 37°C incubator humidified with 5% CO2, and grown in complete medium (DMEM, with 10% Fetal Bovine Serum, 100 units/ml Penicillin, 100 μg/ml Streptomycin, and 250 ng/ml Amphotercin B). Cells were transiently transfected with GeneCellin transfection reagent (Bulldog Bio), using 2 μg of DNA, 8 μl of GeneCellin and 2 ml of serum-free DMEM per 24-well plate. Cells were plated at a density of 100,000 or 50,000 cells/well and assayed either 24 hours or 48 hours later.
2.4 Transport assays
2.4.1 [3H]5-HT uptake in oocytes
To assess neurotransmitter flux in oocytes we carried out [3H]5-HT transport assays as previously described (Beckman and Quick, 2001). Briefly, oocytes were placed in a multiwell plate in ND96. At timed intervals radiolabeled neurotransmitter or radiolabeled neurotransmitter plus drug was added to initiate the transport assay. The final concentration of [3H]5-HT was 200 nM. The reaction was terminated by rapid removal of the oocyte from the assay media followed by six washes in ND96. The oocyte was than solubilized in 10% SDS at 60°C for 2 h, scintillation fluid was added and [3H]5-HT uptake was determined by liquid scintillation counting. Specific uptake was determined by subtracting from total uptake the non-specific uptake measured in uninjected oocytes from the same batch.
2.4.2 Competitive uptake assays in hSERT expressing HeLa cells
Cells were washed twice with MKRHG buffer (5mM Tris, 7.5 mM HEPES, 120 mM NaCl, 5.4 mM KCL, 1.2 mM CaCl2, 1.2 mM MgSO4, 1 mM glucose, pH 7.4) prewarmed to 37°C and incubated with a range of inhibitor concentrations for 10 min. [3H]5-HT (PerkinElmer) was added to a final concentration of 50 nM in pargyline, and ascorbic acid for 10 min at 37°C. Assays were terminated with three washes of ice-cold MKRHG. [3H]5-HT uptake was determined by addition of 0.5 ml of MicroScint 20 (PerkinElmer) and scintillation counting on a TopCount NXT system (PerkinElmer). Specific activity was determined by subtracting non-specific counts obtained from non-transfected samples. Specific uptake was then normalized to percent activity of no drug control of transfected cells. IC50 and Ki values were determined using a one-site non-linear curve fit as a function of the log of the inhibitor concentration (Prism 5 for Mac, Graphpad Software).
2.5 Electrophysiological Recordings
Whole-cell currents were measured using a GeneClamp 500 amplifier (Axon Instruments, Foster City, CA) in a standard two-microelectrode voltage-clamp configuration. Electrodes were filled with 3M KCl and had resistances of 0.5 to 2 MΩ. Unless otherwise indicated, oocytes were clamped at −50 mV and superfused with room temperature buffer via a manual gravity perfusion system with a flow rate of approximately 12 ml/minute. Control recording buffer was ND96. Currents were low-pass filtered at 10–100 Hz and digitized at 100–200 Hz. Steady-state currents in ND96 alone were subtracted from those in 5-HT or 5-HT plus drug to yield the current associated with dSERT (Petersen and DeFelice, 1999). Data were stored to disk and subsequent acquisition and analysis were done with pClamp 8.0 (Axon Instruments).
2.6 Generation hSERT comparative models
The 3D coordinates of the “occluded” and “open-to-out” crystal structures (PDB IDs 2A65 and 3F3A, respectively) of the leucine transporter (LeuT) from Aquifex aeolicus (Uniprot accession no. P23977), were mapped onto hSERT sequence using a comprehensive sequence alignment (Beuming et al., 2006). The missing atomic densities in the loop regions were rebuilt using the kinematic closure (KIC) method (Mandell et al., 2009) in Rosetta3.5 (Leaver-Fay et al., 2011; “Rosetta 3.5,” 2013). The N− and C–termini, which are missing in LeuT, were truncated in the hSERT models to yield a structure consisting of amino acids 79 to 607, which includes TMs 1–12. Side chains for all residues in the protein were built using Rosetta’s Metropolis Monte Carlo rotamer search algorithm (Kuhlman et al., 2003). The hSERT model was subjected to six iterative rounds of relaxation and minimization using Rosetta3.5 to produce an ensemble of 100 hSERT models each for 2A65 and 3F3A. The top 10 ranked models based on the ROSETTA Etotal score for each template were carried forward for docking studies.
2.7 Docking of antimalarial compounds using ROSETTALIGAND
The 3D structure of the compounds in sdf format was obtained from PubChem (http://pubchem.ncbi.nlm.nih.gov), visualized and protonated in VIDA (version 4.2.1, OpenEye Software). The protonation state of each ligand at pH 7.4 was based on analysis using FIXPKA in QUACPAK ver 1.5.0 (QUACPAC, 2011). Conformers of each ligand were generated using Omega (Hawkins et al., 2010; OMEGA, 2013). The ligand parameter file which assigns Rosetta atom types to the molecule for use in RosettaLigand (RL) was generated using the Rosetta3.5 script “molfile_to_params.py”. The conformers were docked to the models as previously described (Combs et al., 2013). Briefly, ligands were placed in the ten hSERT models at coordinates equivalent to the substrate binding site in LeuT and allowed to randomly translate within a 10Å sphere which covers both the S1 and S2 binding sites (Quick et al., 2009). Ligand translations that were acceptable based on the energy score were then optimized by 1000 random rotations to minimize clashes. Residue side chains within 6Å of the ligand were repacked using a Metropolis Monte Carlo simulated annealing algorithm and scored using the knowledge-based Rosetta energy function interface_delta, which is the difference between the total binding energy E(transporter+ligand) and the sum of the energy of the individual components E(transport) + E(ligand)separated by 500Å. A total of 25,000 ligand dockings were conducted for the 10 models from 2A65. The process was repeated for 3F3A-based models. The resulting ligand/hSERT complexes were ranked based on the interface_delta scores and the top 5% of each of the 10 hSERT structures were compiled and evaluated by score and binding site.
2.8 Data analysis and statistics
For experiments in which inhibition constants (IC50) values were determined, data were fit using either Kaleidagraph 3.5 (Synergy Software) or Prism (Graphpad) with the following equation: I=Imax/[I+([antagonist]/IC50)n], where I is the current produced at a given antagonist concentration [antagonist], Imax is the maximal current, and n is the Hill coefficient. Ki values were determined using non-linear regression to a one-site binding model with Prism (Graphpad). For statistical comparison of mean data, Student’s t tests or one-way ANOVAs were performed.
3. Results
3.1 Antimalarial impact on SERT function
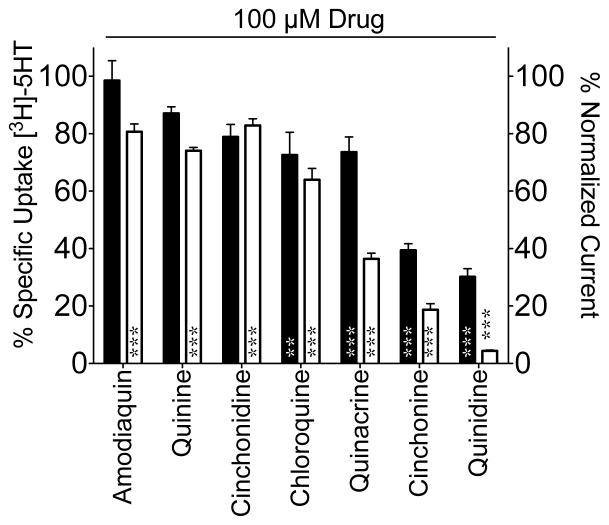
To assess the effect of antimalarial drugs on SERT function, Xenopus oocytes expressing dSERT were exposed to the compounds at a single concentration of 100 μM and evaluated for [3H]5-HT uptake. The analysis revealed the antimalarials tested (Figure 1) differ in their ability to inhibit 5-HT uptake by dSERT with a rank order potency as follows: quinidine-cinchonine > chloroquine = quinacrine = cinchonidine = quinine > amodiaquin (Figure 2, black bars) and ranged from the least effective drug, amodiaquin, which was not significantly different from the vehicle control, to quinidine which inhibited uptake by ~70%.
Figure 2. Antimalarial inhibition of dSERT function.
Inhibition of [3H]5-HT uptake (black bars, left Y-axis) or induced current (I5HT, white bars, right Y-axis) in oocytes expressing dSERT is plotted on the as a percentage of activity measured in vehicle control treated oocytes. Non-specific uptake was calculated from uninjected oocytes and subtracted to yield specific uptake. Data were analyzed using a one-way ANOVA and a Dunnett post-hoc test. *=P<0.05, **=P<0.01, ***=P<0.001 indicate significant difference compared to vehicle control.
In addition to 5-HT uptake, SERT activity was assessed by measuring the substrate-elicited ion conductance. 5-HT flux through dSERT is voltage-dependent (Galli et al., 1997) and electrogenic (Beckman and Quick, 2001; Corey et al., 1994; Galli et al., 1997; Petersen and DeFelice, 1999) where perfusion of voltage-clamped oocytes with substrates such as 5-HT results in an inward current whose magnitude changes with the holding potential. In addition, SERT exhibits a constitutive leak current in the absence of substrates that is revealed by application of SERT antagonists such as SSRIs (Galli et al., 1997; Mager et al., 1994). Therefore, we measured I5HT currents in Xenopus oocytes expressing dSERT upon application of antimalarial compounds at 100μM (Figure 2, white bars and Figure 3). The rank order potency of the compounds to inhibit I5HT was virtually the same as seen with 5-HT uptake (quinidine > cinchonine > quinacrine > chloroquine > quinine = amodiaquin = cinchonidine) except amodiaquin, which did not impact 5-HT uptake, was as affective as quinine, cinchonidine and chloroquine at inhibiting I5HT (Figure 2).
Figure 3. Antimalarial compounds differentially inhibit 5-HT-elicited currents through dSERT.
Raw traces from oocytes under two-electrode voltage clamp at −50 mV superfused with various drugs. 5-HT was applied at 10 μM, and antimalarial compounds at 100 μM in all cases. S indicates application of 5-HT alone, S+D indicates 5-HT plus drug, and D indicates drug alone. Note separate scale bars for each trace. Similar results were obtained in 4–8 oocytes for each compound tested.
Importantly, when tested alone, none of the antimalarials were able to induce inward currents supporting that these compounds are not substrates for dSERT (Figure 3, drug only application). In fact, with the exception of amodiaquin, application of the drug alone resulted in a positive deflection of the current trace indicative of ILeak blockade, a property consistent with SERT blockers. Application of these drugs (at 100 μM) to uninjected oocytes did not result in any significant current (data not shown).
3.2 Structural determinants of antimalarial action on SERT
The pairs of molecules quinine and quinidine, and cinchonine and cinchonidine are enantiomers about carbons 8 and 9 with the former pair differing from the latter only by the presence of a methoxy (CH3O) substituent in place of a proton (H) in the quinolone ring moiety (Figure 1; (Notterman et al., 1986; Oleksyn et al., 1992). The uptake and current analyses in section 3.1 revealed that of the drugs tested, quinidine and cinchonine were the most effective inhibitors for all of the modes, while quinine and cinchonidine were less effective suggesting the stereochemistry around carbons 8 and 9 play a significant role in the potency of the cinchona alkaloids.
3.3 Determination of Ki values for antimalarial agents to block 5-HT uptake by SERT
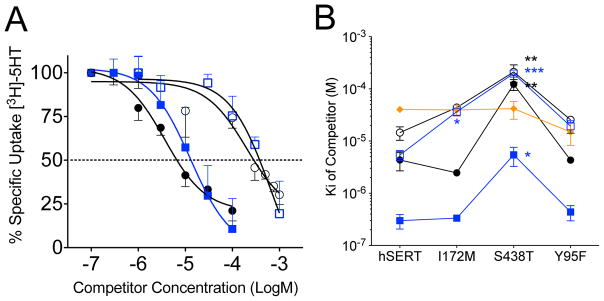
To further explore this structure-activity relationship, [3H]5-HT competition uptake assays were performed on oocytes and HeLa cells expressing dSERT and hSERT, respectively, to determine the Ki for the antimalarial compounds quinidine, quinine, cinchonidine and cinchonine (Figure 4A and B, Table 1 dSERT and hSERT columns). Comparison of the Ki values for the compounds against hSERT and dSERT reveal that all of the drugs with exception of quinidine exhibit greater affinity for hSERT than dSERT with the greatest difference being cinchonidine which exhibits a 79-fold lower Ki against hSERT compared to dSERT. Despite the differences of the Ki for the compounds between dSERT and hSERT, the stereoselective property was maintained where quinidine and cinchonine (both 8S,9R) were significantly more potent in comparison to their enantiomers quinine and cinchonidine (8R,9S). For hSERT, the 4-aminoquinoline compound chloroquine and its analog amodiaquin were 6.9 and 2.7-fold less potent than the least potent cinchona alkaloid, quinine (Table 1). The structurally unrelated sesquiterpene lactone, artemisinin (Figure 1), showed no inhibition of hSERT mediated 5-HT transport at the highest dose tested (1 mM) (Table 1).
Figure 4. Potency of antimalarial compounds to inhibit [3H]5-HT uptake by dSERT and hSERT.
(A) dSERT expressing oocytes were incubated with [3H]5-HT (200 nM) for and increasing concentrations of quinine (○), quinidine (●), cinchonine (
 ), cinchonidine (
), cinchonidine (
 ) for 20 min. Percent uptake was normalized to no drug control and plotted versus log of the competitor concentration. Data were fit to a one-site competition non-linear regression equation. (B) HeLa cells expressing hSERT or hSERT mutants important in high-affinity antidepressant binding were incubated with [3H]5-HT (50 nM) for 10 min in the presence of increasing concentrations of inhibitor quinine (○), quinidine (●), cinchonine (
) for 20 min. Percent uptake was normalized to no drug control and plotted versus log of the competitor concentration. Data were fit to a one-site competition non-linear regression equation. (B) HeLa cells expressing hSERT or hSERT mutants important in high-affinity antidepressant binding were incubated with [3H]5-HT (50 nM) for 10 min in the presence of increasing concentrations of inhibitor quinine (○), quinidine (●), cinchonine (
 ), cinchonidine (
), cinchonidine (
 ) amodiaquin (
) amodiaquin (
 ). Specific uptake was calculated and normalized as a percent of no drug control. The data were fit to a one-site competition non-linear regression equation and converted to Ki using the Km for 5-HT. The Ki values are plotted versus the mutant background. Data represent at least three independent experiments. Data were analyzed using a one-way ANOVA and a Bonferroni post-hoc test. *=P<0.05, **=P<0.01, ***=P<0.001 indicate significant difference compared to native hSERT.
). Specific uptake was calculated and normalized as a percent of no drug control. The data were fit to a one-site competition non-linear regression equation and converted to Ki using the Km for 5-HT. The Ki values are plotted versus the mutant background. Data represent at least three independent experiments. Data were analyzed using a one-way ANOVA and a Bonferroni post-hoc test. *=P<0.05, **=P<0.01, ***=P<0.001 indicate significant difference compared to native hSERT.
Table 1.
Ki values of antimalarial compounds in dSERT, hSERT and hSERT S1 site mutants.
| Compound | Ki (μM ± SEM) | ||||
|---|---|---|---|---|---|
|
| |||||
| dSERT | hSERT | hSERT I172M | hSERT S438T | hSERT Y95F | |
| Cinchonidine | 330 ± 19 | 4.2 ± 0.2 | 36 ± 4.8 | 196 ± 20 | 15 ± 5 |
| Cinchonine | 6.4 ± 0.05 | 0.2 ± 0.1 | 0.31 ± 0.002 | 5.4 ± 2.2 | 0.3 ± 0.05 |
| Quinidine | 1.8 ± 0.11 | 4.3 ± 1.7 | 2.5 ± 0.22 | 64 ± 29 | 4 ± 0.3 |
| Quinine | 102 ± 24 | 15 ± 4.2 | 43 ± 2.9 | 211 ± 77 | 26 ± 3 |
| Amodiaquin | ND | 40 ± 4.5 | 39 ± 9.3 | 42 ± 16 | 22 ± 2.3 |
| Chloroquine | ND | 122 ± 55 | 56 ± 24 | 208 ± 71 | 83 ± 13 |
| Artemisinin | ND | NE | NE | NE | NE |
ND – not determined
NE – no detectable effect on 5-HT transport
3.4 Docking of cinchona alkaloids to hSERT comparative models
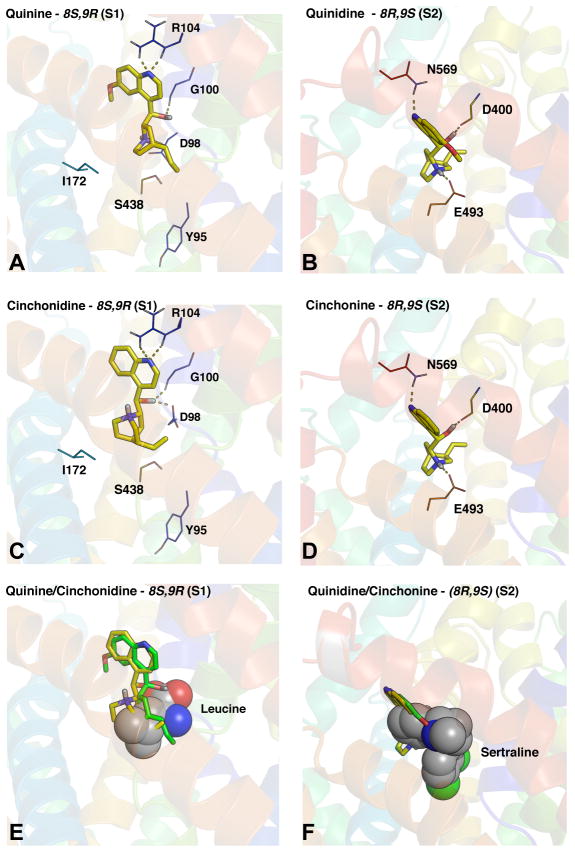
Based on the stereochemical patterns in the potency of the cinchona alkaloids, quinine, quinidine, cinchonine and cinchonidine were computationally docked (in their monoprotonated forms) into hSERT comparative models constructed from the ‘open-to-out’ and ‘outward-occluded’ LeuT structures (Singh et al., 2008; Yamashita et al., 2005) (which represent competitor and substrate bound complexes, respectively) using RosettaLigand (Combs et al., 2013; Kaufmann and Meiler, 2012; Kaufmann et al., 2009). The analysis generated 25,000 decoys/ligand/hSERT model. The top scoring ligand/transporter complexes for both the ‘open-to-out, 3F3A’ and ‘outward-occluded, 2A65’ gave essentially the same results and revealed that the vast majority and best scoring poses for quinine and cinchonidine were located in the S1 binding pocket which corresponds to the primary substrate binding pocket in LeuT (Figure 5A, C, E). In contrast, quinidine and cinchonine were predicted to bind to a region more extracellularly located in hSERT (Figure 5B, D, F). This pocket is essentially the S2 site which corresponds to the location of antidepressant binding in LeuT co-crystals (Singh et al., 2007; Zhou et al., 2009, 2007) and the controversial second substrate binding site (Piscitelli et al., 2010; Quick et al., 2009) (Figure 5F). Notably, quinine and quinidine were found to dock higher up in the S1 site compared to inhibitors like (S) citalopram that usually are positioned deeper down in the S1 pocket (Combs et al., 2011; Kaufmann et al., 2009) (Figure 5A,C,E). These findings indicate that correlation between potency and stereochemistry may be attributed to binding to distinct sites on SERT.
Figure 5. RosettaLigand docking analysis of antimalarial compounds suggest preference for the S1 and S2 sites on hSERT are directly related to stereochemistry.
Compounds were computationally docked into hSERT comparative models (based on LeuT ‘open-to-out’; PDBID 3F3A and ‘outward-occluded’; PDBID 2A65 crystal structures) using Rosetta version 3.5. As both models yielded essentially the same results we only show data from 3F3A as it represents an inhibitor bound conformation. The best-scoring models predict (A) quinine and (C) cinchonidine with S and R stereo centers around Carbons 8 and 9, respectively, bind to S1 whereas (B) quinidine and (D) cinchonine which have the opposite stereochemistry (8R, 9S) bind to S2. hSERT helices with key residues interacting with the ligand represented as lines. The ligand (yellow) is depicted in stick mode. Dashed lines indicated points of interaction. (E) Overlap of quinine (green) and cinchonidine (yellow) with leucine (spheres) in S1 and (F) quinidine (green) and cinchonine (yellow) with sertraline (spheres) in LeuT.
3.5 Impact of SERT mutants on the inhibitory potency of cinchona alkaloids
To further investigate the predictions from the computational docking studies, the potency of quinine, quinidine, cinchonine and cinchonidine was determined in hSERT mutants I172M, S438T and Y95F. These mutations, which are located in the S1 binding site, have been previously identified to dramatically alter the binding affinity of many antidepressants and cocaine (Andersen et al., 2009; Barker et al., 1998; Henry et al., 2006; Thompson et al., 2011). Interestingly, quinine and cinchonidine, which have the (8S,9R) chirality and we predict to bind to S1 exhibited reduced potency in the I172M and S438T mutant backgrounds (Figure 4B, Table 1). In contrast, only the S438T mutation was found to affect potency of quindine and cinchonine which both have the (8R,9S) stereochemistry. The Y95F mutation did not significantly alter potency in any of the four compounds. However, this is not surprising given that Y95 is at the base of the S1 binding pocket and quinine and quinidine are position higher up in this binding site (Figure 5A,C,E, see Sec 3.4). The differential impact of these mutations on potency implies that quinidine and cinchonine share the same binding mode, which is distinct from the mode shared by quinine and cinchonidine. These observations are consistent with our computational docking results. The finding that the S438T mutant affected the compounds that bind to S2 seems surprising given the distance between S438 and the S2 site (~7Å). However, by homology to LeuT, S438 coordinates Na+ at the Na2 site and there is increasing evidence that the Na1, Na2 and Cl binding sites are tightly coordinated where changes at one of these sites can impact coordination and presumably structure at the other sites (Henry et al., 2011; Kantcheva et al., 2013; Yu et al., 2010).
4. Discussion
4.1 Antimalarial impact on monoamine neurotransmission
Pharmacological modulation of monoamine neurotransmitter transporter function is relevant in the treatment of human diseases and disorders (Bröer and Gether, 2012; Hahn et al., 2003; Pramod et al., 2013) as well as to addiction and drug abuse in regards to cocaine, phenylethylamines and amphetamines and their derivatives (Dean et al., 2013; Volkow and Li, 2004). Though poorly studied, several antimalarial drugs have been found to modulate serotonin levels in the synapse and in platelets. Though some of the antimalarial-mediated increase in neuronal 5-HT levels can be attributed to their activation of K+ channels, there are some antimalarial agents that bind to SERT and inhibit 5-HT reuptake (Bridges and Baldini, 1966; Clement et al., 1998; Davis and Wilson, 1969; Oz et al., 2012).
4.2 Interaction of antimalarial compounds with SERT
In this study, we investigated the effect of a panel of antimalarial compounds on two heterologously-expressed 5-HT transporters, dSERT and hSERT, in an attempt to determine the effect of these compounds on the functional properties of the transporter, to elucidate the mechanism of inhibition, and to identify structural features of these molecules important in their ability to bind and inhibit SERT.
Initial characterization of the antimalarial agents was performed in dSERT using TEVC studies to measure 5-HT-elicited currents, and substrate-independent leak currents as well as [3H]5-HT uptake under a membrane clamp to ensure the drug’s inhibition of uptake was not due to simple altering of membrane potential. hSERT was used to evaluate the effects of the antimalarials on the human transporter by focusing on competitive uptake analysis to compare the potency of the cinchona alkaloids between hSERT and dSERT. These types of comparisons can identify species-specific differences in inhibition potency which have proven useful in identifying molecular determinants in antagonist/transporter interactions (Barker et al., 1998; Henry et al., 2006; Kaufmann et al., 2009).
4.2.1 Quantification of antimalarial-mediated inhibition of SERT
Our results suggest that many antimalarial agents contain structural aspects that permit binding of these compounds to SERTs as exposure of dSERT to a single concentration of quinidine, quinine, cinchonine, cinchonidine, amodiaquine, quinacrine or chloroquine resulted in inhibition of 5-HT transport and I5HT with virtually the same rank order potency. In general, the compounds inhibited I5HT to a greater extent than 5-HT transport, which is consistent with previous data showing that 5-HT transport and I5HT can be affected differentially by various experimental manipulations such as substrate concentration and temperature (Beckman and Quick, 2001). This single point analysis showed that the cinchona alkaloids, quinidine and cinchonine were the most potent dSERT antagonists, whereas their respective enantiomers, quinine and cinchonidine, were less effective. Notably, 5-HT uptake Ki values for these compounds revealed that quinine, cinchonine and cinchonidine were 6.8, 32 and 79-fold more potent, respectively, at inhibiting hSERT than dSERT, suggesting molecular distinctions between the binding sites of these compounds in these transporters. Future studies can focus on identifying the molecular determinants that contribute to the increased potency of some antimalarial agents for hSERT versus dSERT. Importantly, the specificity of many of the antimalarial compounds to inhibit 5-HT uptake by SERT are supported by the fact that the structurally distinct antimalarial compound artemisinin exhibited no inhibitory effect on SERT function.
4.2.2 Computational docking of antimalarial agents to SERT homology models
Computational docking of the cinchona alkaloids to hSERT homology models predicts the differences in potency between the enantiomers originates from their interaction at two distinct binding sites on the transporter. The best-scoring poses for quinine and cinchonidine were located in the S1 binding pocket, which corresponds to the accepted substrate-binding site in SERT (Bröer and Gether, 2012; Kristensen et al., 2011; Pramod et al., 2013). Quinidine and cinchonine bound preferentially to the S2 site (the site of SSRI and TCA binding in co-crystals of LeuT). A similar mechanism is proposed to account for the different potencies between the (R) and (S) enantiomers of the SSRI citalopram (Plenge et al., 2007).
Analysis of the Ki values for 5-HT uptake inhibition by the cinchona alkaloids in mutant hSERT backgrounds known to influence high-affinity antidepressant binding revealed that the potency of the (8S,9R) compounds quinine and cinchonidine are impacted by two of the three S1 site mutants (I172M and S438T). In contrast, the (8R,9S) drugs, quinidine and cinchonine, were only affected by the S438T mutation. At first, this was an unexpected finding given that the S438T mutation has been reported to greatly impact inhibitor potency at S1 (Andersen et al., 2009). However, a recent study, which engineered the Cl− site into LeuT, showed that organization of Na+ at the Na1 site and Cl− at the Cl site arrange their coordinating residues to form a H-bond network between these sites and residues involved in the outer gate (Kantcheva et al., 2013). Given that binding of quinine and cinchonidine at the S2 site directly involves outer gate residues, we argue that mutations at S438 could impact both the potency of compounds binding either to S1 or S2.
4.3 Conclusion
Previous studies have shown: (1) that SSRI and TCA efficacy is enhanced following quinine treatment in an animal model used to assess antidepressants (Guo et al., 1995), (2) improvement in apathy and sleep indices in patients with myotonic dystrophy following hydroquinidine treatment (Di Costanzo et al., 2000) and (3) that co-administration of a monoamine oxidase inhibitor and quinine to rats results in the 5-HT syndrome (Wang and Grahame-Smith, 1992). Whereas, it has been reported that these effects by malarial compounds are due to activation of K channels (Guo et al., 1995), our studies suggest clinically relevant direct interactions between antimalarial compounds and SERT which can impact serotonergic system. Estimates of both quinidine and quinine concentrations calculated from measured blood levels in humans suggest that blood quinidine concentrations can rise to 9.5 μM in patients treated for arrhythmia (Berry et al., 1988) and in humans treated with quinine blood levels can range from 6.2–32.1 μM (Pussard et al., 1999; White, 1985). Based on our studies, these levels are sufficient to inhibit the serotonin transporter.
Interestingly, in contrast to most SERT antagonists, the most potent SERT-inhibiting antimalarial compounds tested in this study appear to prefer binding to the S2 site. This finding may warrant further investigation in drug development as the discovery and use of compounds that preferentially bind the S2 site over the S1 site has been proposed as an adjunctive therapy to S1-preferring antidepressants by allosterically slowing their dissociation from the transporter (Plenge et al., 2012).
In conclusion, the results from our experiments identify structural elements found in several antimalarial compounds that may be important in the design of SERT inhibitors with clinical efficacy, or at minimum can aid in the determination of the structural aspects of the interaction between 5-HT transporters and their ligands.
Acknowledgments
We thank Dr. R. Paul Malchow for suggesting that the effect of quinine on dSERT currents be tested. The initial studies of antimalarial inhibition of dSERT were conducted at the Marine Biological Laboratory (Woods Hole, MA) and supported by a Grass Fellowship to MLB. This work was funded in part by an NSF-EPSCOR Faculty Startup Award to LKH. We thank Dr. Michael Wentzel for discussions on stereochemistry of antimalarials.
Abbreviations
- 5-HT
serotonin
- dSERT
Drosophila serotonin transporter
- hSERT
human serotonin transporter
- SERT
serotonin transporter
- SSRI
selective serotonin reuptake inhibitor
- TCA
tricyclic antidepressant
- TEVC
two-electrode voltage clamp
References
- Andersen J, Taboureau O, Hansen KB, Olsen L, Egebjerg J, Strømgaard K, Kristensen AS. Location of the antidepressant binding site in the serotonin transporter: importance of Ser-438 in recognition of citalopram and tricyclic antidepressants. J Biol Chem. 2009;284:10276–10284. doi: 10.1074/jbc.M806907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker EL, Perlman MA, Adkins EM, Houlihan WJ, Pristupa ZB, Niznik HB, Blakely RD. High affinity recognition of serotonin transporter antagonists defined by species-scanning mutagenesis. An aromatic residue in transmembrane domain I dictates species-selective recognition of citalopram and mazindol. J Biol Chem. 1998;273:19459–19468. doi: 10.1074/jbc.273.31.19459. [DOI] [PubMed] [Google Scholar]
- Beckman M, Quick M. Substrates and temperatures differentiate ion flux from serotonin flux in a serotonin transporter. Neuropharmacology. 2001;40:526–535. doi: 10.1016/s0028-3908(00)00191-x. [DOI] [PubMed] [Google Scholar]
- Berry NS, Bauman JL, Gallastegui JL, Bauma W, Beckman KJ, Hariman RJ. Analysis of antiarrhythmic drug concentrations determined during electrophysiologic drug testing in patients with inducible tachycardias. Am J Cardiol. 1988;61:922–924. doi: 10.1016/0002-9149(88)90376-1. [DOI] [PubMed] [Google Scholar]
- Beuming T, Shi L, Javitch J, Weinstein H. A comprehensive structure-based alignment of prokaryotic and eukaryotic neurotransmitter/Na+ symporters (NSS) aids in the use of the LeuT structure to probe NSS structure and function. Mol Pharmacol. 2006;70:1630–42. doi: 10.1124/mol.106.026120. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Bridges JM, Baldini M. Effect of quinidine and related compounds on uptake and release of serotonin by blood platelets. Nature. 1966;210:1364–1365. doi: 10.1038/2101364a0. [DOI] [PubMed] [Google Scholar]
- Bröer S, Gether U. The solute carrier 6 family of transporters. Br J Pharmacol. 2012;167:256–278. doi: 10.1111/j.1476-5381.2012.01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AS, Chang SM, Starnes DM, Schroeter S, Bauman AL, Blakely RD. Cloning and expression of the mouse serotonin transporter. Brain Res Mol Brain Res. 1996;43:185–192. doi: 10.1016/s0169-328x(96)00172-6. [DOI] [PubMed] [Google Scholar]
- Chen JG, Sachpatzidis A, Rudnick G. The third transmembrane domain of the serotonin transporter contains residues associated with substrate and cocaine binding. J Biol Chem. 1997;272:28321–28327. doi: 10.1074/jbc.272.45.28321. [DOI] [PubMed] [Google Scholar]
- Chen JX, Pan H, Rothman TP, Wade PR, Gershon MD. Guinea pig 5-HT transporter: cloning, expression, distribution, and function in intestinal sensory reception. Am J Physiol. 1998;275:G433–448. doi: 10.1152/ajpgi.1998.275.3.G433. [DOI] [PubMed] [Google Scholar]
- Clement E, Grahame-Smith D, Elliott J. Investigation of the presynaptic effects of quinine and quinidine on the release and uptake of monoamines in rat brain tissue. Neuropharmacology. 1998;37:945–951. doi: 10.1016/s0028-3908(98)00075-6. [DOI] [PubMed] [Google Scholar]
- Combs S, Kaufmann K, Field JR, Blakely RD, Meiler J. Y95 and e444 interaction required for high-affinity s-citalopram binding in the human serotonin transporter. ACS Chem Neurosci. 2011;2:75–81. doi: 10.1021/cn100066p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs SA, Deluca SL, Deluca SH, Lemmon GH, Nannemann DP, Nguyen ED, Willis JR, Sheehan JH, Meiler J. Small-molecule ligand docking into comparative models with Rosetta. Nat Protoc. 2013;8:1277–1298. doi: 10.1038/nprot.2013.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey J, Quick M, Davidson N, Lester H, Guastella J. A cocaine-sensitive Drosophila serotonin transporter: cloning, expression, and electrophysiological characterization. Proc Natl Acad Sci U S A. 1994;91:1188–92. doi: 10.1073/pnas.91.3.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JW, Wilson SJ. The effect of quinidine on the uptake and release of serotonin by platelets in vitro. Proc Soc Exp Biol Med Soc Exp Biol Med New York N. 1969;131:1107–1110. doi: 10.3181/00379727-131-34048. [DOI] [PubMed] [Google Scholar]
- Dean BV, Stellpflug SJ, Burnett AM, Engebretsen KM. 2C or not 2C: phenethylamine designer drug review. J Med Toxicol Off J Am Coll Med Toxicol. 2013;9:172–178. doi: 10.1007/s13181-013-0295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demchyshyn LL, Pristupa ZB, Sugamori KS, Barker EL, Blakely RD, Wolfgang WJ, Forte MA, Niznik HB. Cloning, expression, and localization of a chloride-facilitated, cocaine-sensitive serotonin transporter from Drosophila melanogaster. Proc Natl Acad Sci U S A. 1994;91:5158–5162. doi: 10.1073/pnas.91.11.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Costanzo A, Mottola A, Toriello A, Di Iorio G, Tedeschi G, Bonavita V. Does abnormal neuronal excitability exist in myotonic dystrophy? II Effects of the antiarrhythmic drug hydroquinidine on apathy and hypersomnia. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2000;21:81–86. doi: 10.1007/s100720070100. [DOI] [PubMed] [Google Scholar]
- Fuller RW, Wong DT. Serotonin uptake and serotonin uptake inhibition. Ann N Y Acad Sci. 1990;600:68–78. doi: 10.1111/j.1749-6632.1990.tb16873.x. discussion 79–80. [DOI] [PubMed] [Google Scholar]
- Galli A, Petersen CI, deBlaquiere M, Blakely RD, DeFelice LJ. Drosophila serotonin transporters have voltage-dependent uptake coupled to a serotonin-gated ion channel. J Neurosci Off J Soc Neurosci. 1997;17:3401–3411. doi: 10.1523/JNEUROSCI.17-10-03401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo WY, Todd KG, Bourin M, Hascoet M. The additive effects of quinine on antidepressant drugs in the forced swimming test in mice. Psychopharmacology (Berl) 1995;121:173–179. doi: 10.1007/BF02245627. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Robertson D, Blakely RD. A mutation in the human norepinephrine transporter gene (SLC6A2) associated with orthostatic intolerance disrupts surface expression of mutant and wild-type transporters. J Neurosci Off J Soc Neurosci. 2003;23:4470–4478. doi: 10.1523/JNEUROSCI.23-11-04470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins PCD, Skillman AG, Warren GL, Ellingson BA, Stahl MT. Conformer Generation with OMEGA: Algorithm and Validation Using High Quality Structures from the Protein Databank and Cambridge Structural Database. J Chem Inf Model. 2010;50:572–584. doi: 10.1021/ci100031x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry LK, Field JR, Adkins EM, Parnas ML, Vaughan RA, Zou MF, Newman AH, Blakely RD. Tyr-95 and Ile-172 in transmembrane segments 1 and 3 of human serotonin transporters interact to establish high affinity recognition of antidepressants. J Biol Chem. 2006;281:2012–23. doi: 10.1074/jbc.M505055200. [DOI] [PubMed] [Google Scholar]
- Henry LK, Iwamoto H, Field JR, Kaufmann K, Dawson ES, Jacobs MT, Adams C, Felts B, Zdravkovic I, Armstrong V, Combs S, Solis E, Rudnick G, Noskov SY, DeFelice LJ, Meiler J, Blakely RD. A conserved asparagine residue in transmembrane segment 1 (TM1) of serotonin transporter dictates chloride-coupled neurotransmitter transport. J Biol Chem. 2011;286:30823–30836. doi: 10.1074/jbc.M111.250308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman BJ, Mezey E, Brownstein MJ. Cloning of a serotonin transporter affected by antidepressants. Science. 1991;254:579–580. doi: 10.1126/science.1948036. [DOI] [PubMed] [Google Scholar]
- Kantcheva AK, Quick M, Shi L, Winther A-ML, Stolzenberg S, Weinstein H, Javitch JA, Nissen P. Chloride binding site of neurotransmitter sodium symporters. Proc Natl Acad Sci. 2013 doi: 10.1073/pnas.1221279110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann KW, Dawson ES, Henry LK, Field JR, Blakely RD, Meiler J. Structural determinants of species-selective substrate recognition in human and Drosophila serotonin transporters revealed through computational docking studies. Proteins. 2009;74:630–642. doi: 10.1002/prot.22178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann KW, Meiler J. Using RosettaLigand for small molecule docking into comparative models. PloS One. 2012;7:e50769. doi: 10.1371/journal.pone.0050769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jørgensen TN, Sørensen L, Eriksen J, Loland CJ, Strømgaard K, Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- Kuhlman B, Dantas G, Ireton GC, Varani G, Stoddard BL, Baker D. Design of a novel globular protein fold with atomic-level accuracy. Science. 2003;302:1364–1368. doi: 10.1126/science.1089427. [DOI] [PubMed] [Google Scholar]
- Leaver-Fay A, Tyka M, Lewis SM, Lange OF, Thompson J, Jacak R, Kaufman K, Renfrew PD, Smith CA, Sheffler W, Davis IW, Cooper S, Treuille A, Mandell DJ, Richter F, Ban YEA, Fleishman SJ, Corn JE, Kim DE, Lyskov S, Berrondo M, Mentzer S, Popović Z, Havranek JJ, Karanicolas J, Das R, Meiler J, Kortemme T, Gray JJ, Kuhlman B, Baker D, Bradley P. ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Methods Enzymol. 2011;487:545–574. doi: 10.1016/B978-0-12-381270-4.00019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Lester H, Mager S. Single-channel currents produced by the serotonin transporter and analysis of a mutation affecting ion permeation. Biophys J. 1996;71:3126–35. doi: 10.1016/S0006-3495(96)79506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager S, Min C, Henry D, Chavkin C, Hoffman B, Davidson N, Lester H. Conducting states of a mammalian serotonin transporter. Neuron. 1994;12:845–59. doi: 10.1016/0896-6273(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Mandell DJ, Coutsias EA, Kortemme T. Sub-angstrom accuracy in protein loop reconstruction by robotics-inspired conformational sampling. Nat Methods. 2009;6:551–552. doi: 10.1038/nmeth0809-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notterman DA, Drayer DE, Metakis L, Reidenberg MM. Stereoselective renal tubular secretion of quinidine and quinine. Clin Pharmacol Ther. 1986;40:511–517. doi: 10.1038/clpt.1986.216. [DOI] [PubMed] [Google Scholar]
- Oleksyn BJ, Suszko-Purzycka A, Dive G, Lamotte-Brasseur J. Molecular properties of Cinchona alkaloids: a theoretical approach. J Pharm Sci. 1992;81:122–127. doi: 10.1002/jps.2600810204. [DOI] [PubMed] [Google Scholar]
- OMEGA. OpenEye Scientific Software. Santa Fe, NM: 2013. [Google Scholar]
- Oz M, Isaev D, Lorke DE, Hasan M, Petroianu G, Shippenberg TS. Methylene blue inhibits function of the 5-HT transporter. Br J Pharmacol. 2012;166:168–176. doi: 10.1111/j.1476-5381.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CI, DeFelice LJ. Ionic interactions in the Drosophila serotonin transporter identify it as a serotonin channel. Nat Neurosci. 1999;2:605–610. doi: 10.1038/10158. [DOI] [PubMed] [Google Scholar]
- Piscitelli CL, Krishnamurthy H, Gouaux E. Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site. Nature. 2010;468:1129–1132. doi: 10.1038/nature09581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenge P, Gether U, Rasmussen SG. Allosteric effects of R- and S-citalopram on the human 5-HT transporter: evidence for distinct high- and low-affinity binding sites. Eur J Pharmacol. 2007;567:1–9. doi: 10.1016/j.ejphar.2007.03.055. [DOI] [PubMed] [Google Scholar]
- Plenge P, Shi L, Beuming T, Te J, Newman AH, Weinstein H, Gether U, Loland CJ. Steric hindrance mutagenesis in the conserved extracellular vestibule impedes allosteric binding of antidepressants to the serotonin transporter. J Biol Chem. 2012;287:39316–39326. doi: 10.1074/jbc.M112.371765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramod AB, Foster J, Carvelli L, Henry LK. SLC6 transporters: Structure, function, regulation, disease association and therapeutics. Mol Aspects Med. 2013;34:197–219. doi: 10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pussard E, Barennes H, Daouda H, Clavier F, Sani AM, Osse M, Granic G, Verdier F. Quinine disposition in globally malnourished children with cerebral malaria. Clin Pharmacol Ther. 1999;65:500–510. doi: 10.1016/S0009-9236(99)70069-X. [DOI] [PubMed] [Google Scholar]
- QUACPAC. OpenEye Scientific Software. Santa Fe, NM: 2011. [Google Scholar]
- Quick M, Winther AML, Shi L, Nissen P, Weinstein H, Javitch JA. Binding of an octylglucoside detergent molecule in the second substrate (S2) site of LeuT establishes an inhibitor-bound conformation. Proc Natl Acad Sci U S A. 2009;106:5563–5568. doi: 10.1073/pnas.0811322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U S A. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosetta 3.5. 2013 https://www.rosettacommons.org.
- Rudnick G, Wall SC. The molecular mechanism of “ecstasy” [3,4-methylenedioxy-methamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci U S A. 1992;89:1817–1821. doi: 10.1073/pnas.89.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Yamashita A, Gouaux E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature. 2007;448:952–6. doi: 10.1038/nature06038. [DOI] [PubMed] [Google Scholar]
- Singh SK, Piscitelli CL, Yamashita A, Gouaux E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science. 2008;322:1655–1661. doi: 10.1126/science.1166777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ, Jessen T, Henry LK, Field JR, Gamble KL, Gresch PJ, Carneiro AM, Horton RE, Chisnell PJ, Belova Y, McMahon DG, Daws LC, Blakely RD. Transgenic elimination of high-affinity antidepressant and cocaine sensitivity in the presynaptic serotonin transporter. Proc Natl Acad Sci. 2011;108:3785–3790. doi: 10.1073/pnas.1011920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Li T. Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci. 2004;5:963–70. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Wang H, Grahame-Smith DG. The effects of rubidium, caesium and quinine on 5-HT-mediated behaviour in rat and mouse--3. Quinine Neuropharmacology. 1992;31:425–431. doi: 10.1016/0028-3908(92)90079-5. [DOI] [PubMed] [Google Scholar]
- White N. Clinical pharmacokinetics of antimalarial drugs. Clin Pharmacokinet. 1985;10:187–215. doi: 10.2165/00003088-198510030-00001. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Singh S, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437:215–23. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Yu H, Noskov SY, Roux B. Two mechanisms of ion selectivity in protein binding sites. Proc Natl Acad Sci U S A. 2010;107:20329–20334. doi: 10.1073/pnas.1007150107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhen J, Karpowich NK, Goetz RM, Law CJ, Reith MEA, Wang DN. LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science. 2007;317:1390–1393. doi: 10.1126/science.1147614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhen J, Karpowich NK, Law CJ, Reith MEA, Wang DN. Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nat Struct Mol Biol. 2009;16:652–657. doi: 10.1038/nsmb.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]