Primary Sclerosing Cholangitis is an Idiopathic, Heterogeneous, cholestatic liver disease that is characterized by persistent, progressive, biliary inflammation and fibrosis. There is no effective medical therapy for this condition.1 End-stage liver disease necessitating liver transplantation may ultimately develop in affected patients.2
The cause and pathogenesis of primary sclerosing cholangitis are unclear, although it is generally accepted that both genetic and environmental risk factors contribute to the development of the disease as well as to its progression and outcomes.3 Primary sclerosing cholangitis is strongly associated with inflammatory bowel disease (70 to 80% of patients have both conditions), and it is a risk factor for colon, bile-duct, and gallbladder cancers.4
In this review, we discuss the clinical features of primary sclerosing cholangitis. We also summarize the current understanding of its pathogenesis and address the challenges and opportunities associated with its management.
DEMOGRAPHIC AND EPIDEMIOLOGIC CHARACTERISTICS OF PATIENTS
Approximately 60% of patients with primary sclerosing cholangitis are male, and the median age at diagnosis is 41 years.5 The incidence ranges from 0 to 1.3 cases per 100,000 persons per year, and the prevalence ranges from 0 to 16.2 cases per 100,000 persons.6 Studies from northern Europe suggest that both the incidence and the prevalence are increasing.5,6 It is not clear whether these increases reflect true increases in disease occurrence or are due to better detection owing to increased awareness or to the availability of better diagnostic techniques such as endoscopic retrograde cholangiopancreatography (ERCP) and magnetic resonance cholangiopancreatography (MRCP). In the United States, approximately 29,000 patients have this disease.7
CLINICAL MANIFESTATIONS
Primary sclerosing cholangitis is insidious; about half the patients with this condition do not have symptoms but receive a diagnosis after liver-function tests are found to be abnormal.8,9 The most frequent signs at diagnosis are hepatomegaly (in 44% of patients) and splenomegaly (in 39%).9 When symptoms are present, abdominal pain (in 20% of patients), pruritus (in 10%), jaundice (in 6%), and fatigue (in 6%) predominate.8
Diagnostic criteria include an increased serum alkaline phosphatase level that persists for more than 6 months, cholangiographic findings of bile-duct strictures detected by means of either MRCP or ERCP (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org), and exclusion of causes of secondary sclerosing cholangitis (Table 1).1,10 A liver biopsy is not necessary for diagnosis unless small-duct primary sclerosing cholangitis or an overlap with autoimmune hepatitis is suspected.1 Magnetic resonance elastography and transient elastography of the liver are promising noninvasive diagnostic tools that assess mechanical properties of the tissue such as fibrosis, but their specific role in evaluating the degree of liver fibrosis in patients with primary sclerosing cholangitis is unclear.11,12
Table 1.
Causes of Secondary Sclerosing Cholangitis.*
| Cause | Proposed Pathogenesis |
|---|---|
| Abdominal trauma | Damage and subsequent strictures of the biliary tree |
| AIDS-related cholangiopathy | Biliary strictures associated with infection, most commonly due to Cryptosporidium parvum |
| Amyloidosis | Systemic disease involving the biliary tree |
| Cholangiocarcinoma | New development of cancer that mimics clinical presentation of primary sclerosing cholangitis |
| Choledocholithiasis | Strictures due to a stone or stones within the biliary tree |
| Eosinophilic cholangiopathy | Systemic disease involving the biliary tree |
| Graft-versus-host disease | Systemic disease involving the biliary tree |
| Hepatic inflammatory pseudotumor | Inflammatory condition that mimics cholangiographic features of primary sclerosing cholangitis |
| Histiocytosis X | Systemic disease involving the biliary tree |
| Iatrogenic biliary strictures | Strictures due to surgery or ERCP |
| IgG4-associated cholangitis | Systemic disorder that is characterized by high serum IgG4 levels and IgG4-positive lymphoplasmacytic infiltration of affected organs (the pancreas and bile ducts) and that causes biliary strictures |
| Intraarterial chemotherapy | Biliary strictures due to infusion offluorouracil chemotherapy through the hepatic artery |
| Ischemic cholangiopathy | Inadequate arterial supply of the biliary tree |
| Mast-cell cholangiopathy | Systemic disease involving the biliary tree |
| Portal hypertensive biliopathy | Extrahepatic portal venous obstruction causing compression and stricturing of the biliary tree |
| Recurrent pyogenic cholangitis | Progressive and diffuse biliary stricturing, ectasias, and local stone formation; common in East Asia |
| Sarcoidosis | Systemic disease involving the biliary tree |
AIDS denotes acquired immunodeficiency syndrome, and ERCP endoscopic retrograde cholangiopancreatography.
There are several subtypes of primary sclerosing cholangitis (Table 2). The classic subtype, which involves the entire biliary tree, is present in approximately 90% of patients with primary sclerosing cholangitis. Approximately 5% of patients have disease that affects only small intrahepatic bile ducts.13 In addition, an overlap syndrome of primary sclerosing cholangitis with autoimmune hepatitis is present in 35% of children with primary sclerosing cholangitis,14 but this combined entity is seen in only approximately 5% of adults.15
Table 2.
Established Subtypes of Primary Sclerosing Cholangitis.*
| Subtype | Diagnostic Approach and Criteria | Cholangiographie Features | Histopathological Features | Management | Other Features |
|---|---|---|---|---|---|
| Classic | M RCP or ERCP with typical cholangio-graphic features; elevation of alkaline phosphatase level (more than doubled) for >6 mo; exclusion of causes of secondary sclerosing cholangitis | Affects small and large bile ducts | Mixed inflammatory-cell infiltrate, usually more intense around bile ducts; often nonspecific and nondiagnostic | Evaluate and treat coexisting conditions; endoscopic management of dominant stricture; liver transplantation for advanced disease | 70–80% of patients have inflammatory bowel disease; increased risk of colon and gallbladder cancer, cholangiocarcinoma, and hepatocellular carcinoma |
| Small-duct | Liver biopsy; elevation of alkaline phosphatase level (more than doubled) for >6 mo; exclusion of causes of secondary sclerosing cholangitis | Affects only small bile ducts | Mixed inflammatory-cell infiltrate, usually more intense around bile ducts; often nonspecific and nondiagnostic | Evaluate and treat coexisting conditions; liver transplantation for advanced disease | May progress to classic subtype; associated with longer survival and less risk of cholangiocarcinoma than classic subtype |
| Associated with autoimmune hepatitis | Laboratory evidence of autoimmune hepatitis plus MRCP or ERCP findings of primary sclerosing cholangitis; exclusion of causes of secondary sclerosing cholangitis | Affects small and large bile ducts | Lymphoplasmacytic infiltrate, interface hepatitis | Same as for classic sub-type (see above); treatment for autoimmune hepatitis | Better prognosis than with classic subtype but worse prognosis than with autoimmune hepatitis alone |
MRCP denotes magnetic resonance cholangiopancreatography.
The clinical manifestations and progression of primary sclerosing cholangitis may differ according to subtype (Table 2). For example, patients with small bile-duct disease generally have better outcomes than those with classic disease.16,17 Furthermore, patients who have primary sclerosing cholangitis without inflammatory bowel disease may have a different subtype than those with primary sclerosing cholangitis and concurrent inflammatory bowel disease.18 Nevertheless, determining whether this combination is more than a coincidence is challenging, because inflammatory bowel disease may develop years after the diagnosis of primary sclerosing cholangitis and may develop even after liver transplantation.
Approximately 10% of patients with primary sclerosing cholangitis have increased serum IgG4 levels, and these patients have a poorer outcome than those with normal serum IgG4 levels.19 The condition of such patients should not be confused with that of patients who have IgG4-associated cholangitis, which is a systemic disorder characterized by high serum IgG4 levels, IgG4-positive lymphoplasmacytic infiltration of affected organs (such as the pancreas and bile ducts), the abrupt onset of jaundice, biliary strictures that often respond to treatment with glucocorticoids (e.g., prednisolone at a dose of 40 mg daily), and the absence of inflammatory bowel disease.20
No reliable biomarkers have been identified that predict the pace of progression of any form of primary sclerosing cholangitis. One or 2 years after diagnosis, a serum alkaline phosphatase level of less than 1.5 times the upper limit of the normal range has been associated with better outcomes than a level that is equal to or higher than 1.5 times the upper limit of the normal range.21–23 However, it is not clear whether the serum alkaline phosphatase level is a reliable surrogate end point for clinical trials or a useful predictor of the long-term outcome in patients with primary sclerosing cholangitis.24
In general, primary sclerosing cholangitis is slowly progressive, with variable outcomes. In a recent population-based study, the median survival among patients with primary sclerosing cholangitis who were seen at 44 hospitals in the Netherlands was longer than the median survival among patients who were evaluated at three transplantation centers in the Netherlands (21.3 years vs. 13.2 years, P<0.001). This finding probably reflects the referral of more seriously ill patients for evaluation for liver transplantation.17
Bacterial cholangitis, which is the reported initial symptom in approximately 6% of patients with primary sclerosing cholangitis,8 may be recurrent and intractable, and it occasionally necessitates liver transplantation.25 In a study involving a cohort of 273 German patients, in which the median follow-up time was 76 months, a dominant stricture (a narrowing of an extrahepatic duct to <1.5 mm)10 developed in approximately 40% of patients and may have been associated with cancer in 15 to 20% of patients.9 Thus, the occurrence of a dominant stricture should arouse concern and prompt additional studies. Differentiating between benign and ma-lignant dominant strictures is challenging, despite the advent of fluorescence in situ hybridization techniques to evaluate cells retrieved through brush specimens of the bile ducts for cytologic analysis.26
A variety of coexisting conditions are associated with primary sclerosing cholangitis.10 Because inflammatory bowel disease (ulcerative colitis more often than Crohn’s disease) occurs in most patients with primary sclerosing cholangitis, colonoscopy is warranted in all patients who have received a new diagnosis. In one study, virtually all patients with coexisting primary sclerosing cholangitis and inflammatory bowel disease (either ulcerative colitis or Crohn’s disease) had bowel disease affecting the entire colon, with evidence, in rare instances, of backwash ileitis (inflammatory and ulcerative changes seen in the terminal ileum of patients with ulcerative colitis) but with rectal sparing.27 The risk of colon cancer among patients with primary sclerosing cholangitis and concomitant inflammatory bowel disease is four times as high as the risk among patients with inflammatory bowel disease alone and 10 times as high as the risk in the general population.28
Gallbladder disease (stones, polyps, and cancer) is common in patients with primary sclerosing cholangitis. Gallstones have been reported in 25% of patients, and a mass has been reported in 6 to 14% of patients.29,30 Approximately 60% of mass lesions in the gallbladder are adenocarcinomas,29,30 and one study showed that gallbladders that were removed before or at liver transplantation in patients with primary sclerosing cholangitis revealed dysplasia in 37% of patients and adenocarcinoma in 14%.31
In developed countries, primary sclerosing cholangitis is the most common risk factor for cholangiocarcinoma.32 Indeed, the risk of cholangiocarcinoma among patients with primary sclerosing cholangitis is 400 times as high as the risk in the general population.17 Among patients with primary sclerosing cholangitis, the annual risk of cholangiocarcinoma is 2% and the 30-year cumulative incidence is 20%.17,33
Abrupt changes in clinical features such as a new onset of jaundice, fever, or weight loss, or biochemical abnormalities such as a new elevation in the level of alkaline phosphatase, bilirubin, or both (with or without a progressive elevation in the level of the serum tumor marker CA 19-9) should prompt additional evaluation. Useful studies include cross-sectional imaging (usually ultrasonography or magnetic resonance imaging of the liver), often followed by ERCP. Although biomarkers that reliably predict the development of cholangiocarcinoma in patients with primary sclerosing cholangitis have not been identified, many clinicians recommend annual ultrasonographic evaluation of the liver and serum testing for CA 19-9.
Other conditions may occur in patients with this disease. For example, in a large study involving 237 patients with primary sclerosing cholangitis, approximately 15% had osteoporosis (T score of less than −2.5).34 Moreover, an age older than 54 years, a body-mass index (BMI; the weight in kilograms divided by the square of the height in meters) of 24 or lower, and a long duration of inflammatory bowel disease (≥19 years) were correlated with osteoporosis.34
PATHOGENESIS
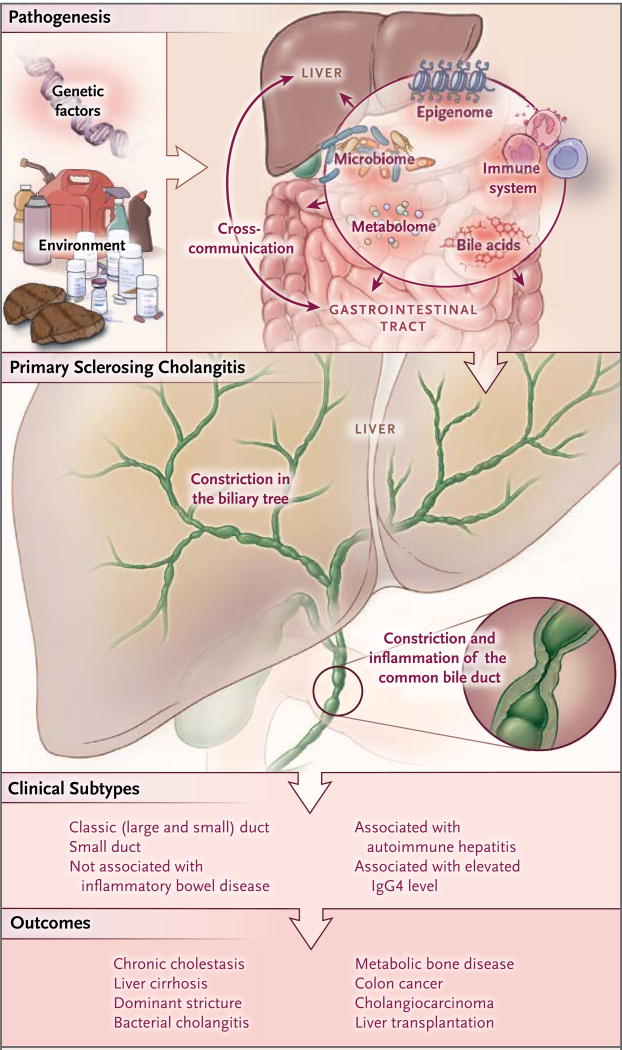
Both hereditary and environmental factors are involved in the cause and pathogenesis of primary sclerosing cholangitis1,10 (Fig. 1). A current working hypothesis postulates that after an unidentified environmental exposure, several genetically predisposed pathways contribute to persistent injury of cholangiocytes (the cells lining the bile ducts). This injury results in biliary inflammation and fibrosis.
Figure 1. Pathogenesis, Clinical Subtypes, and Outcomes of Primary Sclerosing Cholangitis.
Primary sclerosing cholangitis is caused by the interaction of predisposing genetic factors and environmental exposures in local biologic processes that occur at the level of the gut (i.e., gut lymphocyte homing to liver and chronic mucosal inflammation) and the liver (i.e., activation of cholangiocytes and the bile milieu). This condition is influenced by the intestinal microbiome and other dynamic elements, including the epigenome and metabolome. The stochastic effect of environmental factors on germline and somatic biologic events leads to a variety of clinical subtypes of primary sclerosing cholangitis. The persistence and progressive nature of biologic dysfunction result in multifaceted, often interrelated, outcomes of primary sclerosing cholangitis. These outcomes range from chronic liver cholestasis and inflammation to malignant transformation of the liver and gut and to metabolic bone disease, and they may necessitate liver transplantation.
Initial evidence of genetic susceptibility was derived from studies showing that the relative risk of primary sclerosing cholangitis among siblings with the disease is 9 to 39 times as high as the risk in the general population.35 Validated genomewide association studies have shown 16 risk loci that are associated with primary sclerosing cholangitis (Table S1 in the Supplementary Appendix).36–38 The HLA locus on chromosome 6p21 appears to contain regions associated with increased susceptibility to this disease.36 Primary sclerosing cholangitis is strongly associated with HLA class I, II, and III regions (i.e., HLA-B*08, HLA-DRB1 alleles, and a locus near NOTCH4, respectively).36–39 Determination of causative genetic variants in these loci may lead to identification of the putative antigens, which in turn might lead to new therapeutic targets.
Moreover, genes of the interleukin-2 pathway (CD28, interleukin-2, and the alpha subunit of the interleukin-2 receptor) have also been associated with susceptibility to primary sclerosing cholangitis.37,38 Such data and functional studies suggest that adaptive immunity and regulatory T cells may be important in the pathogenesis of the disease.40,41 The contribution of B cells to the pathogenesis of primary sclerosing cholangitis is unclear, although, as mentioned, elevated serum levels of IgG4 have been described in approximately 10% of patients with this condition.42
As of this writing, the environmental trigger or triggers for primary sclerosing cholangitis are only associational. Of interest, smoking appears to be protective,43 and patients with the disease consume less coffee than do controls.43,44 Patients with primary sclerosing cholangitis have reported a higher frequency of exposure to farm animals, but not domestic animals, during childhood than controls.43 In one study, fewer female patients than female controls reported the use of contraceptive hormones.43 In another study, women with primary sclerosing cholangitis had more frequent urinary tract infections than did controls.45 Finally, patients with primary sclerosing cholangitis, regardless of their sex or associated inflammatory bowel disease, were less likely than controls to consume fish but were more likely to consume well-done steak or hamburgers.45 These findings might imply that dietary intake and methods of food preparation may contribute to the development of the disease, perhaps through changes in the gut microbiome.
The strong association of primary sclerosing cholangitis with inflammatory bowel disease has led to a “microbiota hypothesis” that is supported by observations both in vitro46 and in animal models.47 This hypothesis postulates that microbial molecules arising from the intestine and reflecting microbial dysbiosis reach the liver through the portal circulation and initiate an aberrant cholangiocytic response, such as induction of cholangiocyte senescence (see below). In humans, genetic variants of fucosyl transferase 2, a molecule expressed in gut and cholangiocytes, participate in the synthesis of the H antigen oligosaccharide, which serves as a binding moiety for some intestinal bacteria. These variants are linked to differences in the microbial composition of bile — specifically, decreases in Proteobacteria and increases in Firmicutes.48,49
An additional theory regarding the pathogenesis of primary sclerosing cholangitis is the “gut lymphocyte homing” hypothesis, which postulates that activated T cells from the intestine home in on the liver and initiate immune-mediated damage.50 Such a scenario assumes that intestinal T cells are stimulated within intestine-associated lymphoid tissue, express the cell-surface receptors integrin α4β7 and CCR9, and then are recruited to the hepatic tissue as a result of abnormal expression in the liver of their associated ligands such as the adhesion protein mucosal addressin-cell adhesion molecule 1 (MAdCAM-1) and the chemotactic protein CCL25, which are typically limited to the gut.51,52 The expression of these ligands on periportal endothelial cells and the subsequent homing of α4β7-positive and CCR9-positive T cells to liver parenchyma have been reported in patients with primary sclerosing cholangitis.53
It is now thought that in addition to extrinsic mechanisms, through which cells are targeted for injury, cholangiocytes themselves may be actively involved in the cause and pathogenesis of primary sclerosing cholangitis.54 For example, in response to recognition of pathogen-associated molecular patterns and other stimuli, cholangiocytes express a number of proinflammatory cytokines such as tumor necrosis factor α, interleukin-6, interleukin-8, and other bioactive molecules.55 Cholangiocyte synthesis and secretion of such signaling mediators compose part of the bile-duct innate immune and repair response and mediate recruitment and stimulation of T cells, macrophages, neutrophils, natural killer cells, and other resident and recruited cells.54 Dysregulation of such “activated cholangiocytes,” particularly in genetically susceptible persons, may confer a predisposition to the development and progression of primary sclerosing cholangitis.
Recent data show that in patients with primary sclerosing cholangitis, cholangiocytes undergo the process of cellular senescence. The phenotype of cellular senescence is linked to both cell-cycle arrest and vigorous secretion of various molecules, including cytokines. This process is called the senescence-associated secretory phenotype.56,57 Cells with this phenotype can modify their microenvironment (e.g., the extracellular matrix), bolster the senescent phenotype, induce proinflammatory cellular reactions, and accelerate neoplastic transformation.56,58–60 Investigators are focusing on the relationship between cholangiocyte senescence and the pathogenesis and progression of primary sclerosing cholangitis in order to develop new therapeutic interventions called “senolytics.” Unfortunately, an animal model of primary sclerosing cholangitis that recapitulates the cardinal features of the disease is lacking; this will hamper preclinical drug testing once targets are identified.61
MANAGEMENT
Caring for patients with primary sclerosing cholangitis is challenging and complicated. It necessitates treatment of both the primary liver disease and coexisting conditions, as well as subsequent therapy for potential complications of end-stage liver disease. In our view, patients with advanced primary sclerosing cholangitis, such as those with refractory symptoms or cholangiocarcinoma, are best treated at specialized centers that offer an integrated, multidisciplinary approach with a team that includes hepatologists, gastroenterologists, endoscopists, radiologists, and liver transplantation surgeons.
As of this writing, no effective medical therapy exists for primary sclerosing cholangitis, despite multiple clinical trials that have been conducted over the course of decades. Because the pathogenesis of primary sclerosing cholangitis is poorly understood, the identification of therapeutic targets and the design of targeted therapies have been difficult. Moreover, primary sclerosing cholangitis is uncommon, heterogeneous, and lacks reliable biomarkers. Consequently, patients cannot be stratified properly, and well-defined disease end points for adequately powered clinical trials are lacking.
Ursodeoxycholic acid has been widely studied as a therapy for primary sclerosing cholangitis.62 In one randomized, double-blind, placebo-controlled trial, patients who received ursodeoxycholic acid had decreased levels of serum liver enzymes, but they did not have higher rates of survival than the rates among patients who received placebo.63 In a randomized, double-blind, placebo-controlled trial, the risk of the primary end point (death, liver transplantation, minimal listing criteria for liver transplantation, cirrhosis, esophageal or gastric varices, and cholangiocarcinoma) was 2.3 times higher among patients who received high-dose ursodeoxycholic acid (at a dose of 25 mg per kilogram of body weight) than among those who received placebo (P < 0.01).64 Thus, treatment guidelines for primary sclerosing cholangitis are conflicting: the American Association for the Study of Liver Diseases and the American College of Gastroenterology do not support the use of ursodeoxycholic acid,10,65 whereas the European Association for the Study of the Liver endorses the use of moderate doses (13 to 15 mg per kilogram).62 Given the conflicting guidelines, it may be reasonable, in our view, to prescribe ursodeoxycholic acid (at a dose of 13 to 15 mg per kilogram) for 6 months and to monitor the patient’s liver-enzyme levels. If no decrease in alkaline phosphatase levels occurs within that time frame, we would suggest discontinuation of therapy and observation of the patient or enrollment of the patient in a clinical trial.
Several new treatments are being assessed in ongoing clinical trials (Table S2 in the Supplementary Appendix). For example, obeticholic acid is a semisynthetic analogue of chenodeoxycholic acid and a potent ligand for the farsenoid X receptor that has an antifibrotic effect.66 A clinical trial of that agent in patients with primary sclerosing cholangitis is under way (ClinicalTrials.gov number, NCT02177136). Another trial involves the use of simtuzumab (NCT01672853), a monoclonal antibody against lysyl oxidase–like 2 (Loxl2), an enzyme that functions as a profibrotic protein in primary sclerosing cholangitis.67 In addition, 24-nor-ursodeoxycholic acid, a synthetic bile acid that is known to produce a bile acid–dependent bicarbonate-rich choleresis, may have beneficial effects in patients with liver fibrosis68 and is currently under study (NCT01755507).
Reducing exposure of cholangiocytes to putatively toxic bile acids can be achieved by inhibiting the apical sodium-dependent bile acid transporter in the ileum. In an animal model, such inhibition has been shown to reduce hepatic profibrogenic gene expression, up-regulate anti-inflammatory and antifibrogenic genes, and decrease the pool size and composition of bile acid, resulting in improved histologic features in the liver.69 A clinical trial of an apical sodium-dependent bile acid transporter inhibitor, LUM001, is currently under way (NCT02061540).
Another clinical trial is evaluating BTT1023, a human monoclonal antibody that targets the vascular adhesion protein 1 (an adhesion molecule that is important for gut homing of T cells) (NCT02239211). An additional approach tests the concept that oral antibiotics such as vancomycin could alter the gut microbiota and reduce innate immune responses that could be pivotal in the development of biliary inflammation and fibrosis. Indeed, a small study of vancomycin showed a reduction in alkaline phosphatase levels at 12 weeks,70 and an ongoing trial is evaluating its usefulness in children with primary sclerosing cholangitis (NCT01802073). Finally, because the intestinal microbiome may be involved in the development of primary sclerosing cholangitis, a pilot study of fecal microbiota transplantation is in the planning stages (NCT02424175).
The treatment of complications of end-stage liver disease in patients with primary sclerosing cholangitis and the management of associated and coexisting conditions are summarized in Table 3. In patients with both primary sclerosing cholangitis and inflammatory bowel disease, inflammatory bowel disease should be treated according to the relevant guidelines.10 Even if patients with inflammatory bowel disease have undergone liver transplantation, they should undergo annual colonoscopy with surveillance biopsies, given the increased risk of colon cancer.
Table 3.
Management of Manifestations of End-Stage Liver Disease and Coexisting Conditions of Primary Sclerosing Cholangitis.
| Condition | Manifestation | Test | Treatment |
|---|---|---|---|
| Cirrhosis | Esophageal or gastric varices or hepatocellular cancer | Screening for esophageal or gastric varices with esophagogastroduodenoscopy every 2–3 yr; screening for hepatocellular cancer with abdominal ultrasonographic examination yearly with or without measurement of alpha-fetoprotein | Patients with no varices: observe, repeat esophagogastroduodenoscopy; patients with small varices: treat with nadolol (at a dose of 40 mg orally at bedtime); patients with large varices: ligation; patients with hepatocellular cancer: assess for ablation, resection, or transplantation |
| New elevation in level of bilirubin, CA 19-9, or both, or clinical cholangitis | Dominant stricture (benign or malignant) | MRCP, ERCP, or both | Patients with benign dominant stricture: endoscopic dilation; patients with malignant dominant stricture: assess for transplantation or palliative biliary stenting |
| Gallbladder disease | Polyp or polyps, or mass | Abdominal ultrasonography | Patients with polyps or mass: cholecystectomy; consider use of chemotherapy if cancer extends beyond gallbladder wall |
| Inflammatory bowel disease | Colon cancer | Colonoscopy and surveillance biopsies; yearly screening, even after liver transplantation | Patients with colon cancer: colectomy; consider use of chemotherapy according to stage guidelines for colon cancer |
| Metabolic bone disease | Osteopenia or osteoporosis | Bone-mineral-density testing; screening every 2–3 yr | Patients with osteopenia: calcium, 1.0–1.5 g/day and vitamin D, 1000 IU/day; patients with osteoporosis: bisphosphonate plus calcium, 1.0–1.5 g/day and vitamin D, 1000 IU/day |
Patients who do not have evidence of inflammatory bowel disease should undergo colonoscopy every 5 years, given their risk of colonic lesions. An annual ultrasonographic evaluation of the gallbladder for assessment of polyps or other mass lesions is also recommended.10 Because of the risk of cancer, patients with gallbladder masses of any size should undergo cholecystectomy. A 5-year recurrence-free survival rate of 65% has been reported among selected patients with primary sclerosing cholangitis and perihilar cholangiocarcinoma who have undergone liver transplantation after neoadjuvant chemotherapy and radiation therapy.71
Because of the progressive nature of primary sclerosing cholangitis, approximately 40% of patients with this disease will ultimately require liver transplantation.9 In fact, primary sclerosing cholangitis was the indication for approximately 6% of liver transplantations performed in the United States from 1988 through 2015 (www.unos.org). This is a remarkable statistic given the rarity of the disease (1 case per 10,000 persons), and it underscores both the financial burden of primary sclerosing cholangitis (the cost of all liver transplantations for primary sclerosing cholangitis in the United States is approximately $125 million each year) and the urgent need for effective medical therapies for this condition.
After liver transplantation for primary sclerosing cholangitis, the 1-year survival rate is approximately 85% and the 5-year survival rate is approximately 72% (www.unos.org). Nevertheless, the disorder may recur in approximately 25% of patients after transplantation.72 Recurrence of primary sclerosing cholangitis is diagnosed on the basis of cholangiographic evidence of the disease in the absence of chronic liver rejection or vascular offenses (such as ischemia) to the transplanted liver. One study showed that colectomy before liver transplantation in patients with inflammatory bowel disease may decrease the frequency of recurrence after transplantation.73
UNMET NEEDS AND FUTURE DIRECTIONS
Primary sclerosing cholangitis remains a poorly understood disease for which medical therapy is lacking. Both unbiased “-omics” approaches (e.g., genomics, epigenomics, and proteomics) in large, well-phenotyped cohorts of patients and hypothesis-driven experiments on model systems such as animal models and organoids are needed to better delineate the pathogenesis of the disease and to identify new therapies.
Acknowledgments
Dr. LaRusso reports holding a patent related to methods of inhibiting the growth of bile-duct epithelium with the use of a somatostatin or a somatostatin agonist (US 08/163,277), a pending patent related to treating liver disease (US 1,885,384, licensed to Novartis), and issued and pending patents related to vitamin C and chromium-free vitamin K and compositions thereof for treating an NF-κB–mediated condition (AU 2011279808, licensed to IC-MedTech).
We thank Jenn Rud for secretarial support.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521–36. doi: 10.1053/j.gastro.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013;382:1587–99. doi: 10.1016/S0140-6736(13)60096-3. [DOI] [PubMed] [Google Scholar]
- 3.Lazaridis KN, LaRusso NF. The cholangiopathies. Mayo Clin Proc. 2015;90:791–800. doi: 10.1016/j.mayocp.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jussila A, Virta LJ, Pukkala E, Färkkilä MA. Malignancies in patients with inflammatory bowel disease: a nationwide register study in Finland. Scand J Gastroenterol. 2013;48:1405–13. doi: 10.3109/00365521.2013.846402. [DOI] [PubMed] [Google Scholar]
- 5.Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and metaanalysis. Hepatology. 2011;53:1590–9. doi: 10.1002/hep.24247. [DOI] [PubMed] [Google Scholar]
- 6.Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56:1181–8. doi: 10.1016/j.jhep.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Bambha K, Kim WR, Talwalkar J, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125:1364–9. doi: 10.1016/j.gastro.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan GG, Laupland KB, Butzner D, Urbanski SJ, Lee SS. The burden of large and small duct primary sclerosing cholangitis in adults and children: a population-based analysis. Am J Gastroenterol. 2007;102:1042–9. doi: 10.1111/j.1572-0241.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- 9.Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007;102:107–14. doi: 10.1111/j.1572-0241.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 10.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–78. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 11.Corpechot C, Gaouar F, El Naggar A, et al. Baseline values and changes in liver stiffness measured by transient elastography are associated with severity of fibrosis and outcomes of patients with primary sclerosing cholangitis. Gastroenterology. 2014;146:970–9. doi: 10.1053/j.gastro.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 12.Eaton JE, Dzyubak B, Venkatesh SK, et al. Performance of magnetic resonance elastography in primary sclerosing cholangitis. J Gastroenterol Hepatol. 2016;31:1184–90. doi: 10.1111/jgh.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angulo P, Maor-Kendler Y, Lindor KD. Small-duct primary sclerosing cholangitis: a long-term follow-up study. Hepatology. 2002;35:1494–500. doi: 10.1053/jhep.2002.33202. [DOI] [PubMed] [Google Scholar]
- 14.Feldstein AE, Perrault J, El-Youssif M, Lindor KD, Freese DK, Angulo P. Primary sclerosing cholangitis in children: a longterm follow-up study. Hepatology. 2003;38:210–7. doi: 10.1053/jhep.2003.50289. [DOI] [PubMed] [Google Scholar]
- 15.Kaya M, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary sclerosing cholangitis: an evaluation of a modified scoring system. J Hepatol. 2000;33:537–42. doi: 10.1034/j.1600-0641.2000.033004537.x. [DOI] [PubMed] [Google Scholar]
- 16.Björnsson E, Olsson R, Bergquist A, et al. The natural history of small-duct primary sclerosing cholangitis. Gastroenterology. 2008;134:975–80. doi: 10.1053/j.gastro.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 17.Boonstra K, Weersma RK, van Erpecum KJ, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045–55. doi: 10.1002/hep.26565. [DOI] [PubMed] [Google Scholar]
- 18.Naess S, Björnsson E, Anmarkrud JA, et al. Small duct primary sclerosing cholangitis without inflammatory bowel disease is genetically different from large duct disease. Liver Int. 2014;34:1488–95. doi: 10.1111/liv.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendes FD, Jorgensen R, Keach J, et al. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2006;101:2070–5. doi: 10.1111/j.1572-0241.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 20.Björnsson E, Chari ST, Smyrk TC, Lindor K. Immunoglobulin G4 associated cholangitis: description of an emerging clinical entity based on review of the literature. Hepatology. 2007;45:1547–54. doi: 10.1002/hep.21685. [DOI] [PubMed] [Google Scholar]
- 21.Stanich PP, Björnsson E, Gossard AA, Enders F, Jorgensen R, Lindor KD. Alkaline phosphatase normalization is associated with better prognosis in primary sclerosing cholangitis. Dig Liver Dis. 2011;43:309–13. doi: 10.1016/j.dld.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al Mamari S, Djordjevic J, Halliday JS, Chapman RW. Improvement of serum alkaline phosphatase to <1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2013;58:329–34. doi: 10.1016/j.jhep.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Rupp C, Rössler A, Halibasic E, et al. Reduction in alkaline phosphatase is associated with longer survival in primary sclerosing cholangitis, independent of dominant stenosis. Aliment Pharmacol Ther. 2014;40:1292–301. doi: 10.1111/apt.12979. [DOI] [PubMed] [Google Scholar]
- 24.Ponsioen CY, Chapman RW, Chazouillères O, et al. Surrogate endpoints for clinical trials in primary sclerosing cholangitis: review and results from an International PSC Study Group consensus process. Hepatology. 2016;63:1357–67. doi: 10.1002/hep.28256. [DOI] [PubMed] [Google Scholar]
- 25.Abraham SC, Kamath PS, Eghtesad B, Demetris AJ, Krasinskas AM. Liver transplantation in precirrhotic biliary tract disease: portal hypertension is frequently associated with nodular regenerative hyperplasia and obliterative portal venopathy. Am J Surg Pathol. 2006;30:1454–61. doi: 10.1097/01.pas.0000213286.65907.ea. [DOI] [PubMed] [Google Scholar]
- 26.Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology. 2011;54:1842–52. doi: 10.1002/hep.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boonstra K, van Erpecum KJ, van Nieuwkerk KM, et al. Primary sclerosing cholangitis is associated with a distinct phenotype of inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2270–6. doi: 10.1002/ibd.22938. [DOI] [PubMed] [Google Scholar]
- 28.Khaderi SA, Sussman NL. Screening for malignancy in primary sclerosing cholangitis (PSC) Curr Gastroenterol Rep. 2015;17:17. doi: 10.1007/s11894-015-0438-0. [DOI] [PubMed] [Google Scholar]
- 29.Buckles DC, Lindor KD, Larusso NF, Petrovic LM, Gores GJ. In primary sclerosing cholangitis, gallbladder polyps are frequently malignant. Am J Gastroenterol. 2002;97:1138–42. doi: 10.1111/j.1572-0241.2002.05677.x. [DOI] [PubMed] [Google Scholar]
- 30.Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48:598–605. doi: 10.1016/j.jhep.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Lewis JT, Talwalkar JA, Rosen CB, Smyrk TC, Abraham SC. Prevalence and risk factors for gallbladder neoplasia in patients with primary sclerosing cholangitis: evidence for a metaplasia-dysplasiacarcinoma sequence. Am J Surg Pathol. 2007;31:907–13. doi: 10.1097/01.pas.0000213435.99492.8a. [DOI] [PubMed] [Google Scholar]
- 32.Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11(1):13–21.e1. doi: 10.1016/j.cgh.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizvi S, Eaton JE, Gores GJ. Primary sclerosing cholangitis as a premalignant biliary tract disease: surveillance and management. Clin Gastroenterol Hepatol. 2015;13:2152–65. doi: 10.1016/j.cgh.2015.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angulo P, Grandison GA, Fong DG, et al. Bone disease in patients with primary sclerosing cholangitis. Gastroenterology. 2011;140:180–8. doi: 10.1053/j.gastro.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergquist A, Montgomery SM, Bahmanyar S, et al. Increased risk of primary sclerosing cholangitis and ulcerative colitis in first-degree relatives of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2008;6:939–43. doi: 10.1016/j.cgh.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Karlsen TH, Franke A, Melum E, et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology. 2010;138:1102–11. doi: 10.1053/j.gastro.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 37.Melum E, Franke A, Schramm C, et al. Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nat Genet. 2011;43:17–9. doi: 10.1038/ng.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu JZ, Hov JR, Folseraas T, et al. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet. 2013;45:670–5. doi: 10.1038/ng.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Næss S, Lie BA, Melum E, et al. Refinement of the MHC risk map in a Scandinavian primary sclerosing cholangitis population. PLoS One. 2014;9(12):e114486. doi: 10.1371/journal.pone.0114486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liaskou E, Jeffery LE, Trivedi PJ, et al. Loss of CD28 expression by liver-infiltrating T cells contributes to pathogenesis of primary sclerosing cholangitis. Gastroenterology. 2014;147(1):221–232.e7. doi: 10.1053/j.gastro.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sebode M, Peiseler M, Franke B, et al. Reduced FOXP3(+) regulatory T cells in patients with primary sclerosing cholangitis are associated with IL2RA gene polymorphisms. J Hepatol. 2014;60:1010–6. doi: 10.1016/j.jhep.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 42.Björnsson E, Chari S, Silveira M, et al. Primary sclerosing cholangitis associated with elevated immunoglobulin G4: clinical characteristics and response to therapy. Am J Ther. 2011;18:198–205. doi: 10.1097/MJT.0b013e3181c9dac6. [DOI] [PubMed] [Google Scholar]
- 43.Andersen IM, Tengesdal G, Lie BA, Boberg KM, Karlsen TH, Hov JR. Effects of coffee consumption, smoking, and hormones on risk for primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2014;12:1019–28. doi: 10.1016/j.cgh.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 44.Lammert C, Juran BD, Schlicht E, et al. Reduced coffee consumption among individuals with primary sclerosing cholangitis but not primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1562–8. doi: 10.1016/j.cgh.2013.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eaton JE, Juran BD, Atkinson EJ, et al. A comprehensive assessment of environmental exposures among 1000 North American patients with primary sclerosing cholangitis, with and without inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:980–90. doi: 10.1111/apt.13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mueller T, Beutler C, Picó AH, et al. Enhanced innate immune responsiveness and intolerance to intestinal endotoxins in human biliary epithelial cells contributes to chronic cholangitis. Liver Int. 2011;31:1574–88. doi: 10.1111/j.1478-3231.2011.02635.x. [DOI] [PubMed] [Google Scholar]
- 47.Haruta I, Kikuchi K, Hashimoto E, et al. Long-term bacterial exposure can trigger nonsuppurative destructive cholangitis associated with multifocal epithelial inflammation. Lab Invest. 2010;90:577–88. doi: 10.1038/labinvest.2010.40. [DOI] [PubMed] [Google Scholar]
- 48.Rausch P, Rehman A, Künzel S, et al. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc Natl Acad Sci U S A. 2011;108:19030–5. doi: 10.1073/pnas.1106408108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Folseraas T, Melum E, Rausch P, et al. Extended analysis of a genome-wide association study in primary sclerosing cholangitis detects multiple novel risk loci. J Hepatol. 2012;57:366–75. doi: 10.1016/j.jhep.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grant AJ, Lalor PF, Salmi M, Jalkanen S, Adams DH. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet. 2002;359:150–7. doi: 10.1016/S0140-6736(02)07374-9. [DOI] [PubMed] [Google Scholar]
- 51.Eksteen B, Mora JR, Haughton EL, et al. Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology. 2009;137:320–9. doi: 10.1053/j.gastro.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta A, Bowlus CL. Primary sclerosing cholangitis: etiopathogenesis and clinical management. Front Biosci (Elite Ed) 2012;4:1683–705. doi: 10.2741/e490. [DOI] [PubMed] [Google Scholar]
- 53.Eksteen B, Grant AJ, Miles A, et al. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. 2004;200:1511–7. doi: 10.1084/jem.20041035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Hara SP, Tabibian JH, Splinter PL, LaRusso NF. The dynamic biliary epithelia: molecules, pathways, and disease. J Hepatol. 2013;58:575–82. doi: 10.1016/j.jhep.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Hara SP, Splinter PL, Trussoni CE, Gajdos GB, Lineswala PN, LaRusso NF. Cholangiocyte N-Ras protein mediates lipopolysaccharide-induced interleukin 6 secretion and proliferation. J Biol Chem. 2011;286:30352–60. doi: 10.1074/jbc.M111.269464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tabibian JH, O’Hara SP, Splinter PL, Trussoni CE, LaRusso NF. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology. 2014;59:2263–75. doi: 10.1002/hep.26993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–72. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Acosta JC, O’Loghlen A, Banito A, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–18. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 59.Coppé JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–68. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuilman T, Michaloglou C, Vredeveld LC, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–31. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 61.Pollheimer MJ, Trauner M, Fickert P. Will we ever model PSC? — “it’s hard to be a PSC model!”. Clin Res Hepatol Gastroenterol. 2011;35:792–804. doi: 10.1016/j.clinre.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 62.European Association for the Study of the Liver. EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–67. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 63.Lindor KD. Ursodiol for primary sclerosing cholangitis. N Engl J Med. 1997;336:691–5. doi: 10.1056/NEJM199703063361003. [DOI] [PubMed] [Google Scholar]
- 64.Lindor KD, Kowdley KV, Luketic VA, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808–14. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lindor KD, Kowdley KV, Harrison ME. ACG clinical guideline: primary sclerosing cholangitis. Am J Gastroenterol. 2015;110:646–59. doi: 10.1038/ajg.2015.112. [DOI] [PubMed] [Google Scholar]
- 66.Fiorucci S, Rizzo G, Antonelli E, et al. A farnesoid X receptor-small heterodimer partner regulatory cascade modulates tissue metalloproteinase inhibitor-1 and matrix metalloprotease expression in hepatic stellate cells and promotes resolution of liver fibrosis. J Pharmacol Exp Ther. 2005;314:584–95. doi: 10.1124/jpet.105.084905. [DOI] [PubMed] [Google Scholar]
- 67.Nakken KE, Nygård S, Haaland T, et al. Multiple inflammatory-, tissue remodelling- and fibrosis genes are differentially transcribed in the livers of Abcb4 (−/−) mice harbouring chronic cholangitis. Scand J Gastroenterol. 2007;42:1245–55. doi: 10.1080/00365520701320521. [DOI] [PubMed] [Google Scholar]
- 68.Halilbasic E, Fiorotto R, Fickert P, et al. Side chain structure determines unique physiologic and therapeutic properties of norursodeoxycholic acid in Mdr2−/− mice. Hepatology. 2009;49:1972–81. doi: 10.1002/hep.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miethke AG, Zhang W, Simmons J, et al. Pharmacological inhibition of apical sodium-dependent bile acid transporter changes bile composition and blocks progression of sclerosing cholangitis in multidrug resistance 2 knockout mice. Hepatology. 2016;63:512–23. doi: 10.1002/hep.27973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tabibian JH, Weeding E, Jorgensen RA, et al. Randomised clinical trial: vancomycin or metronidazole in patients with primary sclerosing cholangitis — a pilot study. Aliment Pharmacol Ther. 2013;37:604–12. doi: 10.1111/apt.12232. [DOI] [PubMed] [Google Scholar]
- 71.Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143(1):88–98.e3. doi: 10.1053/j.gastro.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fosby B, Karlsen TH, Melum E. Recurrence and rejection in liver transplantation for primary sclerosing cholangitis. World J Gastroenterol. 2012;18:1–15. doi: 10.3748/wjg.v18.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alabraba E, Nightingale P, Gunson B, et al. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15:330–40. doi: 10.1002/lt.21679. [DOI] [PubMed] [Google Scholar]