Abstract
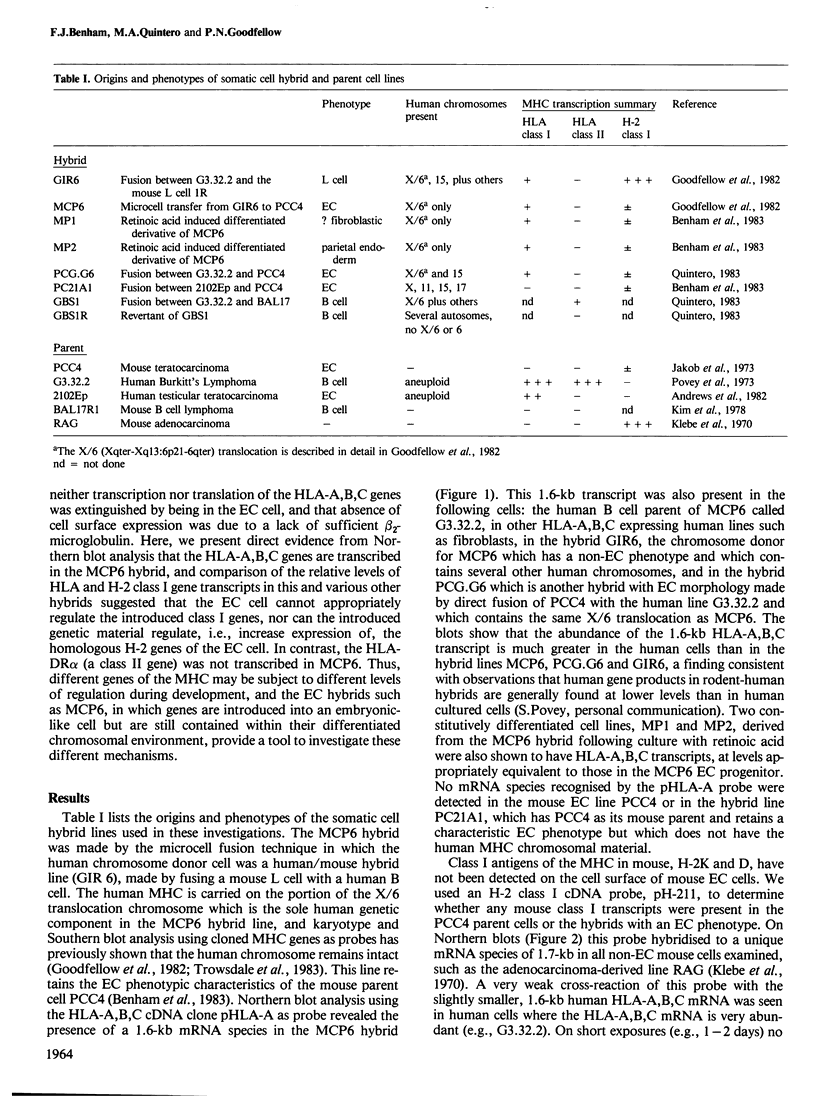
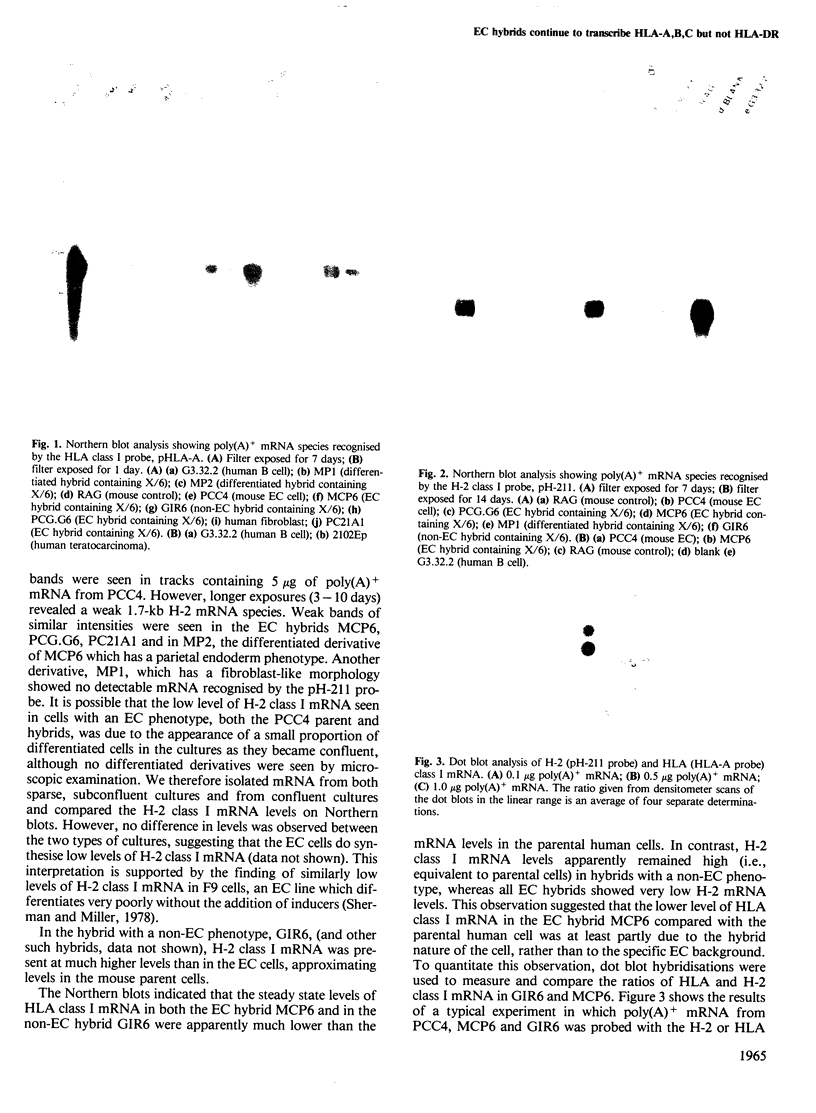
We previously constructed a hybrid cell line, MCP6, which contains an X/6 translocation chromosome as its sole human genetic component in a mouse embryonal carcinoma (EC) cell background. This chromosome, which carries the major histocompatibility complex (MHC) originated from a human B cell which expresses class I and class II MHC antigens. EC cells do not express class I or class II antigens on their cell surface. Northern blot analysis has now shown that in the MCP6 hybrid, human class I genes, i.e., HLA-A,B,C, continued to be transcribed, and cellular levels of the transcripts were similar to, or only slightly lower than, levels in hybrids with a non-EC phenotype. However, very low levels of mRNA species recognised by a mouse class I gene (H-2) probe were also detected in EC cells and EC hybrids. Comparison of the relative levels of H-2 and HLA class I gene transcripts in the EC hybrids and non-EC hybrids indicated that the introduced HLA-A,B,C genes were not appropriately regulated in the EC cell but were subject at least in part to cis control. In contrast to the class I genes, no class II gene (i.e. HLA-DR alpha) transcripts were detected in MCP6. Hybrid EC lines thus provide a system to investigate the different levels of control of MHC gene expression during development and may help to elucidate mechanisms whereby the embryonic genome programs expression of differentiated cell functions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. W., Goodfellow P. N. Antigen expression by somatic cell hybrids of a murine embryonal carcinoma cell with thymocytes and L cells. Somatic Cell Genet. 1980 Mar;6(2):271–284. doi: 10.1007/BF01538801. [DOI] [PubMed] [Google Scholar]
- Artzt K., Jacob F. Letter: Absence of serologically detectable H-2 on primitive teratocarcinoma cells in culture. Transplantation. 1974 Jun;17(6):632–634. doi: 10.1097/00007890-197406000-00015. [DOI] [PubMed] [Google Scholar]
- Boccara M., Kelly F. Etude de la sensibilité au virus du polyome et à SV40 de plusieurs lignées cellulaires de tératocarcinome. Ann Microbiol (Paris) 1978 Feb-Mar;129(2):227–238. [PubMed] [Google Scholar]
- Bucchini D., Lasserre C., Kunst F., Lovell-Badge R., Pictet R., Jami J. Stable transformation of mouse teratocarcinoma stem cells with the dominant selective marker Eco.gpt and retention of their developmental potentialities. EMBO J. 1983;2(2):229–232. doi: 10.1002/j.1460-2075.1983.tb01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone O. R., Milstein C. Control of HLA-A,B,C synthesis and expression in interferon-treated cells. EMBO J. 1982;1(3):345–349. doi: 10.1002/j.1460-2075.1982.tb01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Correani A., Croce C. M. Expression of the teratocarcinoma phenotype in hybrids between totipotent mouse teratocarcinoma and myeloma cells. J Cell Physiol. 1980 Oct;105(1):73–79. doi: 10.1002/jcp.1041050110. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., Sanderson A. R., Temple A. Distribution of MHC antigens in human placental chorionic villi. Transplant Proc. 1977 Jun;9(2):1379–1384. [PubMed] [Google Scholar]
- Gautsch J. W., Wilson M. C. Delayed de novo methylation in teratocarcinoma suggests additional tissue-specific mechanisms for controlling gene expression. Nature. 1983 Jan 6;301(5895):32–37. doi: 10.1038/301032a0. [DOI] [PubMed] [Google Scholar]
- Gmür R., Solter D., Knowles B. B. Independent regulation of H-2K and H-2D gene expression in murine teratocarcinoma somatic cell hybrids. J Exp Med. 1980 Jun 1;151(6):1349–1359. doi: 10.1084/jem.151.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow P. N., Banting G., Trowsdale J., Chambers S., Solomon E. Introduction of a human X-6 translocation chromosome into a mouse teratocarcinoma: investigation of control of HLA-A, B, C expression. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1190–1194. doi: 10.1073/pnas.79.4.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow P. N., Barnstable C. J., Bodmer W. F., Snary D., Crumpton M. J. Expression of HLA system antigens on placenta. Transplantation. 1976 Dec;22(6):595–603. doi: 10.1097/00007890-197612000-00009. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Jähner D., Nobis P., Simon I., Löhler J., Harbers K., Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981 May;24(2):519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Retroviruses and embryogenesis: microinjection of Moloney leukemia virus into midgestation mouse embryos. Cell. 1980 Jan;19(1):181–188. doi: 10.1016/0092-8674(80)90399-2. [DOI] [PubMed] [Google Scholar]
- Jakob H., Boon T., Gaillard J., Nicolas J., Jacob F. Tératocarcinome de la spuris: isolement, culture et propriétés de cellules a potentialités multiples. Ann Microbiol (Paris) 1973 Oct;124(3):269–282. [PubMed] [Google Scholar]
- Jähner D., Stuhlmann H., Stewart C. L., Harbers K., Löhler J., Simon I., Jaenisch R. De novo methylation and expression of retroviral genomes during mouse embryogenesis. Nature. 1982 Aug 12;298(5875):623–628. doi: 10.1038/298623a0. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Weinbaum F. I., Mathieson B. J., McKeever P. E., Asofsky R. Characteristics of BALB/C T cell lymphomas grown as continuous in vitro lines. J Immunol. 1978 Jul;121(1):339–344. [PubMed] [Google Scholar]
- Klebe R. J., Chen T., Ruddle F. H. Controlled production of proliferating somatic cell hybrids. J Cell Biol. 1970 Apr;45(1):74–82. doi: 10.1083/jcb.45.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Trowsdale J., Bodmer W. F. cDNA clones coding for the heavy chain of human HLA-DR antigen. Proc Natl Acad Sci U S A. 1982 Jan;79(2):545–549. doi: 10.1073/pnas.79.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas and mammalian embryogenesis. Science. 1980 Aug 15;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- McBurney M. W. Hemoglobin synthesis in cell hybrids formed between teratocarcinoma and Friend erythroleukemia cells. Cell. 1977 Nov;12(3):653–662. doi: 10.1016/0092-8674(77)90265-3. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Jones-Villeneuve E. M., Edwards M. K., Anderson P. J. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature. 1982 Sep 9;299(5879):165–167. doi: 10.1038/299165a0. [DOI] [PubMed] [Google Scholar]
- Miller R. A., Ruddle F. H. Pluripotent teratocarcinoma-thymus somatic cell hybrids. Cell. 1976 Sep;9(1):45–55. doi: 10.1016/0092-8674(76)90051-9. [DOI] [PubMed] [Google Scholar]
- Miller R. A., Ruddle F. H. Teratocarcinoma X friend erythroleukemia cell hybrids resemble their pluripotent embryonal carcinoma parent. Dev Biol. 1977 Mar;56(1):157–173. doi: 10.1016/0012-1606(77)90159-2. [DOI] [PubMed] [Google Scholar]
- Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978 May;14(1):133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Povey S., Gardiner S. E., Watson B., Mowbray S., Harris H., Arthur E., Steel C. M., Blenkinsop C., Evans H. J. Genetic studies on human lymphoblastoid lines: isozyme analysis on cell lines from forty-one different individuals and on mutants produced following exposure to a chemical mutagen. Ann Hum Genet. 1973 Jan;36(3):247–266. doi: 10.1111/j.1469-1809.1973.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rousset J. P., Jami J., Dubois P., Avilès D., Ritz E. Developmental potentialities and surface antigens of mouse teratocarcinoma x lymphoid cell hybrids. Somatic Cell Genet. 1980 May;6(3):419–433. doi: 10.1007/BF01542793. [DOI] [PubMed] [Google Scholar]
- Sherman M. I., Miller R. A. F9 embryonal carcinoma cells can differentiate into endoderm-like cells. Dev Biol. 1978 Mar;63(1):27–34. doi: 10.1016/0012-1606(78)90110-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Frelinger J. G., Fisher D., Hunkapiller T., Pereira D., Weissman S. M., Uehara H., Nathenson S., Hood L. Three cDNA clones encoding mouse transplantation antigens: homology to immunoglobulin genes. Cell. 1981 Apr;24(1):125–134. doi: 10.1016/0092-8674(81)90508-0. [DOI] [PubMed] [Google Scholar]
- Stewart C. L., Stuhlmann H., Jähner D., Jaenisch R. De novo methylation, expression, and infectivity of retroviral genomes introduced into embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4098–4102. doi: 10.1073/pnas.79.13.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzendruber D. E., Lehman J. M. Neoplastic differentiation: interaction of simian virus 40 and polyoma virus with murine teratocarcinoma cells in vitro. J Cell Physiol. 1975 Apr;85(2 Pt 1):179–187. doi: 10.1002/jcp.1040850204. [DOI] [PubMed] [Google Scholar]
- Teich N. M., Weiss R. A., Martin G. R., Lowy D. R. Virus infection of murine teratocarcinoma stem cell lines. Cell. 1977 Dec;12(4):973–982. doi: 10.1016/0092-8674(77)90162-3. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowsdale J., Lee J., Carey J., Grosveld F., Bodmer J., Bodmer W. Sequences related to HLA-DR alpha chain on human chromosome 6: restriction enzyme polymorphism detected with DC alpha chain probes. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1972–1976. doi: 10.1073/pnas.80.7.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. F., Mintz B. Transfer of nonselectable genes into mouse teratocarcinoma cells and transcription of the transferred human beta-globin gene. Mol Cell Biol. 1982 Feb;2(2):190–198. doi: 10.1128/mcb.2.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]