This analysis updates the results of two phase II studies of translocation‐related sarcomas to evaluate the efficacy of trabectedin against histological subtype and analyze overall survival.
Keywords: Translocation‐related sarcoma, Trabectedin, Histological subtype, Lymphocyte count, Number of previous chemotherapy regimen
Abstract
Background.
Trabectedin is reported as effective, especially against translocation‐related sarcomas (TRSs) after failure of or intolerance to standard chemotherapy. We conducted two phase II studies of TRS, confirming high efficacy of 1.2 mg/m2 trabectedin. The updated data of 66 patients in these studies was integrated to evaluate the efficacy of trabectedin against each histological subtype, and analyze final overall survival (OS).
Methods.
Trabectedin was administered on day one of a 21‐day cycle. Efficacy was assessed using progression‐free survival (PFS), OS, and best overall response. An analysis of OS and PFS was performed for subgroups divided by baseline lymphocyte count (<1,000/μL, ≥1,000/μL) or number of previous chemotherapy regimens (0, 1, 2, ≥3 regimens), and a Weibull parametric model was used to estimate the numerical relationship between lymphocyte count and PFS and OS.
Results.
Median PFS and OS in overall patients were 5.6 (95% confidence interval [CI]: 4.1–7.3) and 17.5 months (95% CI: 12.6–23.6), respectively. PFS in the myxoid and round‐cell liposarcoma (MRCL) group (7.4 months [95% CI: 5.6–11.1]) was longer than in the other subtypes. The response rate was also highest in the MRCL group. Median OS was longer in patients with baseline lymphocyte counts ≥1,000/μL than in those with counts of <1,000/μL, but median PFS was not different between the two subgroups.
Conclusion.
Our updated and pooled data showed that trabectedin exerted prolonged disease control and antitumor effects in patients with advanced TRS, especially in MRCL. We consider that the subgroup analyses also provide important information for trabectedin treatment in patients with TRS.
Implications for Practice.
The progression‐free survival (PFS) for the integrated data of 66 patients with translocation‐related sarcomas (TRSs) in two phase II studies of trabectedin 1.2 mg/m2 was 5.6 months (95% confidence interval: 4.1–7.3). PFS and response rate in myxoid/round‐cell liposarcoma was longer than that of other subtypes. The overall survival (OS) in all TRS subtypes was similar to previous data of TRS patients. In subgroup analysis, the patients with baseline lymphocyte count ≥1,000/μL exhibited better OS, although PFS was not different by baseline lymphocyte count. Our data are considered important information for trabectedin treatment in TRS patients.
Introduction
Trabectedin, a marine‐derived antitumor agent, is reported to exert its antitumor activity by inhibiting DNA repair through its binding to the DNA minor groove, and it is used for treating soft tissue sarcomas (STSs) worldwide. STSs are very rare, accounting for less than 1% of all adult tumors, and comprise a heterogeneous group of more than 50 histological subtypes. Interestingly, trabectedin is reported to inhibit transcription factors derived from fusion proteins [1], [2], and it is considered effective, especially against translocation‐related sarcomas (TRSs) [3]. Approximately one‐third of all STS subtypes are classified as TRS.
Surgical resection is the standard treatment for STS, and chemotherapy is used for patients with unresectable advanced STS. Doxorubicin‐based chemotherapy is recognized as the standard first‐line treatment in STS [4]. However, few therapeutic options exist for patients with STS who previously received doxorubicin. For patients with STS after failure of conventional chemotherapy, the median overall survival (OS) has been reported to be approximately 12 months, with little advance in the previous years; specifically, van der Graaf et al. [5] reported a median OS of 12.5 months for pazopanib treatment, Schöffski et al. [6] reported a median OS of 13.5 months among eribulin‐treated patients with liposarcoma and leiomyosarcoma, and Demetri GD et al. [7] reported a median OS of 12.4 months among trabectedin‐treated patients with liposarcoma and leiomyosarcoma.
We conducted two phase II studies, namely a randomized phase II study (comparative study) evaluating the efficacy and safety of trabectedin 1.2 mg/m2 in patients with TRS compared with best supportive care (BSC) and a single‐arm study evaluating the safety and efficacy of trabectedin in patients randomized to BSC in the comparative study and crossed over to trabectedin after disease progression. The trabectedin dose of 1.2 mg/m2 was chosen on the basis of the results of a phase I study of patients with STS in Japan [8], although this dose was lower than the approved initial dose of 1.5 mg/m2 for the treatment of advanced STS in the European Union. In the comparative study, 73 patients (37 in the trabectedin group and 36 in the BSC group) were included in the efficacy analysis. The results illustrated that trabectedin reduced the risk of disease progression or death compared with BSC in patients with TRS. The median progression‐free survival (PFS) was 5.6 months [95% confidence interval (CI): 4.1–7.5] in the trabectedin group versus 0.9 months (95% CI: 0.7–1.0) in the BSC group (hazard ratio 0.07, 95% CI: 0.03–0.16; p < .0001) [9]. Additionally, the single‐arm study, in which 30 patients with TRS were crossed over to trabectedin, revealed a similar PFS curve (29 patients were analyzed; median: 7.3 months; 95% CI: 2.9–9.1) and safety profile to that of the trabectedin group in the comparative study [10].
In each study, patients with any of 14 different TRS subtypes were eligible for entry, and the efficacy of trabectedin possibly differs according to the histological subtype. However, because of the infrequency of TRS, neither study included sufficient numbers of patients to evaluate the difference in efficacy by subtype. Moreover, it is necessary to evaluate the efficacy for more patients with TRS subtypes.
Additionally, the results of both studies were based on the data accumulated at the cut‐off date (February 8, 2014), and the median OS of trabectedin‐treated patients was not reached (95% CI: 12.8–not estimable) in the comparative study, whereas the median survival time of 10.3 months (95% CI: 6.6–not estimable) was recorded in the single‐arm study [9], [10]. The proportions of patients who died in the two studies were 33% and 38%, respectively. These results suggest that longer follow‐up data are required to evaluate the efficacy of trabectedin regarding OS.
In this analysis, we updated the results of the two phase II studies and integrated the data of 1.2 mg/m2 trabectedin to evaluate the efficacy of the drug by each histological subtype and to investigate factors affecting the OS and PFS.
Materials and Methods
Patients
A post hoc analysis was conducted using pooled data of the efficacy analysis population (66 patients) from two phase II studies of trabectedin 1.2 mg/m2, the comparative study and the single‐arm study. In the comparative study, patients were randomly assigned to either the trabectedin or the BSC group (1:1), and in the single‐arm study, patients in the BSC group of the comparative study were allowed to enroll after their disease progressed (Fig. 1). In both studies, the eligibility criteria were identical [9], [10]. The main inclusion criterion of both studies was a histopathological diagnosis at the study site of TRS (myxoid/round‐cell liposarcoma [MRCL], synovial sarcoma [SS], alveolar rhabdomyosarcoma, extraskeletal Ewing sarcoma/primitive neuroectodermal tumor, dermatofibrosarcoma protuberans, low‐grade fibromyxoid sarcoma, alveolar soft part sarcoma, clear cell sarcoma, angiomatoid fibrous histiocytoma, desmoplastic small‐round‐cell tumor, extraskeletal myxoid chondrosarcoma, mesenchymal chondrosarcoma, giant cell fibroblastoma, or endometrial stromal sarcoma) after failure of or intolerance to standard chemotherapy (for patients with MRCL, SS, and extraskeletal Ewing sarcoma/primitive neuroectodermal tumors, anthracycline‐containing chemotherapy regimen should be included). Histopathological diagnosis was confirmed by central pathological review after enrollment of each patient.
Figure 1.
Disposition of patients.
Abbreviations: BSC, best supportive care; TRS, translocation‐related sarcoma.
†From the comparative study (n = 37) and the single‐arm study (n = 29), a total of 66 patients was defined as the pooled population.
In Figure 1, 37 of the 39 patients who received trabectedin in the comparative study were included in this pooled analysis. Thirty‐six of the 37 patients who received BSC were analyzed in the comparative study. Three patients were excluded from the efficacy analysis of the comparative study because they were diagnosed by central pathological review as non‐TRS. Thirty‐one patients were enrolled in the single‐arm study; five patients in the BSC group were not enrolled because they did not meet the inclusion criteria of the single‐arm study after the end of the comparative study. Twenty‐nine were included in the efficacy analysis of the single‐arm study after excluding two patients (one did not receive trabectedin and one had a diagnosis by central pathological review of non‐TRS). The final data cut‐off date for all data in this analysis was March 5, 2015.
The study protocol and the informed consent documents were approved by the institutional review board at each study site. All patients gave written informed consent before the initiation of any study‐specific procedures. The study was conducted in accordance with the ethical principles originating in or derived from the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice Guidelines, and locally applicable laws and regulations.
Study Treatment
In both studies, dexamethasone and a 5‐HT3 receptor antagonist were intravenously administered on day 1 of a 21‐day treatment cycle. At 30 minutes after the administration of dexamethasone, trabectedin (1.2 mg/m2) was administered via the central vein over 24 hours, followed by a 20‐day monitoring period. Patients in the BSC group of the comparative study received treatments to alleviate symptoms and improve quality of life, but not anticancer therapies. Regarding the efficacy evaluation in each study, the antitumor effect was assessed via a central radiology imaging review according to the Response Evaluation Criteria in Solid Tumors version 1.1 [11]. Tumor assessments via computed tomography or magnetic resonance imaging were repeated at weeks 4, 8, 12, 18, and 24 and every 8 weeks thereafter in the comparative study and at weeks 6, 12, 18, and 24 and every 8 weeks thereafter in the single‐arm study.
Statistical Analysis
Efficacy was evaluated using PFS (the time from the day of enrollment in each study until radiologic progression assessed via a central radiology imaging review or death by any cause), progression‐free rates at 3 and 6 months (Kaplan‐Meier estimate at each time point), time to progression (the time from the day of enrollment in each study until radiologic progression assessed via a central radiology imaging review), the disease control rate (the proportion of patients with complete response [CR], partial response [PR], or stable disease [SD]), the response rate (the proportion of patients with CR or PR), and OS (the time from the day of enrollment until death by any cause). We used the Kaplan‐Meier method to calculate the median OS/PFS and to estimate survival rates at various time points. The distribution of the maximum tumor response rate was presented by waterfall plot. To evaluate the relationship between patient background factors and efficacy of trabectedin, subgroup analyses for PFS and OS were performed. The cut‐off point of baseline lymphocyte count (1,000/μL) was determined according to a previous report by Ray‐Coquard et al. [12]. In addition, to investigate the effect of the lymphocyte count numerically, we conducted a model analysis. A Weibull parametric model was used for the subgroup analysis assuming the following model: S (t) = exp (−exp (log (t) − α − βx)/σ), where t is OS or PFS (months), α is an intercept, β is a vector of regression coefficient, x is the lymphocyte count, and σ is a scale parameter. In the subgroup analysis by lymphocyte count, we used the sum of the diameter of target lesions and performance status (PS) to adjust for the difference between the populations of the comparative and single‐arm studies because greater tumor volume and worse PS would reflect the difference in risk between the two study populations. The model included the baseline lymphocyte count with the sum of the diameters of target lesions (divided by 100 mm) or PS (0 or 1) as covariates. Each parameter was estimated by the maximum‐likelihood method with the Newton‐Raphson algorithm using the LIFEREG procedure in SAS version 9.2 (SAS Institute, Inc., Cary, NC, http://www.sas.com).
Results
Updated Data of Each Study
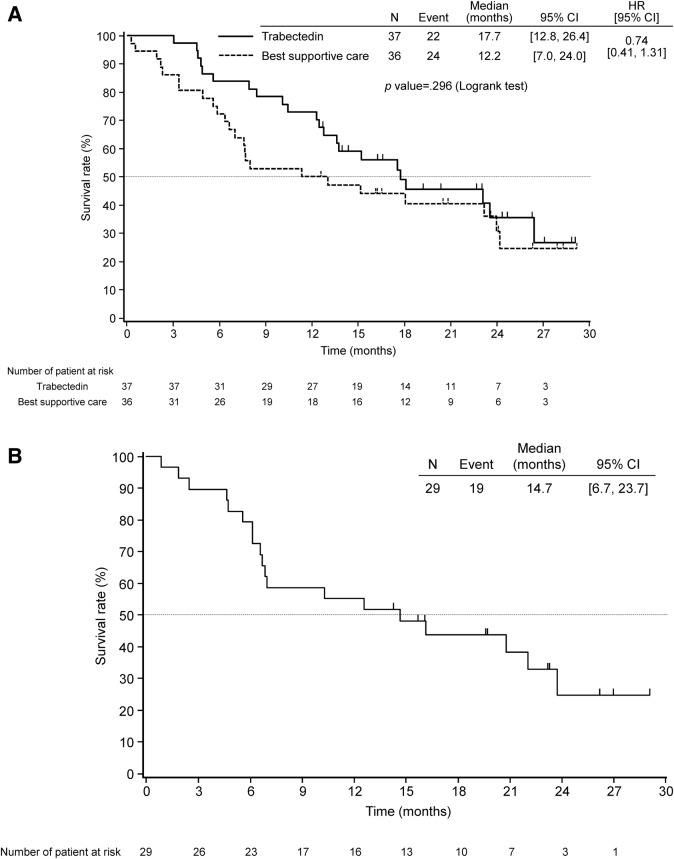
The median follow‐up time was 22.7 months at the final data cut‐off date. At the final cut‐off date, the median PFS was 5.6 months (95% CI: 4.1–7.4) in the trabectedin group, 0.9 months (95% CI: 0.7–1.0) in the BSC group of the comparative study (hazard ratio = 0.11; 95% CI: 0.05–0.21, p < .0001), and 5.5 months (95% CI: 2.9–7.4) in the single‐arm study. The median OS was 17.7 months (95% CI: 12.8–26.4) in the trabectedin group and 12.2 months (95% CI: 7.0–24.0) in the BSC group of the comparative study (hazard ratio = 0.74; 95%CI: 0.41–1.31, p = .296), and 14.7 months (95% CI: 6.7–23.7) in the single‐arm study (Fig. 2A, 2B).
Figure 2.
Kaplan‐Meier plot of overall survival in (A) patients in the comparative study and (B) patients in the single‐arm study.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Pooled Data Analysis
The baseline demographics and disease characteristics of the pooled population are shown in Table 1. The median age of the patients was 38.0 (range: 21–77) years. The most dominant subtype of TRS was MRCL, being detected in 22 patients (33%), followed by SS (17 patients, 26%) and mesenchymal chondrosarcoma (6 patients, 9%). The number of patients with TRS subtypes other than MRCL and SS (other TRS) was 27 (41%). The median total number of trabectedin treatment cycles was 4.0 (range, 1–22). The median relative dose intensity was 79.4% (range, 48.7–100.0).
Table 1. Baseline demographics and disease characteristics.
Trabectedin group of comparative study versus cross‐over to trabectedin.
Anthracycline includes doxorubicin, adriamycin, epirubicin, pirarubicin, pinorubin, and therarubicin.
Abbreviations: BSC, best supportive care; C, Chi‐square test; ECOG, Eastern Cooperative Oncology Group; F, Fisher's exact test; t, t test; W, Wilcoxon's rank sum test.
In the pooled population, median time to progression and PFS was 5.7 months (95% CI: 4.2–7.4) (supplemental online Fig. 1) and 5.6 months (95% CI: 4.1–7.3).
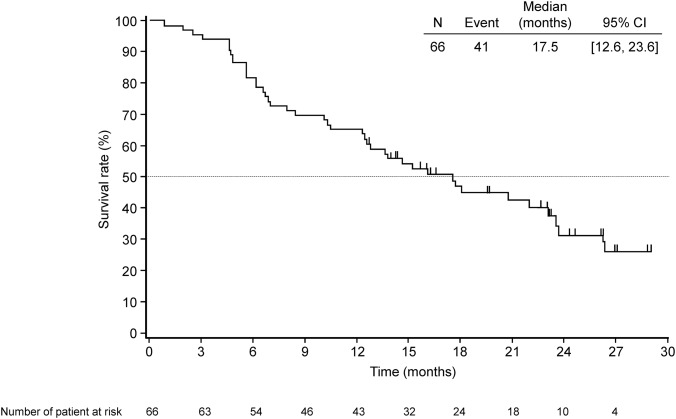
The median OS was 17.5 months (95% CI: 12.6–23.6). Figure 3 shows the Kaplan‐Meier curve of OS. At the final data cut‐off date, 25 patients (37.9%) remained alive. Of the 51 post‐treated patients (77%), 31 (47%) received chemotherapy and 14 (21%) underwent radiotherapy (Table 2).
Figure 3.
Kaplan‐Meier plot of overall survival in pooled population.
Abbreviation: CI, confidence interval.
Table 2. Post‐study treatment.
Abbreviation: BSC, best supportive care.
Subgroup Analysis of the Pooled Data
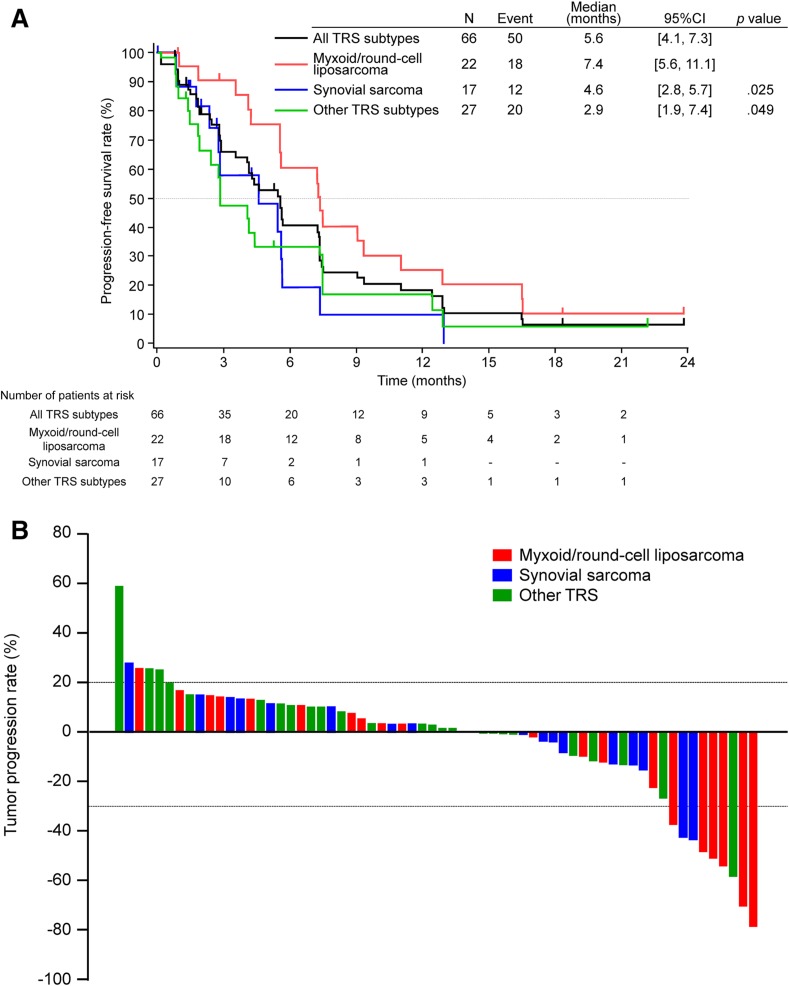
Kaplan‐Meier curves of PFS by TRS subtype (MRCL, SS, and other TRS) are shown in Figure 4A. In patients with MRCL, the median PFS was 7.4 months (95% CI: 5.6–11.1), and the 3‐ and 6‐month progression‐free rates were 91% and 60%, respectively (supplemental online Table 1). A waterfall plot is shown in Figure 4B. The response and disease control rates were 12% and 71%, respectively, in all patients; 27% and 86%, respectively, in the MRCL group; 6% and 82%, respectively, in the SS group; and 4% and 52%, respectively, in the other TRS group, with the highest response rate recorded in the MRCL group (supplemental online Table 2). The more tendencies toward improvement in response rate and PFS were observed in MRCL group than in the other groups (PFS in MRCL, vs. SS, p = .025; vs. other TRS, p = .049).
Figure 4.
Kaplan‐Meier plot of progression‐free survival by histological subtype (A). Waterfall plot by histological subtype (B).
Abbreviations: CI, confidence interval; other TRS, all patients with TRS subtypes excluding myxoid/round‐cell liposarcoma and synovial sarcoma; PFS, progression‐free survival; TRS, translocation‐related sarcoma.
The results of subgroup analyses by lymphocyte count are summarized in supplemental online Table 3. The Kaplan‐Meier curves of PFS and OS by baseline lymphocyte count (<1,000/μL and ≥1,000/μL) are shown in Figures 5A and 5B, respectively. The log‐rank tests for PFS stratified by the sum of the diameter of target lesions and PS were not significant (p = .785 for the sum of the diameter of target lesions and p = .781 for PS, respectively). The result from the model analysis was similar to the stratified log‐rank test (p = .573 for the sum of the diameter of target lesions and p = .710 for PS, respectively). The log‐rank test for OS stratified by the sum of the diameter of target lesions and PS did not show a significant effect of the baseline lymphocyte count (p = .075 for the sum of the diameter of target lesions and p = .197 for PS, respectively). However, the result from the model analysis of OS showed a significant effect of the baseline lymphocyte count, regardless of the adjustment variables (p =.029 for the sum of the diameter of target lesions and p = .05 for PS, respectively). The model analyses indicated the possibility of the importance of the lymphocyte count for OS although the stratified log‐rank tests for OS were not significant. Response by baseline lymphocyte count is summarized in supplemental online Table 4. The Kaplan‐Meier curves of PFS and OS according to the number of previous chemotherapy regimens (0, 1, 2, and ≥3) are shown in supplemental online Figure 2. Adverse drug reactions occurred in ≥10% of patients are shown in supplemental online Table 5.
Figure 5.
Kaplan‐Meier plot of (A) progression‐free survival and (B) overall survival by baseline lymphocyte count (<1,000/μL and ≥1,000/μL).
Abbreviation: CI, confidence interval; HR, hazard ratio.
Discussion
The efficacy results for trabectedin in two phase II clinical studies involving patients with advanced TRS were combined and retrospectively analyzed. In the pooled population, trabectedin consistently exerted prolonged disease control and antitumor effects in patients with advanced TRS. The drug was more effective against MRCL than other histological subtypes.
In the present analysis, we were able to integrate the data of two phase II studies, because we set the eligibility criteria of the two studies accordingly, thus ensuring the patient characteristics in both studies were similar.
In the comparative study, there was no significant difference in updated OS. One of the reasons could be high rate of post‐study treatment in the BSC group; 29 of 36 patients (81%) in the BSC group received trabectedin in the subsequent single‐arm study after disease progression in the BSC group in the comparative study. For this reason, data enabling comparisons with the survival results of trabectedin‐treated patients were limited. However, we compared the results of our pooled data with previously reported data of patients with TRS, and the median OS in the present pooled population (17.5 months, 95% CI: 12.6–23.6) was similar to the retrospective data of patients with TRS who received trabectedin 0.58‐1.5 mg/m2 reported by Le Cesne et al. [3] (17.4 months, 95% CI: 11.1–23.2), which were concluded to be longer than the OS in patients with other STS subtypes. Median PFS in the pooled population was also similar to the retrospective data given above for TRS (4.1 months, 95% CI: 2.8–6.1) [3] and the previous data of trabectedin 1.5 mg/m2 reported by Demetri GD et al. in patients with liposarcoma and leiomyosarcoma after failure of standard chemotherapy (4.2 months) [7]. The response and disease control rates among patients with TRS were also similar to previous data [3], which means that present analysis confirms the efficacy to advanced TRS patients prospectively. Disease control was consistently observed among patients who received trabectedin 1.2 mg/m2 in this analysis based on the results of both the comparative and single‐arm studies [9], [10]. We categorized TRS into three histological subtypes, and the MRCL group displayed a more pronounced improvement of PFS in response to trabectedin than the other groups, as previously reported [3], [13]. It is notable that the response rate was 27% in the pretreated MRCL patients.
The number of previous chemotherapy regimens showed a tendency for correlation with OS, although the correlation between PFS of trabectedin and the number of previous chemotherapy regimens was not clear. In the subgroup analysis of PFS and OS by lymphocyte count, we accounted for the sum of the diameter of the target lesions or PS because they were considered to be the difference in the risk of progression and death between each study. The baseline lymphocyte count appears to correlate with survival time but not with PFS. These results were consistent with the previous report by Ray‐Coquard et al. [12]. In addition, the discrepancy between the results of the stratified log‐rank test and the model analysis indicated that a better cut‐off point of lymphocyte count than 1,000/μL might be needed.
The difference in the time intervals for tumor assessment between the two studies is considered to be a limitation of integrating the data of the two studies.
The safety profile of 1.2 mg/m2 trabectedin of the updated data was as expected from the data of each study at the previous cut‐off date [9], [10] and from previous reported data of 1.5 mg/m2 trabectedin [7].
Conclusion
Our updated and pooled data showed that trabectedin prolonged PFS in patients with TRS, especially those with MRCL, after failure of or intolerance to standard chemotherapy. In addition, we consider that the subgroup analyses provide important information regarding the benefit of trabectedin treatment in particular subgroups of patients with TRS.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
The comparative phase II study and the single‐arm phase II study were sponsored by Taiho Pharmaceutical Co., Ltd. All authors, excluding Masashi Shimura, were the investigators of the two clinical studies. National Institute of Biomedical Innovation (Osaka, Japan) promoted Taiho Pharmaceutical Co., Ltd. in development of trabectedin under orphan designation in Japan. Editorial and writing assistance was provided by Honyaku Center Inc. and funded by Taiho Pharmaceutical Co., Ltd. The costs of publication of this article were defrayed in part by the payment of page charges. Clinical trial registration numbers are JapicCTI‐121850 (the comparative study) and JapicCTI‐121853 (the single‐arm study).
Author Contributions
Conception/design: Mitsuru Takahashi, Shunji Takahashi, Nobuhito Araki, Hideshi Sugiura, Takafumi Ueda, Tsukasa Yonemoto, Hideo Morioka, Hiroaki Hiraga, Toru Hiruma, Toshiyuki Kunisada, Akihiko Matsumine, Akira Kawai
Provision of study material or patients: Mitsuru Takahashi, Shunji Takahashi, Nobuhito Araki, Hideshi Sugiura, Takafumi Ueda, Tsukasa Yonemoto, Hideo Morioka, Hiroaki Hiraga, Toru Hiruma, Toshiyuki Kunisada, Akihiko Matsumine, Akira Kawai
Collection and/or assembly of data: Mitsuru Takahashi, Shunji Takahashi, Nobuhito Araki, Hideshi Sugiura, Takafumi Ueda, Tsukasa Yonemoto, Hideo Morioka, Hiroaki Hiraga, Toru Hiruma, Toshiyuki Kunisada, Akihiko Matsumine, Akira Kawai
Data analysis and interpretation: Mitsuru Takahashi, Shunji Takahashi, Nobuhito Araki, Hideshi Sugiura, Takafumi Ueda, Tsukasa Yonemoto, Hideo Morioka, Hiroaki Hiraga, Toru Hiruma, Toshiyuki Kunisada, Akihiko Matsumine, Masashi Shimura, Akira Kawai
Final approval of manuscript: Mitsuru Takahashi, Shunji Takahashi, Nobuhito Araki, Hideshi Sugiura, Takafumi Ueda, Tsukasa Yonemoto, Hideo Morioka, Hiroaki Hiraga, Toru Hiruma, Toshiyuki Kunisada, Akihiko Matsumine, Masashi Shimura, Akira Kawai
Disclosures
Mitsuru Takahashi: Taiho (RF, H); Shunji Takahashi: Taiho, Eisai, Boehringer Ingelheim, Novartis, Bayer, Daiichi‐Sankyo, Merck, Astellas Pharma, AstraZeneca (RF, H), GSK, Chugai, Zenyaku Kogyo, Sanofi, Otsuka Pharmaceutical, Pfizer, Japan Clinical Oncology Group (RF); Nobuhito Araki: Taiho, GSK, Eisai, Japan Clinical Oncology Group, MSD (RF); Hideshi Sugiura: Taiho (RF), GSK, Eisai, MSD (RF); Takafumi Ueda: Taiho, Eisai, MSD (RF); Daiichi‐Sankyo, GSK (RF, H); Tsukasa Yonemoto: Taiho (RF, H), Daiichi‐Sankyo (H), GSK, Merck (RF); Hideo Morioka: Taiho, Daiichi‐Sankyo, GSK (RF, H), Eisai (RF), Novartis (H); Hiroaki Hiraga: Taiho, GSK, Eisai, MSD, Ono Pharmaceutical, Daiichi‐Sankyo, Ministry of Health, Labour and Welfare, Center for Clinical Trials, Japan Medical Association, National Cancer Center (RF); Toru Hiruma: Taiho (RF, H), GSK (H); Toshiyuki Kunisada: Taiho (RF); Akihiko Matsumine: Taiho, GSK, Eisai, MSD, Japan Clinical Oncology Group (RF); Masashi Shimura: Taiho (E); Akira Kawai: Taiho, GSK, Eisai, MSD (RF, H), Japan Clinical Oncology Group (RF).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Grohar PJ, Griffin LB, Yeung C et al. Ecteinascidin 743 interferes with the activity of EWS‐FLI1 in Ewing sarcoma cells. Neoplasia 2011;13:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Giandomenico S, Frapolli R, Bello E et al. Mode of action of trabectedin in myxoid liposarcomas. Oncogene 2014;33:5201–5210. [DOI] [PubMed] [Google Scholar]
- 3. Le Cesne A, Cresta S, Maki RG et al. A retrospective analysis of antitumour activity with trabectedin in translocation‐related sarcomas. Eur J Cancer 2012;48:3036–3044. [DOI] [PubMed] [Google Scholar]
- 4.ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol; 2014;25:iii102–iii112. [DOI] [PubMed] [Google Scholar]
- 5. van der Graaf WT, Blay JY, Chawla SP et al. Pazopanib for metastatic soft‐tissue sarcoma (PALETTE): A randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet 2012;379:1879–1886. [DOI] [PubMed] [Google Scholar]
- 6. Schöffski P, Chawla S, Maki RG et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open‐label, multicentre, phase 3 trial. Lancet 2016;387:1629–1637. [DOI] [PubMed] [Google Scholar]
- 7. Demetri GD, von Mehren M, Jones RL et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J Clin Oncol 2015;34:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ueda T, Kakunaga S, Ando M et al. Phase I and pharmacokinetic study of trabectedin, a DNA minor groove binder, administered as a 24‐h continuous infusion in Japanese patients with soft tissue sarcoma. Invest New Drugs 2014;32:691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kawai A, Araki N, Sugiura H et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation‐related sarcoma: A randomised, open‐label, phase 2 study. Lancet Oncol 2015;16:406–416. [DOI] [PubMed] [Google Scholar]
- 10. Araki N, Takahashi S, Sugiura H et al. Retrospective inter‐ and intra‐patient evaluation of trabectedin after best supportive care for patients with advanced translocation‐related sarcoma after failure of standard chemotherapy. Eur J Cancer 2016;56:122–130. [DOI] [PubMed] [Google Scholar]
- 11. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 12. Ray‐Coquard I, Cropet C, Van Glabbeke M et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res 2009;69:5383–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grosso F, Jones RL, Demetri GD et al. Efficacy of trabectedin (ecteinascidin‐743) in advanced pretreated myxoid liposarcomas: A retrospective study. Lancet Oncol 2007;8:595–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.