This study builds on analyses performed as part of an original comprehensive National Cancer Institute Community Cancer Centers Program evaluation and examines improvements in quality of care. The following research questions are addressed: (a) have improvements in concordance rates with the five quality of care measures been sustained since 2010 and (b) how does the change in concordance for minority/underserved patients compare to the change for nonminority/nonunderserved patients through 2013?
Keywords: Quality improvement, Quality indicators, Health care, Health care disparities, Breast neoplasms, Colonic neoplasms
Abstract
Background.
The National Cancer Institute Community Cancer Centers Program (NCCCP) pilot was designed to improve quality of cancer care and reduce disparities at community hospitals. The NCCCP's primary intervention was the implementation of the Commission on Cancer Rapid Quality Reporting System (RQRS). The RQRS is a hospital‐based data collection and evaluation system allowing near real‐time assessment of selected breast and colon cancer quality of care measures. Building on previous NCCCP analyses, this study examined whether improvements in quality cancer care within NCCCP hospitals early in the program were sustained and whether improvements were notable for minority or underserved populations.
Methods.
We compared changes in concordance with three breast and two colon cancer quality measures approved by the National Quality Forum for patients diagnosed at NCCCP hospitals from 2006 to 2007 (pre‐RQRS), 2008 to 2010 (early‐RQRS), and 2011 to 2013 (later‐RQRS). Data were obtained from NCCCP sites participating in the Commission on Cancer Rapid Quality Reporting System. Logistic regression analyses were performed to identify predictors of concordance with breast and colon cancer quality measures.
Results.
The sample included 13,893 breast and 5,546 colon cancer patients. After RQRS initiation, all five quality measures improved significantly and improvements were sustained through 2013. Quality of care measures showed sustained improvements for both breast and colon cancer patients and for vulnerable patient subgroups including black, uninsured, and Medicaid‐covered patients.
Conclusions.
Quality improvements in NCCCP hospitals were sustained throughout the duration of the program, both overall and among minority and underserved patients. Because many individuals receive cancer treatment at community hospitals, facilitating high‐quality care in these environments must be a priority.
Implications for Practice.
Quality improvement programs often improve practice, but the methods are not maintained over time. The implementation of a real‐time quality reporting system and a network focused on improving quality of care sustained quality improvement at select community cancer centers. The NCCCP pilot increased numbers of patients receiving guideline‐concordant care for breast and colon cancer in community settings, and initial improvements noted in earlier years of RQRS were sustained into later years, both overall and among minority and underserved patients. National initiatives that improve care for diverse patient groups are important for reducing and eliminating barriers to care.
Introduction
In 2007, the National Cancer Institute (NCI) funded the NCI Community Cancer Centers Program (NCCCP) pilot, an initiative at hospital‐based community cancer centers designed to help build a community‐based research platform with the goals of improving quality of care, reducing cancer disparities, and increasing participation in clinical trials. As the NCCCP sites developed over time, they began to function as a network, collaborating to improve patient care, share best practices, and develop new tools to achieve program goals [1]. The NCCCP implemented an intervention that involved sites participating in the Commission on Cancer (CoC) Rapid Quality Reporting System (RQRS). The RQRS is a hospital‐based data collection and evaluation system allowing near real‐time assessment of selected breast and colon cancer quality of care measures [2]. The RQRS began as a pilot with a few sites testing a beta version of the dashboard. In 2008, the official dashboard was released and participation expanded to include CoC‐accredited hospitals.
Previous studies have examined the impact of the NCCCP program on quality of cancer care. A comprehensive, multimethod evaluation of the NCCCP was performed, and one component of the evaluation focused on how participation in the NCCCP changed the quality of cancer care provided at these hospitals before versus after NCCCP initiation between 2007 and 2010 [3]. The study showed that NCCCP sites had significant improvements in quality of care for a subset of measures. In a follow‐up study, Halpern and Spain et al. examined the effects of the NCCCP pilot on quality of care for patients from underserved populations during the same period and found that while quality of care improvements were similar for all patients, the percent of patients with guideline‐concordant care was lower among certain underserved patient groups [4].
The aims of the current study were twofold. First, we built on analyses performed as part of the original comprehensive NCCCP evaluation, going through 2010, and examined whether improvements in quality of care have been sustained through 2013. Second, because reduction in cancer disparities was an NCCCP goal, we assessed whether sustained quality changes were observed among underserved populations. Specifically, this research addressed the following research questions: (a) have improvements in concordance rates with the five quality of care measures been sustained since 2010 and (b) how does the change in concordance for minority/underserved patients compare to the change for nonminority/nonunderserved patients through 2013?
Materials and Methods
Data and Analytic Sample
Analyses were performed using data from the RQRS [2]. The RQRS was designed to improve the timeliness and reporting of National Quality Forum (NQF)‐endorsed quality indicators and to provide a platform for quality improvement based on those measures [1]. Participating NCCCP sites uploaded RQRS data to a secure server on a monthly basis as an NCCCP‐quality reporting deliverable. Details on the NCCCP and the RQRS have been previously published [3], [4], [5].
The study sample consisted of 19,439 patients diagnosed with breast or colon cancer between 2006 and 2013 at 12 NCCCP sites [2]. Patients diagnosed in 2006 or 2007 are considered to have been diagnosed “pre‐RQRS” initiation, those diagnosed between 2008 and 2010 are classified as having been diagnosed “early RQRS,” and those diagnosed between 2011 and 2013 are classified as “later RQRS.”
Measures
The study examined changes over time in the following breast and colon cancer measures that were endorsed by the National Quality Forum (NQF) and CoC and were included in the RQRS initiated by the National Cancer Institute [3]:
Breast‐breast‐conserving surgery (BCS): Radiation therapy administered within 1 year of diagnosis for females under age 70 receiving BCS for breast cancer.
Breast‐hormone therapy (HT): Tamoxifen or third generation aromatase inhibitor considered or administered within 1 year of diagnosis for females with American Joint Committee on Cancer (AJCC) T1cN0M0, or stage II or III hormone‐receptor‐positive breast cancer.
Breast‐multi‐agent chemotherapy (MAC): Combination (multi‐agent) chemotherapy considered or administered within 4 months of diagnosis for females under 70 with AJCC T1cN0M0 or stage II or III hormone‐receptor‐negative breast cancer.
Colon‐adjuvant chemotherapy (ACT): Adjuvant chemotherapy considered or administered within 4 months of diagnosis for patients under age 80 with AJCC stage III colon cancer.
Colon‐12RLN: At least 12 regional lymph nodes are removed and pathologically examined for resected colon cancer.
For our study sample, we implemented the above preidentified measure specifications from the NQF and used them with the patient population specified by the measure developer. Patient characteristics were obtained from the RQRS data, including patient race (white or nonwhite); patient sex (for colon cancer only); patient primary insurance (Medicare, Medicaid, private insurance, other insurance, or uninsured); and patient age group (categorized as <50, 50–59, 60–69, and 70 and older). Cases were included in the analysis if they received all or part of their treatment at an NCCCP site.
Analyses
All analyses were performed using Stata version 13 [6]. We examined concordance rates for the five quality of care measures separately across three time periods: pre‐RQRS (2006–2007), early RQRS (2008–2010), and later RQRS (2011–2013) (Tables 1 and 2). For each quality measure, we examined concordance rates overall and separately by patient characteristics. We performed logistic regression models to identify predictors of concordance with breast and colon cancer quality measures. Since NCCCP sites are heterogeneous in terms of urban/rural status, data management, infrastructure, and funding source [7], we accounted for intragroup correlation within community hospitals using Stata's cluster option. We performed logistic regression models for the main effects of RQRS time period and patient characteristics for each quality measure (Model 1 in Table 3). To assess whether the likelihood of sustained concordance with quality measures differed among patient subgroups, we also performed logistic regression models for interaction effects between RQRS time period and patient characteristics, including patient race (Model 2 in Table 3), patient sex (for colon cancer; Model 3 in Table 3), primary patient insurance (Fig. 1), and patient age group (Fig. 2). All variables in logistic regression analyses were examined for multicollinearity (variance inflation factor > 5) prior to inclusion in the final models.
Table 3. Odds ratios (95% CI) from multivariable regression analyses of the likelihood of concordance with breast and colon cancer quality measures.
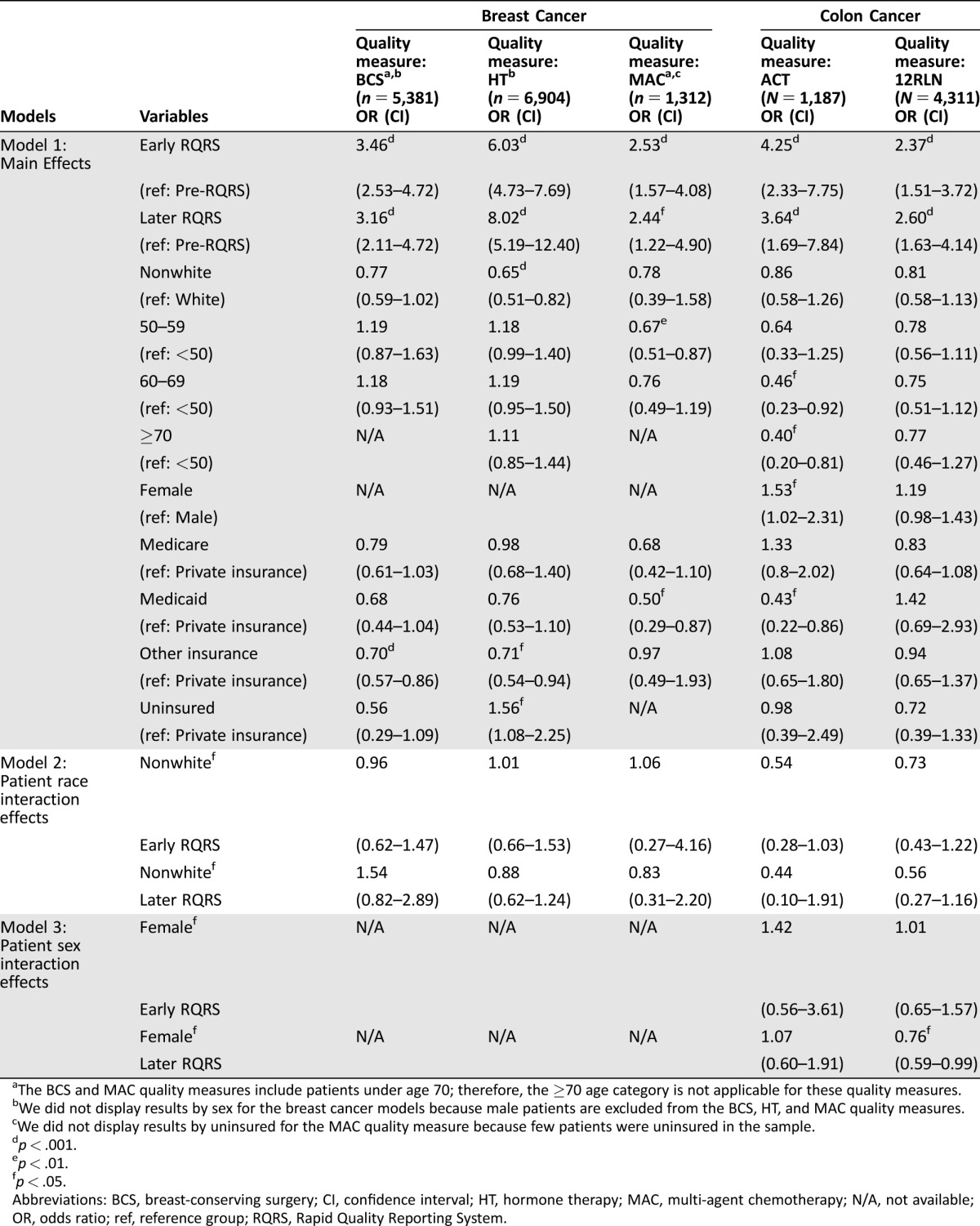
The BCS and MAC quality measures include patients under age 70; therefore, the ≥70 age category is not applicable for these quality measures.
We did not display results by sex for the breast cancer models because male patients are excluded from the BCS, HT, and MAC quality measures.
We did not display results by uninsured for the MAC quality measure because few patients were uninsured in the sample.
p < .001.
p < .01.
p < .05.
Abbreviations: BCS, breast‐conserving surgery; CI, confidence interval; HT, hormone therapy; MAC, multi‐agent chemotherapy; N/A, not available; OR, odds ratio; ref, reference group; RQRS, Rapid Quality Reporting System.
Figure 1.
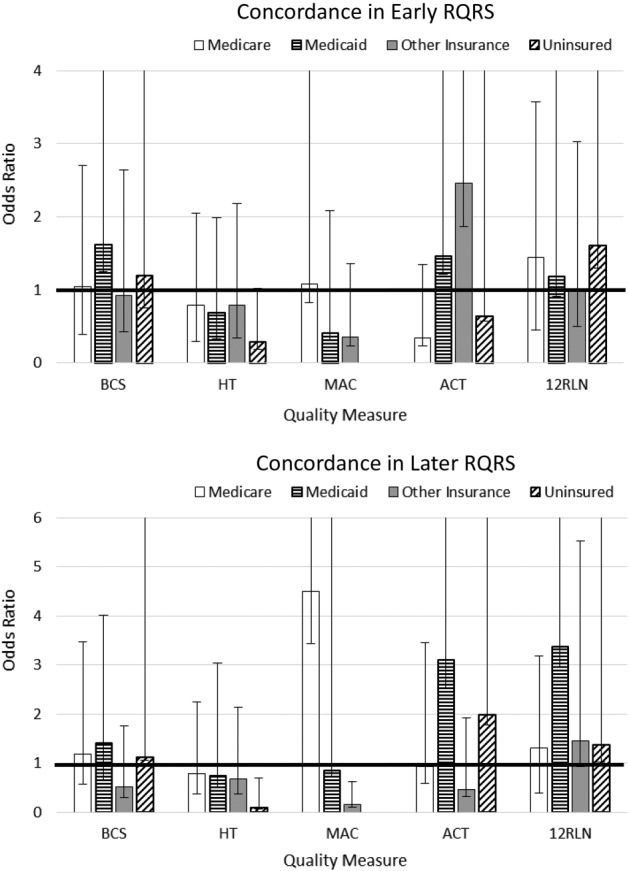
Effects of patient insurance and Rapid Quality Reporting System time period interactions on likelihood of concordance with breast and colon cancer quality measures. The full extent of the upper confidence interval may not be shown.
Abbreviations: 12RLN, regional lymph nodes; ACT, adjuvant chemotherapy; BCS, breast‐conserving surgery; HT, hormone therapy; MAC, multi‐agent chemotherapy; NCCCP, National Cancer Institute Community Cancer Centers Program.
Figure 2.
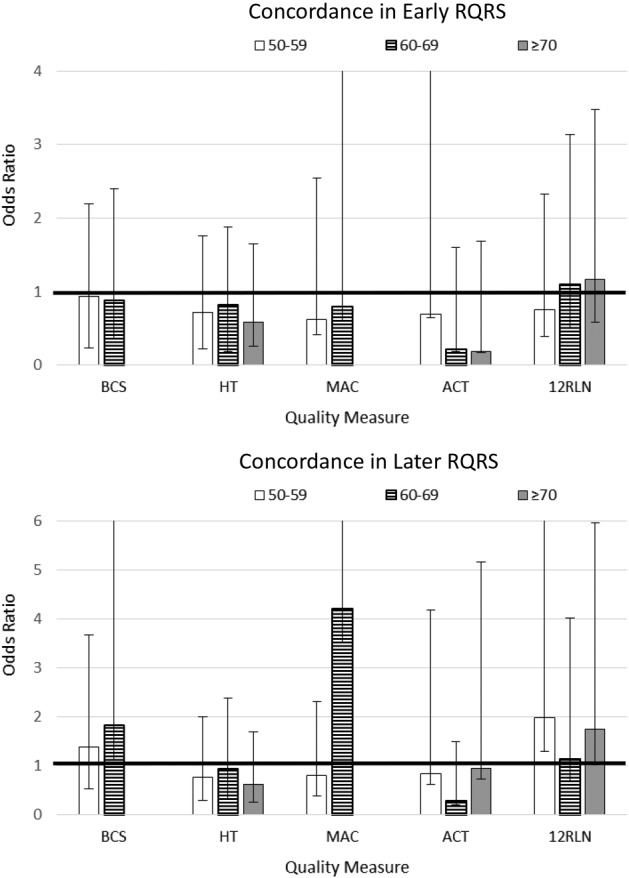
Effects of patient age and Rapid Quality Reporting System time period interactions on likelihood of concordance with breast and colon cancer quality measures. The full extent of the upper confidence interval may not be shown.
Abbreviations: 12RLN, regional lymph nodes; ACT, adjuvant chemotherapy; BCS, breast‐conserving surgery; HT, hormone therapy; MAC, multi‐agent chemotherapy; NCCCP, National Cancer Institute Community Cancer Centers Program.
Results
Patient Sample
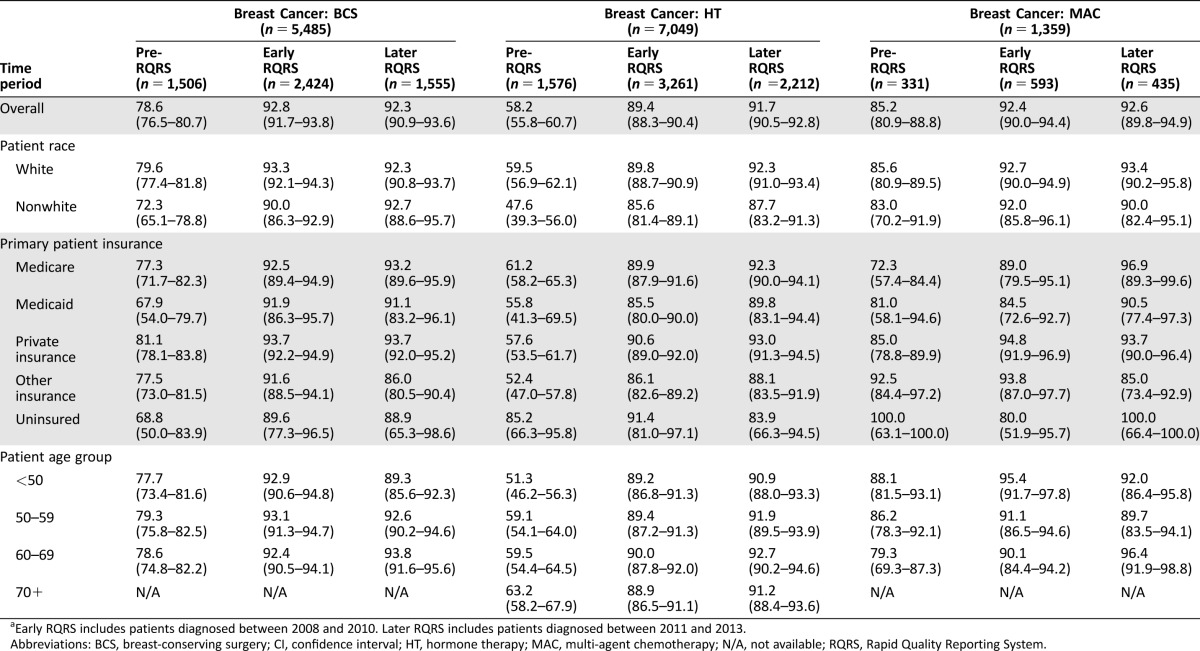
The breast cancer sample included 13,893 patients, 25% diagnosed during the pre‐RQRS time period, 45% from the early RQRS time period, and 30% from the later RQRS time period (Table 1). Of the breast cancer patients, 87% were white. Insurance groups for females with breast cancer included 25% with Medicare, 6% with Medicaid, 50% with private insurance, 17% with other insurance, and 2% uninsured. Age groups included 26% younger than 50 years of age, 32% 50–59 years, 30– 60–69 years, and 12% 70 years of age and older. See supplemental online Table 1 for patient characteristics by breast and colon cancer quality measures.
Table 1. Concordance rates percentage, (95% CI): pre‐RQRS, early RQRS, and later RQRSa: breast cancer quality measures, by patient characteristics.
Early RQRS includes patients diagnosed between 2008 and 2010. Later RQRS includes patients diagnosed between 2011 and 2013.
Abbreviations: BCS, breast‐conserving surgery; CI, confidence interval; HT, hormone therapy; MAC, multi‐agent chemotherapy; N/A, not available; RQRS, Rapid Quality Reporting System.
The colon cancer sample included 5,546 patients; 27% from the pre‐RQRS time period, 42% from the early RQRS time period, and 31% from the later RQRS time period (Table 2). The sample of colon cancer patients was approximately evenly split between males and females. Of the colon cancer sample, 85% were white. Insurance groups for patients with colon cancer included 56% Medicare, 3% Medicaid, 27% private insurance, 12% with other insurance, and 3 percent uninsured. Age groups included 10% younger than 50 years of age, 19% 50–59 years, 25% 60–69 years, and 46% 70 years of age and older.
Table 2. Concordance rates percentage (95% CI): pre‐RQRS, early RQRS, and later RQRSa: colon cancer quality measures, by patient characteristics.
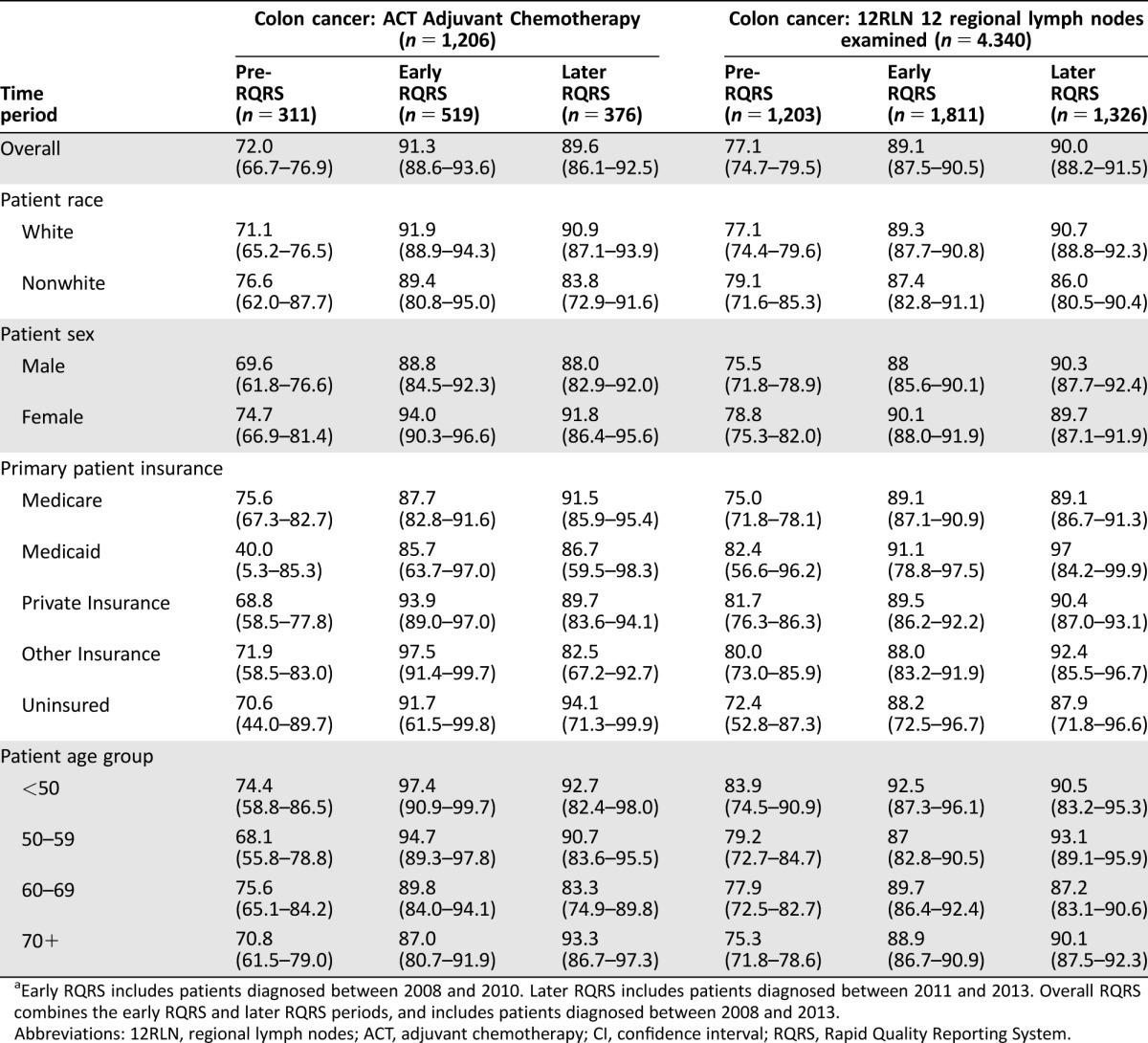
Early RQRS includes patients diagnosed between 2008 and 2010. Later RQRS includes patients diagnosed between 2011 and 2013. Overall RQRS combines the early RQRS and later RQRS periods, and includes patients diagnosed between 2008 and 2013.
Abbreviations: 12RLN, regional lymph nodes; ACT, adjuvant chemotherapy; CI, confidence interval; RQRS, Rapid Quality Reporting System.
Sustained Concordance with Quality of Care Measures
The rate of concordance with each of the five quality of care measures increased substantially from the early RQRS period and were sustained through the later RQRS period (Tables 1, 2). Regression analyses controlling for patient sociodemographic characteristics indicate that the likelihood of concordance with all five quality measures increased significantly in both the early RQRS and later RQRS periods compared with the pre‐RQRS period (Table 3). Furthermore, there were no significant differences in the likelihood of concordance for the early RQRS versus the later RQRS periods (Table 3). That is, the increase in concordance observed in the early RQRS period was sustained during the later RQRS period.
Concordance with Breast Cancer Measures by Patient Sociodemographic Characteristics
Across the entire study period, females with “other” insurance were significantly less likely to receive guideline‐concordant radiation therapy following breast‐conserving surgery compared with females with private insurance (Model 1 in Table 3; odds ratio [OR] = 0.70, 95% confidence interval [CI] [0.57, 0.86]). However, during the early and later RQRS time periods, females with other insurance no longer had disparities in concordance with radiation therapy (Fig. 1).
Across the entire study period, nonwhite females and females with other insurance were significantly less likely to receive guideline‐concordant hormone therapy compared to white females and females with private insurance (Model 1 in Table 3; nonwhite compared to white: OR = 0.65, 95% CI [0.51, 0.82]; other Insurance compared to private Insurance: OR = 0.71, 95% CI [0.54, 0.94]). These disparities were not statistically significant during the early and later RQRS periods (interaction terms not significant in Model 2 in Table 3 and in Fig. 1). However, uninsured females were significantly less likely to receive guideline‐concordant hormone therapy during both the early and later RQRS time periods (Fig. 1; early RQRS: OR = 0.28, 95% CI [0.10, 0.74]; later RQRS: OR = 0.10, 95% CI [0.02, 0.61]). This suggests that, as the concordance rate for the Breast‐HT measure increased from the pre‐RQRS period to the early and later RQRS periods, the rate among uninsured women did not increase as rapidly.
Across the entire study period, Medicaid‐enrolled females were significantly less likely to receive guideline‐concordant multi‐agent chemotherapy (MAC) for breast cancer compared to females with private insurance (Model 1 in Table 3; OR = 0.50, 95% CI [0.29, 0.87]), and females 50–59 years of age were significantly less likely to receive guideline‐concordant MAC compared to females who were younger than age 50 (Model 1 in Table 3; OR = 0.67, 95% CI [0.51, 0.87]). During the early and later RQRS time periods, Medicaid‐enrolled females and females who were 50–59 years of age no longer had disparities in concordance with chemotherapy for breast cancer (interaction terms not significant in Figs. 1, 2). However, during the early RQRS time period, Medicare‐enrolled females were significantly more likely to receive guideline‐concordant MAC (Fig. 1; OR = 1.08, 95% CI [0.25, 4.61]), while females with other insurance were significantly less likely to receive guideline‐concordant MAC (Fig. 1; OR = 0.36, 95% CI [0.13, 1.00]).
Concordance with Colon Cancer Measures by Patient Sociodemographic Characteristics
Across the entire study period, patients 60 or more years of age were significantly less likely to receive guideline‐concordant adjuvant chemotherapy (ACT) for stage III colon cancer compared with patients who were younger than age 50 (Model 1 in Table 3; 60–69 compared to <50 age: OR = 0.46, 95% CI [0.23, 0.92]; ≥ 70 compared to <50 age: OR = 0.40, 95% CI [0.20, 0.81]). In addition, female patients were significantly more likely to receive guideline‐concordant ACT compared with male patients (Model 1 in Table 3; OR = 1.53, 95% CI [1.02, 2.31]), and Medicaid‐enrolled patients were significantly less likely to receive guideline‐concordant ACT compared with patients with private insurance (Model 1 in Table 3; OR = 0.43, 95% CI [0.22, 0.86]). During the early and later RQRS time periods, there were no longer disparities in concordance with ACT by patient sex, insurance, or age group (interaction terms were not significant in Model 3 in Table 3 and in Figs. 1, 2). During the Later RQRS time period, females were significantly less likely to be concordant with Colon‐12RLN (interaction term was significant in Model 3 in Table 3; OR = 0.76, 95% CI [0.59, 0.99]).
Discussion
The goals of this study were to examine whether improvements in guideline‐concordant breast and colon cancer care during the first 3 years following RQRS implementation noted by Halpern et al. [3] had been sustained throughout the program, and to examine whether significant quality improvements were also noted for minority/underserved patients. Our results indicate the improvements in quality of care were sustained through 2013 for all five measures examined. Significant differences in the likelihood of concordance with quality of care measures were identified among specific patient subgroups during the early and later RQRS periods. For example, uninsured females with hormone receptor positive breast cancer were significantly less likely to receive hormonal therapy (quality measure HT) than were women with private insurance. These results indicate that while the RQRS did improve quality of breast and colon cancer care, and these improvements in the quality of care were sustained through the later RQRS period, not all patients benefited equally. However, not all of these differences indicate traditional disparities in care such as privately insured patients receiving better quality of care than patients insured by Medicare or Medicaid. Female Medicare beneficiaries with breast cancer were significantly more likely to receive MAC than were females with private insurance in the later RQRS period.
Concurrent NCCCP Quality of Care Subcommittee activities included a working group to improve RQRS reporting and twice annual presentations of the NCCCP results to the network, which may have helped sites improve and sustain quality cancer care. Also, all sites had at least one affiliated or employed oncology practice participating in the American Society of Clinical Oncology Quality Oncology Practice Initiative (QOPI) abstraction during the reporting period, which may have led to an increased focus on quality improvement at the sites since many RQRS measures are also QOPI Measures. Furthermore, the NCCCP program design required management engagement and co‐investment (e.g., support for RQRS, increased navigation), which led to enhanced infrastructure [8].
As noted by Halpern et al. [4], quality improvement processes, sharing of exemplary practices, and other initiatives instituted as part of the NCCCP benefitted all population groups, including those from minority or underserved populations. For example, the NCCCP program focused on reducing health care disparities through a number of initiatives, such as patient navigation, which was used by some sites to address patient barriers to concordant care.
This study has several important limitations. The analysis aggregates results from all NCCCP sites that had substantial variation in baseline (pre‐RQRS) quality of care (for example, the concordance rate for radiation after breast conserving surgery during the pre‐RQRS period was 30% for one NCCCP site and 91 percent for another NCCCP site). While we analyzed patient‐level data, the effect was site‐specific. The NCCCP sites have substantial heterogeneity, which increases the generalizability of results. We considered variation across NCCCP sites by accounting for clustering of patients within NCCCP sites. Separate analyses found site effects on concordance for all five quality measures (data not shown). This limits our ability to identify which specific NCCCP sites or aspects of the NCCCP were most effective in improving quality of care overall and for underserved populations. This analysis was also limited to available data and did not cover the entire universe of factors that could influence quality of care; for example, we did not have access to data on therapy omission and whether treatments were actually completed. Furthermore, this analysis did not examine improvements in quality of care in non‐NCCCP hospitals, so it is unknown how much improvement can be attributed to the NCCCP. More specifically, it is unknown how much improvement in quality of cancer care can be attributed to the NCCCP's focus on improved quality and collaborative sharing of best practices versus improved recording of treatment administration due to the RQRS requirements. Finally, outcomes for this analysis were limited to five quality‐of‐care process measures collected by the RQRS and constitute a fairly small component of the entire universe of structures, processes, and outcomes potentially influenced by the NCCCP. Nevertheless, these analyses provide important information on sustaining high quality of breast and colorectal cancer care for patients treated at community hospitals.
Conclusion
The NCCCP pilot has resulted in increased numbers of patients receiving guideline‐concordant care for breast and colon cancer in community settings, and the initial improvements that were noted in the first few years of the RQRS have been sustained into later years. The NCCCP pilot has been successful, in part, because NCCCP sites functioned as a network, engaged as a learning collaborative, and shared lessons learned and best practices. Future programs should explore elements of hospital collaboratives that are most salient in terms of sustaining quality and reducing disparities. Increased focus on national initiatives that improve care for diverse patient groups will continue to be important as part of programs for reducing and eliminating barriers to care, including Medicaid expansion as part of the Affordable Care Act as well as The Centers for Medicare and Medicaid Services' new Oncology Care Model. Additional research is needed to identify the key components of local, state, and national policies that significantly impact cancer care improvements for population groups served by community cancer centers.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank the National Cancer Institute (NCI) for their funding of this study and guidance during the study. We are grateful to the NCI Community Cancer Centers Program (NCCCP) site Principal Investigators and staff for their contributions to this effort. This work was completed while Dr. Clauser was employed at the NCI and does not reflect the policy or position of the Patient Centered Outcomes Research Institute. This project has been funded in whole or in part with Federal funds from the NCI, NIH, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Author Contributions
Conception/design: Kathleen M Castro, Pamela C Spain, Stephanie Teixeira‐Poit, Michael Halpern, Irene Prabhu Das, Brenda Adjei, Steven B Clauser
Collection and/or assembly of data: Kathleen M Castro, Pamela C Spain, Stephanie Teixeira‐Poit,
Data analysis and interpretation: Kathleen M Castro, Pamela C Spain, Stephanie Teixeira‐Poit, Michael Halpern, Irene Prabhu Das, Rebecca Lewis, Steven B Clauser
Manuscript writing: Kathleen M Castro, Pamela C Spain, Stephanie Teixeira‐Poit, Michael Halpern, Irene Prabhu Das, Brenda Adjei, Steven B Clauser
Final approval of manuscript: Kathleen M Castro, Pamela C Spain, Stephanie Teixeira‐Poit, Michael Halpern, Irene Prabhu Das, Brenda Adjei, Steven B Clauser
Disclosures
Michael Halpern: AbbVie (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Clauser SB, Johnson MR, O'Brien DM et al. Improving clinical research and cancer care delivery in community settings: Evaluating the NCI community cancer centers program. Implement Sci 2009;24:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stewart AK, McNamara E, Gay EG et al. The Rapid Quality Reporting System – A New Quality of Care Tool for CoC‐Accredited Cancer Programs. J Registry Manag 2011;38:61–63. [PubMed] [Google Scholar]
- 3. Halpern MT, Spain PC, Holden DJ et al. Improving quality of cancer care at community hospitals: Impact of the National Cancer Institute Community Cancer Centers Program Pilot. J Oncol Pract 2013;9:e298–e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halpern MT, Spain PC, Holden DJ. Evaluation of the NCI's Community Cancer Centers Program (NCCCP): Impact on Disparities in Quality of Cancer Care. J Health Dispar Res Pract 2015;8:63–80. [Google Scholar]
- 5. Clauser, ST, Johnson, MR, O'Brien DM et al. Improving clinical research and cancer care delivery in community settings: Evaluating the NCI community cancer centers program. Implement Sci 2009;4:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP. [Google Scholar]
- 7. Zaren HA, Nair S, Go RS et al. Early‐phase clinical trials in the community: Results from the national cancer institute community cancer centers program early‐phase working group baseline assessment. J Oncol Pract 2013;9:e55–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Brien DM, Kaluzny AD. The role of a public‐private partnership: Translating science to improve cancer care in the community. J Healthc Manag 2014;59:17–29. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.