This study investigated the relationship between the degree of spinal instability, as defined by the Spinal Instability Neooplastic Score, and response to radiotherapy in patients with symptomatic spinal metastases in a multi‐institutional cohort.
Keywords: Spinal instability, Spinal neoplastic instability score, Spinal metastases, Pain response, Palliative radiotherapy
Abstract
Background.
A substantial number of patients with spinal metastases experience no treatment effect from palliative radiotherapy. Mechanical spinal instability, due to metastatic disease, could be associated with failed pain control following radiotherapy. This study investigates the relationship between the degree of spinal instability, as defined by the Spinal Instability Neoplastic Score (SINS), and response to radiotherapy in patients with symptomatic spinal metastases in a multi‐institutional cohort.
Methods and Materials.
The SINS of 155 patients with painful thoracic, lumbar, or lumbosacral metastases from two tertiary hospitals was calculated using images from radiotherapy planning CT scans. Patient‐reported pain response, available for 124 patients, was prospectively assessed. Pain response was categorized, according to international guidelines, as complete, partial, indeterminate, or progression of pain. The association between SINS and pain response was estimated by multivariable logistic regression analysis, correcting for predetermined clinical variables.
Results.
Of the 124 patients, 16 patients experienced a complete response and 65 patients experienced a partial response. Spinal Instability Neoplastic Score was associated with a complete pain response (adjusted odds‐radio [ORadj] 0.78; 95% confidence interval [CI] 0.62–0.98), but not with an overall pain response (ORadj 0.94; 95% CI 0.81–1.10).
Conclusions.
A lower SINS, indicating spinal stability, is associated with a complete pain response to radiotherapy. This supports the hypothesis that pain resulting from mechanical spinal instability responds less well to radiotherapy compared with pain from local tumor activity. No association could be determined between SINS and an overall pain response, which might indicate that this referral tool is not yet optimal for prediction of treatment outcome.
Implications for Practice.
Patients with stable painful spinal metastases, as indicated by a Spinal Instability Neoplastic Score (SINS) of 6 or lower, can effectively be treated with palliative external beam radiotherapy. The majority of patients with (impending) spinal instability, as indicated by a SINS score of 7 or higher, will achieve a (partial) response after palliative radiotherapy; however, some patients might require surgical intervention. Therefore, it is recommended to refer patients with a SINS score of 7 or higher to a spine surgeon to evaluate the need for surgical intervention.
Introduction
The incidence of patients with spinal metastases is increasing due to the increasing cancer incidence and the improved life expectancy of cancer patients [1], [2], [3]. Spinal metastases may cause debilitating pain and neurological deficits, impairing quality of life [4], [5]. Radiotherapy has been the standard of care for the treatment of uncomplicated painful spinal metastases. However, up to 70% of the patients treated with radiotherapy are resistant to treatment or experience only a partial response [6]. Surgery is offered to patients with mechanical spinal instability and patients with persisting or progressive neurological deficits.
To ensure fast and effective symptom relief, optimal treatment selection is crucial considering the limited life expectancy of these patients. Previous studies have tried to identify predictive factors for response to palliative radiotherapy, but have shown inconsistent results [7], [8], [9], [10], [11], [12], [13]. Therefore, to optimize treatment and/or patient selection we need to identify new factors to predict treatment outcome. Pain from spinal metastases can result from local tumor activity, pressure on neurological structures, and/or impaired mechanical integrity [14]. The Spine Oncology Study Group developed the Spinal Instability Neoplastic Score (SINS) to assess the degree of spinal instability in order to guide patient referral [15]. In two retrospective studies, it was shown that a higher SINS, reflecting a higher degree of spinal instability, was associated with radiotherapy failure [16], [17]. This suggests that discriminating mechanical pain from tumor pain could help in identifying patients at increased risk of radiation treatment failure. In order to confirm the retrospective data, a prospective cohort study was conducted to investigate the relationship between the degree of spinal (in)stability and response to radiotherapy.
Materials and Methods
Study Design
An observational cohort study including patients with spinal metastases treated with palliative radiotherapy was conducted between July 2013 and January 2015 in two tertiary referral centers in North America and Europe. Institutional review board approval was obtained for both institutions. Patients were prospectively enrolled and followed longitudinally for up to 6 weeks (time window −2/+2 weeks) after treatment and all patients provided written informed consent to participate in this study. All patients from the department of radiation oncology with painful (i.e., a pain score of at least 2, on a scale of 0–10) thoracic, lumbar, or lumbosacral metastases without invalidating neurological deficits (American Spinal Injury Association [18] E or D without progression) were eligible for inclusion. Patients with multiple myeloma, lymphoma, or a history of surgery to the same anatomic level were excluded. Patient characteristics were collected from medical records at baseline and governmental databases were accessed to retrieve vital statistics. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for reporting of observational cohort studies was used [19].
Measurements
A single senior spine surgeon, specialized in spine oncology, calculated the SINS score using images from routine treatment planning computed tomography (CT) scans, while blinded for radiotherapy outcome. The CT scans were obtained by a 16‐detector row CT scanner (Brilliance, Philips Medical Systems, Eindhoven, The Netherlands) or GE LightSpeed RT16 (GE Healthcare, Mississauga, Canada) and were reviewed in standardized settings (window level at 300 Hounsfield Units [HU] and window width at 1,000 HU). The SINS was calculated by the sum of five radiological and one clinical component: spine location, pain, bone lesion quality, spinal alignment, vertebral body collapse, and posterolateral involvement of spinal elements (Table 1) [15]. In case of multiple spinal metastases, the SINS of all lesions within the radiation field was calculated and the highest SINS score was used for analysis. Clinical notes of the radiation oncologist or the referring specialist were reviewed to indicate whether pain was movement‐related, occasional, or absent.
Table 1. Spinal Instability Neoplastic Score (SINS)a.

0–6 points: stable; 7–12 points: impending unstable; 13–18 points: unstable. In patients with a score of 7 or higher, consultation of a spine surgeon is recommended.
Data adapted from Fisher et al. [16].
Pain improvement with recumbency and/or pain with movement/loading of spine.
Facet, pedicle, or costovertebral joint fracture or replacement with tumor.
Abbreviation: SINS, Spinal Instability Neoplastic Score.
Pain scores were reported as a number between 0 (indicating no pain) and 10 (worst pain imaginable) at baseline and at fixed points in time after palliative radiotherapy. In addition, analgesic use for the preceding 24 hours was collected at time of recording the pain score. A daily total oral morphine dose was calculated from the reported opioid analgesic consumption. In case a patient did not return the pain questionnaire in time, a trained research nurse contacted the patient by phone after 2 weeks. The response to radiotherapy was determined 4 to 8 weeks after palliative radiotherapy according to the international consensus criteria [20] summarized in Table 2. Patients were classified as overall responders if a complete or partial response was achieved. Patients with progressive pain or undetermined response were classified as non‐responders. Patients who died within 4 weeks after radiotherapy and patients with unknown pain response were excluded from the final analysis.
Table 2. Response rate to radiotherapy according to the international consensus [20].
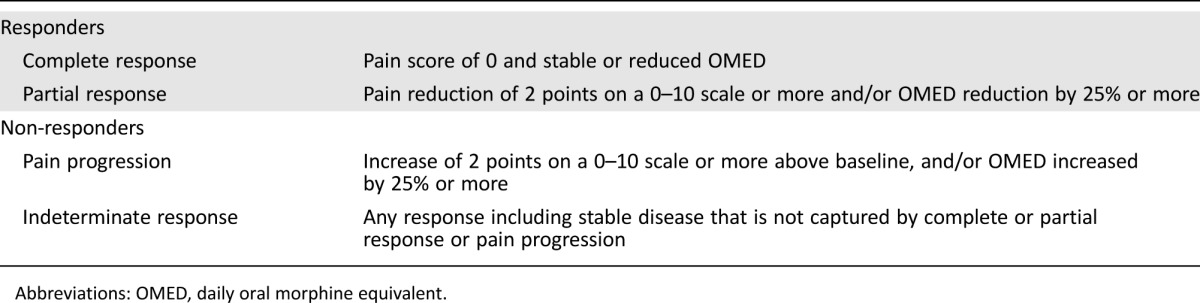
Abbreviations: OMED, daily oral morphine equivalent.
Statistical Analysis
Categorical variables were expressed as count and proportions; continuous variables were expressed as mean ± standard deviation or median with ranges. Chi‐Square tests were used to assess differences in baseline characteristics between responders and non‐responders. One‐way analysis of variance (ANOVA) tests were used for continuous variables. Logistic regression was used to assess whether SINS (continuous, or binary [SINS ≤6 or ≥7]) was related to pain response. First, overall pain response (i.e., complete and partial responses combined) was assessed, followed by in‐depth analysis selecting only complete pain responders, as these patients may represent a distinct group of patients with tumor pain only. Variables related to pain response, predefined based on literature and clinical experience, were entered in a multivariable logistic regression model to obtain adjusted odds ratios. These variables were gender, primary tumor (breast/prostate/lung/kidney/other) and performance score (World Health Organization [WHO] 0–2/3–4). A worst‐case scenario analysis was performed as sensitivity analysis, assuming that all patients who died or were lost to follow‐up experienced no response. We estimated the ability of the preselected clinical variables (i.e., gender, primary tumor, and performance score) to discriminate between patients with and without pain response by using the area under the receiver operating characteristics (ROC) curve (AUC), and compared this to the area under the ROC curve when SINS was added to these preselected variables. An AUC of 0.5 indicates no discriminating ability, in contrast to perfect discrimination with an AUC of 1 [21]. The database was analyzed using IBM SPSS statistics for Windows version 23.0 (IBM Corporation, Armonk, NY). Results were considered significant if p < .05.
Results
Between January 2013 and September 2014, 103 patients from the European center and 52 patients from the North American center with painful thoracic, lumbar, or lumbosacral metastases were included. Except for WHO performance score, no significant differences were found regarding patients and disease characteristics between responders and non‐responders within the cohort (Table 3). A favorable performance score was associated with a positive treatment response (p = .017). Thirteen patients (8%) died within the first 4 weeks after palliative radiotherapy and 18 patients (12%) were lost to follow‐up. Of the patients who died within 4 weeks after radiotherapy, six patients had a SINS score of 7 or higher. In the lost to follow‐up group, 16 patients had a SINS score of 7 or higher. Of all assessable patients, 73 (59%) patients had a SINS higher than 7, of which 10 (8%) patients had a SINS higher than 13.
Table 3. Baseline characteristics for responders, non‐responders, and patients with an unknown outcome.

Pearson Chi‐Square.
One‐way ANOVA.
Abbreviations: ANOVA, analysis of variance; WHO, World Health Organization; Gy, Gray.
The association between SINS and pain response was studied in the remaining 124 patients. Of these 124 patients, 16 (13%) patients experienced a complete response, 65 (52%) patients experienced a partial response, and 43 (35%) patients did not experience a response.
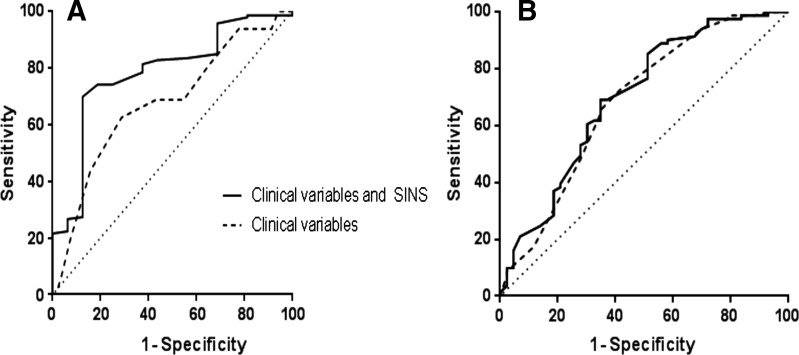
In the multivariate analysis (Table 4) relating SINS to complete versus partial and non‐responders demonstrated a significant and independent association when considered as binary variable. Considering the SINS as continuous variable, the association remained significant and independent, yet the association may be marginal given the width of the confidence interval (CI) (adjusted odds ratio 0.78 [95% CI 0.62–0.98]). The median SINS in responders was lower compared with the median SINS in partial or non‐responders (6 and 8, respectively, p = .030). Sensitivity analysis also showed a significant and independent association between SINS and complete pain response. Spinal Instability Neoplastic Score improved the area under the ROC curve of complete response in addition to other clinical variables from 0.68 (0.53–0.82) to 0.78 (0.66–0.90) (Fig. 1A).
Table 4. Association between Spinal Instability Neoplastic Score (SINS) and complete response status in patients with symptomatic spinal metastases.

Adjusted for gender, tumor, and performance status.
SINS modeled as continuous variable ranging from 0–18.
SINS modeled as binary variable 0–6 points vs. 7–18 points.
Abbreviations: CI, confidence interval; OR, odds ratio; SINS, spinal instability neoplastic score.
Figure 1.
Receiver operating characteristics curves for the discriminative value of clinical variables (gender, primary tumor, and performance status, dotted line), and Spinal Instability Neoplastic Score in addition to those clinical variables (solid line) in predicting complete pain response (A) and overall pain response (B).
Abbreviations: SINS, Spinal Instability Neoplastic Score.
In contrast, the multivariate analysis relating SINS to overall pain response versus no response demonstrated no significant and independent association, whether considered continuous or binary (Table 5). The median SINS was similar in non‐responders compared to responders (7 and 8, respectively, p = .449). Analyzing the six components of the SINS, no significant differences were found between the responders and the non‐responders (location, p = .107; pain, p = .751; lesion, p = .642; alignment, p = .323; collapse, p = 0.587; and involvement, p = .908). Sensitivity analysis showed similar results, with no association between SINS and radiotherapy failure. Spinal Instability Neoplastic Score improved the prediction of overall response in addition to other clinical variables only marginally: the area under the ROC curve increased from 0.68 (0.60–0.79) to 0.70 (0.60–0.80) (Fig. 1B).
Table 5. Association between Spinal Instability Neoplastic Score (SINS) and overall response status in patients with symptomatic spinal metastases.

Adjusted for gender, tumor, and performance status.
SINS modeled as continuous variable ranging from 0–18.
Abbreviations: CI, confidence interval; OR, odds ratio; SINS, spinal instability neoplastic score.
Discussion
In this prospective multi‐institutional cohort study, we found an association between spinal stability, as reflected by a SINS score lower than 7, and a complete pain response after radiotherapy. When considered as a continuous variable, the association between SINS and a complete pain response might be marginal, given the width of the CI. However, the association of the binary SINS score with a complete pain response is more important, as it is advised that patients with a SINS score of 7 or higher are to be referred to a spinal surgeon for evaluation. No association between SINS and overall pain response could be demonstrated. These results are in line with two previous retrospective studies demonstrating a statistically significant relationship between an increasing SINS score and a higher risk of radiotherapy failure [16], [17]. In the retrospective study of Huisman et al., the odds of radiotherapy failure for a potentially unstable (SINS 7—12) or unstable (SINS 13—18) lesion was respectively 5.9 and 12.8 times higher compared with a stable lesion [16]. Their study, however, used radiotherapy failure, defined as the need for retreatment on the index site, as the main treatment outcome. Patients who did not achieve a response but were physically not fit enough to receive retreatment or patients who declined retreatment were therefore not accounted for. In the current work, a cohort study was performed with prospectively measured radiotherapy response at 4–8 weeks post‐treatment according to the international consensus guidelines for palliative radiotherapy [20]. Therefore, all patients, even the patients with debilitating physical condition, could have been followed prospectively. Lam et al. investigated predictive factors for the occurrence of spinal adverse events (SAE) after palliative radiotherapy in a retrospective study including 299 patients [17]. Spinal adverse events were defined as interventions to achieve pain relief after fractures or uncontrolled pain despite radiation treatment, indicating a failed pain response after conventional radiotherapy. During the study period, 98 SAEs were reported in 51 patients. A SINS of 11 or higher was shown to be independently associated with a higher incidence of SAEs with a hazard ratio for first SAE of 2.52 (95% CI 1.29–4.92) [17]. These results underscore the importance of the assessment of spinal instability in patients who receive palliative radiation treatment.
In contrast to the two retrospective studies, Mitera et al. prospectively investigated the relationship between radiological features on computed tomography imaging and overall pain response after conventional external beam radiotherapy [10]. They investigated radiological parameters partially overlapping the parameters of the SINS score, including lesion type, presence of kyphosis, vertebral body collapse, and involvement of the posterior elements. A total of 33 patients were included and pain response was measured using the international consensus guidelines [20]. Six patients showed a response at 1 month, but no (significant) relationship between radiological parameters and overall pain response was found.
An impaired performance status is a known risk factor for decreased radiotherapy response [7], [13], as was confirmed in our study. Moreover, Yates et al. demonstrated that a lower performance status was associated with short‐term survival, explained by a rapid decline in performance status within the last 2 months of life [22]. The rapid decline in performance status might be due to a widespread burden of disease in the terminal phase of cancer. The association between a low performance status and impaired radiotherapy response might, therefore, be explained by this widespread burden of disease; as a local treatment modality, radiotherapy will not provide systemic control and subsequently pain control. Patients with a complete pain response might differ from patients with a partial pain response in their burden of disease. This, therefore, might explain why we found an association between a low SINS score and complete response, but not between SINS and overall response. Another explanation why we found an association between a low SINS score and a complete response might be that these patients represent a true group of patients without (pain due to) mechanical instability, supporting our hypothesis that pain mainly caused by mechanical instability responds less well to radiotherapy than pain caused by local tumor effects.
The current study has several methodological strengths. First, this study was conducted prospectively using an international multicenter cohort design enhancing generalizability of the results. There were some differences in fractionation schedule between the two institutions, but these are unlikely to have influenced the results, as single‐fraction and multi‐fraction have shown to be equally effective for the treatment of spinal metastases [6]. Secondly, international guidelines for the measurement of radiotherapy response were used, ensuring comparability to other studies. Lastly, SINS was assessed in a standardized way, by an experienced observer who was blinded for treatment outcome. One observer was deemed sufficient as the literature demonstrated excellent reproducibility of the SINS score [23].
We acknowledge that this study has some limitations as well. First, there were a limited number of cases with a high SINS score indicating spinal instability (SINS ≥13), which may be due to a low incidence of spinal instability in radiotherapy practice. The low number of cases with a high SINS score limited extensive statistical analyses, but adjusting for the known confounding factors of gender, tumor type, and performance status was performed. Adjusting for these factors demonstrated no significant difference in the CI, confirming the association between a low SINS score and complete radiotherapy response. Another important reason could be the introduction of the SINS in our institutions approximately 2 years before the start of inclusion. Recently, our group demonstrated that after introduction of the SINS, the mean SINS score in a radiotherapy and surgical cohort decreased [24]. This can be explained by increased awareness of the radiation oncologist for spinal instability and subsequent earlier referral to a spine surgeon, resulting in fewer patients with a high SINS score in the radiotherapy cohort. However, this study sample is a realistic representation of the radiotherapy population after introduction of SINS in clinical practice. Second, a substantial number of patients died within the first 4 weeks after radiotherapy or were lost to follow‐up. Despite maximized efforts to obtain follow‐up information, becoming lost to follow‐up is inherent to the study population, resulting in a relatively large number of patients with an unknown response. Notably, in the current study these patients had high SINS scores. However, the worst‐case scenario analysis, assuming no response in all patients with an unknown pain response, confirmed the results of the primary analysis. Lastly, in the current study, only patients with thoracic, lumbar, or lumbosacral metastases were included, limiting generalizability to these locations. The cervical spine has unique biomechanical characteristics compared with the thoracic and lumbar spine, providing stability for the head while simultaneously allowing for a wide range of motion. As a result, the current composition of the SINS score may be less reliable at detecting instability of the cervical spine.
Conclusion
The present study used the SINS score, reflecting the degree of spinal (in)stability, as a tool to predict radiotherapy response in patients with spinal metastases. A low SINS score (<7) was associated with a complete pain response to palliative radiotherapy. However, no relation could be demonstrated between the SINS (whether continuous or binary) and overall pain response (i.e., complete and partial combined) to radiotherapy. Spinal Instability Neoplastic Score was developed to help identify spinal neoplastic‐related instability, with the main purpose of guiding referrals and improving communication rather than providing a prognostic tool for treatment outcome. As necessary for a referral tool, the SINS score includes both components quantifying the present degree of spinal instability (e.g., spinal malalignment) as well as components reflecting the future risk of spinal instability (e.g., lytic aspect of the lesion). This decreases, however, the applicability of the SINS as a prediction tool. Translating the results of the current study in clinical practice, patients with a low SINS score indicating no spinal instability can effectively be treated with palliative conventional external beam radiotherapy. However, it is advised that patients with a SINS score of 7 or higher be referred to a spinal surgeon to evaluate if surgical intervention is indicated as currently recommended by the SINS [15]. Although the majority of these patients will achieve a (partial) response after radiotherapy, some patients might benefit more from surgical intervention, whether or not combined with postoperative irradiation, as radiation therapy outcomes in these patients is less predictable. Future studies should be directed at optimizing the definition of spinal neoplastic‐related (in)stability if it is to be used as a tool to predict treatment outcome.
†Contributed equally
Author Contributions
Conception/design: Anne L. Versteeg, Joanne M. van der Velden, Helena Verkooijen, Cumhur Oner, Jorrit‐Jan Verlaan
Provision of study material or patients: Anne L. Versteeg, Charles Fisher, Marco van Vulpen, Lorna Weir
Collection and/or assembly of data: Anne L. Versteeg, Joanne M. van der Velden
Data analysis and interpretation: Anne L. Versteeg, Joanne M. van der Velden, Helena Verkooijen, Edward Chow, Jorrit‐Jan Verlaan
Manuscript writing: Anne L. Versteeg, Joanne M. van der Velden, Helena Verkooijen, Charles Fisher, Edward Chow, Cumhur Oner, Marco van Vulpen, Lorna Weir, Jorrit‐Jan Verlaan
Final approval of manuscript: Anne L. Versteeg, Joanne M. van der Velden, Helena Verkooijen, Charles Fisher, Edward Chow, Cumhur Oner, Marco van Vulpen, Lorna Weir, Jorrit‐Jan Verlaan
Disclosures
Charles Fisher: Medtronic, Nuvasive (C/A), Orthopaedic Research and Education Foundation (RF), AOSpine, Medtronic (Other: Fellowship support paid to institution). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Hayat MJ, Howlader N, Reichman ME et al. Cancer Statistics, Trends, and Multiple Primary Cancer Analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. The Oncologist 2007;12:20–37. [DOI] [PubMed] [Google Scholar]
- 2. Harel R, Angelov L. Spine metastases: Current treatments and future directions. Eur J Cancer 2010;46:2696–2707. [DOI] [PubMed] [Google Scholar]
- 3. Poon M, Zeng L, Zhang L et al. Incidence of skeletal‐related events over time from solid tumour bone metastases reported in randomised trials using bone‐modifying agents. Clin Oncol (R Coll Radiol) 2013;25:435–444. [DOI] [PubMed] [Google Scholar]
- 4. Henry DH, Costa L, Goldwasser F et al. Randomized, Double‐Blind Study of Denosumab Versus Zoledronic Acid in the Treatment of Bone Metastases in Patients With Advanced Cancer (Excluding Breast and Prostate Cancer) or Multiple Myeloma. J Clin Oncol 2011;29:1125–1132. [DOI] [PubMed] [Google Scholar]
- 5. Weinfurt KP. The significance of skeletal‐related events for the health‐related quality of life of patients with metastatic prostate cancer. Ann Oncol 2005;16:579–584. [DOI] [PubMed] [Google Scholar]
- 6. Chow E, Zeng L, Salvo N et al. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol) 2012;24:112–124. [DOI] [PubMed] [Google Scholar]
- 7. Arcangeli G, Giovinazzo G, Saracino B et al. Radiation therapy in the management of symptomatic bone metastases: The effect of total dose and histology on pain relief and response duration. Int J Radiat Oncol Biol Phys 1998;42:1119–1126. [DOI] [PubMed] [Google Scholar]
- 8. Hird A, Chow E, Yip D et al. After radiotherapy, do bone metastases from gastrointestinal cancers show response rates similar to those of bone metastases from other primary cancers? Curr Oncol 2008;15:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirou‐Mauro A, Hird A, Wong J et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys 2008;71:1208–1212. [DOI] [PubMed] [Google Scholar]
- 10. Mitera G, Probyn L, Ford M et al. Correlation of Computed Tomography Imaging Features with Pain Response in Patients with Spine Metastases After Radiation Therapy. Int J Radiat Oncol Biol Phys 2011;81:827–30. [DOI] [PubMed] [Google Scholar]
- 11. Nguyen J, Chow E, Zeng L et al. Palliative response and functional interference outcomes using the brief pain inventory for spinal bony metastases treated with conventional radiotherapy. Clin Oncol (R Coll Radiol) 2011;23:485–491. [DOI] [PubMed] [Google Scholar]
- 12. Zeng L, Chow E, Zhang L et al. Comparison of pain response and functional interference outcomes between spinal and non‐spinal bone metastases treated with palliative radiotherapy. Support Care Cancer 2012; 20:633–639. [DOI] [PubMed] [Google Scholar]
- 13. Westhoff PG, de Graeff A, Monninkhof EM et al. Quality of life in relation to pain response to radiotherapy for painful bone metastases. Int J Radiat Oncol Biol Phys 2015;19:1–22. [DOI] [PubMed] [Google Scholar]
- 14. Laufer I, Sciubba DM, Madera M et al. Surgical management of metastatic spinal tumors. Cancer Control 2012;19:122–128. [DOI] [PubMed] [Google Scholar]
- 15. Fisher CG, DiPaola CP, Ryken TC et al. A novel classification system for spinal instability in neoplastic disease: An evidence‐based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010;22:E1221–1229. [DOI] [PubMed] [Google Scholar]
- 16. Huisman M, Van der Velden JM, van Vulpen M et al. Spinal instability as defined by the spinal instability neoplastic score is associated with radiotherapy failure in metastatic spinal disease. Spine J 2014;14(12):2835–40. [DOI] [PubMed] [Google Scholar]
- 17. Lam TC, Uno H, Krishnan M et al. Adverse Outcomes After Palliative Radiation Therapy for Uncomplicated Spine Metastases: Role of Spinal Instability and Single‐Fraction Radiation Therapy. Int J Radiat Oncol Biol Phys 2015;93(2):373–81. [DOI] [PubMed] [Google Scholar]
- 18. Kirshblum SC, Burns SP, Biering‐Sorensen F et al. International standards for neurological classification of spinal cord injury (Revised 2011). J Spinal Cord Med 2011; 34:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. von Elm E, Altman DG, Egger M et al. STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 20. Chow E, Hoskin P, Mitera G et al. International Bone Metastases Consensus Working Party. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys 2012;5:1730–1737. [DOI] [PubMed] [Google Scholar]
- 21. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 22. Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer 1980;45:2220–2224. [DOI] [PubMed] [Google Scholar]
- 23. Fourney DR, Frangou EM, Ryken TC et al. Spinal Instability Neoplastic Score: An Analysis of Reliability and Validity From the Spine Oncology Study Group. J Clin Oncol 2011;29:3072–3077. [DOI] [PubMed] [Google Scholar]
- 24. Versteeg AL, van der Velden JM, Verkooijen HM et al. The Effect of Introducing the Spinal Instability Neoplastic Score in Routine Clinical Practice for Patients With Spinal Metastases. The Oncologist 2016;21(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]