Disparities in breast cancer care in the U.S. are multifactorial, arising from differences in income, education, cultural beliefs, and social support. This article describes the social networks of newly diagnosed breast cancer patients and explores the contributing role of patient navigators.
Keywords: Breast cancer, Cancer health disparities, Patient navigation, Social networks, Social support
Abstract
Background.
Minority women in the U.S. continue to experience inferior breast cancer outcomes compared with white women, in part due to delays in care delivery. Emerging cancer care delivery models like patient navigation focus on social barriers, but evidence demonstrating how these models increase social capital is lacking. This pilot study describes the social networks of newly diagnosed breast cancer patients and explores the contributing role of patient navigators.
Materials and Methods.
Twenty‐five women completed a one hour interview about their social networks related to cancer care support. Network metrics identified important structural attributes and influential individuals. Bivariate associations between network metrics, type of network, and whether the network included a navigator were measured. Secondary analyses explored associations between network structures and clinical outcomes.
Results.
We identified three types of networks: kin‐based, role and/or affect‐based, or heterogeneous. Network metrics did not vary significantly by network type. There was a low prevalence of navigators included in the support networks (25%). Network density scores were significantly higher in those networks without a navigator. Network metrics were not predictive of clinical outcomes in multivariate models.
Conclusion.
Patient navigators were not frequently included in support networks, but provided distinctive types of support. If navigators can identify patients with poorly integrated (less dense) social networks, or who have unmet tangible support needs, the intensity of navigation services could be tailored. Services and systems that address gaps and variations in patient social networks should be explored for their potential to reduce cancer health disparities.
Implications for Practice.
This study used a new method to identify the breadth and strength of social support following a diagnosis of breast cancer, especially examining the role of patient navigators in providing support. While navigators were only included in one quarter of patient support networks, they did provide essential supports to some individuals. Health care providers and systems need to better understand the contributions of social supports both within and outside of health care to design and tailor interventions that seek to reduce health care disparities and improve cancer outcomes.
Introduction
Racial and ethnic minority women in the U.S. continue to experience inferior breast cancer outcomes compared with white women [1]. Studies show that black and low‐income women are less likely to complete cancer treatment, suggesting health care delivery models may not be responsive to their needs or social context [2]. As a result, the gap in mortality rates between advantaged and disadvantaged segments of the U.S. population is widening [3], [4], [5], [6]. Persistence of these disparities cannot be attributed solely to racial differences in disease characteristics. Evidence is clear that reasons for disparities are multifactorial, arising from differences in income, education, cultural beliefs, and social support [7], [8].
Low social capital among individuals is a known contributor to poor health outcomes, likely playing a role in racial and ethnic health disparities [9], [10]. Social capital is the resources imbedded in social networks that can be accessed through ties between members [11]. Research shows that social networks can promote participation in health care and/or healthy behaviors [12], [13], [14]. Being socially connected to those who can provide different types of support ensures a stable social network [15]. There is a robust literature on how elements of social support like marital status, social integration, and social network size contribute to cancer outcomes [16], [17], [18], [19].
Delivery models to improve disparities in cancer care and outcomes related to increasing social capacity have been developed. One such model is patient navigation, which uses lay health workers integrated in the healthcare team to target disadvantaged patients across a defined episode of cancer care [20]. The goal of navigation is to reduce delays in care by identifying and addressing patient barriers within and beyond the health care system. Patient navigation targeting low‐income, minority patients have demonstrated an impact on receiving timely diagnosis and initiation of care [21], [22], [23], [24], [25], [26], contributing to the widespread dissemination of navigation [27], [28]. While patient navigation shows promise, research demonstrates that there is variation in how navigation is practiced across the country, including models using both lay and professional (i.e., nurse) navigator models [29]. In addition, research on the impact of navigation throughout the course of treatment has demonstrated mixed results [30]. These factors complicate the assessment of the impact of patient navigation on individual patients. One approach to understanding the mechanism of effect of navigation is to explore how navigators and the health care team are integrated into the social networks of patients to provide support during cancer treatment. However, there is little evidence documenting how the navigator role increases social capital. Therefore, the purpose of this exploratory study is to describe the social networks of newly diagnosed breast cancer patients undergoing treatment and explore the contributing role of patient navigators.
Materials and Methods
We implemented a social network study among a subset of breast cancer patients enrolled in an active randomized‐controlled trial (RCT) comparing two forms of navigation. The RCT compares clinical and patient‐reported outcomes between newly diagnosed breast cancer patients receiving institutional standard navigation (control) versus those whose patient navigator collaborates with legal advocates to address health‐related social needs (intervention). Women seeking care at an urban, safety‐net hospital with a new diagnosis of breast cancer are approached within 30 days of diagnosis and invited to participate in the study, randomized to receive one of the two modes of navigation above, and participate in the collection of survey and clinical data at diagnosis, and then 3, 6, and 12 months later. Both intervention and control navigators are lay navigators, responsible for reaching out to patients via phone or in person within one week of study enrollment, identifying barriers to care, and providing referrals and resources to address stated barriers during the course of treatment. The intervention navigator is further trained to assess health‐related social needs and, when needed, collaborate with legal advocates to ameliorate these barriers.
This study attempts to understand, from the patient perspective, who patients rely on for help in obtaining treatment, how the navigator may be integrated into the patient support network, and how these networks impact clinical outcomes. Participants were eligible if they were enrolled in the parent RCT and completed baseline surveys, spoke English or Spanish, and agreed to an interview 3–7 months after diagnosis. Over 7 months, a convenience sample of 31 consecutive patients who met the above criteria were invited for an in‐person interview about their social network, 25 of whom agreed (81% response rate). Once the target sample of 25 interviews had been conducted, we ceased recruitment. The interview elicited information from the patient (“ego”) about who she relied upon for care, advice, and support during cancer treatment [31]. Participants generated names of all of the people who made up their social networks (“alters”) using an open‐ended approach [32]. Participants then provided basic demographic and background information about alters, described how they knew alters, and how often they communicated with each. This interview approach provides a more comprehensive picture of social networks through discussion and collects detailed information about alters’ contributions to illness‐relevant tasks, in comparison to the use of a structured survey [14].
Variable Specification
Social Network Analysis (SNA) allows for the nature of social ties to vary between individuals and includes using network‐specific metrics to assess the influence of individuals within networks. This study sought to describe patient networks using four network metrics: density, diversity, load, and knowledge exclusivity.
Network density measured the number of direct ties among alters to whom the patient is connected, divided by the number of theoretical connections possible among these ties [32], [33]. Density essentially indicates how closely knit the support network of patients is (range: 0–1). A strength of SNA is that it allows for the analysis of not only individuals within the network, but also the types of support they provide. Examining two‐mode network graphics, modeling both the individuals within the network along with the types of support (e.g., emotional/moral, household, access to services), we were able to measure diversity, load, and knowledge exclusivity.
Diversity determined the distribution of different types of support, measuring whether support was equally distributed across the network (range: 0–1). Load is the average number of supports provided per person in the network. Knowledge exclusivity, described here as support exclusivity, represented the connection of alters who provided types of support that were shared by no or few others. For example, if a spouse was the only person in the network providing personal care and transportation support, then he or she may have the highest support exclusivity. We identified the individual within each network with the highest support exclusivity.
In a secondary analysis, we linked network data to treatment outcomes using clinical data abstracted from the medical record. Clinical data included the patient's age, race, insurance, type of cancer, stage at diagnosis, type of treatment, and dates of treatment. The clinical outcome of time to initiation of primary cancer treatment was used as an exploratory outcome metric. Using the date of diagnosis and the date of first treatment, the number of days to initiation of treatment was calculated. We also categorized treatment as timely when initiated within 90 days of diagnosis, as delays beyond this have been shown to affect mortality outcomes [34].
Analysis
Relationships (“ties”) between individuals, types of support provided, and demographics were compiled directly from audio‐recordings independently by two trained research assistants and entered into a database. Using two independent coders to translate data from narrative to discrete variables enhanced reliability. Any discrepancies in interpretation were resolved through a consensus process between the principal investigator and coders. All networks were binary, meaning ties were considered either present and/or absent (nondirectional), and were not weighted in the analysis.
Network analysis was conducted using ORA Net‐Scenes, a software package designed for the analysis of social network data [35]. Preliminary analysis consisted of visualizing network structures in ORA. Because we were interested in the types of individuals that comprised support networks, each network was visualized by coloring nodes by relation to the ego. We used three broad categories of social relations: (a) kinship ties, which included family members; (b) role‐based ties, including those connected to the ego through a specific role, such as pastor or doctor; and (c) affective ties, which were relationships based on feelings (i.e., friends) [36]. We excluded perceptual ties, meaning, statements like “Samantha knows of Jane,” as these were not meaningful ties when probed further. In conducting interviews, women named groups, such as breast cancer support groups, as a source of support. Therefore, a fourth type of relation was added to include ties to groups. After coloring the tie types, four members of the research team independently examined networks for possible patterns, qualitatively classifying networks into typologies.
The primary analysis used network metrics generated in ORA to quantify important structural attributes and influential individuals using the network variables described above (density, diversity, load, and support exclusivity). Bivariate associations between network metrics and the type of network and whether the network included a navigator were measured. A secondary analysis explored associations between network structures and time to initiation of cancer treatment using Chi‐Square (adjusting with Fisher's Exact test to account for the small sample size) or analysis of variance (with Tukey's Studentized Range), as appropriate. In order to account for the intensity of navigation services provided, we also examined associations between the number of contacts a navigator had with a participant prior to the interview (via phone or in person) and outcomes. Statistical analyses were performed using SAS version 9.3.
Results
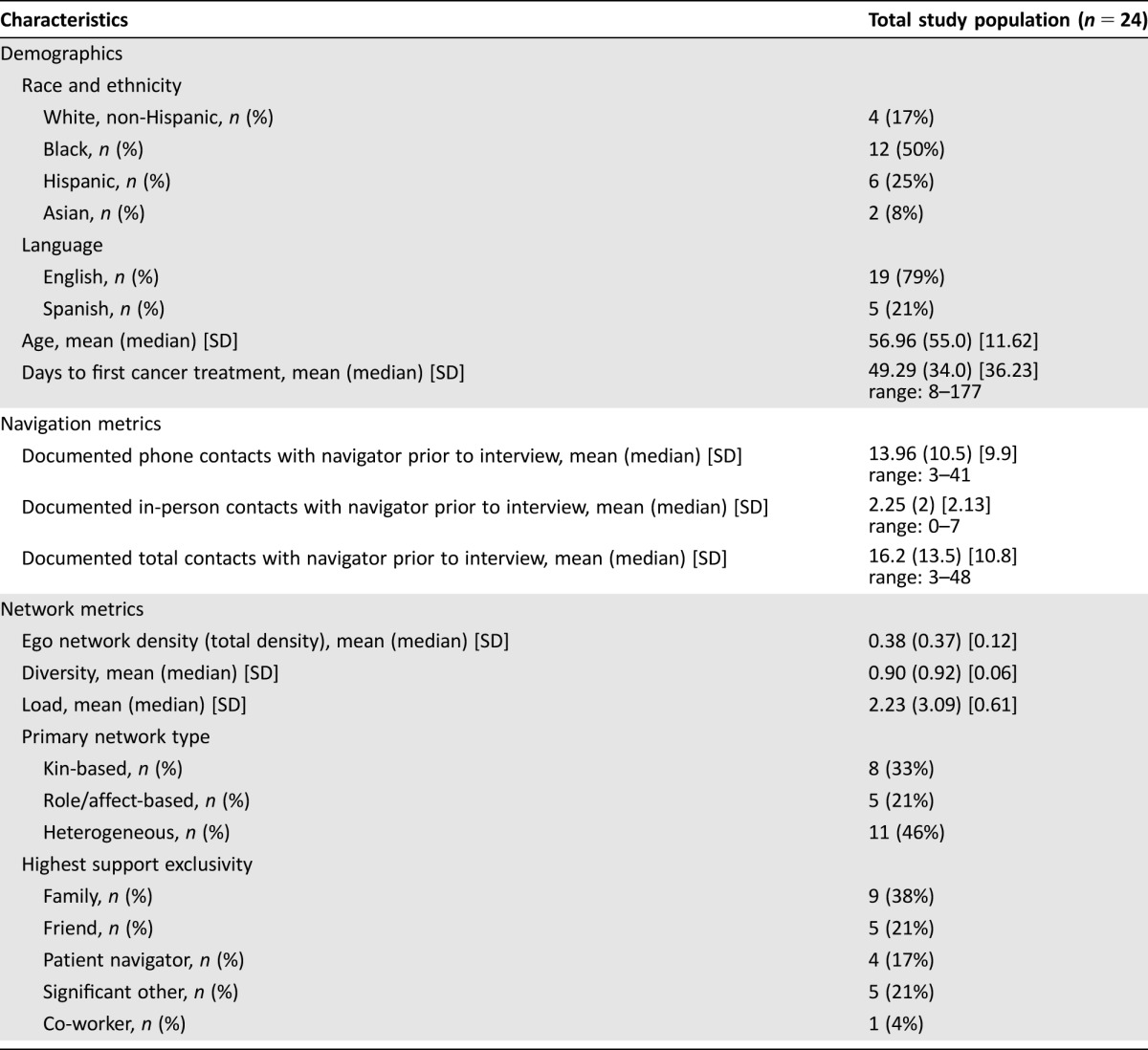
Twenty‐four women completed social network interviews. One interview was partially completed and excluded from further analyses. Nineteen interviews were conducted in English and five in Spanish. Interviews were conducted, on average, 171 days after diagnosis (range: 93–284). Table 1 displays the demographic profile of sampled women. Mean time from diagnosis to first cancer treatment was 49.3 days (range: 8–177 days). All participants had at least three contacts with a navigator between the time of diagnosis and the social network interview.
Table 1. Characteristics of study sample.
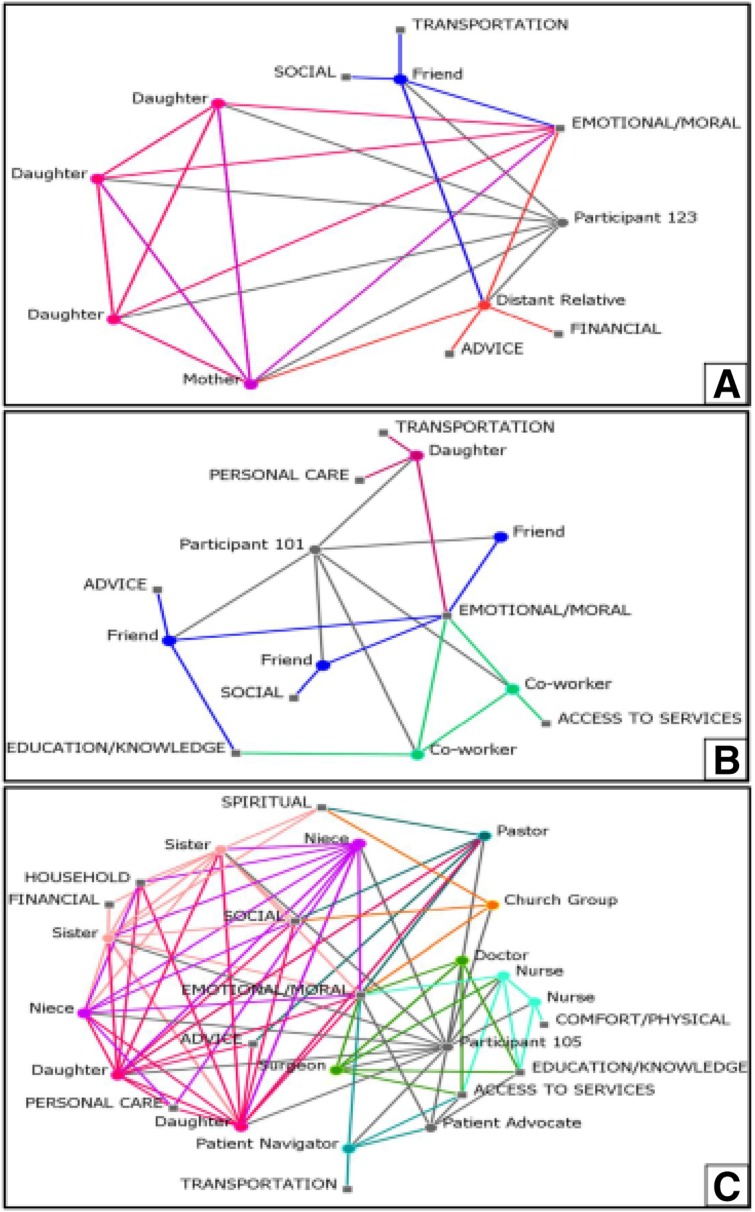
Network Types
Qualitatively examining network structures identified three broad types, based on the predominant type of ties that comprised patient support networks. The network graphs in Figure 1 are examples of each network type and display the individuals within the patient network and the types of support provided. Kin‐based networks had family members as the main source of support during cancer treatment (Fig. 1A, n = 8). Role and/or affect‐based networks involved women relying mainly on friends, colleagues, and/or health care workers for support (Fig. 1B, n = 5). Finally, heterogeneous networks were characterized by the presence of all three tie types, and were often larger (Fig.1C, n = 11).
Figure 1.
Sample patient social networks by network type.
Network Metrics
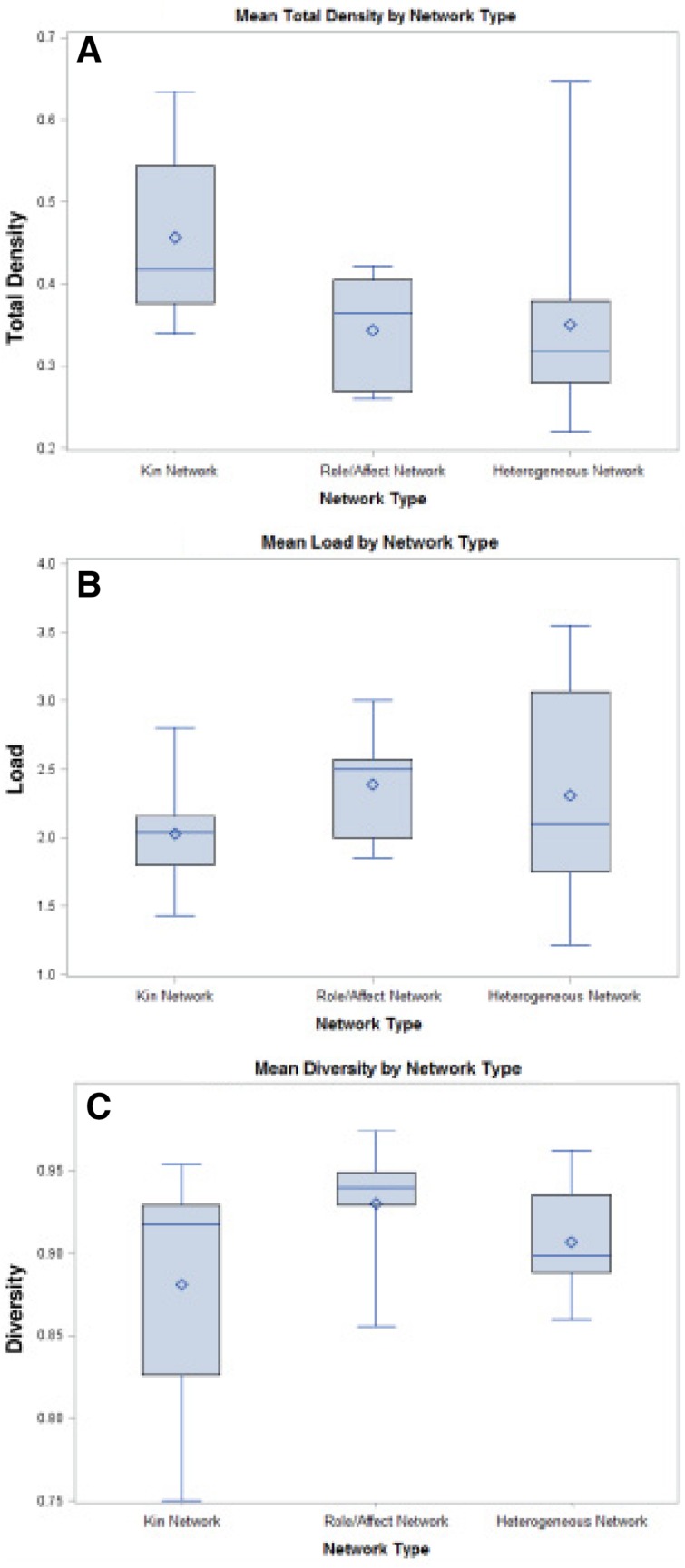
Primary analyses sought to describe the networks using four network metrics. The boxplots (Fig. 2) display the median values (middle bar), 25th, and 27th percentile (lower and upper bounds of boxes), and range (bars) for total density, load, and diversity. The diamond in the box represents the mean value.
Figure 2.
Network metrics by patient network type.
First, total network density assessed the average structural density of networks within each qualitative network type. Kin networks had the highest density at 0.46, meaning 46% of possible ties were actually connected. This is unsurprising, as we would expect networks comprised of family members to have more connections between alters than those made of diverse and nonfamilial ties. However, no significant differences in mean total density between groups were identified (p = .09). Second, load was used to describe the average number of supports each person provided across the network. The average load was 2.03 (SD = .41) types of support per alter in kin networks, 2.39 (SD = .46) in role and/or affect networks, and 2.31 (SD = .78) in heterogeneous networks, suggesting no significant differences across network types (p = .52). Diversity measured the distribution of different types of support (i.e., household, emotional and/or moral, personal care) within the network, gauging whether support was equally distributed. Diversity did not differ between network types (p = .33). Emotional and moral support was the most common support type mentioned across all networks and was distributed across many members of the network.
Navigator Role
Navigators were mentioned by participants in only 6 of the 24 interviews (25%). When navigators were mentioned, they often contributed exclusive types of support to patients. In four of the six networks where navigators were mentioned (67%), they represented the role with the highest support exclusivity. The unique types of support that navigators provided included: transportation, access to services, and help with paperwork. Examining network metrics by the presence of a navigator within the network, we found that only total network density varied (p = .0093) between those with and without navigators mentioned as part of their social network. The mean difference in density was 0.137 (95% confidence interval [CI]: 0.037–0.236) using Tukey's Studentized Rand test, with patient networks without navigators mentioned displaying higher density than those with navigators.
We further examined the relationship between number of contacts (phone, in person) and salient network metrics (navigator presence in network, density). In‐person contacts with the navigator did not significantly predict whether a navigator was mentioned as part of the social network. The difference in the mean number of in‐person contacts between those with and without navigators in the network was 1.89 contacts (95% CI: −0.07, 3.85, p = .06). Conversely, higher numbers of phone contacts were associated with including a navigator in one's network. The difference in the mean number of phone contacts between those with and without navigators in the network was 10.5 (95% CI: 1.76, 19.24, p = .02). However, this may have been driven by outliers with very high numbers of contacts and support provided being more likely to mention navigators within their networks. No significant associations were found between total network density and contacts with the navigator.
Network Metrics and Outcomes
In secondary analyses, the qualitative network types (kin, affective, heterogeneous) identified had no statistically significant relationship with timeliness of treatment, measured dichotomously (yes = received within 90 days) (p = .79). Looking at the distribution of the continuous time to treatment variable, the data were not distributed normally. Transforming the variable using the natural log resulted in a normal distribution. In a multivariate linear regression model including age, network type, load, density, diversity, and patient race, the model was not statistically significant in predicting time to treatment (Model R2 = 0.52, p = .18), nor were individual associations. Given the small sample size and exploratory nature of these analyses, models are likely underpowered.
Discussion
This exploratory pilot study describing the social networks of breast cancer patients and the contributing role of patient navigators revealed a heterogeneity of support structures among a diverse sample of women. Many of the participants described emotional and moral support as the most common type of support provided, and it was often provided by many alters within networks. We found a low prevalence of navigators included in the support networks (25%), particularly given that all participants were assigned a patient navigator at the time of diagnosis. Furthermore, network density scores were higher in those networks without a navigator, suggesting that navigators played a larger role in supporting women with less well‐integrated social support networks.
The varying social network structures identified in this study suggest there may be identifiable patterns of support that contribute to patient outcomes, which our study was not designed to identify. Others have shown that the degree to which individuals are socially isolated or integrated contributes to health‐related quality of life measures [37], [38], treatment choices [39], and, ultimately, mortality [40]. This study goes beyond scaled, unidimensional measures of social isolation to identify potential patterns of network structures and influential types of ties. While further research is required to understand how these network types, among others, might contribute to outcomes, they provide a more comprehensive picture of social support during cancer treatment.
Our findings further suggest relatively diverse types of supports are distributed across many individuals in a network. Efforts focused narrowly on patient‐caregiver dyads [41], [42] (e.g., a spouse or adult child) may miss the broader context and complexity of a patient's lay caregiving team that contributes diverse types of support. Participants in this study most frequently identified emotional and/or moral support (e.g., listening, talking, comforting) as spread widely across network members, consistent with other research in breast cancer. Kroenke et al. ([19]) found that emotional support was important to women, but, importantly, that it only impacted social and emotional well‐being, and not physical or clinical outcomes [37]. Tangible supports, however, were associated with physical quality of life, particularly among women with later stage disease. These findings point to the importance of having multiple individuals in one's social network that can provide tangible supports (i.e., house assistance) to help one achieve positive health outcomes.
Prior to this study, we expected that patient navigators would be a prominent source of such tangible support, given their mandate is to reduce barriers to care and help women complete cancer treatment. Our findings did not directly support this hypothesis since only one quarter of participants included their patient navigator as a source of support during cancer treatment. Reasons for the low prevalence of navigators within networks are speculative, but could include participants having little contact with navigators, as we found a significant association between the number of phone contacts with the navigator and inclusion of a navigator within the network. The small sample size precludes a more nuanced analysis of the number and quality of navigator contacts required before a participant includes them as part of their social support network. Examining how the intensity of navigation services influences perceived social support and interacts with network density is an important area for future study.
Importantly, when navigators were mentioned, they were often instrumental in providing a type of support that was not included elsewhere within the network. These included supports such as transportation, access to services, or assistance with paperwork. These types of tangible supports that facilitate coordinating complex cancer treatments were unique to navigators and valued by patients who required them, as others have described [43]. However, further work is required to characterize the interpersonal relationships formed between navigators, patients, and other network members to understand the mechanism by which navigator support can influence cancer care outcomes [44].
Finally, this study observed higher network density among those networks without navigators versus those with navigators. This suggests that there may be opportunities to tailor navigation services based on an assessment of social networks if the health system, or navigators themselves [45], can identify patients who may have poorly integrated (less dense) social networks or unmet tangible support needs. If such a screening tool could identify those with less dense networks and/or higher social needs, navigators could target such patients earlier to provide tailored services in a more efficient manner. Alternatively, navigators could offer a less‐intensive intervention to those with robust social networks, ensuring the navigator's time is focused on those with fewer social resources. These strategies would maximize the impact of navigators by providing additional time for those with the highest needs, while helping to maintain a manageable case load.
This study has several limitations. Given the pilot nature of the study, there was limited power to detect a difference in clinical outcomes. Further, as patients were part of a larger RCT, patients may have received a different level of navigation depending upon which arm of the RCT they were randomized to. Employing a qualitative, open‐ended approach to eliciting social networks was useful in identifying a broad range of social supports, but it may have influenced who was included in networks. For example, we did not prompt participants to include health care workers, including navigators, and these roles may have not been conceptualized as part of an individual's “social network.” We attempted to mitigate this by asking women to include all “people who you really count on for physical and emotional support and/or advice with your cancer care.” Finally, we did not assess whether the individuals and types of support met all of patient's needs, or whether some types of support were lacking. Unmet needs and/or support provided that is not aligned with patient needs are both important contributors to breast cancer outcomes [46], and may have influenced results.
Conclusion
We demonstrated the feasibility of collecting social network data to assist in identifying the contributing role of patient navigators in providing social support during breast cancer treatment. While navigators were not included in all networks, they did provide essential supports to some individuals. Further research on understanding how navigators are integrated in to social networks and how to tailor navigation to patient needs shows promise. The contributions of social supports both within and outside of health care require further attention to better design and tailor interventions that seek to reduce health care disparities and improve cancer outcomes.
Author Contributions
Conception/design: Christine Marie Gunn, Victoria A Parker, Sharon M Bak
Provision of study material or patients: Tracy A Battaglia
Collection and/or assembly of data: Christine Marie Gunn, Sharon M Bak
Data analysis and interpretation: Christine Marie Gunn, Victoria A Parker, Naomi Ko, Kerrie P Nelson, Tracy A Battaglia
Manuscript writing: Christine Marie Gunn, Victoria A Parker, Sharon M Bak, Naomi Ko, Kerrie P Nelson, Tracy A Battaglia
Final approval of manuscript: Christine Marie Gunn, Victoria A Parker, Sharon M Bak, Naomi Ko, Kerrie P Nelson, Tracy A Battaglia
Disclosures
The authors indicated no financial relationships.
References
- 1. Hunt BR, Whitman S, Hurlbert MS. Increasing black:white disparities in breast cancer mortality in the 50 largest cities in the United States. Cancer Epidemiol 2014;38:118–123. [DOI] [PubMed] [Google Scholar]
- 2. Hershman D, McBride R, Jacobson JS et al. Racial disparities in treatment and survival among women with early‐stage breast cancer. J Clin Oncol 2005;23:6639–6646. [DOI] [PubMed] [Google Scholar]
- 3. Elmore JG, Nakano CY, Linden HM et al. Racial inequities in the timing of breast cancer detection, diagnosis, and initiation of treatment. Med Care 2005;43:141–148. [DOI] [PubMed] [Google Scholar]
- 4. Maly RC, Umezawa Y, Ratliff CT et al. Racial/ethnic group differences in treatment decision‐making and treatment received among older breast carcinoma patients. Cancer 2006;106:957–965. [DOI] [PubMed] [Google Scholar]
- 5. Mason C, Yokubaitis K, Howard E et al. Impact of Henda's law on the utilization of screening breast magnetic resonance imaging. Proc (Bay Uni Med Cent) 2015;28:7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hicks LK. Reframing overuse in health care: Time to focus on the harms. J Oncol Pract 2015;11:168–170. [DOI] [PubMed] [Google Scholar]
- 7. DeNavas‐Walt C, Proctor BD, Smith JC. Income, Poverty, and Health Insurance Coverage in the United States: 2008. Available at http://www.census.gov/prod/2009pubs/p60-236.pdf. Accessed April 6, 2017.
- 8. Ell K, Nishimoto R, Mediansky L, et al. Social relations, social support and survival among patients with cancer. Journal Psychosom Res 1992;36:531–541. [DOI] [PubMed] [Google Scholar]
- 9. Petrou S, Kupek E. Social capital and its relationship with measures of health status: Evidence from the Health Survey for England 2003. Health Econ 2008;17:127–143. [DOI] [PubMed] [Google Scholar]
- 10. van Hooijdonk C, Droomers M, Deerenberg IM et al. The diversity in associations between community social capital and health per health outcome, population group and location studied. Int J Epidemiol 2008;37:1384–1392. [DOI] [PubMed] [Google Scholar]
- 11. Lin N. Social capital: A theory of structure and action. New York: Cambridge University Press; 2001. [Google Scholar]
- 12. Thoits PA. Mechanisms linking social ties and support to physical and mental health. J Health Soc Behav 2011;52:145–161. [DOI] [PubMed] [Google Scholar]
- 13. Pescosolido BA. Beyond rational choice: The social dynamics of how people seek help. Am J Sociol 1992;97:1096–1138. [Google Scholar]
- 14. Vassilev I, Rogers A, Blickem C, et al. Social networks, the ‘work' and work force of chronic illness management: A survey analysis of personal communities. PLoS One 2013;8:e59723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wellman B, Wortley S. Different strokes from different folks: Community ties and social support. Am J Sociol 1990;96:558–588. [Google Scholar]
- 16. Allen JD, Sorensen G, Stoddard AM et al. The relationship between social network characteristics and breast cancer screening practices among employed women. Ann Behav Med 1999;21(3):193–200. [DOI] [PubMed] [Google Scholar]
- 17. Yang YC, Li T, Frenk SM. Social network ties and inflammation in US adults with cancer. Biodemography Soc Bio 2014;60:21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beasley JM, Newcomb PA, Trentham‐Dietz A et al. Social networks and survival after breast cancer diagnosis. J Cancer Surviv 2010;4:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kroenke CH, Quesenberry C, Kwan ML et al. Social networks, social support, and burden in relationships, and mortality after breast cancer diagnosis in the Life After Breast Cancer Epidemiology (LACE) Study. Breast Cancer Res Treat 2013;137:261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Freeman H, Muth B, Kerner J. Expanding access to cancer screening and clinical follow‐up among the medically underserved. Cancer Pract 1995;3:19–30. [PubMed] [Google Scholar]
- 21. Battaglia TA, Bak SM, Heeren T et al. Boston Patient Navigation Research Program: The impact of navigation on time to diagnostic resolution after abnormal cancer screening. Cancer Epidemiol Biomarkers Prev 2012;21:1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Battaglia TA, Santana MC, Bak S et al. Predictors of timely follow‐up after abnormal cancer screening among women seeking care at urban community health centers. Cancer 2010;116:913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Battaglia TA, Roloff K, Posner MA et al. Improving follow‐up to abnormal breast cancer screening in an urban population. A patient navigation intervention. Cancer 2007;109(suppl 6):359–367. [DOI] [PubMed] [Google Scholar]
- 24. Phillips CE, Rothstein JD, Beaver K et al. Patient navigation to increase mammography screening among inner city women. J Gen Intern Med 2011;26:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Battaglia TA, Burhansstipanov L, Murrell SS et al. Assessing the impact of patient navigation: Prevention and early detection metrics. Cancer 2011;117(suppl 15):3553–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clark JA, Parker VA, Battaglia TA et al. Patterns of task and network actions performed by navigators to facilitate cancer care. Health Care Manage Rev 2013; 39:90–101. [DOI] [PubMed] [Google Scholar]
- 27. Eng E. The Save our Sisters Project. A social network strategy for reaching rural black women. Cancer. 1993;72(S3):1071–1077. [DOI] [PubMed] [Google Scholar]
- 28.Commission On Cancer. Cancer Program Standards 2012: Ensuring Patient‐Centered Care. Available at https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed on April 3, 2013.
- 29. Gunn C, Clark J, Battaglia T et al. An assessment of patient navigator activities in breast cancer patient navigation programs using a nine‐principle framework. Health Serv Res 2014;49:1555–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fiscella K, Whitley E, Hendren S et al. Patient navigation for breast and colorectal cancer treatment: A randomized trial. Cancer Epidemiology Biomarkers Prev 2012;21:1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hanneman R, Riddle M. Introduction to social network methods. Available at http://faculty.ucr.edu/~hanneman/nettext/Introduction_to_Social_Network_Methods.pdf Accessed July 31, 2014.
- 32. Borgatti SP, Mehra A, Brass DJ et al. Network analysis in the social sciences. Science 2009;323:892–895. [DOI] [PubMed] [Google Scholar]
- 33. Burt R. Social contagion and innovation: Cohesion versus structural equivalence. Am J Sociol 1987;92:1287–1335. [Google Scholar]
- 34. Richards M, Westcombe A, Love S et al Influence of delay on survival in patients with breast cancer: A systematic review. Lancet 1999;353:1119–1126. [DOI] [PubMed] [Google Scholar]
- 35.ORA NetScenes [computer program]. Version 3.0.8.5: Center for Computational Analysis of Social and Organizational Systems (CASOS), Institute for Software Research International (ISRI), School of Computer Science. Pittsburgh, PA: Carnegie Mellon University; 2013.
- 36. Borgatti SP, Brass DJ, Halgin DS. Social Network Research: Confusions, Criticisms, and Controversies. In: D.J. Brass GL, A. Mehra, D.S. Haglin et al., eds. Research in the Sociology of Organizations. Vol 40. Bradford, United Kingdom: Emerald Publishing; 2014.
- 37. Kroenke CH, Kwan ML, Neugut AI et al. Social networks, social support mechanisms, and quality of life after breast cancer diagnosis. Breast Cancer Res Treat 2013;139:515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Michael YL, Berkman LF, Colditz GA et al. Social networks and health‐related quality of life in breast cancer survivors: A prospective study. J Psychosom Res 2002;52:285–293. [DOI] [PubMed] [Google Scholar]
- 39. Kroenke CH, Michael YL, Shu X‐O et al. Post‐diagnosis social networks, and lifestyle and treatment factors in the After Breast Cancer Pooling Project. Psychooncology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kroenke CH, Kubzansky LD, Schernhammer ES et al. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol 2006;24:1105–1111. [DOI] [PubMed] [Google Scholar]
- 41. Shin DW, Park J‐H, Shim E‐J et al. The development of a comprehensive needs assessment tool for cancer‐caregivers in patient–caregiver dyads. Psychooncology 2011;20:1342–1352. [DOI] [PubMed] [Google Scholar]
- 42. DuBenske LL, Gustafson DH, Namkoong K et al. CHESS improves cancer caregivers’ burden and mood: Results of an eHealth RCT. Health Psychol 2014;33:1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carroll JK, Humiston SG, Meldrum SC et al. Patients’ experiences with navigation for cancer care. Patient Educ Couns 2010;80:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jean‐Pierre P, Cheng Y, Wells KJ et al. Satisfaction with cancer care among underserved racial‐ethnic minorities and lower‐income patients receiving patient navigation. Cancer 2016;122:1060–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carroll JK, Winters PC, Purnell JQ et al. Do navigators’ estimates of navigation intensity predict navigation time for cancer care? J Cancer Educ 2011;26:761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reynolds JS, Perrin NA. Mismatches in social support and psychosocial adjustment to breast cancer. Health Psychol 2004;23:425–430. [DOI] [PubMed] [Google Scholar]