Given the age‐related disparities in cancer care and the expanding role of PD‐1/PD‐L1 inhibitors, it is critical to determine whether patient age impacts the effectiveness and toxicity of these treatments in the geriatric oncology population. In this retrospective study, data from 254 patients, treated at two academic cancer centers with anti‐PD‐1 or PD‐L1 therapy, was analyzed. Progression‐free survival (PFS), overall survival (OS), and immune‐related toxicity was compared between older and younger patients with melanoma.
Keywords: Immunotherapy, Melanoma, Geriatrics, Toxicity
Abstract
Background.
Monoclonal antibodies (mAb) targeting PD‐1/PD‐L1 have revolutionized melanoma treatment, yet data regarding effectiveness and tolerability across age groups is limited. We sought to determine the impact of age on overall survival (OS), progression‐free survival (PFS), and rates of immune‐mediated toxicities in patients treated with anti–PD‐1/anti‐PD‐L1 mAb at two academic medical centers.
Methods.
We retrospectively collected data on all patients with metastatic melanoma treated with anti‐PD‐1/PD‐L1 mAb between May 2009 and April 2015. We used Kaplan‐Meier and Cox regression analyses to assess OS and PFS and identify factors associated with these outcomes. We also compared rates of autoimmune toxicity across age groups.
Results.
Of 254 patients, 57 (22.4%) were <50 years old, 85 (33.5%) were age 50–64, 65 (25.6%) were age 65–74, and 47 (18.5%) were ≥75 years. Across age groups, no differences existed in median OS (age <50: 22.9 months, age 50–64: 25.3 months, age 65–74: 22.0 months, age ≥75: 24.3 months) or PFS (age <50: 4.1 months, age 50–64: 6.5 months, age 65–74: 5.4 months, age ≥75: 7.9 months). The presence of liver metastases and elevated pre‐treatment lactate dehydrogenase (LDH) were associated with reduced OS. Presence of liver metastasis, pretreatment LDH, BRAF mutation, and type of melanoma correlated with PFS. Overall, 110 patients (43.3%) experienced immune‐mediated toxicities; 25 (9.8%) had colitis and 26 (10.2%) had endocrine toxicity. Rates of colitis, hepatitis, and pneumonitis did not differ across age groups.
Conclusion.
We demonstrated that patients could safely tolerate anti‐PD1/PDL‐1 mAb therapy and achieve similar outcomes regardless of their age.
Implications for Practice.
Immunotherapy has revolutionized treatment for patients with metastatic melanoma, yet data are lacking regarding the effectiveness and tolerability of these treatments for older patients. In this study, we demonstrated that patients with melanoma safely tolerate immunotherapy and achieve similar outcomes regardless of their age. Specifically, we utilized data from two academic cancer centers and found no significant difference in overall survival, progression free survival, or immune‐related toxicities, other than arthritis, across age groups. As the population ages, studies such as this will become critical to help us understand how best to treat older adults with cancer.
Introduction
The past 5 years have brought a revolution in the treatment of unresectable and/or metastatic melanoma. In that time, eight agents and three combinations have received regulatory approval. These include the BRAF inhibitors vemurafenib and dabrafenib, the MEK inhibitors trametinib and cobimetinib, the combinations of dabrafenib and trametinib and vemurafenib and cobimetinib, the anti‐cytotoxic T lymphocyte antigen 4 (CTLA‐4) antibody ipilimumab, the two anti‐programmed cell death 1 (PD‐1) antibodies pembrolizumab and nivolumab, the combination of ipilimumab and nivolumab, and the oncolytic viral vaccine talimogene laherparepvec (T‐VEC) [1], [2], [3], [4], [5], [6], [7], [8], [9], [10]. These agents have had a profound impact on patient outcomes, with both single‐agent anti–PD‐1 antibody therapy (in patients independent of BRAF mutation status) and combined BRAF and MEK inhibitor therapy (in patients with BRAF mutations) improving median survival from approximately 6 months [11], [12] to over 2 years [7], [13].
Perhaps the most remarkable advance has been the emergence of monoclonal antibodies targeting PD‐1 and its ligand PD‐L1. These agents show marked efficacy in patients with unresectable or metastatic melanoma, with response rates in the 35%–45% range in first‐line therapy and median overall survival of over 2 years [6], [7], [14]. Further, inhibitors of PD‐1 and PD‐L1 have activity in a number of other malignancies, gaining regulatory approval in non‐small cell lung cancer (nivolumab and pembrolizumab), renal cell carcinoma (nivolumab), and urothelial bladder cancer (atezolizumab) [15], [16], [17]. Immune checkpoint inhibitors have also exhibited impressive activity in triple‐negative breast cancer, gastric cancer, squamous cell carcinoma of head and neck, and Hodgkin lymphoma [18], [19], [20], [21], [22], [23]. Notably, these agents are relatively well tolerated, as evidenced by the low grade 3–4 adverse event rate (10%–15%) [6], [7], [9]. Toxicity is commonly mediated by immune activation triggering tissue‐specific autoimmunity. The most common events are fatigue, rash, pruritus, thyroid dysfunction (both hyper‐ and hypothyroidism), and diarrhea, though more severe immune‐specific adverse events are observed, including colitis, pneumonitis, hepatitis, uveitis, myositis, nephritis, and type 1 diabetes mellitus [7], [9].
Although clinical trials have shown remarkable survival outcomes with relatively mild toxicity profiles for PD‐1/PD‐L1 inhibitors, little research has focused on the impact of these treatments for older cancer patients. Previous studies have demonstrated greater toxicity and decreased tolerability with some systemic chemotherapy regimens for older patients with cancer. For example, breast cancer studies suggest that older patients are more likely to experience toxicity and treatment‐related deaths than younger patients [24], [25]. Evidence suggests that older patients with colon cancer are more likely than younger patients to experience early discontinuation of chemotherapy [26]. Additionally, age has been shown to affect the treatment regimens that patients receive, resulting in undertreatment in older patients [27]. Further, older patients and those with more comorbid conditions are often underrepresented in oncology clinical trials, thus limiting the data available to assess these patients’ tolerance to certain therapies [28], [29], [30], [31].
Given the age‐related disparities in cancer care and the expanding role of PD‐1/PD‐L1 inhibitors, it is critical to determine whether patient age impacts the effectiveness and toxicity of these treatments in the geriatric oncology population. In this retrospective study, we sought to analyze the data from 254 patients treated at two academic cancer centers with anti‐PD‐1 or PD‐L1 therapy and compared progression‐free survival (PFS), overall survival (OS), and immune‐related toxicity between older and younger patients with melanoma.
Methods
Patient Population and Study Design
We retrospectively collected data on all patients with metastatic melanoma treated with anti–PD‐1 and/or PD‐L1 monoclonal antibodies at Massachusetts General Hospital (MGH) and Vanderbilt University Medical Center from May 2009 to April 2015. The Partners Institutional Review Board approved the study protocol.
We obtained data from the electronic medical record regarding patients’ sociodemographic and clinical characteristics. Specifically, we collected information about patients’ age, sex, education, marital status, and employment. We also assessed patients’ Eastern Cooperative Oncology Group (ECOG) performance status and comorbid conditions in order to calculate Charlson Comorbidity Index (CCI) scores at the time of first presentation to an oncologist regarding their melanoma. Additionally, we collected patients’ laboratory values, cancer mutation status, sites of metastases, and cancer therapy, including whether they had received ipilimumab before or after their anti–PD‐1 or PD‐L1 therapy. We obtained the date of cancer progression (using documentation from the medical record by the treating clinician of disease progression), death date, and date of last follow‐up for those still alive. We assessed whether patients had experienced the following toxicities: colitis, hypophysitis, pneumonitis, thyroiditis, dermatitis, duodenitis, gastritis, nephritis, meningitis, encephalitis, uveitis, myocarditis, pericarditis, arthritis, and hepatitis. We also evaluated the combined endpoint of endocrine‐related toxicities, defined as the combination of hypophysitis and thyroiditis.
Statistical Analysis
We first calculated descriptive statistics to analyze the frequencies, means, and SDs of the study variables stratified by age (age <50 years, age 50–64 years, age 65 to 74 years, and age ≥75 years). Progression‐free survival was defined as the time from initiation of anti–PD‐1 or PD‐L1 therapy until date of progression or death, whichever occurred earlier. Overall survival was defined as the time from initiation of PD‐1 therapy until date of death from any cause. Follow‐up of patients without outcome events was censored at the date of their last follow‐up visit. The distributions of PFS and OS were estimated using the Kaplan‐Meier method; median follow‐up time was based on the reverse Kaplan‐Meier method [32]. We fit multivariable Cox proportional hazard models for both PFS and OS to assess important predictors of these outcomes. Standard model‐building techniques were used for model development. Candidate predictors for the models were patient demographic, disease, and prior treatment factors that were assessed to be different between younger and older patients based on univariate comparisons (p < .3). For the models, we dichotomized lactate dehydrogenase (LDH) as abnormal versus normal/unknown based on a cut point of 333 UI/L; categorized treatment with ipilimumab as having received it before, after, or during versus never having received it; and dichotomized BRAF mutation as mutated versus not mutated/unknown. We also used the CCI, unadjusted for age, as a model covariate. Cox models were stratified by ECOG performance status to accommodate differences in the underlying baseline hazard. Final models included patient age, gender, and CCI in addition to those predictors that were statistically significantly related to outcome (Wald chi‐squared p < .05). We compared the presence or absence of toxicities across age groups using Fisher's exact tests.
Results
Patient Characteristics
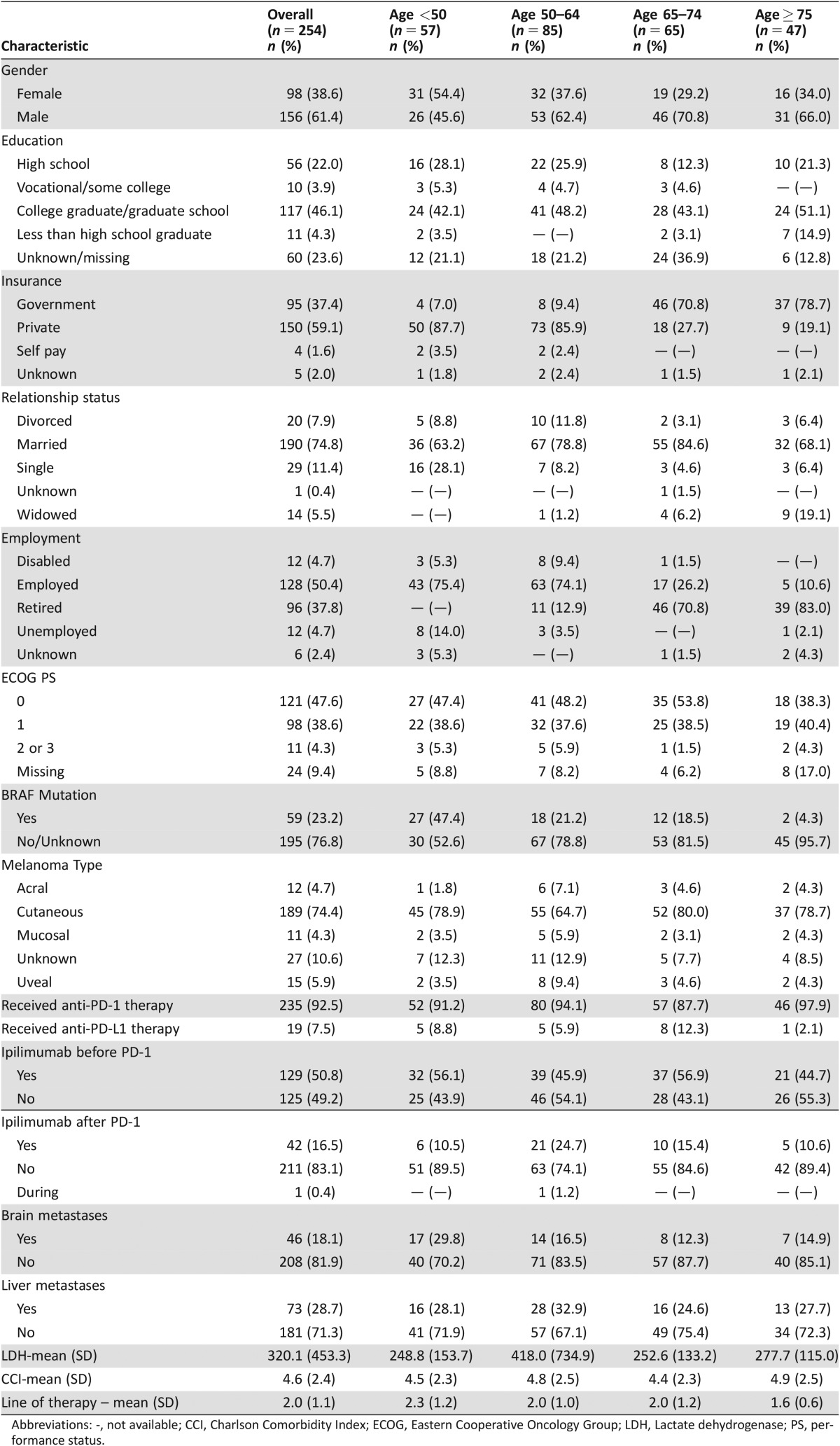
Of the 254 patients we analyzed, 57 (22.4%) were less than 50 years old, 85 (33.5%) were age 50–64, 65 (25.6%) were age 65–74, and 47 (18.5%) were 75 years or older (Table 1). A larger proportion of patients in all age groups over age 50 were male gender (p = .03). Younger patients were more likely to have a BRAF mutation (p = .0001), and of 59 patients with a BRAF mutation, 42 (71.2%) received BRAF‐targeted therapy prior to beginning PD‐1 inhibitor therapy. Notably, CCI scores did not differ between the older and younger patients (mean CCI age <50: 4.5, mean CCI age 50–64: 4.8, mean CCI age 65–74: 4.4, mean CCI age ≥75: 4.9, p = .48). Further, there were no significant differences in ECOG performance status between groups at the time of first presentation to an oncologist. Overall, the majority of patients received anti–PD‐1 therapy (92.5%). Our cohort included 120 (47.2%) patients from MGH and 134 (52.8%) from Vanderbilt University Medical Center (supplemental online Table 1). Patients from MGH were more likely to have education beyond high school, lower ECOG performance status, lower CCI, and to receive PD‐1 inhibitor therapy as an earlier line of therapy compared with patients from Vanderbilt University Medical Center.
Table 1. Baseline characteristics of patients.
Abbreviations: ‐, not available; CCI, Charlson Comorbidity Index; ECOG, Eastern Cooperative Oncology Group; LDH, Lactate dehydrogenase; PS, performance status.
Overall Survival
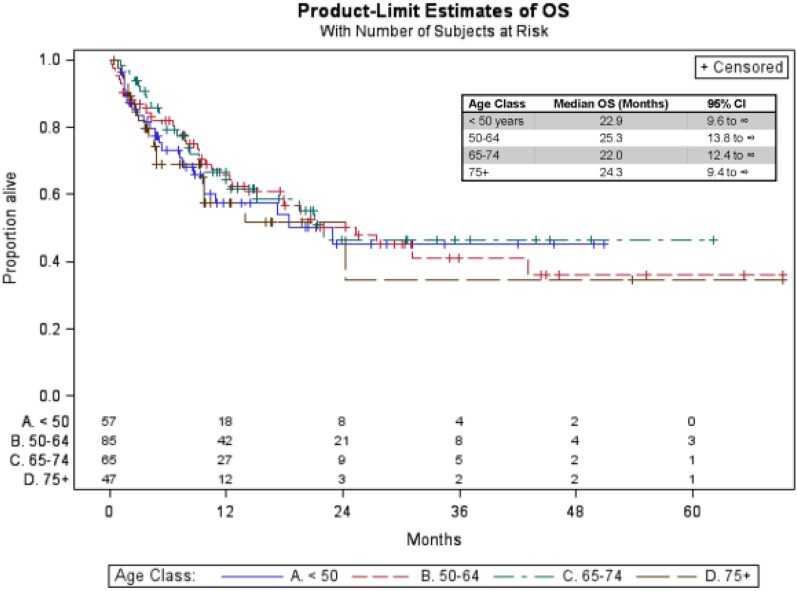
Median follow‐up time in this analysis was 16.0 months (95% confidence interval [CI]: 13.1–20.4 months). After beginning PD‐1 inhibitor therapy, the median OS for the entire cohort was 22.0 months (95% CI: 17.2–43.0 months). Median OS was comparable between younger and older patients (age <50: 22.9 months, age 50–64: 25.3 months, age 65–74: 22.0 months, age ≥75: 24.3 months). Kaplan‐Meier estimates of OS are shown in Figure 1.
Figure 1.
Kaplan‐Meier overall survival curves across age groups.
Abbreviations: OS, overall survival; CI, confidence interval.
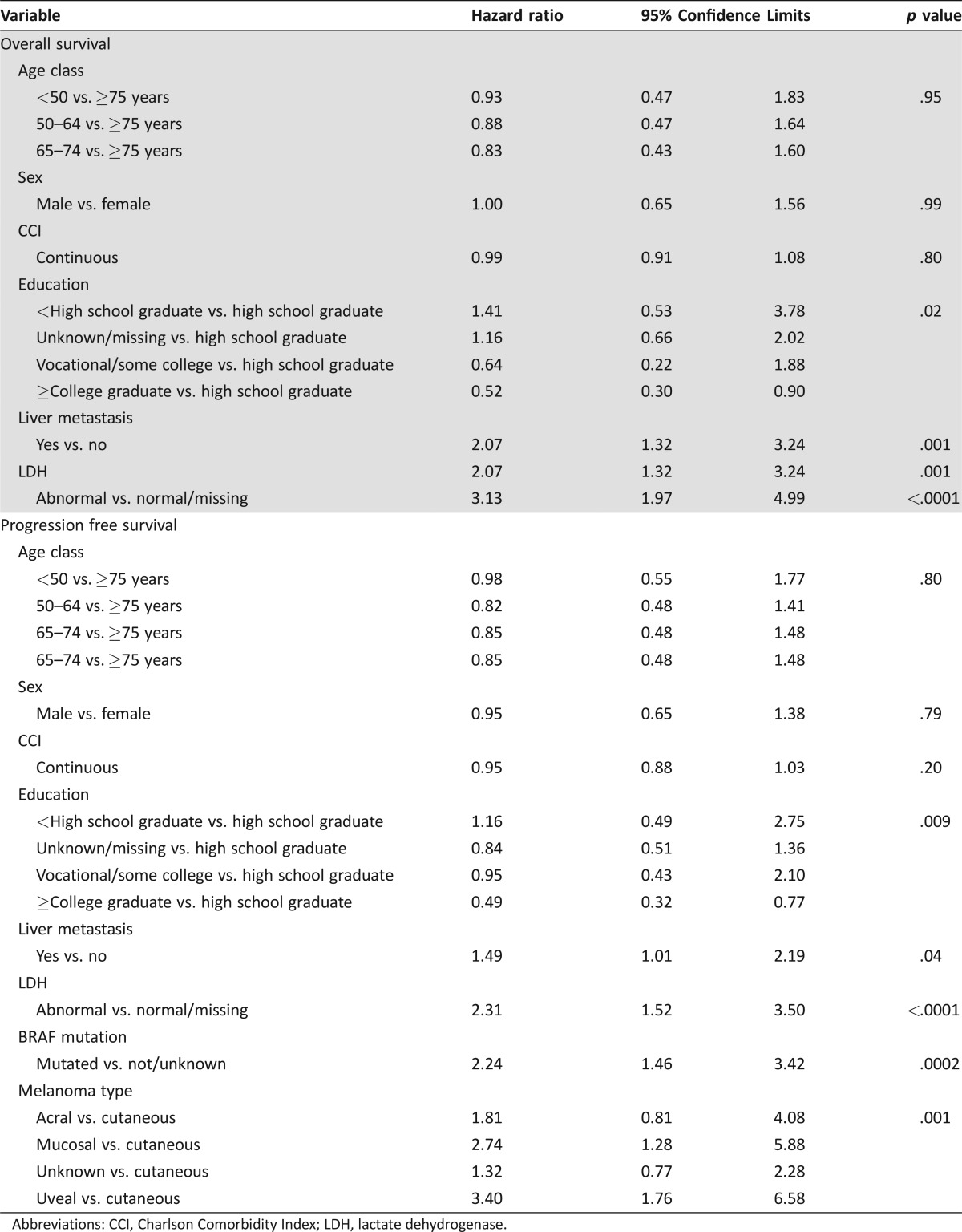
Multivariable Cox proportional hazard model results (reference group ≥75 years) showed no significant difference in OS according to age (hazard ratio [HR] age <50: 0.9 [95% CI 0.5–1.8]; HR age 50–64: 0.9 [95% CI 0.5–1.6]; HR age 65–74: 0.8 [95% CI 0.6–1.6) (Table 2). Survival was significantly influenced by the presence of liver metastasis and pretreatment LDH. The presence of liver metastasis at the time of first presentation to an oncologist doubled the hazard for death (HR: 2.1, 95% CI: 1.3 to 3.2, p = .001), and abnormal pretreatment LDH tripled the hazard of death (HR: 3.1, 95% CI: 2.0 to 5.0, p < .0001).
Table 2. Multivariable model of characteristics associated with overall survival and progression‐free survival.
Abbreviations: CCI, Charlson Comorbidity Index; LDH, lactate dehydrogenase.
Progression‐Free Survival
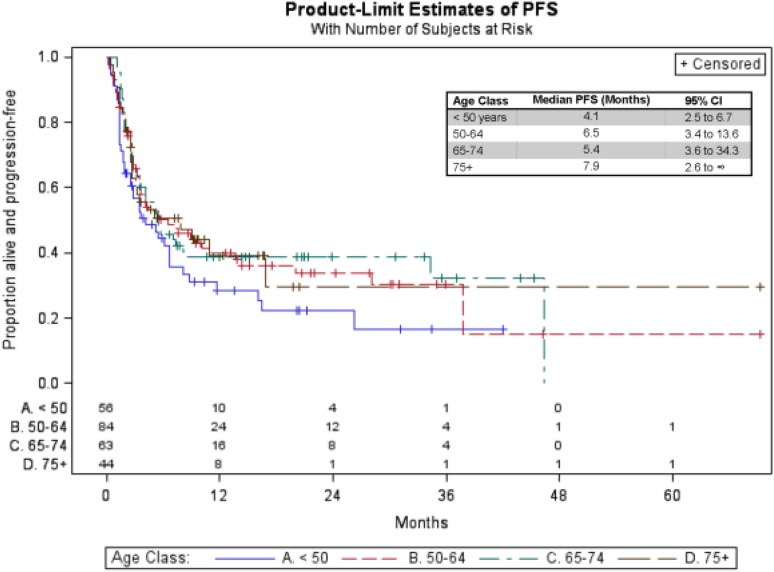
The median PFS for the entire sample was 5.4 months (95% CI: 3.9–7.5 months) after initiation of PD‐1 inhibitor therapy and was similar between age groups (age <50: 4.1 months, age 50–64: 6.5 months, age 65–74: 5.4 months, age ≥75: 7.9 months). Kaplan‐Meier estimates of PFS are shown in Figure 2.
Figure 2.
Kaplan‐Meier progression‐free survival curves across age groups.
Abbreviations: PFS, progression free survival; CI, confidence interval.
Although patients age ≥75 had nearly double the PFS of patients age <50, we found no significant difference in PFS for older versus younger patients using multivariable Cox regression (HR age <50: 1.0 [95% CI 0.5–1.8]); HR age 50–64: 0.8 [95% CI 0.5–1.4]; HR age 65–74: 0.8 [95% CI 0.5–1.5]). Progression‐free survival was significantly influenced by presence of liver metastasis, pretreatment LDH, BRAF mutation, and the type of melanoma. Patients with liver metastasis at the time of the first oncology visit experienced shorter PFS (HR: 1.5, 95% CI: 1.0–2.2, p = .04). Those with abnormal pretreatment LDH also experienced worse PFS (HR: 2.3, 95% CI: 1.5–3.5, p < .0001). Having a BRAF mutation was significantly associated with shorter PFS (HR: 2.3, 95% CI: 1.5–3.5, p < .0001). Compared with patients diagnosed with cutaneous melanoma, those with mucosal (HR: 3.1, 95% CI: 1.5–6.4, p < .001) or uveal melanomas (HR: 3.6, 95% CI: 1.9–7.0, p < .001) also had significantly worse PFS.
Toxicities
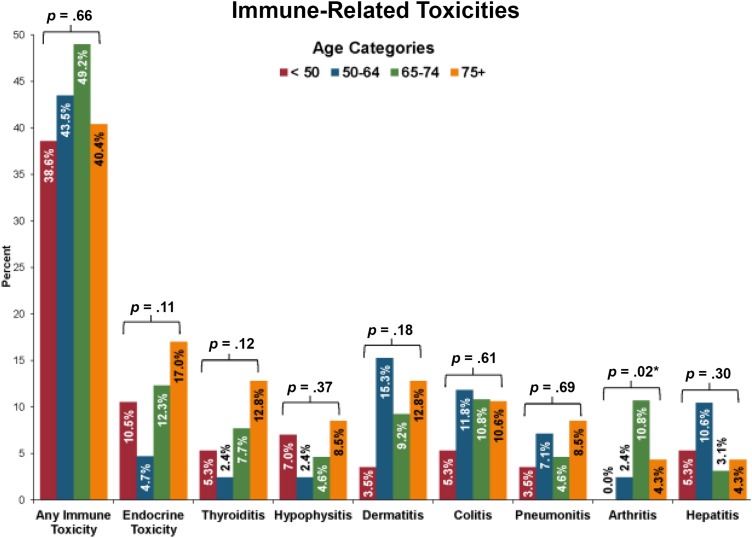
In our sample, 110 patients (43.3%, 95% CI: 37.1%–49.7%) experienced any immune‐related adverse events, as shown in Figure 3. The most common toxicities were dermatitis (n = 27, 10.6%) and colitis (n = 25, 9.8%). The incidence of arthritis was significantly higher among patients aged 65–74 years (10.8%, p = .02). The data suggest that patients who are ≥75 years old may have higher incidences of thyroiditis or endocrine‐related toxicity; however, neither of these comparisons reached statistical significance. Notably, there were no significant differences in colitis, hepatitis, or pneumonitis between age groups.
Figure 3.
Immune‐related toxicities across age groups.
p values obtained using Fisher's exact tests to compare the presence or absence of toxicities across age groups.
Patient Sociodemographic Characteristics
To further assess differences between older and younger patients, we assessed sociodemographic characteristics that could influence treatment outcomes. We found that OS was significantly influenced by education status. Compared with patients who were high school graduates, those who completed college or beyond had reduced hazard ratios for death (HR: 0.5, 95% CI: 0.3–0.9, p = .01). Similarly, PFS was influenced by level of education, and completion of college or beyond reduced the hazard of a PFS event by approximately half (HR: 0.5, 95% CI: 0.3–0.8, p = .009).
Discussion
The emergence of anti–PD‐1 therapy has revolutionized the treatment of advanced melanoma and other malignancies, but little has been reported about the effects of these agents in the geriatric oncology population [33]. Age‐related immunosenescence could have meaningful implications on the efficacy of checkpoint inhibitors in older patients with cancer, theoretically reducing the effectiveness of these therapies, although older patients often have tumors with higher mutational loads (e.g., association of age and desmoplastic melanoma), which may predict a higher chance of tumor PD‐L1 expression, T‐cell infiltration, and increased responsiveness to anti–PD‐1/PD‐L1 therapy [34], [35]. Our data suggest that PD‐1 inhibitors are effective in patients with advanced melanoma independent of age.
In addition to highlighting that patient age did not affect OS and PFS outcomes, we were able to determine additional factors that influence these outcomes. Consistent with prior research, elevated LDH and presence of liver metastases were independent predictors of worse OS and PFS outcomes [12]. Interestingly, we found that the presence of BRAF mutation was associated with worse PFS, but nearly three‐fourths of patients with a known BRAF mutation had received BRAF‐targeted therapy prior to beginning PD‐1 inhibitor therapy. Our findings also suggest that patient education was associated with both OS and PFS. Patients who had not completed high school appeared to have worse OS and PFS. Although this is hypothesis‐generating, we lack information regarding other measures of socioeconomic, status such as income and employment, which may be important confounders [36], and further research is needed to confirm this finding.
Safety and toxicity profiles are also of primary concern when considering whether and how to treat older patients with cancer. Specific to immune‐related adverse events is the decision to treat with steroids, which can be particularly morbid in the elderly population. This is the first report on whether age affects the frequency or severity of immune‐related adverse events. In our sample from two large academic centers, we did not find significant differences in toxicity between older and younger age groups, although more arthritis was observed in older patients. When hypophysitis and thyroiditis were combined, there was a trend toward more endocrinopathies in older patients, but this did not reach statistical significance. This latter finding is consistent with our previous observation that ipilimumab‐induced hypophysitis is more common in older patients [37]. The incidence and severity of these adverse events stratified by age should be assessed in future, larger clinical trials.
There are several important limitations to this work. First, this was a retrospective study with relatively few patients over age 75, and, thus, the results merit further confirmation in prospective trials with larger numbers of older adults. Second, although this was a multisite study, all patients were treated at academic cancer centers and, thus, our results may not generalize to patients treated at community sites. Third, we were unable to grade the severity of the toxicities, as these data were often not recorded in the medical record. We also lack information regarding treatment discontinuation due to toxicity. Therefore, we are only able to comment on the rate of toxicity occurrence. Future studies should seek to better understand age‐related differences in the severity of toxicities experienced by patients treated with PD‐1 inhibitors and the impact of steroids on both the toxicity and the patient.
Our study has several strengths. We included a relatively large cohort of patients, which allowed us to compare outcomes between older and younger patients with melanoma who received treatment with immunotherapy. In addition, this was a multisite study that included patients from different geographical locations and allowed for differing practice patterns at each site. Lastly, with this study we aimed to better understand the effectiveness and tolerability of novel immunotherapy agents in the geriatric oncology population. As the population ages, older adults represent the majority of patients seen in routine cancer practices [38], [39]. Thus, studies such as this will become critical to help us understand how best to treat older adults with cancer and improve the evidence base in geriatric oncology.
Conclusion
Our data suggest that older patients can safely tolerate anti–PD1/PD‐L1 monoclonal antibody therapy and achieve similar outcomes to younger patients. Furthermore, toxicity profiles are comparable between older and younger patients, providing additional support for the use of these effective therapies in the geriatric oncology population. As the population ages and the landscape of cancer treatments continues to expand, efforts to understand how novel chemotherapeutic agents impact older patients in real‐world settings will become increasingly important.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgment
Previously presented as an abstract at the 2015 Society for Melanoma Research Congress, San Francisco, CA.
Footnotes
For Further Reading: Deborah Kuk, Alexander N. Shoushtari, Christopher A. Barker et al. Prognosis of Mucosal, Uveal, Acral, Nonacral Cutaneous, and Unknown Primary Melanoma From the Time of First Metastasis. The Oncologist 2016;21:848–854.
Implications for Practice: Uveal, acral, and mucosal melanoma are assumed to result in a worse prognosis than nonacral cutaneous melanoma or unknown primary melanoma. No studies, however, have been conducted assessing the overall survival of patients with these melanoma subtypes starting at the time of distant metastatic disease. The present study found that patients with uveal, acral, nonacral cutaneous, and unknown primary melanoma have similar overall survival after distant metastases have been diagnosed. These findings provide information for oncologists to reconsider previously held assumptions and appropriately counsel patients. Patients with mucosal melanoma have worse overall survival and are thus a group in need of specific research and advocacy.
Author Contributions
Conception/design: Ryan J Sullivan, Allison S Betof, Ryan D Nipp, Krista M Rubin, Doug Johnson
Provision of study material or patients: Ryan J Sullivan, Allison S Betof, Ryan D Nipp, Romany A Johnpulle, Keith Flaherty, Donald Lawrence, Doug Johnson
Collection and/or assembly of data: Ryan J Sullivan, Allison S Betof, Ryan D Nipp, Romany A Johnpulle, Samuel M Rubinstein, Doug Johnson
Data analysis and interpretation: Ryan J Sullivan, Allison S Betof, Ryan D Nipp, Anita Giobbie‐Hurder, Doug Johnson
Manuscript writing: Ryan J Sullivan, Allison S Betof, Ryan D Nipp, Anita Giobbie‐Hurder, Romany A Johnpulle, Krista M Rubin, Samuel M Rubinstein, Keith Flaherty, Donald Lawrence, Doug Johnson
Final approval of manuscript: Ryan J Sullivan, Allison S Betof, Ryan D Nipp, Anita Giobbie‐Hurder, Romany A Johnpulle, Krista M Rubin, Samuel M Rubinstein, Keith Flaherty, Donald Lawrence, Doug Johnson
Disclosures
Keith Flaherty: Bristol‐Myers Squibb, Merck, Roche (C/A); Doug Johnson: Bristol‐Myers Squibb, Genoptix (C/A), Incyte (RF); Ryan J Sullivan: Merck, Genentech, Bristol‐Myers Squibb (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Chapman PB, Hauschild A, Robert C et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hauschild A, Grob JJ, Demidov LV et al. Dabrafenib in BRAF‐mutated metastatic melanoma: A multicentre, open‐label, phase 3 randomised controlled trial. Lancet 2012;380:358–365. [DOI] [PubMed] [Google Scholar]
- 3. Flaherty KT, Robert C, Hersey P et al. Improved survival with MEK inhibition in BRAF‐mutated melanoma. N Engl J Med 2012;367:107–114. [DOI] [PubMed] [Google Scholar]
- 4. Long GV, Stroyakovskiy D, Gogas H et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877–1888. [DOI] [PubMed] [Google Scholar]
- 5. Robert C, Karaszewska B, Schachter J et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30–39. [DOI] [PubMed] [Google Scholar]
- 6. Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 7. Robert C, Schachter J, Long GV et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 8. Larkin J, Ascierto PA, Dreno B et al. Combined vemurafenib and cobimetinib in BRAF‐mutated melanoma. N Engl J Med 2014;371:1867–1876. [DOI] [PubMed] [Google Scholar]
- 9. Larkin J, Chiarion‐Sileni V, Gonzalez R et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andtbacka RH, Kaufman HL, Collichio F et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 33:2780–2788, 2015. [DOI] [PubMed] [Google Scholar]
- 11. Korn EL, Liu PY, Lee SJ et al. Meta‐analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression‐free and overall survival benchmarks for future phase II trials. J Clin Oncol 2008;26:527–534. [DOI] [PubMed] [Google Scholar]
- 12. Balch CM, Gershenwald JE, Soong SJ et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Long GV, Stroyakovskiy D, Gogas H et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF‐mutant melanoma: A multicentre, double‐blind, phase 3 randomised controlled trial. Lancet 2015;386:444–451. [DOI] [PubMed] [Google Scholar]
- 14. Herbst RS, Soria JC, Kowanetz M et al. Predictive correlates of response to the anti‐PD‐L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non‐Small‐Cell Lung Cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 17. Motzer RJ, Escudier B, McDermott DF et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Powles T, Eder JP, Fine GD et al. MPDL3280A (anti‐PD‐L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558–562. [DOI] [PubMed] [Google Scholar]
- 19. Rosenberg JE, Hoffman‐Censits J, Powles T et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: A single‐arm, multicentre, phase 2 trial. Lancet 2016; 387:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ansell SM, Lesokhin AM, Borrello I et al. PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nanda R, Chow LQ, Dees EC et al. Pembrolizumab in patients with advanced triple‐negative breast cancer: Phase Ib KEYNOTE‐012 study. J Clin Oncol, 2016;34:2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seiwert TY, Haddad RI, Gupta S et al. Antitumor activity and safety of pembrolizumab in patients (pts) with advanced squamous cell carcinoma of the head and neck (SCCHN): Preliminary results from KEYNOTE‐012 expansion cohort. J Clin Oncol 2015;33(suppl):LBA6008. [Google Scholar]
- 23. Cetin B, Gumusay O, Cengiz M et al. Advances of molecular targeted therapy in gastric cancer. J Gastrointest Cancer 2016;47:125–134. [DOI] [PubMed] [Google Scholar]
- 24. Muss HB, Berry DA, Cirrincione C et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node‐positive breast cancer: the Cancer and Leukemia Group B Experience. J Clin Oncol 2007;25:3699–3704. [DOI] [PubMed] [Google Scholar]
- 25. Muss HB, Woolf S, Berry D et al. Adjuvant chemotherapy in older and younger women with lymph node‐positive breast cancer. JAMA 2005;293:1073–1081. [DOI] [PubMed] [Google Scholar]
- 26. Neugut AI, Matasar M, Wang X et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J Clin Oncol 2006;24:2368–2375. [DOI] [PubMed] [Google Scholar]
- 27. Bouchardy C, Rapiti E, Fioretta G et al. Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol 2003;21:3580–3587. [DOI] [PubMed] [Google Scholar]
- 28. Hutchins LF, Unger JM, Crowley JJ et al. Underrepresentation of patients 65 years of age or older in cancer‐treatment trials. N Engl J Med 1999;341:2061–2067. [DOI] [PubMed] [Google Scholar]
- 29. Sateren WB, Trimble EL, Abrams J et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol 2002;20:2109–2017. [DOI] [PubMed] [Google Scholar]
- 30. Scher KS, Hurria A. Under‐representation of older adults in cancer registration trials: Known problem, little progress. J Clin Oncol 2012;30:2036–2038. [DOI] [PubMed] [Google Scholar]
- 31. Nipp RD, Yao NA, Lowenstein LM et al. Pragmatic study designs for older adults with cancer: Report from the U13 conference. J Geriatr Oncol, 2016;7:234–241. [DOI] [PubMed] [Google Scholar]
- 32. Altman DG, De Stavola BL, Love SB et al. Review of survival analyses published in cancer journals. Br J Cancer 1995;72:511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rogiers A, van den Oord JJ, Garmyn M et al. Novel therapies for metastatic melanoma: An update on their use in older patients. Drugs Aging 2015;32:821–834. [DOI] [PubMed] [Google Scholar]
- 34. Malaguarnera L, Cristaldi E, Malaguarnera M. The role of immunity in elderly cancer. Crit Rev Oncol Hematol 2010;74:40–60. [DOI] [PubMed] [Google Scholar]
- 35. Montecino‐Rodriguez E, Berent‐Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest 2013;123:958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cella DF, Orav EJ, Kornblith AB et al. Socioeconomic status and cancer survival. J Clin Oncol 1991;9:1500–1509. [DOI] [PubMed] [Google Scholar]
- 37. Faje AT, Sullivan R, Lawrence D et al. Ipilimumab‐induced hypophysitis: A detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab 2014;99:4078–4085. [DOI] [PubMed] [Google Scholar]
- 38. Parry C, Kent EE, Mariotto AB et al. Cancer survivors: A booming population. Cancer Epidemiol Biomarkers Prev 2011;20:1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith BD, Smith GL, Hurria A et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758–2765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.