This study focuses on whether male breast cancer as a second primary cancer is increasing and what, if any, relationships this might have with the index malignancy and associated therapy. These findings might offer insights into surveillance interventions that could benefit male cancer survivors.
Keywords: Male breast neoplasms; Surveillance, Epidemiology, and End Results program; Incidence; Second primary neoplasm
Abstract
Background.
Male breast cancer (MBC) as a second primary cancer (SPC) has a known association with prior MBC. However, its association with non‐breast index malignancies, relative to population risk, has not been previously reported.
Materials and Methods.
Using Surveillance, Epidemiology, and End Results program (9 catchment area) data, we identified MBCs diagnosed from 1973–2012 as their SPC. Information regarding the index malignancy was also obtained. Standardized incidence ratios (SIR) of MBC as SPC were estimated, along with incidence rates and trends. Kaplan‐Meier curves were used to estimate survival.
Results.
Over a 38‐year period, 464 MBCs were identified as SPC. The most common index malignancies were breast (SIR 30.86, 95% confidence interval [CI] 21.50–42.92, p < .001), lymphoma (SIR 1.58, 95% CI 1.08–2.22, p = .014), melanoma (SIR 1.26, 95% CI 0.80–1.89), urinary (SIR 1.05, 95% CI 0.74–1.43), colorectal (SIR 0.94, 95% CI 0.69–1.24), and prostate (SIR 0.93 95% CI 0.81–1.07). Apart from the known association with prior breast cancer, the only significant association was with lymphoma as an index cancer, although not significant with a Bonferroni correction. From 1975–2012, incidence of breast cancer as a first cancer increased at an annual percentage change of 1.3% while breast cancer as a SPC increased at 4.7% (both p values < .001).
Conclusion.
Male breast cancer as a SPC has increased markedly over 4 decades. Men with a history of lymphoma may experience higher‐than‐expected rates of breast SPC. These observations warrant further research, and suggest possible etiologic connections with disease biology, prior therapy, or genetics.
Implications for Practice.
This study reports that men are presenting more frequently to the clinic with breast cancer, both as an initial cancer and as a second cancer following an earlier malignancy. We also report the novel observation that men who survive lymphoma are at increased risk of developing a subsequent breast cancer. Further work is needed to better understand possible treatment or biologic causes of this association. More immediately, these findings suggest the need for heightened vigilance for male breast cancer overall and, in particular, for male lymphoma survivors.
Introduction
Male breast cancer is a rare diagnosis that affects 1 in 100,000 men [1] and accounts for approximately 1% of all breast cancer [2]. More than half of male breast cancers are diagnosed at stage II or higher compared to approximately 35% of women with breast cancer [2], [3]; this speaks to the low index of suspicion of the patient and the physician [4]. While stage‐ and age‐matched breast cancer between men and women have the same prognosis [5], a less favorable overall outcome is seen in men due to more advanced age and stage at presentation [6], [7]. Further, the overall incidence of male breast cancer continues to increase [3]. The reasons for this trend remain unclear. Etiologic factors could include increasing lifespan, greater awareness, or as yet undescribed factors.
Risk factors for male breast cancer include genetic predisposition, such as breast cancer susceptibility gene (BRCA1/2) mutations and Klinefelter's syndrome; radiation exposure; and increased estrogen exposure from gonadal dysfunction, obesity, liver disorders, or occupational hazards [2], [8]. A history of breast cancer in a male also places him at a 30‐fold excess risk of having a second breast cancer [9]. Less is known about the risk of breast cancer in men as a second primary cancer (SPC) following other, non‐breast malignancies. Cancer treatments or other factors relating to a prior malignancy may be overlooked as a risk factor for developing subsequent male breast cancer.
We sought to understand if male breast cancer as SPC is also increasing and what, if any, relationships this might have with the index malignancy and the related therapy. We further hypothesized that these findings might offer insights into surveillance interventions that could benefit male cancer survivors.
Materials and Methods
We used data from the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) program, limiting to the original SEER 9 registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco‐Oakland, Seattle‐Puget Sound, and Utah). From this, we identified males diagnosed from 1973–2012 with breast cancer as their second primary cancer. Single (vs. second) “primary” are defined by SEER and refer to the number of reportable cases in the database. A latency exclusion period was set at 2 months. These breast cancers were characterized by patient age at diagnosis, stage (according to breast adjusted American Joint Committee on Cancer 6th staging for those diagnosed 1988 and later), grade, and hormone receptor status (for those diagnosed 1990 and later).
Information regarding the index malignancy was also obtained and patients were categorized by the five most common index primary sites: prostate, colorectal (which included cancers of the cecum, appendix, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon, rectosigmoid junction, and rectum), ureter and urinary bladder (henceforth “urinary”), breast, lymphoma (which included Hodgkin lymphoma, non‐Hodgkin lymphoma [NHL] and chronic lymphocytic leukemia [CLL]), and melanoma of the skin. Patient age at diagnosis, grade and stage of tumor, and initial local therapy (radiation and surgery) were also identified for the index cancer. Surveillance, Epidemiology, and End Results defines receipt of radiation and surgery (for each malignancy) as that which was part of first course of treatment and performed within 1 year of diagnosis (for that malignancy).
Standardized incidence ratios (SIR) and 95% confidence intervals (CI) of breast cancer as an SPC in men were calculated based on observed rates divided by expected rates (SEER*Stat software, version 8.2.1; Information Management Services, Incorporated; Calverton, MD). Expected rates of breast cancer were provided by NCI in the multiple primary‐SIR session of SEER*Stat, calculated for the general male population living in the same SEER 9 registries for each year during our study period. The accumulated person‐time at risk was also calculated for our study cohort and used to to estimate excess risk (expressed per 10,000 men). Time between the index cancer and the breast cancer diagnosis was calculated in months and bootstrap methods were used to compare differences in the medians across groups. Yearly incidence rates and their associated standard errors were calculated for diagnoses between 1975 and 2012, to allow for a consistent population for both breast cancer as the SPC (per million men) and the index cancer (per 100,000 men). Changes in incidence rates over time were assessed with joinpoint models to allow for multiple trends across the study period, using NCI's Joinpoint Regression Program (version 4.2.0.2, Information Management Services, Incorporated; Calverton, MD). Six log‐linear models were fitted for each set of incidence data, allowing between zero and five joinpoints. The final model was selected using two methods: Monte Carlo permutation tests to identify the number of joinpoints and the Bayesian Information Criterion to identify the optimal model. Annual percentage change (APC) was also calculated for each trend segment of the final, selected model. Kaplan‐Meier curves were used to estimate five‐year overall survival and median survival.
For those with breast cancer as their index and second primary cancer, laterality was also assessed. Those with known laterality for both cancers were included in this subanalysis. Rates of ipsilateral and contralateral cancers were calculated and compared to women with breast cancer as their index and second primary cancer. Median time to subsequent breast cancer diagnosis was estimated using the Kaplan‐Meier estimator, and CIs for the differences in median time to subsequent breast cancer diagnosis for males and females were estimated with bootstrap methods. All tests were two‐sided and analyses conducted using STATA/MP, version 12.0 (StataCorp LP, College Station, TX). This study was considered not human subjects research by the University of Iowa Institutional Review Board.
Results
Breast Cancer as Second Primary Malignancy
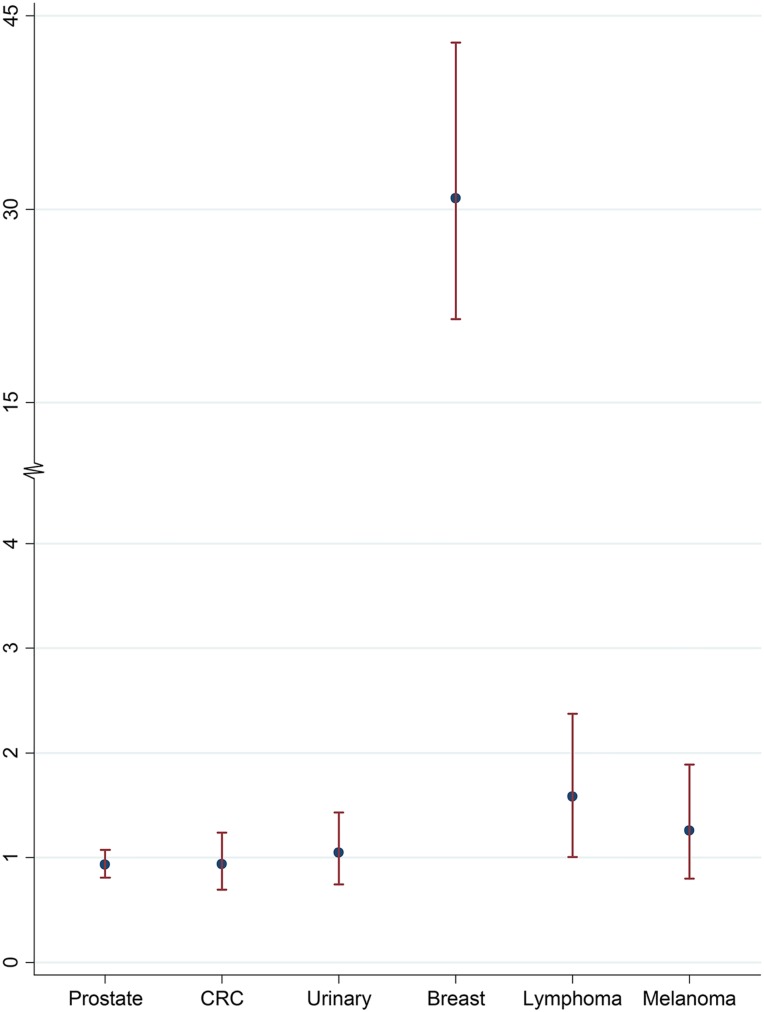
Over a 38‐year period, 464 male breast cancers were identified as second primary cancer (SPC) (Table 1). The five most common index malignancies in descending order were prostate (42.9%), colorectal (10.6%), urinary (8.4%), breast (7.5%), lymphoma (6.9%), and melanoma (5.0%). Standardized incidence ratios were the following by index site: breast (SIR 30.86, 95% CI 21.50–42.92), lymphoma (SIR 1.58, 95% CI 1.08–2.22), melanoma (SIR 1.26, 95% CI 0.80–1.89), urinary (SIR 1.05, 95% CI 0.74–1.43), colorectal (SIR 0.94, 95% CI 0.69–1.24), and prostate (SIR 0.93, 95% CI 0.81–1.07) (Fig. 1). Apart from the known association with prior breast cancer, the only significant association was with lymphoma as an index cancer (p = .014), with an excess risk of 0.17 per 10,000 men. Although significant as a single test, the p value would not be considered statistically significant at the 5% level with a Bonferroni correction for five comparisons.
Table 1. Characteristics of men with breast cancer as their second primary cancer, by site of index cancer.
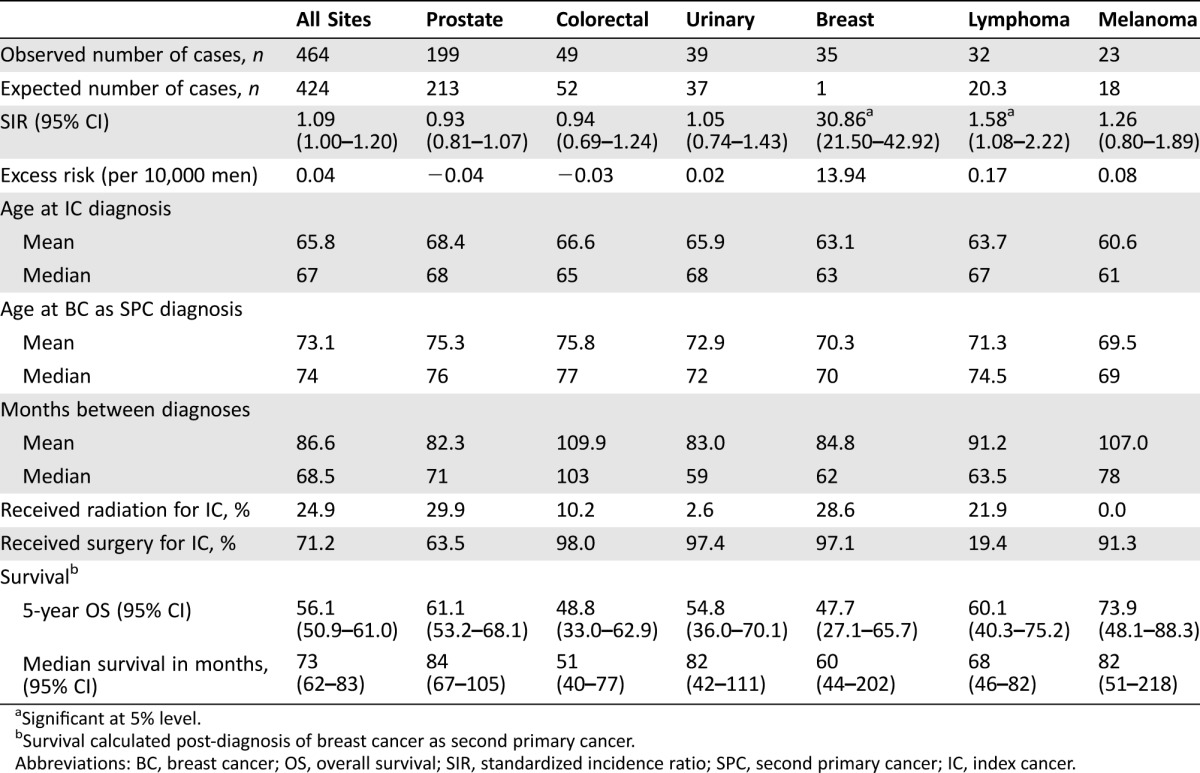
Significant at 5% level.
Survival calculated post‐diagnosis of breast cancer as second primary cancer.
Abbreviations: BC, breast cancer; OS, overall survival; SIR, standardized incidence ratio; SPC, second primary cancer; IC, index cancer.
Figure 1.
Estimated standardized incidence ratios and 95% confidence interval for breast as second malignancy, by site of index cancer.
Abbreviations: CRC, colorectal cancer.
Age and Timing of Index Malignancies
Median age at first cancer diagnosis was in the 7th decade for all of the index cancers. Median age at diagnosis of breast cancer as SPC was in the 8th decade for all cancers, except melanoma, where the median age was 69. The median interval between index malignancy and the breast cancer as a second primary malignancy was the longest for colorectal cancer as the index cancer at 103 months, followed by melanoma at 78 months, prostate at 71 months, lymphoma at 63.5 months, breast at 62 months, and urinary at 59 months (Table 1). Compared to women who also had breast cancer as their index and second breast cancer, the interval time to breast SPC was shorter for men, with a median of 62 months versus 84 months for women (difference of 22 months, 95% CI of difference −1.2–45.2, p = .063) (Table 2). For those with laterality known for both breast cancers, men were also more likely to have the second breast cancer on the contralateral side than women (94.1% vs. 82.3%), although this was not statistically significant (p = .072).
Table 2. Rates of subsequent breast cancer after primary breast cancer for men and women in Surveillance, Epidemiology, and End Results program 9 registries, 1973–2012.
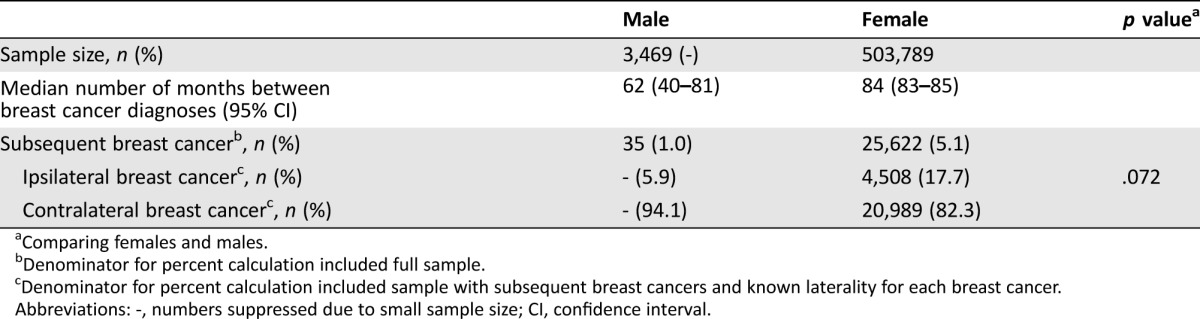
Comparing females and males.
Denominator for percent calculation included full sample.
Denominator for percent calculation included sample with subsequent breast cancers and known laterality for each breast cancer.
Abbreviations: ‐, numbers suppressed due to small sample size; CI, confidence interval.
Treatment for Index Malignancy
Treatment modalities varied by site of the index cancer. Overall, 24.9% of men who developed a SPC had received radiation within 1 year of diagnosis of the initial cancer. Men whose index cancer was prostate cancer received radiation most commonly (29.9%), followed by breast cancer (28.6%) and lymphoma (21.9%). All three of the men who had Hodgkin lymphoma as their index cancer received radiation therapy. Four of the 29 men with index NHL received radiation therapy at the time of lymphoma diagnosis. Radiotherapy to treat the index cancer was least common among those with urinary cancer (2.6%) and colorectal cancer (10.2%). None of the men with melanoma as the index malignancy received radiation. Conversely, men with lymphoma were the least likely to undergo surgery as a primary treatment (19.4%). Men most likely to have surgery for their index cancer were those with colorectal cancer (98.0%), urinary cancer (97.4%), primary breast cancer (97.1%), melanoma (91.3%), and prostate cancer (63.5%) (Table 1).
Survival
The 5‐year overall survival following diagnosis of breast cancer as the SPC was comparable across the index malignancies, but was lowest among men who had breast cancer as both primary and secondary malignancy (47.7%, 95% CI 27.1–65.7%). Median survival from time of breast cancer as SPC diagnosis was 73 months (95% CI 62–83) across all index sites. Median survival following diagnosis of second cancer was shortest for those whose index cancer was colorectal (51 months, 95% CI 40–77) and breast (60 months, 95% CI 44–202), and longest for prostate cancer (84 months) (Table 1).
Incidence
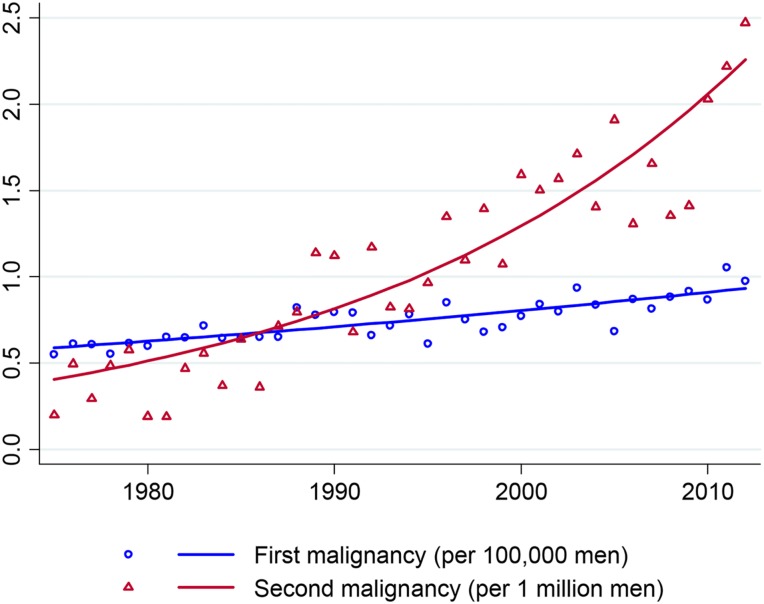
Over the study period, the incidence of breast cancer as a SPC increased from 0.2 to 2.5 per million men, at an average APC of 4.7% (95% CI 3.9%–5.6%, p < .001) (Fig. 2). Male breast cancer as index malignancy grew, albeit at a slower rate, from 5.5 to 9.8 per 100,000 men during the same time period, with an average APC of 1.3% (95% CI 0.97%–1.5%, p < .001).
Figure 2.
Incidence of male breast cancer diagnoses in Surveillance, Epidemiology, and End Results program 9 registries, 1975–2012.
Pathologic Features of Male Breast Cancer as SPC
The histology for male breast cancer as a SPC was most often invasive ductal carcinoma at 72.4%, followed distantly by adenocarcinoma not otherwise specified (NOS) at 6.5%, carcinoma NOS at 2.4%, and papillary carcinoma NOS at 2.6%. This was similar in distribution and ranking to male breast cancer as index cancer. Grade was unknown for 21.3% of SPCs, compared with 32.4% of index breast cancers (p < .001) (Table 3). For SPCs, grade 2 represented the largest portion of patients (38.8%), followed by grade 3 (27.6%) and grade 1 (11.6%) disease. This did not differ from index breast cancers, for those with known grade (p = .083). Estrogen receptor (ER) and progesterone receptor (PR) status was unknown for 17.4%–18.2% of SPCs, a rate similar to index breast cancers. Most of the sample had ER‐positive (77.8%) and PR‐positive (70.2%) tumors, again similar to those whose breast cancer was their index cancer. The majority of breast SPCs were early stage (stage I 35.5% and stage II 33.1%), whereas 13.2% were stage III, 5.6% were stage IV, and 11.2% were unknown. When stage was categorized as individual groups, its distribution did not differ significantly across index and SPCs (p = .055). However, men with SPCs were more likely to present with stage I disease than stage II–IV disease, compared with those with breast cancer as their index cancer (p = .010).
Table 3. Characteristics of male breast cancer as index and second primary cancer.
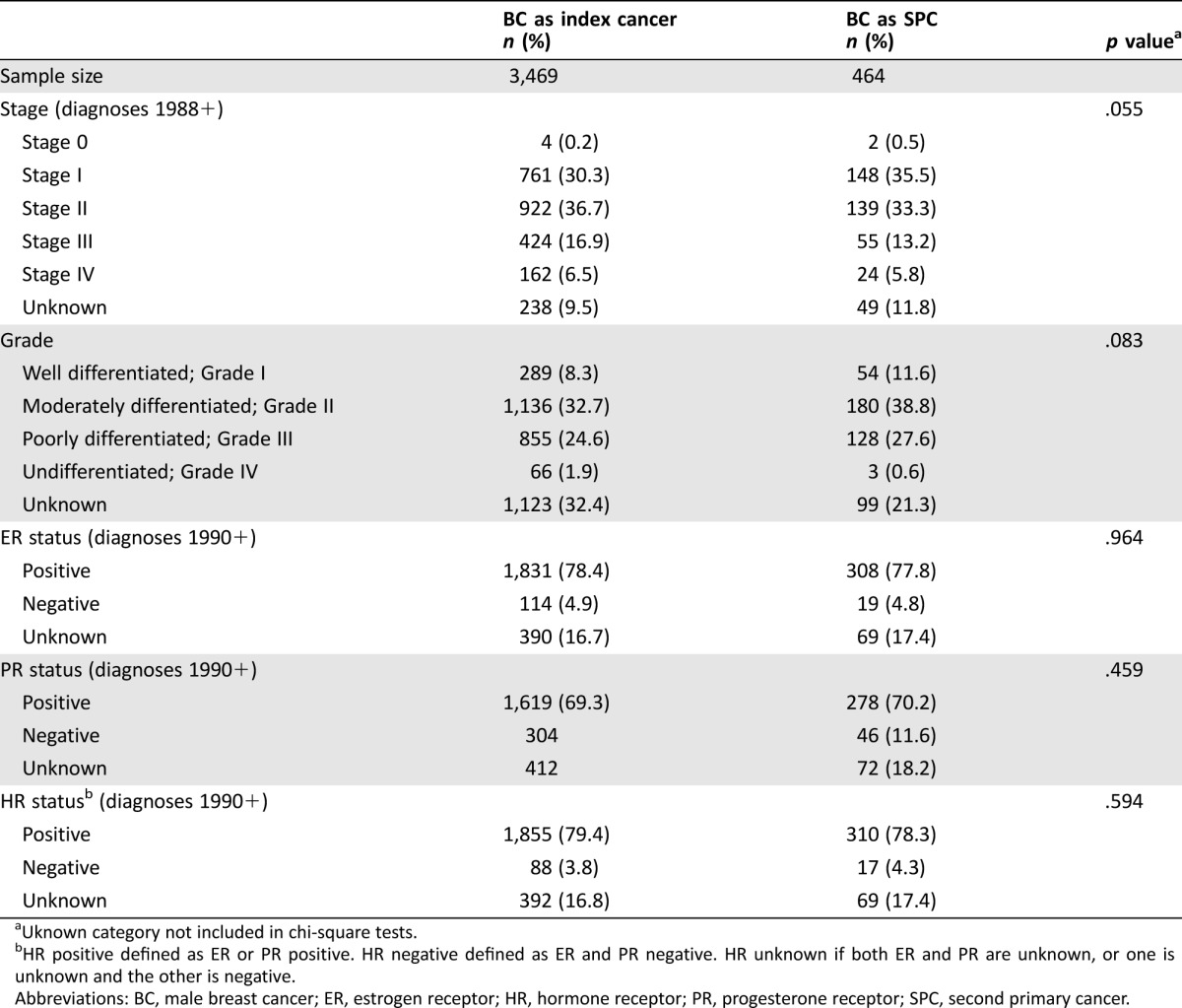
Uknown category not included in chi‐square tests.
HR positive defined as ER or PR positive. HR negative defined as ER and PR negative. HR unknown if both ER and PR are unknown, or one is unknown and the other is negative.
Abbreviations: BC, male breast cancer; ER, estrogen receptor; HR, hormone receptor; PR, progesterone receptor; SPC, second primary cancer.
Discussion
We evaluated patterns of male breast cancer as a second malignancy in the SEER population, with the goal of identifying associations that may improve our understanding of etiology as well as to suggest strategies to better manage male cancer survivors. We found the previously noted strong association of an index breast cancer with breast SPC, as well as a novel association of male breast cancer with index lymphoma, which warrants further examination. These lymphoma patients were among the most likely to receive radiation and the least likely to have surgery for their index cancer. In addition, these men were also younger, had a longer interval from first to second malignancy, and had one of the most favorable 5‐year survival rates, compared with the other index malignancies.
Women treated for Hodgkin lymphoma in earlier eras have a markedly elevated risk of breast cancer, attributable to mantle‐field radiation [10], [11], [12]. In the contemporary era, lower‐risk, involved‐field radiation has largely replaced mantle‐field radiation [13]. Notably, all the men in this study with Hodgkin lymphoma as their index cancer received radiation therapy, although as SEER records neither the anatomic site nor the specific dose of radiation delivered, the details of what these men received are not available. Furthermore, the association of earlier lymphoma with male breast SPC held up when the Hodgkin cases were excluded (data not shown). With regard to NHL, chemotherapy and immunotherapy are generally considered first‐line treatments, with radiation reserved for locoregional control, selected bulky, extranodal, advanced stage, or indolent NHL [14], [15], [16]. Overall, very few men with lymphoma as an index malignancy had Hodgkin lymphoma, and a minority of the men with lymphoma had radiation as part of initial local therapy for the index cancer. Therefore, the breast cancer risk after lymphoma treated with contemporary lymphoma therapy appears unlikely to be driven by radiotherapy in the region of the breast. Of note, the use of upfront radiation therapy was highest in men with lung cancer as index cancer (47.6%), but their risk of second primary breast cancer was not significantly elevated (SIR 1.25, 95% CI 0.78–1.89).
Many indolent lymphomas, most notably chronic lymphocytic leukemia, can induce a mild chronic immune suppression and may increase the risk of a second primary malignancy on this basis [17], [18], [19], [20]. The SIR seen here for male breast cancer following lymphomatous malignancies as a broad category is similar to those for second cancers overall reported in a European trial population and an earlier report on second cancers following CLL in SEER, which only reported on subsequent female breast cancers [17], [18]. Importantly, disease‐specific risks for SPC would be unlikely to change over time and would be less likely to explain the increasing incidence of male breast cancer as SPC. Newer therapies, such as monoclonal antibodies, have been introduced in the last 2 decades and can profoundly alter immune function and potentially increase cancer risk [21], [22]. Could this, in part, explain our findings? Several studies looking at rituximab suggested that this particular agent is not associated with increased cancer risk [18], although others have reported that immune modulation with contemporary NHL therapy appears to be associated with melanoma [23].
The known increased risk of a second male breast cancer after a primary breast cancer was again seen in our data with a SIR of 30.86. This is consistent with a previous study citing a SIR of 29.6 (95% CI 15–52) [9]. The SIR of a second primary breast cancer following a first breast malignancy is markedly higher for men than it is for women (30.86, 95% CI 21.5–42.92 vs. 1.59, 95% CI 1.57–1.61).
The fact that men also tend to have a contralateral cancer rather than a second ipsilateral event may be due to the fact that men were more likely to undergo mastectomies rather than breast conservation; 83% of men diagnosed after 1988 received mastectomies compared with 37% of women. However, it should be noted that the SEER database identifies malignancies occurring greater than 5 years after the primary malignancy as a new cancer rather than a recurrence, regardless of the location. Within 5 years, the second cancer must differ sufficiently within the same primary site of breast (e.g., by histology, laterality) to be categorized as another reportable case in the dataset. Thus, ipsilateral breast events are only designated as SPCs if they occur more than 5 years after the index breast (assuming both events have the same histology).
Over the past 4 decades, there is a clear temporal trend demonstrating a significant increase in incidence of male breast cancer as SPC. This could be due to increased awareness, longer survival after primary treatment of the index cancer, or another as yet not fully characterized cause. Nevertheless, it is a trend that bears close watching, and a more extensive study including additional SEER sites would be helpful. With larger numbers, it may be possible to generate both etiologic hypotheses regarding specific therapies, and the possibility of genetic influences that could guide management and surveillance.
The limitations of our study include its retrospective nature and the limited family and genetic information of the patient population. Furthermore, the impact of systemic treatments, which are not available in the SEER database, could not be included. Lymphoma is a heterogenous disease with diverse treatment. This group of lymphomatous malignancies was defined as our “Lymphoma” categories based on relatively similar treatments and recent World Health Organization Classification [24]. Notably, SIRs for male breast cancer as SPC following lymphoma did not change with the exclusion of CLL as an index cancer (SIR 1.58, 95% CI 1.00–2.38). It is reassuring that the SPC tended to be diagnosed at an earlier stage than initial male breast cancer, suggesting not only some awareness of this risk but also opportunities for enhanced patient education and clinical surveillance.
Conclusion
In this review of the SEER database spanning almost 4 decades, men with a previous lymphoma were more likely than expected to develop breast cancer as a SPC. This increased risk could be related to therapy for the primary malignancy or a yet unknown environmental link. Over time, the incidence of second primary male breast cancer has increased markedly. These findings suggest the need for future work investigating the causes of these incidence trends, and have implications with regard to surveillance for male breast cancer in cancer survivors.
Contributor Information
Seema Ahsan Khan, Email: s-khan2@northwestern.edu.
Mary C. Schroeder, Email: mary-schroeder@uiowa.edu
Author Contributions
Conception/design: Seema Ahsan Khan, Deborah E. Farr, Alexandra Thomas, Mary C. Schroeder
Collection and/or assembly of data: Seema Ahsan Khan, Deborah E. Farr, Alexandra Thomas, Mary C. Schroeder
Data analysis and interpretation: Seema Ahsan Khan, Deborah E. Farr, Alexandra Thomas, Mary C. Schroeder
Manuscript writing: Seema Ahsan Khan, Deborah E. Farr, Alexandra Thomas, Mary C. Schroeder
Final approval of manuscript: Seema Ahsan Khan, Deborah E. Farr, Alexandra Thomas, Mary C. Schroeder
Disclosures
The authors indicated no financial relationships.
References
- 1. Czene K, Bergqvist J, Hall P et al. How to treat male breast cancer. Breast 2007;16(suppl 2):S147–154. [DOI] [PubMed] [Google Scholar]
- 2. Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet 2006;367:595.–. [DOI] [PubMed] [Google Scholar]
- 3. Giordano SH, Cohen DS, Buzdar AU et al. Breast carcinoma in men: A population‐based study. Cancer 2004;101:51.–. [DOI] [PubMed] [Google Scholar]
- 4. Contractor KB, Kaur K, Rodrigues GS et al. Male breast cancer: Is the scenario changing. World J Surg Oncol 2008;6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Willsher PC, Leach IH, Ellis IO et al. A comparison outcome of male breast cancer with female breast cancer. Am J Surg 1997;173:185.–. [DOI] [PubMed] [Google Scholar]
- 6. Ribeiro W, Muscara MN, Martins AR et al. Bioequivalence study of two enalapril maleate tablet formulations in healthy male volunteers. Pharmacokinetic versus pharmacodynamic approach. Eur J Clin Pharmacol 1996;50:399.–. [DOI] [PubMed] [Google Scholar]
- 7. Joshi MG, Lee AK, Loda M et al. Male breast carcinoma: An evaluation of prognostic factors contributing to a poorer outcome. Cancer 1996;77:490.–. [DOI] [PubMed] [Google Scholar]
- 8. Johansen Taber KA, Morisy LR, Osbahr AJ 3rd et al. Male breast cancer: Risk factors, diagnosis, and management (Review). Oncol Rep 2010;24:1115.–. [DOI] [PubMed] [Google Scholar]
- 9. Auvinen A, Curtis RE, Ron E. Risk of subsequent cancer following breast cancer in men. J Natl Cancer Inst 2002;94:1330.–. [DOI] [PubMed] [Google Scholar]
- 10. Schaapveld M, Aleman BM, van Eggermond AM et al. Second cancer risk up to 40 years after treatment for hodgkin's lymphoma. N Engl J Med 2015;373:2499.–. [DOI] [PubMed] [Google Scholar]
- 11. Swerdlow AJ, Cooke R, Bates A et al. Breast cancer risk after supradiaphragmatic radiotherapy for hodgkin's lymphoma in england and wales: A national cohort study. J Clin Oncol 2012;30:2745.–. [DOI] [PubMed] [Google Scholar]
- 12. Intra M, Mattar D, Sangalli C et al. Local therapy for breast cancer in malignant lymphoma survivors. Breast 2011;20(suppl 3):S99.–103. [DOI] [PubMed] [Google Scholar]
- 13. Hodgson DC. Late effects in the era of modern therapy for hodgkin lymphoma. Hematology Am Soc Hematol Educ Program 2011;2011:323.–. [DOI] [PubMed] [Google Scholar]
- 14. Shi Z, Das S, Okwan‐Duodu D et al. Patterns of failure in advanced stage diffuse large b‐cell lymphoma patients after complete response to r‐chop immunochemotherapy and the emerging role of consolidative radiation therapy. Int J Radiat Oncol Biol Phys 2013;86:569.–. [DOI] [PubMed] [Google Scholar]
- 15. Dorth JA, Chino JP, Prosnitz LR et al. The impact of radiation therapy in patients with diffuse large b‐cell lymphoma with positive post‐chemotherapy fdg‐pet or gallium‐67 scans. Ann Oncol 2011;22:405.–. [DOI] [PubMed] [Google Scholar]
- 16. Dorth JA, Prosnitz LR, Broadwater G et al. Impact of consolidation radiation therapy in stage iii‐iv diffuse large b‐cell lymphoma with negative post‐chemotherapy radiologic imaging. Int J Radiat Oncol Biol Phys 2012;84:762.–. [DOI] [PubMed] [Google Scholar]
- 17. Maurer C, Langerbeins P, Bahlo J et al. Effect of first‐line treatment on second primary malignancies and richter's transformation in patients with cll. Leukemia 2016;30:2019–2025. [DOI] [PubMed] [Google Scholar]
- 18. Hisada M, Biggar RJ, Greene MH et al. Solid tumors after chronic lymphocytic leukemia. Blood 2001;98:1979.–. [DOI] [PubMed] [Google Scholar]
- 19. Wadhwa PD and Morrison VA. Infectious complications of chronic lymphocytic leukemia. Semin Oncol 2006;33:240.–. [DOI] [PubMed] [Google Scholar]
- 20. Ravandi F and O'Brien S. Immune defects in patients with chronic lymphocytic leukemia. Cancer Immunol Immunother 2006;55:197.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anolik JH, Friedberg JW, Zheng B et al. B cell reconstitution after rituximab treatment of lymphoma recapitulates b cell ontogeny. Clin Immunol 2007;122:139.–. [DOI] [PubMed] [Google Scholar]
- 22. Stroopinsky D, Katz T, Rowe JM et al. Rituximab‐induced direct inhibition of t‐cell activation. Cancer Immunol Immunother 2012;61:1233.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lam CJ, Curtis RE, Dores GM et al. Risk factors for melanoma among survivors of non‐hodgkin lymphoma. J Clin Oncol 2015;33:3096.–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Campo E, Swerdlow SH, Harris NL et al. The 2008 who classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011;117:5019.–. [DOI] [PMC free article] [PubMed] [Google Scholar]