This prospective study evaluated the efficacy of comprehensive geriatric assessment in predicting survival and discriminating between causes of death in elderly patients with high‐risk stage II and stage III colorectal cancer who underwent curative resection to support the decision‐making process related to adjuvant therapy.
Keywords: Elderly, Colorectal cancer, Adjuvant therapy, Decision‐making, Comprehensive geriatric assessment, Competing risk model
Abstract
Background.
The challenge when selecting elderly patients with colorectal cancer (CRC) for adjuvant therapy is to estimate the likelihood that death from other causes will preclude cancer events from occurring. The aim of this paper is to evaluate whether comprehensive geriatric assessment (CGA) can predict survival and cancer‐specific mortality in elderly CRC patients candidates for adjuvant therapy.
Material and Methods.
One hundred ninety‐five consecutive patients aged ≥75 with high‐risk stage II and stage III CRC were prospectively included from May 2008 to May 2015. All patients underwent CGA, which evaluated comorbidity, polypharmacy, functional status, geriatric syndromes, mood, cognition, and social support. According to CGA results, patients were classified into three groups—fit, medium‐fit, and unfit—to receive standard therapy, adjusted treatment, and best supportive care, respectively. We recorded survival and cause of death and used the Fine‐Gray regression model to analyze competing causes of death.
Results.
Following CGA, 85 (43%) participants were classified as fit, 57 (29%) as medium‐fit, and 53 (28%) as unfit. The univariate 5‐year survival rates were 74%, 52%, and 27%. Sixty‐one (31%) patients died due to cancer progression (53%), non‐cancer‐related cause (46%), and unknown reasons (1%); there were no toxicity‐related deaths. Fit and medium‐fit participants were more likely to die due to cancer progression, whereas patients classified as unfit were at significantly greater risk of non‐cancer‐related death.
Conclusion.
CGA showed efficacy in predicting survival and discriminating between causes of death in elderly patients with high‐risk stage II and stage III resected CRC, with potential implications for shaping the decision‐making process for adjuvant therapies.
Implications for Practice.
Adjuvant therapy in elderly patients with colorectal cancer is controversial due to the high risk for competing events among these patients. In order to effectively select older patients for adjuvant therapy, we have to weigh the risk of cancer‐related mortality and the potential survival benefits with treatment against the patient's life expectancy, irrespective of cancer. This prospective study focused on the prognostic value of geriatric assessment for survival using a competing‐risk analysis approach, providing an important contribution on the treatment decision‐making process and helping clinicians to identify elderly patients who might benefit from adjuvant chemotherapy among those who will not.
Introduction
The rapid aging of the Western population and the higher incidence of cancer associated with old age are leading to an increase in the number of elderly patients who will require appropriate oncological treatment in the coming years [1].
Colorectal cancer (CRC) is the most common cancer affecting both sexes in Europe, and risk increases with each decade of life [2]. It is estimated that in the next decade more than 75% of the cases and 85% of deaths from CRC will occur in patients 65 years or older [3].
Survival for people with CRC continues to improve worldwide, probably because of better screening procedures as well as advances in surgery and adjuvant therapy [4], [5]. For patients with node‐positive CRC (stage III) and also sometimes for node‐negative patients (stage II) at high risk for systemic recurrence, the standard treatment is curative intent surgery followed by adjuvant chemotherapy [6]. There is no doubt that curative surgery should be offered to all elderly patients whenever the surgical risk is assumable. However, adjuvant treatment in this population is controversial because rigorous evidence on efficacy is scarce, and these therapies can be associated with considerable toxicity [7], [8]. As a result, nearly half of elderly patients do not receive postoperative treatment, even though experts generally agree that this population can attain the same benefits as younger adults with no excess toxicity [9], [10].
Aging is a process associated with a gradual loss of physiologic reserves along with a transformation that can be described in three phases, from a state of functional independence (fit) to one of vulnerability (medium‐fit), which finally imposes severe limitations on people, with no possibility of recovering functional reserves and limited life expectancy (unfit). However, aging is also characterized by great physiological heterogeneity, and chronological age can differ significantly from biological or functional age. Traditional tools used by oncologists to assess functional status, such as the Eastern Cooperative Group performance status (ECOG‐PS), are not accurate in older patients [11], and accumulating evidence supports the need for geriatric assessment in elderly cancer patients receiving onco‐specific treatment [12], [13], [14]. Comprehensive geriatric assessment (CGA) is a multidimensional tool that systematically evaluates all aspects of the patient's life that can have an impact on the tolerance and response to treatment. CGA has proven useful in creating a stratification system for patients according to their frailty profile and in predicting toxicity and mortality [15], [16], [17].
The critical challenge when deciding whether adjuvant treatment is appropriate in elderly patients is to estimate the likelihood that death from other causes will preclude cancer events (progression and death). Cancer patients of advanced age are not only at risk for cancer relapse or cancer‐related death; they also are at high risk of dying from non‐cancer‐related causes. This so‐called competing risk scenario may give rise to a competing event that prevents a cancer‐specific event from happening [18]. In order to assess survival gain in this population, it is necessary to consider both the probability of relapse and the risk of death from a competing cause.
The primary aim of this prospective study is to evaluate the efficacy of CGA in predicting survival and discriminating between causes of death in elderly patients with high‐risk stage II and stage III CRC who underwent curative resection in order to support the decision‐making process related to adjuvant therapy.
Materials and Methods
Study Design and Patient Population
This is a prospective cohort study carried out at the Institut Català d'Oncologia‐Hospital Duran i Reynals, a comprehensive cancer center with a catchment area of one million inhabitants and a reference center for six hospitals in the cancer care network of south Barcelona. Patients who underwent colectomy in the other general hospitals are referred to our center to determine the need for complementary adjuvant therapy. In 2008, an oncogeriatric unit was created, and CGA was incorporated as a part of the routine clinical assessment of incident CRC patients aged 75 and older. Our study population comprised all patients referred to our center from May 2008 to May 2015 with high‐risk stage II disease (poorly differentiated histology [G3], lymphovascular invasion, bowel obstruction or perforation at presentation, clinical stage of T4, fewer than 12 resected lymph nodes, and microsatellite‐stable disease) and stage III disease, after curative resection (R0 surgery with lymph node dissection) [19]. A team including a geriatrician and oncologist trained in geriatric oncology assessed all patients within 8 weeks postsurgery, treating them as appropriate. We excluded patients who did not undergo radical resection (R1–2 surgery), those with cancer metastases at diagnosis, and those that did not continue follow‐up for at least 6 months or until death. After approval by the Ethics Committee, all data were recorded by the principal investigators of the study at the oncogeriatrics unit and anonymously analyzed. A flow chart of the enrollment process is presented in supplemental online Figure 1.
Geriatric Assessment
All patients underwent a CGA that incorporated validated instruments to assess health and functional status based on their predictive validity in terms of mortality and morbidity in eight domains: functional status, nutritional status, cognitive status, psychological status, comorbidities, medication review, social support, and geriatric syndromes.
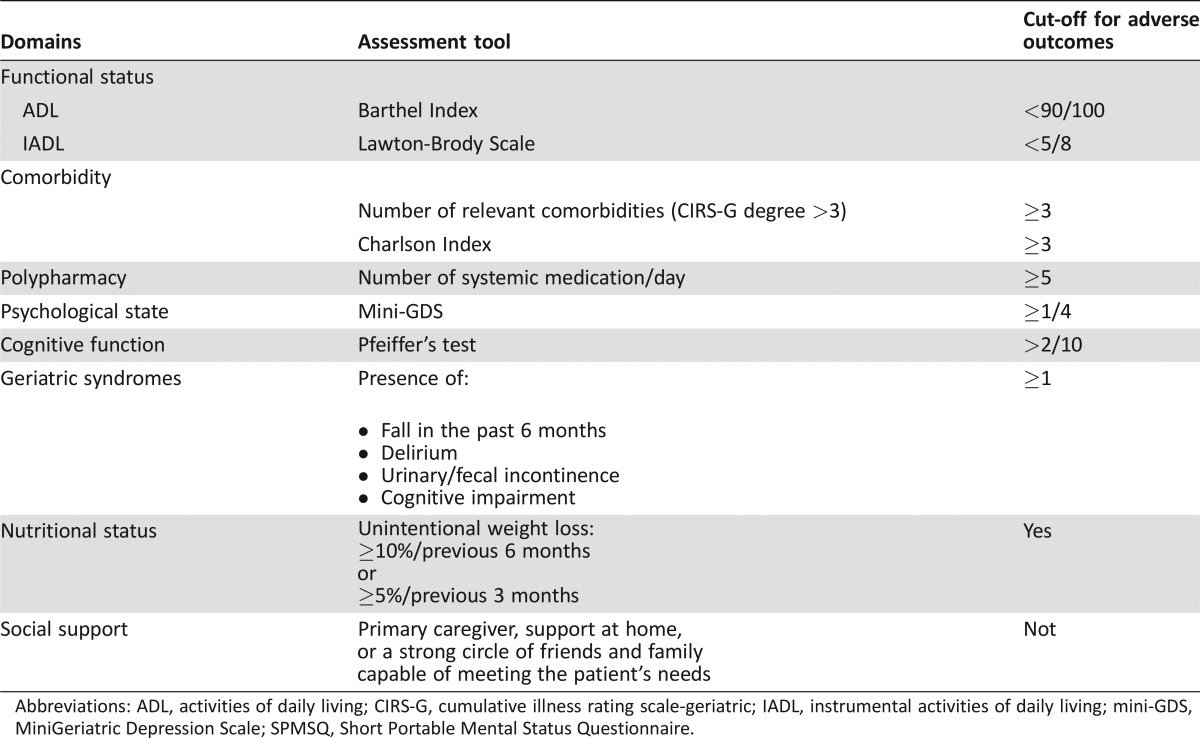
Functional status was measured using the Barthel Activities of Daily Living (ADL) [20] scale and the Lawton Index of Instrumental Activities of Daily Living (IADL) [21]. The Barthel Index includes ten items assessing basic self‐care abilities (transfer, bathing, toileting, dressing, feeding), with scores ranging from 0 to 100. Lawton's eight‐item instrument assesses independence in more complex activities of daily life that require interaction with the external environment: shopping, cooking, using the telephone, handling finances, housekeeping, laundry, self‐managing medication, and using transportation. The summary score ranges from 0 (low function, dependent) to 8 (high function, independent). To assess nutritional status, patients were asked about unintentional weight loss over 5% in the previous 3 months or over 10% in the previous 6 months. Cognitive status was determined using the Short Portable Mental Status Questionnaire (Pfeiffer's test), which assigns a score from 0 to 10 mistakes [22]. Mood was assessed using the four‐item Mini‐Geriatric Depression Scale [23]. Comorbidity was assessed by a modified Charlson Index [24], which includes neither cancer nor age. Because of the limitations of the Charlson index in the elderly, we also recorded the number of comorbid conditions, considered as >3 degrees in Cumulative Illness Rating Scale‐Geriatric (CIRS‐G) [25]. Data on current medication were collected according to self‐report and by reviewing the patient's medical charts; polypharmacy was defined as taking five or more oral medications each day. The social environment was considered good if the patient had a primary caregiver, support at home, or a strong circle of friends and family capable of meeting the patient's needs. A geriatric syndrome was determined by self‐reported number of falls in the last 6 months, cognitive impairment, delirium, and urinary and/or fecal incontinence. If cognitive impairment was detected by Pfeiffer's test, the patient's assessment was completed with a neurocognitive consultation; in case of confirmed cognitive impairment, we considered this a geriatric syndrome, not as a comorbid condition, in order to avoid overlap between comorbidity and geriatric syndrome domains. Incontinence was considered a geriatric syndrome if it wasn't stress incontinence and it was not related to tumor location or surgery. Scores and the cut‐off values of CGA variables are presented in Table 1.
Table 1. Domains, scores and cut‐off values of geriatric assessment.
Abbreviations: ADL, activities of daily living; CIRS‐G, cumulative illness rating scale‐geriatric; IADL, instrumental activities of daily living; mini‐GDS, MiniGeriatric Depression Scale; SPMSQ, Short Portable Mental Status Questionnaire.
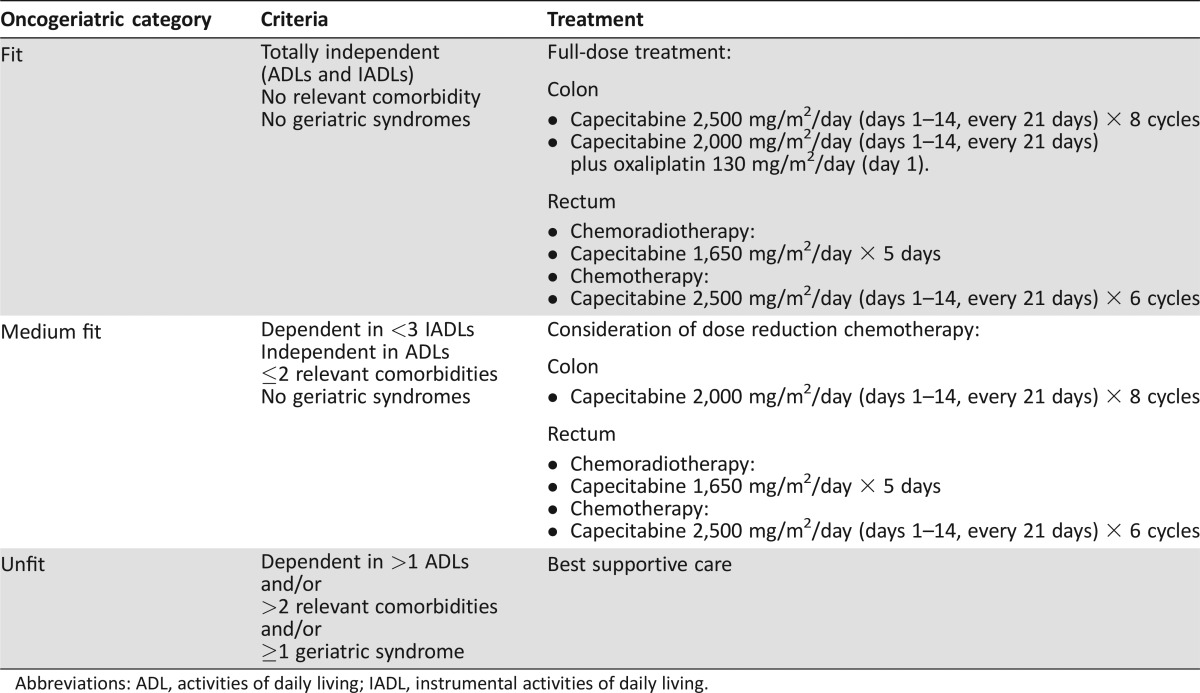
We then classified patients into three groups using a modification of the criteria proposed by Balducci and Extermann [26]. “Fit” patients were independent in all ADL and IADL and had no clinically significant comorbid conditions or geriatric syndromes; “medium‐fit” had fewer than three clinically significant comorbid conditions, fewer than four IADL, no ADL disabilities, and no geriatric syndromes; and “unfit” patients had any of the following characteristics: more than four IADL or ADL disabilities, multiple comorbidities, or geriatric syndromes. Fit subjects received standard therapy, medium‐fit patients received dose‐reduced adjusted treatment, and unfit patients received best supportive care (Table 2). When domains other than functional status, comorbidity, or geriatric syndromes were affected, and specific interventions were deemed appropriate, patients were referred to the social worker, dietician, pharmacist, psycho‐oncologist, or geriatrician to try to improve problems detected. The principal investigators followed up all patients in our unit, even unfit patients referred to the palliative unit care.
Table 2. Geriatric criteria to classify elderly colorectal cancer patients and administer therapy accordingly.
Abbreviations: ADL, activities of daily living; IADL, instrumental activities of daily living.
Outcome Variables
All patients were followed up for at least 6 months or until death. Primary outcome measures were overall survival (OS) and disease‐free survival (DFS), estimated from time of geriatric assessment.
Toxicity was graded before each cycle according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (version 4.0/NCI‐CTC 4.0). We collected data on drug discontinuation for any cause.
We retrieved causes of death for all patients who died in the study period. For patients who had died in a hospital, we obtained detailed mortality data from institutional medical records. For deaths occurring at home, the primary care doctor is responsible in our country for certifying the cause of death. In all cases, we compared the clinical information with that appearing in the official mortality registry, which includes data on the primary and two underlying causes of death. We classified causes of death as CRC‐specific mortality, toxicity, and non‐cancer‐related cause.
Statistical Analyses
OS and DFS probabilities were estimated using Kaplan‐Meier curves; survival functions by oncogeriatric category were compared using the Log Rank test.
The Cox proportional hazards regression model was applied to estimate independent effects associated with different explanatory variables by stepwise selection using p < .10 in univariate analysis. We calculated the incidence rate of dying from any cause, cancer‐related causes, and non‐cancer‐related causes according to oncogeriatric category.
Traditional approaches like Kaplan‐Meier survival analysis and Cox proportional hazards regression can overestimate the risk of disease by failing to consider competing risks of death [18]. To adjust for this competing risk scenario, we used the Fine‐Gray regression method [27], which considers the effect of predictors on the subhazard function and on the cumulative incidence function (CIF) to account for competing events, with cancer mortality treated as a competing event for non‐cancer mortality. When calculating the time to cancer relapse following chemotherapy, the CIF estimates the probability of cancer‐free survival, adjusting for the associated risk of dying from other causes. Raw and adjusted subdistribution hazard ratios (SHRs) and their corresponding CIF were determined using models that included oncogeriatric categories as an independent factor, adjusted for age, sex, stage (II vs. III), pathology (colon vs. rectum), and performance status (PS) score (0–1 vs. >1). Interpretation of parameters from the Fine‐Gray model is not straightforward, as the subdistribution hazard has no resemblance to an epidemiological rate if individuals die from other causes in the risk set. Although SHR sounds like a hazard ratio, it is not the same, mainly because individuals who die from another cause remain in the risk set even though they are no longer at risk of experiencing the cause of interest. Thus, it is advisable to focus on the statistical significance and the direction of the parameter but not on the magnitude of effect [28].
All analyses were conducted using R software (version 3.2.2) [29].
Results
Patient Characteristics
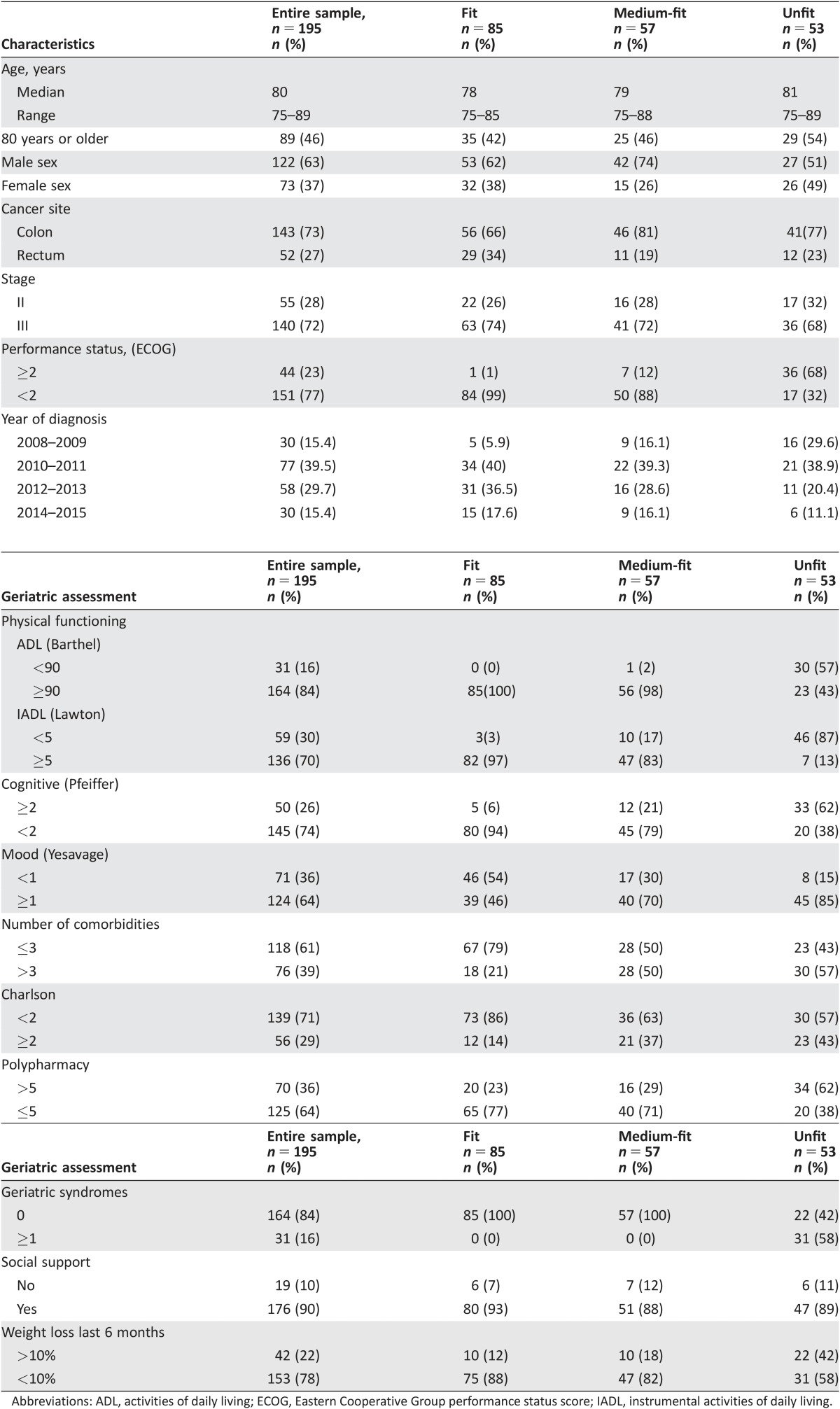
The study population consisted of 195 elderly patients with high‐risk stage II (n = 55) and stage III (n = 140) resected CRC with an indication for adjuvant chemotherapy. The mean age of the patient sample was 80 years (range 75–89), and 46% were over 80. Most of our patients (77%) had a relatively good performance status (ECOG‐PS <2) at study entry. The median follow‐up was 28.7 months (range 0.40–84.37) or 35.5 months if we take into account only patients alive at the moment of the analyses
Geriatric Assessment
Most of the patients were able to independently perform most ADL: the Barthel‐ADL index was ≥90 in 84% of participants, while the Lawton‐IADL index was ≥5 in 70% of the patients. Sixty‐one percent had two or fewer relevant comorbidities, and 84% had no geriatric syndromes. Detailed results of baseline clinical and tumor characteristics, and geriatric assessment are displayed in Table 3.
Table 3. Patient, clinical, and geriatric assessment characteristics at inclusion by onocogeriatric groups.
Abbreviations: ADL, activities of daily living; ECOG, Eastern Cooperative Group performance status score; IADL, instrumental activities of daily living.
On the basis of CGA, 85 (43%) patients were classified as fit, 57 (29%) as medium‐fit, and 53 (28%) as unfit. This result was then applied to provide guidance on the best adjuvant treatment for each individual patient.
A total of 142 (73%) fit and medium‐fit patients were initially considered eligible to receive adjuvant treatment. However, the indication for chemotherapy was rejected due to surgical complications in ten patients and due to a specific contraindication for chemotherapy in seven. Of the remaining 125 patients, 17 refused treatment.
Association Between CGA and Compliance
Of the 108 patients who received adjuvant therapy, 76 (70%) fit patients were administered standard adjuvant therapy, and 32 (30%) medium‐fit patients were treated with a tailored‐dose adjuvant strategy; those not undergoing any postoperative chemotherapy were followed up. Unfit patients received best supportive care. Fifty‐seven (53%) patients receiving chemotherapy were able to complete the scheduled cycles. The remaining 51 patients discontinued treatment before completion because of toxicity (61%), cancer recurrence (6%), aggravation of comorbidities (29%), or patient decision (4%). Eighteen of the 76 (24%) fit patients experienced grade 3 toxicity, compared with 5/32 (16%) of the patients classified as medium‐fit (p > .05).
Association Between CGA and Mortality
At the time of analysis, 52 (27%) patients had experienced tumor recurrence. The fit and medium‐fit groups had not reached the median DFS by study end, while in the unfit group, it was 1,003 days. The DFS rates (95% confidence interval [CI]; number still at risk) at 3 years following surgery were 77% (0.67–0.87; n = 28), 66% (0.51–0.79; n = 22), and 47% (0.28–0.61; n = 10) in the fit, medium‐fit, and unfit groups, respectively.
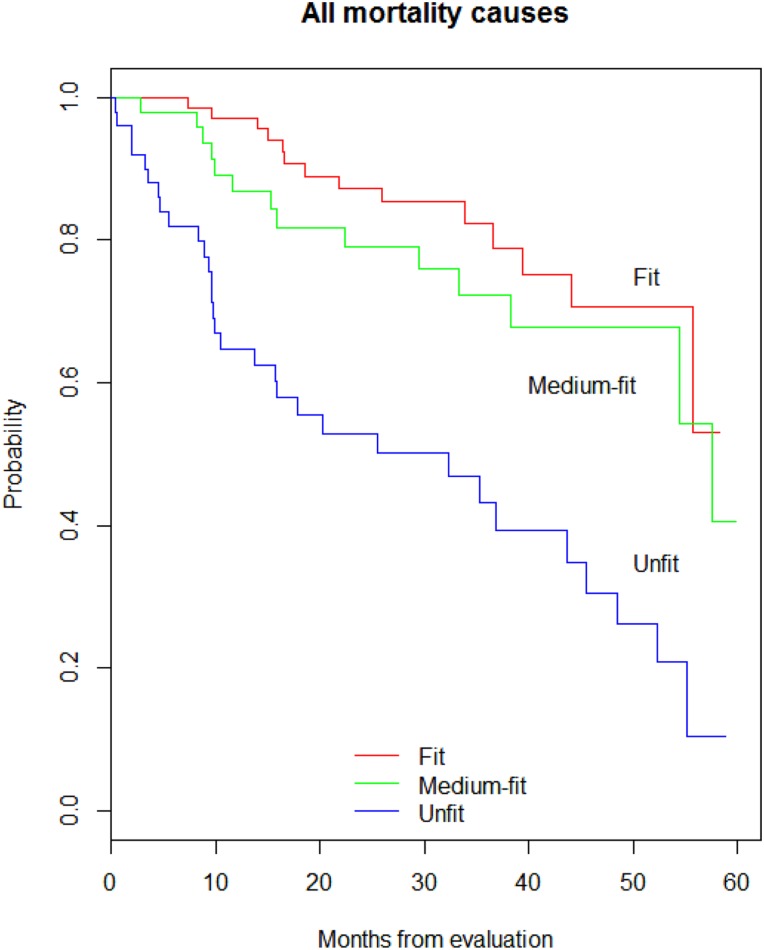
Less than half of the fit and medium‐fit patients had died by study end, while median OS in the unfit patients was 27.6 months. The respective 1‐, 3‐ and 5‐year survival rates were 97%, 85%, and 74% in fit, 90%, 73%, and 52% in medium‐fit, and 71%, 48%, and 27% in unfit participants (log rank, p < .001; Fig. 1).
Figure 1.
Kaplan‐Meier curves of five‐year overall survival in total sample (n = 195) of patients with colorectal cancer by oncogeriatric categories: fit (n = 85), medium‐fit (n = 57) and unfit (n = 53).
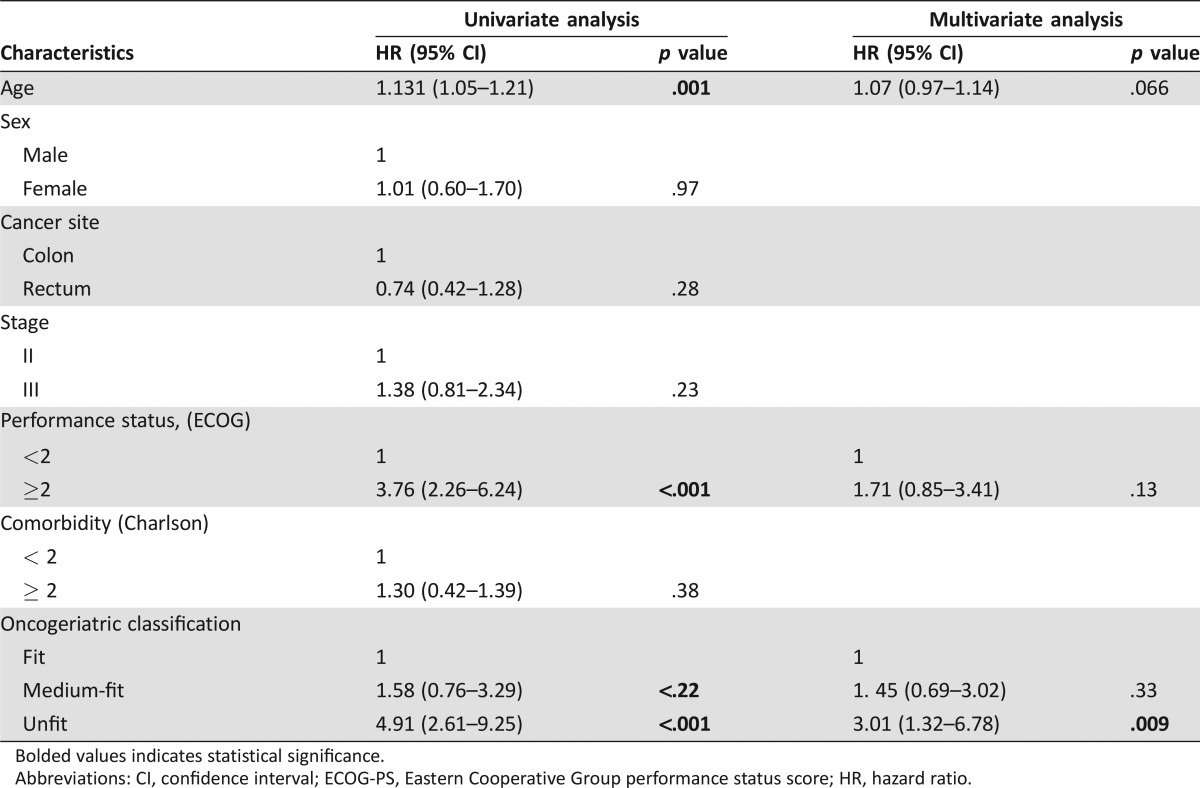
In univariable Cox regression analysis, age, ECOG‐PS, and geriatric classification were prognostic factors for OS. In the multivariable model, geriatric classification was the only independent factor for OS after adjusting for age, tumor site, clinical stage, ECOG‐PS, and comorbidity (Table 4).
Table 4. Univariate and multivariate analysis of covariates associated with the overall survival in the whole cohort.
Bolded values indicates statistical significance.
Abbreviations: CI, confidence interval; ECOG‐PS, Eastern Cooperative Group performance status score; HR, hazard ratio.
At the end of the follow‐up, 61 (31%) patients had died (14 fit, 16 medium‐fit, and 31 unfit) due to cancer progression (53%), non‐cancer‐related cause (46%), and unknown reasons (1%); there were no toxicity‐related deaths.
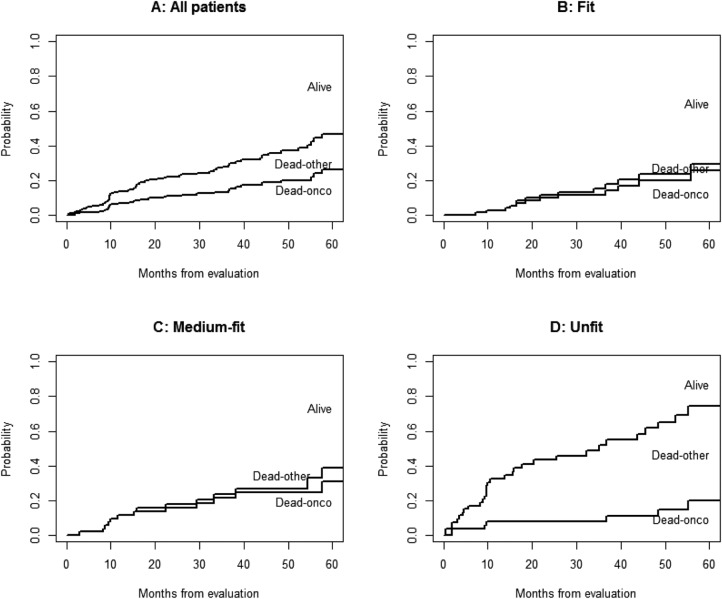
Overall, 53% of patients died of cancer. Figure 2 shows the CIF by cause of death: the proportion of patients alive, patients who died due to cancer, and patients who died from causes other than cancer. Thus, the overall patient population (Fig. 2A) was more likely to die of a cancer‐related cause than a non‐cancer‐related cause. However, stratification showed that fit (Fig. 2B) and medium‐fit (Fig. 2C) patients had an almost null probability of dying from a non‐cancer cause, whereas in unfit patients (Fig. 2D), the risk of dying from other causes exceeded that of dying from cancer.
Figure 2.
Cumulative incidence function of cause of death for all patients and by oncogeriatric category.
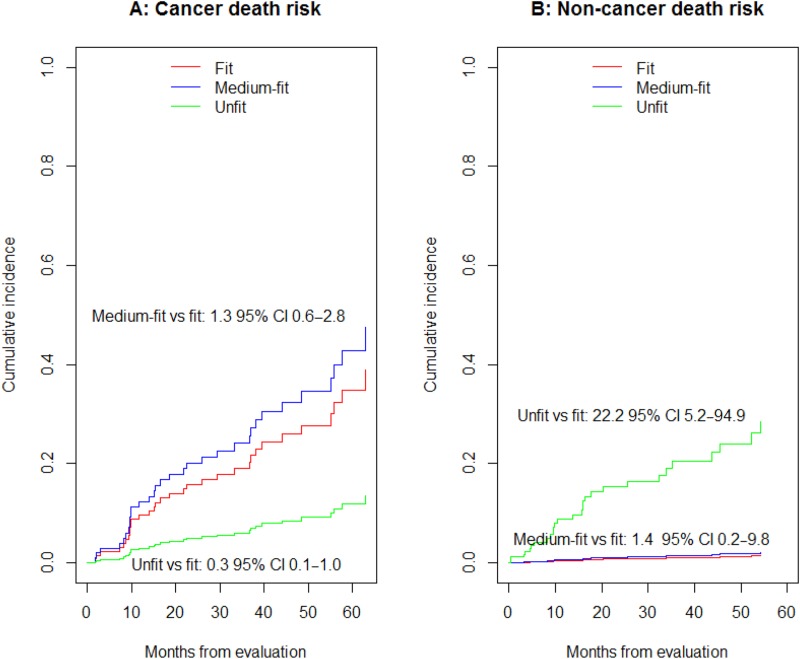
At 3 and 5 respective years following surgery, 23% and 42% fit, 30% and 52% medium‐fit, and 8% and 15% unfit patients died from cancer‐related causes (Fig. 3A). On the contrary, respective 3‐ and 5‐year mortality due to non‐cancer‐related causes was <2% and <3% in fit and medium‐fit patients but 20% and 28% in unfit patients (Fig. 3B).
Figure 3.
Adjusted subdistribution function of cancer‐related mortality (A) and non‐cancer‐related mortality (B) by oncogeriatric category.
Abbreviations: CI, confidence interval.
In the Fine‐Gray adjusted model (Fig. 3), unfit patients were at significantly lower risk of dying from a cancer‐related cause (SHR 0.30, 95% CI 0.09–0.96; Fig. 3A) and at significantly greater risk of dying from a non‐cancer‐related cause (SHR 22.29, 95% CI 5.24–94.78; Fig. 3B) compared with fit patients.
Discussion
Our study reports the results of CGA, carried out within the framework of clinical practice, in a cohort of 195 consecutive elderly patients with high‐risk stage II and stage III CRC, following resection with a curative intent. This study shows the utility of CGA in helping clinicians decide whether or not to administer adjuvant chemotherapy in a well‐defined group of elderly patients with localized CRC for whom therapeutic decision‐making is especially challenging.
Because postoperative chemotherapy reduces the risk of tumor recurrence and improves survival for patients with resected CRC, adjuvant chemotherapy is recommended for patients with stage III disease and sometimes in node‐negative (stage II) when a high risk for systemic recurrence exists. The evidence for elderly patients’ tolerance of chemotherapy suggests that age alone should not determine candidacy for adjuvant therapy [30], [31]. Individuals who reach old age without loss of functional capacity or severe medical conditions should be able to benefit from the most appropriate treatment according to their biological age. A major issue confronting oncologists is how to effectively recognize fit older patients and how to predict whether a competing cause of death might preclude cancer events from occurring. Oncologists have to decide whom to treat after considering the risk of cancer‐related mortality and the potential survival benefits with treatment but also the patient's life expectancy irrespective of cancer.
To date, several studies have described the prognostic impact of geriatric assessment in cancer patients, but most of them were performed in heterogeneous groups of patients with different tumor types and disease stages [32], [33], [34]. To our knowledge, this is the first prospective study focused on the prognostic value of CGA for survival in elderly CRC patients who are candidates to receive adjuvant therapy.
In our study, CGA category was independently associated with survival once adjusted for variables commonly used by oncologists to decide treatment (age, tumor site, TNM stage, PS, and comorbidity).
The present study reported a 5‐year OS in the fit group (74%) that was comparable to those in other population‐based studies [35], demonstrating that CGA is useful in identifying patients who might benefit from adjuvant chemotherapy. In patients with CRC treated with adjuvant therapy, there is a close correlation between 3‐year DFS and 5‐year OS [36]. In our study, we observed this relationship in the fit group (77% and 74%) but, interestingly, not in the unfit group (47% and 27%), probably because cancer relapse was not the main cause of death.
Several competing risk models for predicting breast cancer [37], prostate cancer [38], renal cell carcinoma [39], and thyroid cancer [40] have been published. Recently, a novel competing risk approach to better stratify patients according to their causes of death has been validated in an elderly population with an appreciable risk of competing events (prostate, head and neck, and breast cancer) [41]; however, it hasn't included variables like nutritional or functional status, which are crucial when classifying elderly patients according to their frailty profile. Our competing risk approach showed how CGA helped to stratify elderly CRC patients according to their risk for cancer versus non‐cancer‐related death. Stratifying by oncogeriatric categories, fit and medium‐fit patients had an almost null probability of dying due to a non‐cancer cause, whereas unfit patients were twice as likely to die from non‐cancer‐related causes compared with cancer‐related causes. Life expectancy in unfit patients was explained by their global health status and not just by the oncological disease. Therefore, we can conclude that among unfit patients, the appropriate therapeutic decision based on competing survival analysis was not administering adjuvant treatment.
Major strengths of our study include its prospective design, a standardized CGA, and the fact that the same team both performed CGA and treated patients. CGA parameters were prospectively obtained prior to initiation of chemotherapy, reflecting the patient's baseline health rather than toxicities of therapy. Furthermore, CGA was performed on all recently resected CRC patients who were referred to our center to be considered for adjuvant therapy, without previous selection. Finally, the use of competing risk methodology allows us to assess this crucial factor in analyzing the impact on survival and therefore to best inform the clinical decision‐making process.
At the same time, the study also has some limitations that should be taken into account. It was carried out at a single institution and included a limited number of patients. It is remarkable that most of the patients were fully independent according to evaluations of ADL and IADL, with a probable selection bias, as only patients in apparently good condition were referred to our reference center to be treated. Follow‐up is not complete in all patients for analysis of recurrence rates and longer‐term survival. Furthermore, data collected did not include socioeconomic status, which can influence the availability of medical and support resources.
Stemming from the need for a common tool and methodology, this research adds to a consistent body of evidence showing that CGA improves the way clinicians evaluate and classify elderly cancer patients in order to lead to a more personalized therapy plan. Traditionally, tools used by oncologists to assess functional status have not been considered useful in elderly patients. Provision of competent cancer care to this growing CRC population would require that oncology professionals become familiar with age‐associated changes in organ physiology and their impact on survival and toxicity. Integrating a standardized geriatric assessment tool is the first step for oncologists seeking to better tailor their treatments. In fact, the International Society for Geriatric Oncology [31] and National Comprehensive Cancer Network guidelines [42] recommend that a geriatric assessment be performed in all cancer patients older than 70.
Conclusion
In conclusion, our prospective study adds to the growing body of literature supporting the relevant role of CGA for clinical decision‐making in older adults with cancer. We have shown that CGA is a useful tool in clinically assessing older adults with high‐risk stage II–III CRC, for whom making a therapeutic decision is especially challenging. We would like to highlight that using a competing risk approach is critical to analyzing adjuvant treatment for elderly individuals because it helps to minimize the bias that would be introduced by a traditional survival analysis and therefore best informs clinical decision‐making.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We would like to thank Meggan Harris for her editorial assistance in the preparation of this paper. This is an academic study partially supported by the Fund for Health Research (FIS, PI 060990), AGAUR (2014 SGR 0635), and the Cancer Research Network (RTICC, RD 12/0036/0053) of Carlos III Institute of Health (Instituto de Salud Carlos III/ISCIII; co‐funded by FEDER funds/European Regional Development Funds). This study has been partially presented in ESMO 2016.
Author Contributions
Conception/Design: Maite Antonio, Alberto Carmona‐Bayonas, Valentín Navarro, Marga Nadal, Francesc Formiga, Josep Maria Borràs
Provision of study material or patients: Maite Antonio, Juana Saldaña
Collection and/or assembly of data: Maite Antonio, Juana Saldaña, Cristian Tebé
Data analysis and interpretation: Maite Antonio, Alberto Carmona‐Bayonas, Valentín Navarro, Cristian Tebé, Ramon Salazar, Josep Maria Borràs
Manuscript writing: Maite Antonio, Alberto Carmona‐Bayonas, Marga Nadal, Francesc Formiga, Ramon Salazar, Josep Maria Borràs
Final approval of manuscript: Maite Antonio, Juana Saldaña, Alberto Carmona‐Bayonas, Valentín Navarro, Cristian Tebé, Marga Nadal, Francesc Formiga, Ramon Salazar, Josep Maria Borràs
Disclosures
Cristian Tebé: Boehringer Ingelheim (H); Amgen (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Shih YC, Hurria A. Preparing for an epidemic: Cancer care in an aging population. Am Soc Clin Oncol Educ Book 2014:133–137. [DOI] [PubMed] [Google Scholar]
- 2. Siegel R, Ma J, Zou Z et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute: Surveillance, Epidemiology, and End Results Program: SEER Fact Sheets‐Colon and rectum Cancer. Available at https://seer.cancer.gov/statfacts/html/colorect.html. Accessed April 4, 2017.
- 4. Brenner H, Bouvier AM, Foschi R et al. Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: The EUROCARE study. Int J Cancer 2012;131:1649–1658. [DOI] [PubMed] [Google Scholar]
- 5. Holleczek B, Rossi S, Domenic A et al. On‐going improvement and persistent differences in the survival for patients with colon and rectum cancer across Europe 1999–2007—Results from the EUROCARE‐5 study. Eur J Cancer 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Colon cancer. Version 2.2015. 2015.
- 7. van Erning FN, Janssen‐Heijnen ML, Creemers GJ et al. Deciding on adjuvant chemotherapy for elderly stage III colon cancer patients: A qualitative insight into the perspectives of surgeons and medical oncologists. J Geriatr Oncol 2015;6:219–224. [DOI] [PubMed] [Google Scholar]
- 8. Hung A, Mullins CD. Relative effectiveness and safety of chemotherapy in elderly and nonelderly patients with stage III colon cancer: A systematic review. The Oncologist 2013;18:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aparicio T, Navazesh A, Boutron I et al. Half of elderly patients routinely treated for colorectal cancer receive a sub‐standard treatment. Crit Rev Oncol Hematol 2009;71:249–257. [DOI] [PubMed] [Google Scholar]
- 10. Serra‐Rexach JA, Jimenez AB, García‐Alhambra MA et al. Differences in the therapeutic approach to colorectal cancer in young and elderly patients. The Oncologist 2012;17:1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Repetto L, Fratino L, Audisio RA et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology study. J Clin Oncol 2002;20:494–502. [DOI] [PubMed] [Google Scholar]
- 12. Wildiers H, Heeren P, Puts M et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 2007;25:1824–1831. [DOI] [PubMed] [Google Scholar]
- 14. Puts MT, Hardt J, Monette J et al. Use of geriatric assessment for older adults in the oncology setting: A systematic review. J Natl Cancer Inst 2012;104:1133–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kenis C, Bron D, Libert Y et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: Results of a prospective multicentric study. Ann Oncol 2013;24:1306–1312. [DOI] [PubMed] [Google Scholar]
- 16. Extermann M, Boler I, Reich RR et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer 2012;118:3377–3386. [DOI] [PubMed] [Google Scholar]
- 17. Hurria A, Togawa K, Mohile SG et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol 2011;29:3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berry SD, Ngo L, Samelson EJ et al. Competing risk of death: An important consideration in studies of older adults. J Am Geriatr Soc 2010;58:783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Labianca R, Nordlinger B, Beretta GD et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2013;24(suppl 6):vi64–vi72. [DOI] [PubMed] [Google Scholar]
- 20. Mahoney FI, Barthel DW. Functional evaluation: The Barthel index. Md State Med J 1965;14:61–65. [PubMed] [Google Scholar]
- 21. Lawton MP, Brody EM. Assessment of older people: Self‐maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186. [PubMed] [Google Scholar]
- 22. Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 1975;23:433–441. [DOI] [PubMed] [Google Scholar]
- 23. D'Ath P, Katona P, Mullan E et al. Screening, detection and management of depression in elderly primary care attenders. I: The acceptability and performance of the 15 item Geriatric Depression Scale (GDS15) and the development of short versions. Fam Pract 1994;11:260–266. [DOI] [PubMed] [Google Scholar]
- 24. Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 25. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc 1968;16:622–626. [DOI] [PubMed] [Google Scholar]
- 26. Balducci L, Extermann M. Management of cancer in the older person: A practical approach. The Oncologist 2000;5:224–237. [DOI] [PubMed] [Google Scholar]
- 27. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 28. Andersen PK, Geskus RB, de Witte T et al. Competing risks in epidemiology: Possibilities and pitfalls. Int J Epidemiol 2012;41:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Team RC. R: A language and environment for statistical computing. Vienna ARF for SC 2012. Available at http://cran r‐project org. Accessed April 7, 2017.
- 30. Sargent DJ, Goldberg RM, Jacobson SD et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091–1097. [DOI] [PubMed] [Google Scholar]
- 31. Papamichael D, Audisio RA, Glimelius B et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol 2015;26:463–476. [DOI] [PubMed] [Google Scholar]
- 32. Puts MT, Santos B, Hardt J et al. An update on a systematic review of the use of geriatric assessment for older adults in oncology. Ann Oncol 2014;25:307–315. [DOI] [PubMed] [Google Scholar]
- 33. Hamaker ME, Schiphorst AH, ten Bokkel Huinink D et al. The effect of a geriatric evaluation on treatment decisions for older cancer patients–A systematic review. Acta Oncol 2014;53:289–296. [DOI] [PubMed] [Google Scholar]
- 34. Ommundsen N, Wyller TB, Nesbakken A et al. Frailty is an independent predictor of survival in older patients with colorectal cancer. The Oncologist 2014;19:1268–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Böckelman C, Engelmann BE, Kaprio T et al. Risk of recurrence in patients with colon cancer stage II and III: A systematic review and meta‐analysis of recent literature. Acta Oncol 2015;54:5–16. [DOI] [PubMed] [Google Scholar]
- 36. Sargent DJ, Wieand HS, Haller DG et al. Disease‐free survival versus overall survival as a primary end point for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2005;23:8664–8670. [DOI] [PubMed] [Google Scholar]
- 37. Sun W, Jiang YZ, Liu YR et al. Nomograms to estimate long‐term overall survival and breast cancer‐specific survival of patients with luminal breast cancer. Oncotarget 2016;7:20496–20506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Raldow AC, Zhang D, Chen MH et al. Risk group and death from prostate cancer: Implications for active surveillance in men with favorable intermediate‐risk prostate cancer. JAMA Oncol 2015;1:334–340. [DOI] [PubMed] [Google Scholar]
- 39. Weng SF, Chiu YH, Jan RL et al. Death does matter—Cancer risk in patients with end‐stage renal disease. Medicine (Baltimore) 2016;95:e2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang L, Shen W, Sakamoto N. Population‐based study evaluating and predicting the probability of death resulting from thyroid cancer and other causes among patients with thyroid cancer. J Clin Oncol 2013;31:468–474. [DOI] [PubMed] [Google Scholar]
- 41. Carmona R, Zakeri K, Green G et al. Improved method to stratify elderly patients with cancer at risk for competing events. J Clin Oncol 2016;34:1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hurria A, Wildes T, Blair SL et al. Senior adult oncology, Version 2.2014: Clinical practice guidelines in oncology. J Natl Compr Canc Netw 2014;12:82–126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.