Abstract
Lessons Learned.
Difficulties in translating in vitro results into clinical practice are inevitable.
Further efforts to verify the efficacy of alternative schedules of pemetrexed in solid tumors are encouraged.
Background.
We investigated the cytotoxic activity of pemetrexed in combination with several drugs (gemcitabine, carboplatin, vinorelbine, and mitomycin C) using different exposure schedules in three colon cancer cell lines. The best results were obtained with the following schedule: a prolonged pemetrexed exposure followed by a 48‐hour wash‐out and then gemcitabine. This combination was then advanced to a phase II clinical trial.
Methods.
Patients with metastatic colorectal cancer in progression after standard treatment were included in the study. Adequate bone marrow reserve, normal hepatic and renal function, and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 were required. Treatment consisted of an 8‐hour intravenous infusion of pemetrexed 150 mg/m2 on day 1 and a 30‐minute intravenous infusion of gemcitabine 1,000 mg/m2 on day 3 of each cycle, repeated every 14 days.
Results.
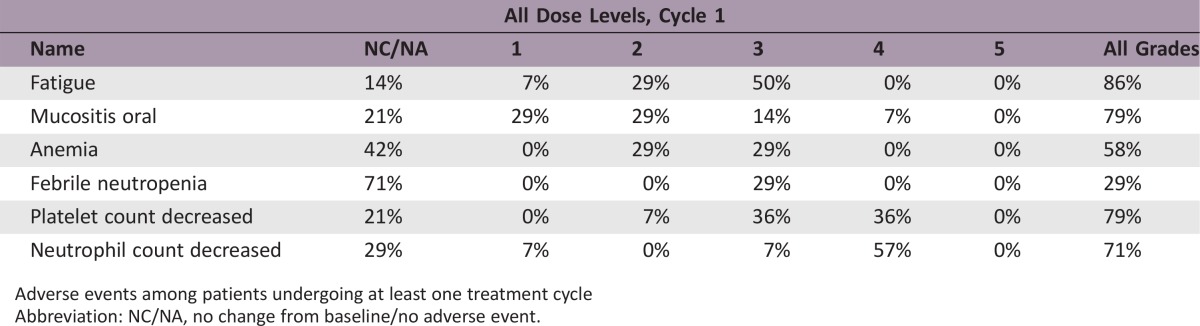
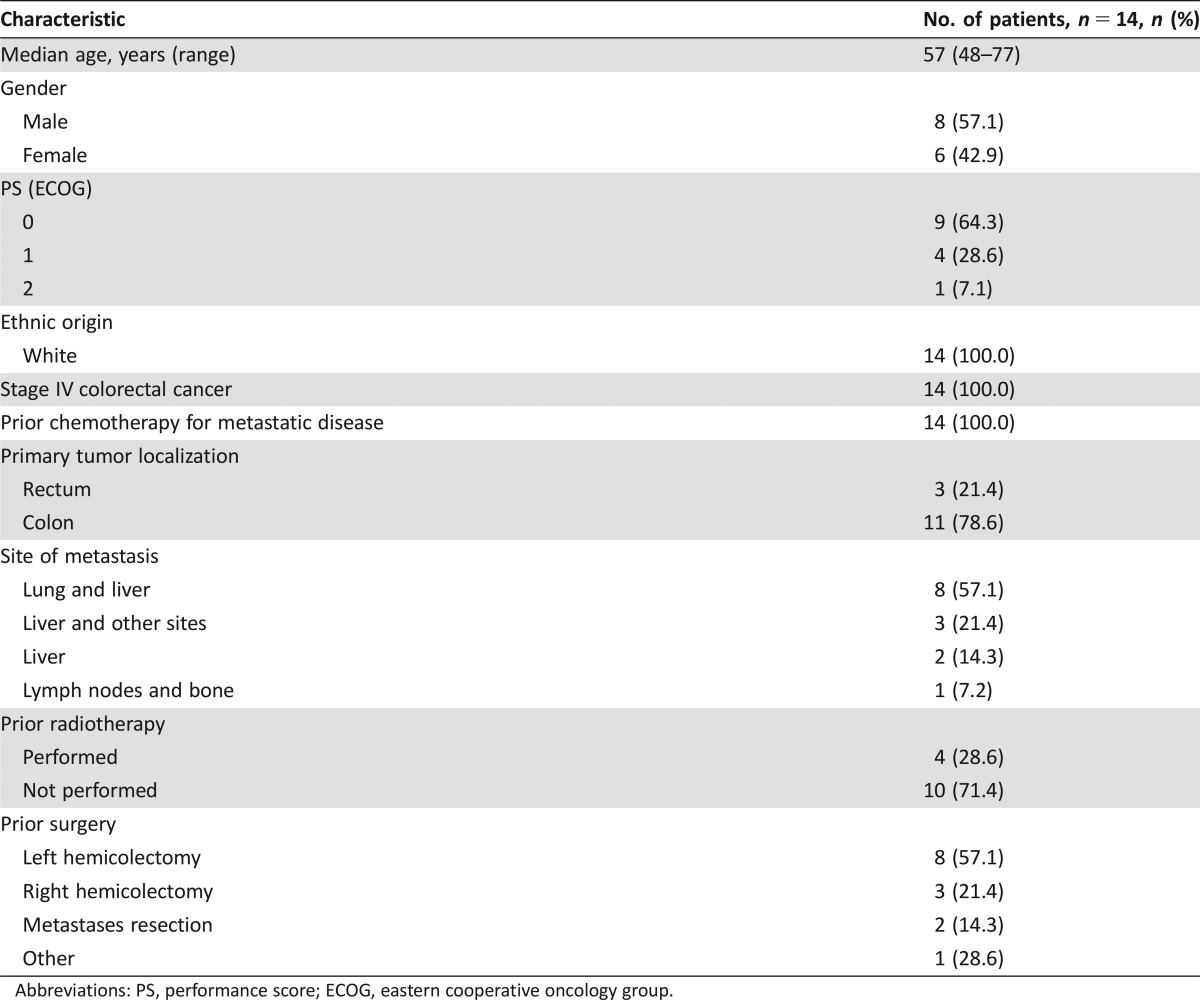
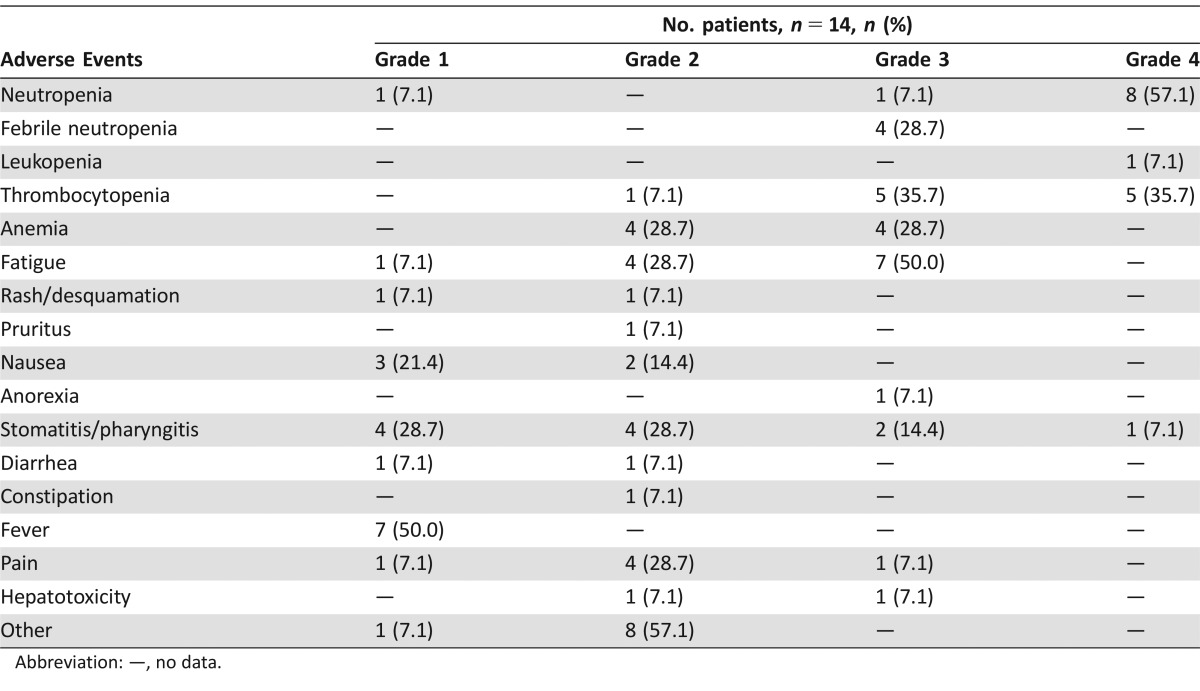
Fourteen patients were enrolled onto the study (first step). No objective responses were seen, and evidence of stable disease was observed in only one of the 12 evaluable patients. The most important grade 3–4 side effects were hematological toxicity (neutropenia 64.2%, thrombocytopenia 71.4%, anemia 28.7%), fatigue (50.0%), and stomatitis (21.5%). Median overall survival and time to progression were 5.8 months (95% confidence interval [CI]: 3.9–7.1) and 2.1 months (95% CI: 1.7–2.8), respectively.
Conclusion.
The experimental pemetrexed‐gemcitabine combination proved to be inactive and moderately toxic.
Abstract
经验总结
• 将体外结果转化为临床实践时, 遇到困难在所难免。
• 鼓励开展进一步工作来验证培美曲塞替代方案治疗实体瘤的疗效。
摘要
背景. 我们在3种结肠癌细胞系中考察了培美曲塞以不同给药方案与数种药物(吉西他滨、卡铂、长春瑞滨和丝裂霉素C)联用时的细胞毒活性。使用以下方案时获得了最佳结果:延长培美曲塞给药时间, 48小时洗脱期之后再给予吉西他滨。随后将这一联合用药方案推进至II期临床试验阶段。
方法. 本研究纳入了标准治疗后疾病进展的转移性结直肠癌患者。要求患者具有适当的骨髓储备、肝肾功能正常且美国东部肿瘤协作组(ECOG)体力状态评分为0‐2。治疗方案为在每个周期的第1天静脉输注培美曲塞150 mg/m2, 输注8小时, 并在第3天静脉输注吉西他滨1 000 mg/m2, 输注30分钟, 每14天为一个周期。
结果. 14例患者入组研究(第1步)。未观察到客观缓解, 12例可评价患者中仅有1例观察到疾病稳定证据。最重要的3‐4级副作用为血液学毒性(中性粒细胞减少症64.2%, 血小板减少症71.4%, 贫血28.7%)、疲乏(50.0%)和口腔炎(21.5%)。中位总生存期和至疾病进展时间分别为5.8个月[95%置信区间(CI):3.9‐7.1]和2.1个月(95% CI:1.7‐2.8)。
结论. 本研究证明, 培美曲塞与吉西他滨的实验性联合治疗方案无效且具有中等毒性。
Discussion
Although gemcitabine and pemetrexed have shown preclinical and clinical activity in patients with metastatic colorectal cancer, clinical data remain inconclusive [1], [2], [3], [4], [5], [6], [7], [8]. A critical review of the available literature shows an evident incoherence between preclinical data and clinical trial design. From in vitro experiments, it seems clear that the administration of pemetrexed should precede all other drugs, with the possible exception of irinotecan, to increase cell kill and induce a synergistic effect [9], [10]. Clinical studies of pemetrexed‐containing regimens have ignored this important finding in that the cytotoxics are generally infused concomitantly. Moreover, pemetrexed is commonly administered intravenously as a 10‐minute infusion, but there is a strong body of evidence that anti‐metabolites such as 5‐fluorouracil and gemcitabine, when given in continuous intravenous infusion, show a different efficacy and toxicity pattern compared with the same dose given as an intravenous bolus [11], [12], [13]. In particular, our preclinical experience suggests that a prolonged exposure (6 or 12 hours) to pemetrexed leads to higher antitumor activity than a short exposure (<6 hours) and that a wash‐out time between each drug administration ranging from 48 to 72 hours is essential for the induction of both cell cycle perturbation and apoptosis [14]. The reasons for this are not clear, and more in‐depth pharmacokinetic studies are warranted.
The experimental regimen was based on these assumptions, in particular the administration of pemetrexed as a continuous intravenous infusion followed by a 48‐hour wash‐out and then gemcitabine infusion. The chosen dose of pemetrexed derived from a phase Ib trial performed at our institute, which showed the feasibility of a 12‐hour continuous infusion of pemetrexed 200 mg/m2 every 2 weeks in patients with cancer who were previously treated (data not published). However, clinical results of the present study were not in line with the preclinical rationale, and the study was closed at the first step because of important toxicity and the absence of proven significant activity.
Trial Information
- Disease
Colorectal cancer
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
More than two prior regimens
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Single arm
- Primary Endpoint
Overall response rate
- Secondary Endpoint
Toxicity
- Secondary Endpoint
Time to progression
- Secondary Endpoint
Overall survival
- Additional Details of Endpoints or Study Design
- A minimax two‐stage Simon design was employed. A 10% response would preclude further study, whereas a 30% response rate would indicate that further study would be warranted. Using α and β errors of 0.10 and 0.10, respectively, 12 patients were required in the first stage, and if 1 or 0 responses were observed, the trial had to be terminated. Otherwise, an additional 23 patients were to be enrolled. If 5 or fewer responses were observed in 35 patients, the combination would not have been considered worthy of further study; however, if 6 or more responses were observed, the combination would have been considered sufficiently active to warrant further testing.
- Investigator's Analysis
Level of activity did not meet planned endpoint
Drug Information for Phase II Pemetrexed/Gemcitabine
- Drug 1
- Generic/Working name
Pemetrexed
- Trade name
Alimta
- Company name
Lilly
- Drug type
- Drug class
Antimetabolite
- Dose
150 milligrams (mg) per squared meter (m2)
- Route
Continuous intravenous infusion (cIV)
- Schedule of Administration
Eight‐hour intravenous infusion of pemetrexed 150 mg/m2 on day 1 and a 30‐minute intravenous infusion of gemcitabine 1,000 mg/m2 on day 3 of each cycle, repeated every 14 days
- Drug 2
- Generic/Working name
Gemcitabine
- Drug type
- Drug class
Antimetabolite
- Dose
1,000 milligrams (mg) per squared meter (m2)
- Route
IV
- Schedule of Administration
Eight‐hour intravenous infusion of pemetrexed 150 mg/m2 on day 1 and a 30‐minute intravenous infusion of gemcitabine 1000 mg/m2 on day 3 of each cycle, repeated every 14 days.
Patient Characteristics for Phase II Pemetrexed/Gemcitabine
- Number of patients, male
8
- Number of patients, female
6
- Stage
Stage IV colorectal cancer
- Age
Median (range): 57 (48–77)
- Number of prior systemic therapies
Median (range): ≥2 in all patients
- Performance Status: ECOG
-
0 — 9
1 — 4
2 — 1
3 — 0
Unknown — 0
- Other
Not Collected
- Cancer Types or Histologic Subtypes
Primary Assessment Method for Phase II Pemetrexed/Gemcitabine
- Number of patients screened
14
- Number of patients enrolled
14
- Number of patients evaluable for toxicity
14
- Number of patients evaluated for efficacy
12
- Evaluation method
RECIST 1.0
- Response assessment CR
n = 0
- Response assessment PR
n = 0
- Response assessment SD
n = 1
- Response assessment PD
n = 11
- Response assessment OTHER
n = 0
- (Median) duration assessments PFS
2.1 months, CI: 1.7–2.8
- (Median) duration assessments OS
5.8 months, CI: 3.9–7.1
Adverse Events: Phase II Pemetrexed/Gemcitabine
Adverse events among patients undergoing at least one treatment cycle
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Terminated reason
Toxicity
- Pharmacokinetics/Pharmacodynamics
Not collected
- Investigator's Assessment
Level of activity did not meet planned endpoint
Metastatic colorectal cancer (mCRC) patients are usually treated with 5‐fluorouracil, oxaliplatin, irinotecan, bevacizumab‐based regimens, and, in selected cases, cetuximab. Regorafenib and TAS‐102 have recently been introduced for the treatment of refractory disease. There are no further treatment options for fit patients who are resistant to these drugs. Although gemcitabine and pemetrexed have shown preclinical and clinical activity in patients with mCRC, clinical data remain inconclusive [1], [2], [3], [4], [5], [6], [7], [8].
Gemcitabine has recognized broad‐spectrum activity and is recommended for treatment in an increasing number of tumors. However, there is still little clinical proof of its efficacy in mCRC. A review by Merl et al. evaluating the efficacy and safety of fluoropyrimidine plus gemcitabine in patients with mCRC reported objective response rates (ORR) of 30%–38.3%, median time to progression (TTP) of 4–8.3 months, and median overall survival (OS) of 9.8–18 months [1].
Pemetrexed is a pyrrolopyrimidine‐based antifolate with proven in vitro activity against folate‐requiring enzymes, including thymidylate synthase, dihydrofolate reductase, and glycinamide ribonucleotide formyltransferase [2]. In vitro studies have shown that pemetrexed is active against a wide range of human cell lines, including colon cells and also VRC5 and HXGC3 human colon xenografts. Phase I clinical trials established pemetrexed 600 mg/m2 once every 21 days as a suitable schedule for phase II trials [3]. Phase II studies of single‐agent pemetrexed in mCRC reported an overall response rate of about 15% in the first‐line setting and no observable responses in refractory patients [4], [5]. In preclinical models, a synergism between pemetrexed, oxaliplatin, and irinotecan was observed, attesting to the potential for using these combination regimens in clinical trials [6]. Pemetrexed has been studied in combination with oxaliplatin as first‐line in mCRC, showing ORR of 29.6%, TTP of 5.3 months, and OS of 12.3 months [7]. The drug has also produced interesting ORRs when used in combination with irinotecan as first‐ and second‐line treatment [8], [9].
A critical review of the available literature shows an evident incoherence between preclinical data and clinical trial design. From in vitro experiments, it seems clear that the administration of pemetrexed should precede all other drugs, with the possible exception of irinotecan, to increase cell kill and induce a synergistic effect [10], [11]. Clinical studies of pemetrexed‐containing regimens have ignored this important indication in that the cytotoxics are generally infused concomitantly. Moreover, pemetrexed is commonly administered intravenously as a 10‐minute infusion, but there is a strong body of evidence that anti‐metabolites such as 5‐fluorouracil and gemcitabine, when given in continuous intravenous infusion, show a different efficacy and toxicity pattern compared to the same dose given as an intravenous bolus [12], [13], [14]. In particular, our preclinical experience suggests that a prolonged exposure (6 or 12 hours) to pemetrexed leads to higher antitumor activity than a short exposure (<6 hours) and that a wash‐out time between each drug administration ranging from 48 to 72 hours is essential for the induction of both cell cycle perturbation and apoptosis [15]. The reasons for this are not clear; it can be argued that a longer exposure of tumor cells to lower doses of the drug may optimize the carrier system much more than higher doses administered over a shorter time. More sophisticated pharmacokinetic studies (intracellular drug concentration) are warranted.
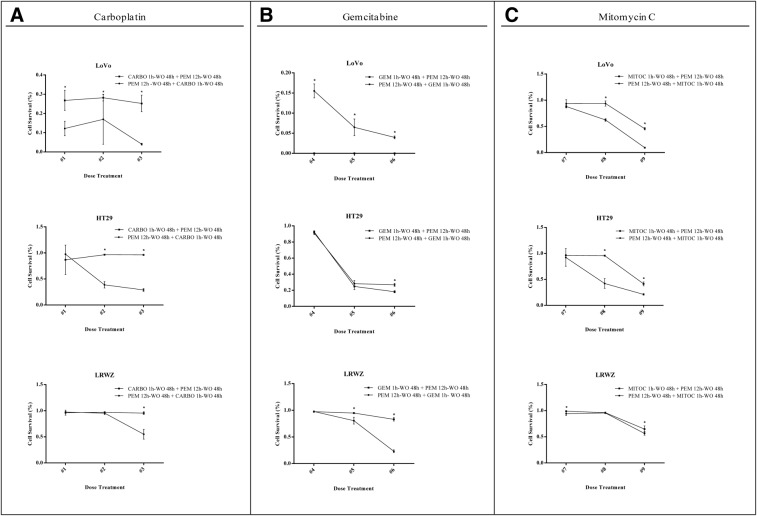
The experimental regimen was based on these assumptions, in particular the administration of pemetrexed as a continuous intravenous infusion followed by a 48‐hour wash‐out and then gemcitabine infusion (Fig. 1). The chosen dose of pemetrexed was derived from a phase Ib trial performed at our institute that showed the feasibility of a 12‐hour continuous infusion of pemetrexed 200 mg/m2 every 2 weeks in pretreated cancer patients (data not published). However, clinical results were not in line with the preclinical rationale and the study was closed at the first step because of important toxicity and the absence of proven significant activity. In particular, no objective responses or even minor tumor shrinkage (enough to satisfy partial response (PR) criteria) were seen. The only patient with stable disease after 8 weeks’ treatment showed clinical progression immediately after the radiological evaluation. One reason for such a negative result might be the low activity of gemcitabine and pemetrexed in mCRC. Enrolled patients had also received several treatments and were presumed to have chemoresistant disease (Table 1). In addition, the toxicity profile (Table 2) was not favorable. Grade 4 neutropenia and thrombocytopenia were observed in 57.1% and 35.7% of patients, respectively, and 4 (28.7%) patients experienced febrile neutropenia. The most important grade 3–4 nonhematological adverse events were fatigue (50%) and stomatitis (21.5%).
Figure 1.
Effect of different schedules of combination of pemetrexed (PEM) with carboplatin (CARBO) (A), gemcitabine (GEM) (B), or mitomycin C (MITOC) (C) on three colon cancer cells lines. Cell viability was determined by SRB assay, and the results were expressed as the mean of an octuplicate from three independent experiments. Bars represent standard deviation (*p < .05). Statistical significance was determined using the GraphPad Prism Software Holm‐Sidak method. Treatment dose: #1 (PEM 1 mM ‐ CARBO 0.8 mM), #2 (PEM 10 mM ‐ CARBO 8 mM), #3 (PEM 100 mM ‐ CARBO 80 mM), #4 (PEM 1 mM ‐ GEM 0.4 mM), #5 (PEM 10 mM ‐ GEM 4 mM), #6 (PEM 100 mM ‐ GEM 40 mM), #7 (PEM 1 mM ‐ MITOC 0.03 mM), #8 (PEM 10 mM ‐ MITOC 0.3 mM), and #9 (PEM 100 mM ‐ MITOC 3 mM).
Abbreviations: CARBO, carboplatin; GEM, gemcitabine, MITOC, mitomycin C; SRB, sulforhodamine B; WO, wash‐out.
Table 1. Patient characteristics.
Abbreviations: PS, performance score; ECOG, eastern cooperative oncology group.
Table 2. Adverse events among patients undergoing at least one treatment cycle.
Abbreviation: —, no data.
Difficulties in translating in vitro results into clinical practice are inevitable. In our study the duration of pemetrexed continuous infusion and the length of the washout period between treatments were defined on an empirical basis. This is an important aspect to consider when conducting translational research, as it may affect clinical results. Taking into account the low efficacy and high toxicity associated with the experimental regimen, clinical development is not recommended. However, further efforts are warranted to verify the efficacy of alternative schedules of pemetrexed in solid tumors.
Figures and Tables
Footnotes
ClinicalTrials.gov Identifier: NCT01909830
Sponsor: none
Principal Investigator: Alessandro Passardi
IRB Approved: Yes
Disclosures
The authors indicated no financial relationships.
References
- 1. Merl M, Hoimes C, Pham T et al. Is there a palliative benefit of gemcitabine plus fluoropyrimidines in patients with refractory colorectal cancer? A review of the literature previously presented: Poster at the 2008 Gastrointestinal Cancer Symposium (Abstract No. 512). Expert Opin Investig Drugs 2009;18:1257–1264. [DOI] [PubMed] [Google Scholar]
- 2. Shih C, Chen VJ, Gossett LS et al. LY231514, a pyrrolo[2,3‐d]pyrimidine‐based antifolate that inhibits multiple folate‐requiring enzymes. Cancer Res 1997;57:1116–1123. [PubMed] [Google Scholar]
- 3. Cripps C, Burnell M, Jolivet J et al. Phase II study of first‐line LY231514 (multi‐targeted antifolate) in patients with locally advanced or metastatic colorectal cancer: An NCIC Clinical Trials Group study. Ann Oncol 1999;10:1175–1179. [DOI] [PubMed] [Google Scholar]
- 4. John W, Picus J, Blanke CD et al. Activity of multitargeted antifolate (pemetrexed disodium, LY231514) in patients with advanced colorectal carcinoma: Results from a phase II study. Cancer 2000;88:1807–1813. [PubMed] [Google Scholar]
- 5. Paulson R, Hainsworth J, Geyer C et al. A phase II trial of MTA (multiple targeted antifolate, LY231514) in patients with 5‐FU and irinotecan‐refractory colorectal cancer. Proc Am Soc Clin Oncol 1999;18:297:1140a. [Google Scholar]
- 6. Raymond E, Louvet C, Tournigand C et al. Pemetrexed disodium combined with oxaliplatin, SN38, or 5‐fluorouracil, based on the quantitation of drug interactions in human HT29 colon cancer cells. Int J Oncol 2002;21:361–367. [PubMed] [Google Scholar]
- 7. Atkins JN, Jacobs SA, Wieand HS et al. Pemetrexed/oxaliplatin for first‐line treatment of patients with advanced colorectal cancer: A phase II trial of the National Surgical Adjuvant Breast and Bowel Project Foundation Research Program. Clin Colorectal Cancer. 2005;5:181–187. [DOI] [PubMed] [Google Scholar]
- 8. Hochster H, Kettner E, Kroning H et al. Phase I/II dose‐escalation study of pemetrexed plus irinotecan in patients with advanced colorectal cancer. Clin Colorectal Cancer 2005;5:257–262. [DOI] [PubMed] [Google Scholar]
- 9. Underhill C, Goldstein D, Gorbounova VA et al. A randomized phase ii trial of pemetrexed plus irinotecan (ALIRI) versus leucovorin‐modulated 5‐FU plus irinotecan (FOLFIRI) in first‐line treatment of locally advanced or metastatic colorectal cancer. Oncology 2007;73:9–20. [DOI] [PubMed] [Google Scholar]
- 10. Giovannetti E, Mey V, Danesi R et al. Synergistic cytotoxicity and pharmacogenetics of gemcitabine and pemetrexed combination in pancreatic cancer cell lines. Clin Cancer Res 2004;10:2936–2943. [DOI] [PubMed] [Google Scholar]
- 11. Tonkinson JL, Worzalla JF, Teng CH et al. Cell cycle modulation by multitargeted antifolate, LY 231514, increases the cytotoxicity and antitumor activity of gemcitabine in HT29 colon carcinoma. Cancer Res 1999;59:3671–3676. [PubMed] [Google Scholar]
- 12. Sobrero AF, Aschele C, Guglielmi AP et al. Synergism and lack of cross‐resistance between short‐term and continuous exposure to fluorouracil in human colon adenocarcinoma cells. J Natl Cancer Inst 1993;85:1937–1944. [DOI] [PubMed] [Google Scholar]
- 13. Rajdev L, Goldberg G, Hopkins U et al. A phase I of gemcitabine administered as a 96‐h continuous intravenous infusion in patients with advanced carcinoma and lymphoma. Med Oncol 2006;23:369–376. [DOI] [PubMed] [Google Scholar]
- 14. Qin B, Tanaka R, Ariyama H et al. In‐vitro differential metabolism and activity of 5‐fluorouracil between short‐term, high‐dose and long‐term, low‐dose treatments in human squamous carcinoma cells. Anticancer Drugs 2006;17:439–443. [DOI] [PubMed] [Google Scholar]
- 15. Marangolo M, Fanini F, Turci L et al. Pemetrexed in human cancer cell lines: Is its activity dose and time dependent? A preliminary preclinical experience on pancreatic cancer (AACR Annual Meeting conference paper). Cancer Research 2008;68(suppl 9):2299a. [Google Scholar]