Abstract
Lessons Learned.
Accrual to renal cell carcinoma trials remains a challenge despite the lack of prolonged response to the available treatments.
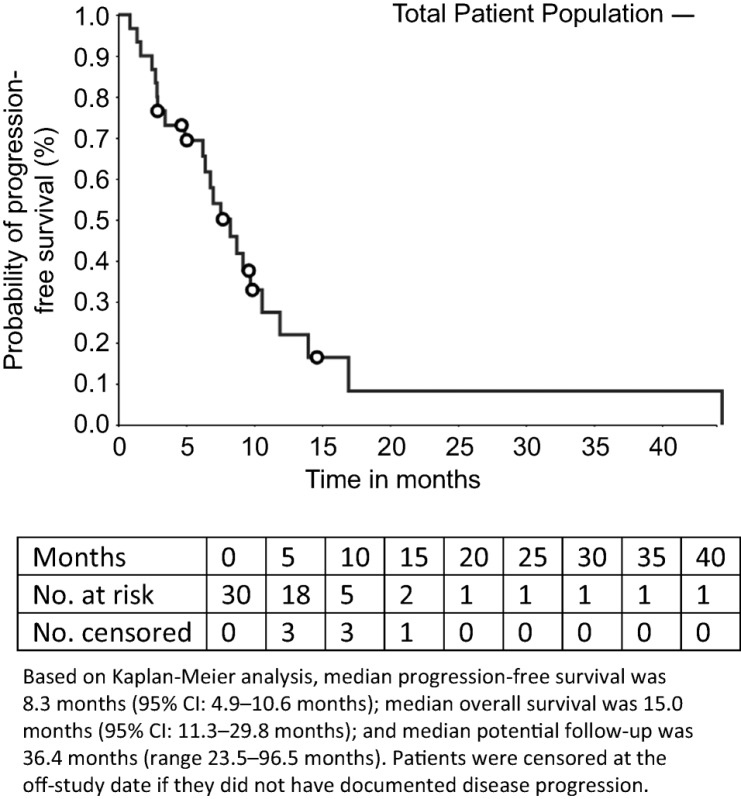
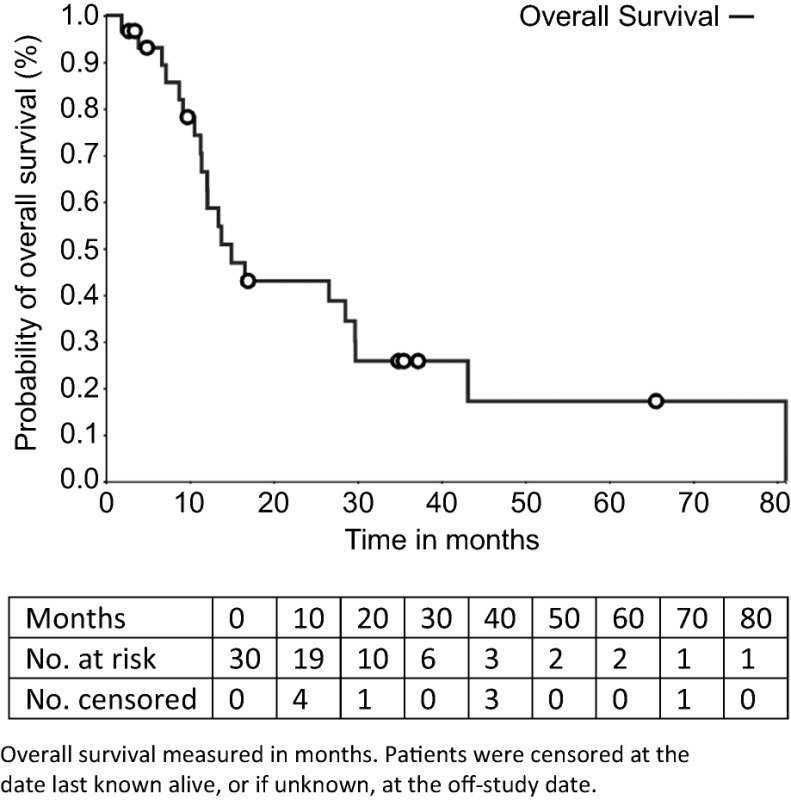
The observation of three responses among the 30 patients with median progression‐free survival and overall survival of 8.3 and 15 months, respectively, indicates the combination has some activity, but it is not sufficient for further development.
Background.
Treatment of metastatic renal cell carcinoma (mRCC) remains suboptimal. Preclinical data have previously shown that ixabepilone, a microtubule‐stabilizing agent approved for the treatment of breast cancer, is active in taxane‐sensitive and ‐resistant cells. In this single‐arm phase II trial, we investigated a combination of ixabepilone plus bevacizumab in patients with refractory mRCC.
Methods.
We enrolled 30 patients with histologically confirmed mRCC, clear cell subtype, who had not been previously treated with ixabepilone or bevacizumab but had received at least one prior U.S. Food and Drug Administration (FDA)‐approved treatment for renal cell carcinoma (RCC). The treatment regimen consisted of 6 mg/m2 ixabepilone per day for 5 days and 15 mg/kg bevacizumab every 21 days. After 6 cycles, the treatment interval could be extended to every 28 days. The primary endpoint was the objective response rate according to the Response Evaluation Criteria in Solid Tumors (RECIST). Secondary endpoints were progression‐free survival (PFS), overall survival (OS), and the toxicity of the combination.
Results.
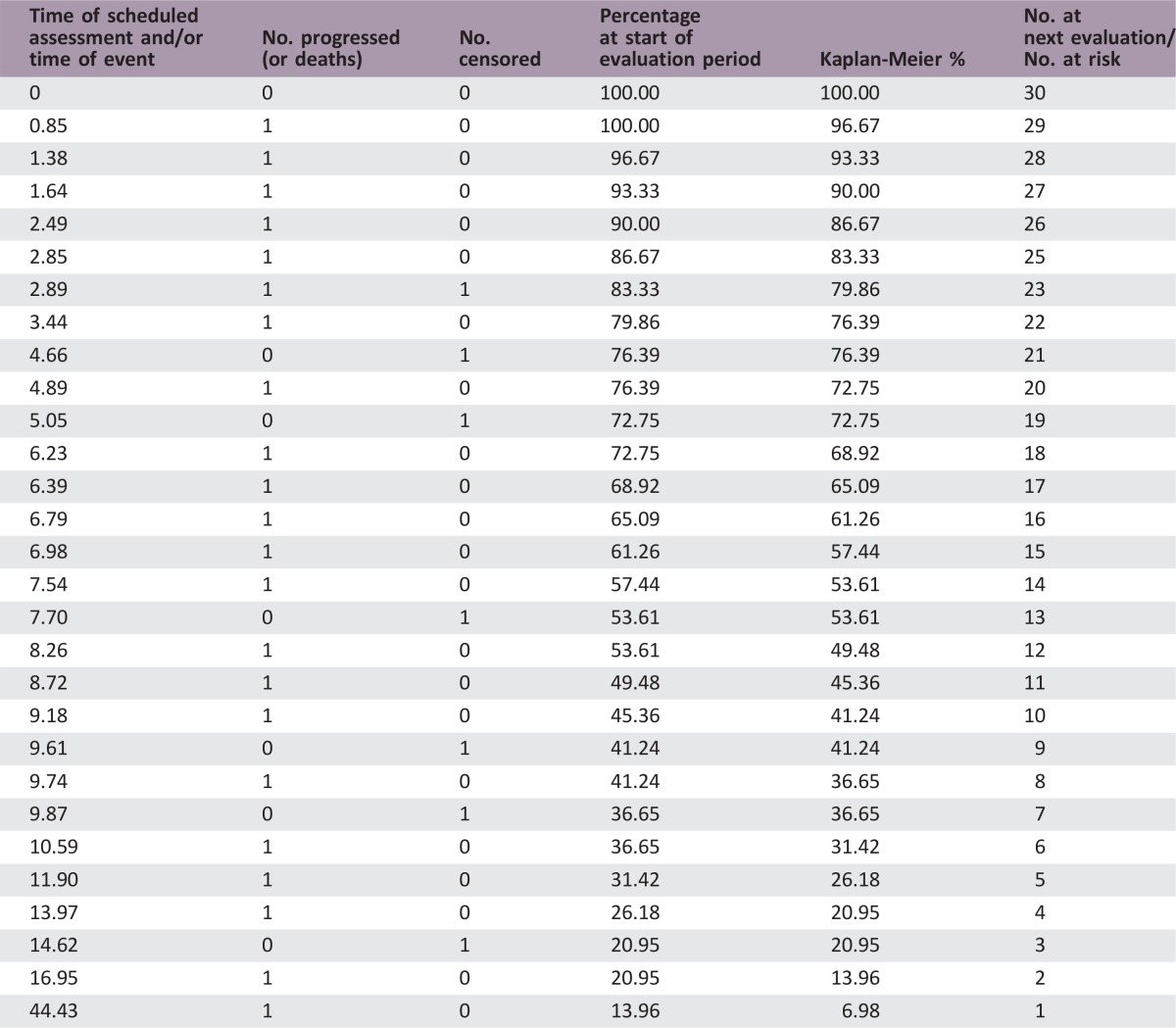
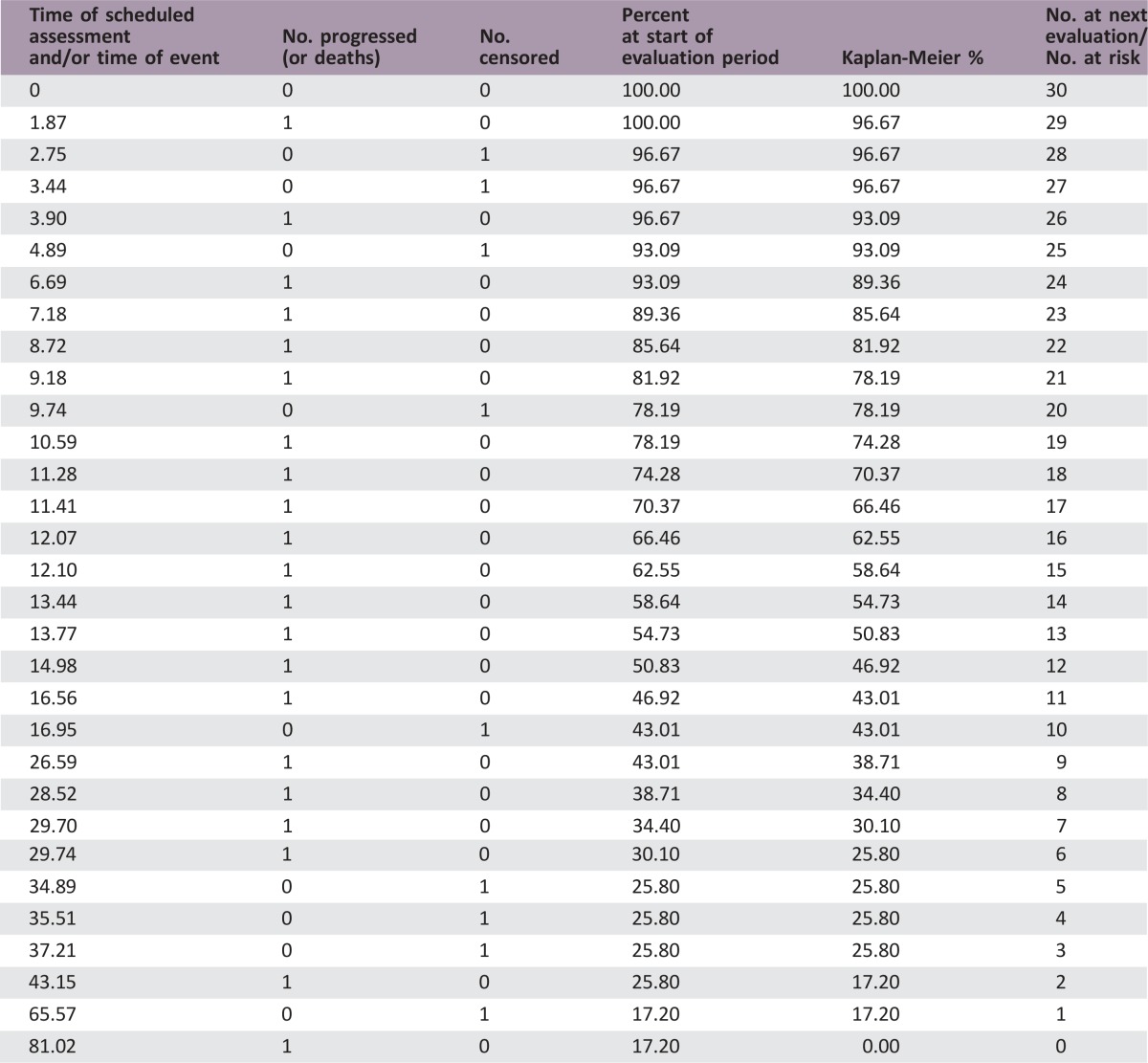
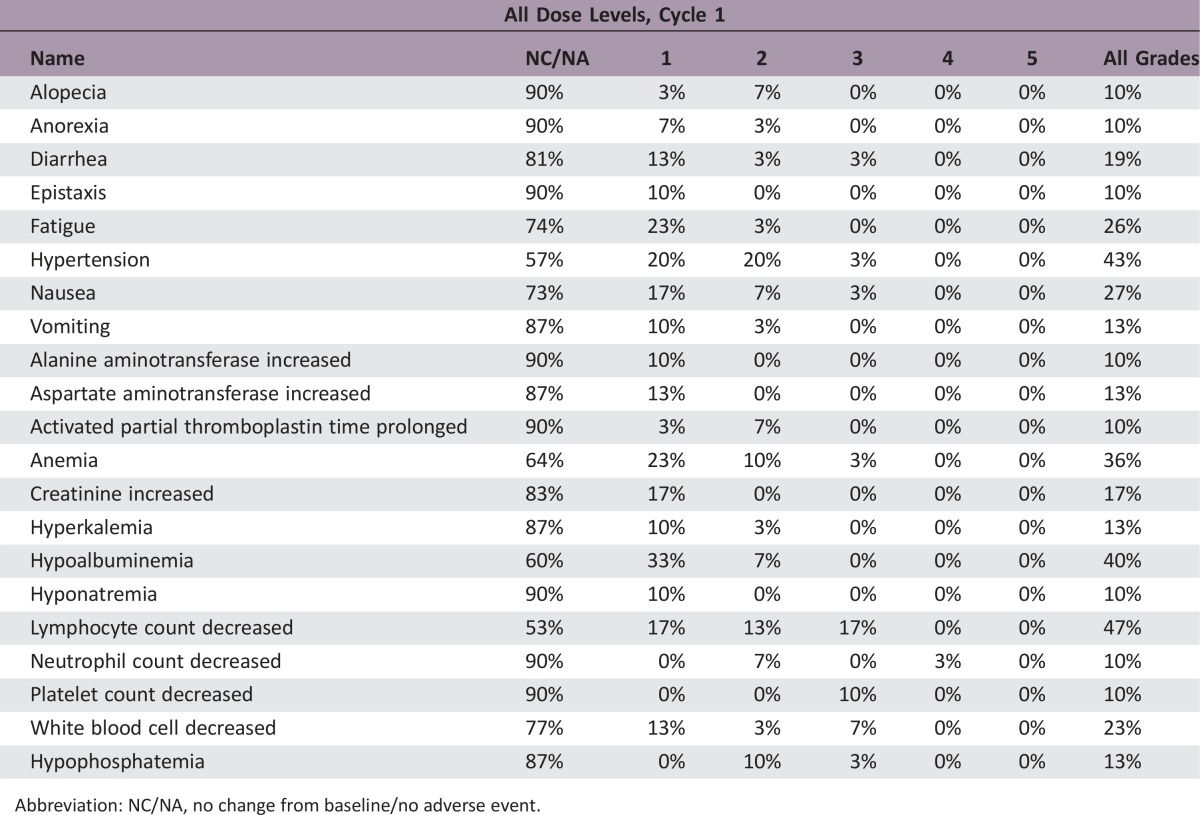
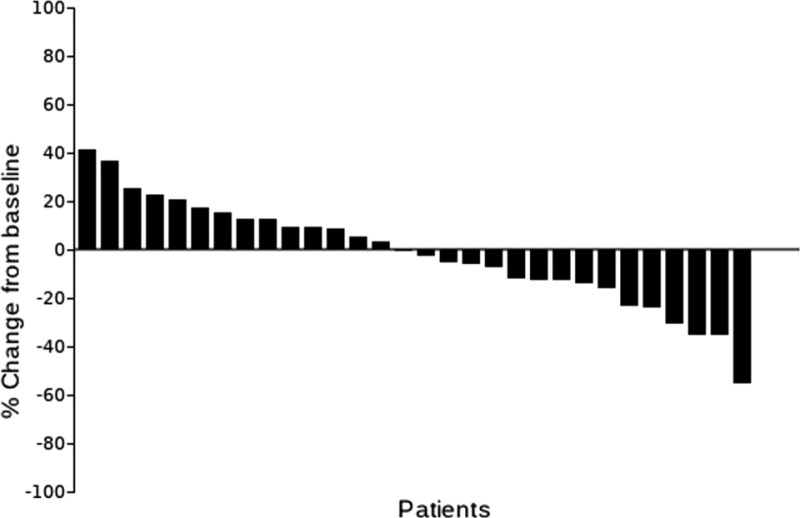
The median number of prior therapies was two (range per patient one to five). Patients received a median of 8 cycles of ixabepilone plus bevacizumab (range 2–54). The median follow‐up was 36.4 months (range 23.5–96.5). Nineteen patients (63.3%) had stable disease as a best response. Three patients (10%) had a partial response. The median PFS was 8.3 months (95% confidence interval [CI], 4.9–10.6) and the median OS was 15.0 months (95% CI, 11.3–28.8). The total number of cycle for safety evaluation was 289. Grade 3/4 adverse events (>5% incidence) included lymphopenia (16.7%), hypertension (6.7%), and leukopenia (6.7%).
Conclusion.
The combination of ixabepilone and bevacizumab was well tolerated, with modest activity in second ‐ or later‐line mRCC, but it is not recommended as a therapy without further clinical development. Alternative combinations with these agents could be explored in future studies.
Abstract
经验总结
• 尽管对已有治疗的长期反应不足, 但肾细胞癌试验的招募仍具有挑战性。
• 在30例中位无进展生存期和总生存期分别为8.3个月和15个月的患者中观察到三种缓解, 表明联合治疗具有部分效果, 但不足以进行进一步开发。
摘要
背景.转移性肾细胞癌(mRCC)的治疗效果不佳。既往临床前数据显示, 伊沙匹隆(一种获批用于治疗乳腺癌的微管稳定剂)在紫杉烷敏感和耐药性细胞中具有活性。在本项单组、II期试验中, 在难治性mRCC患者中考察了贝伐珠单抗联合伊沙匹隆的治疗效果。
方法.研究入组了30例经组织学证实的mRCC的患者, 这些患者具有透明细胞亚型, 此前未接受过伊沙匹隆或贝伐珠单抗治疗, 但至少接受过一次经美国食品药品监督管理局(FDA)批准的肾细胞癌(RCC)药物治疗。治疗方案为:伊沙匹隆6 mg/m2/天, 共5天, 以及每21天接受贝伐珠单抗15 mg/kg。6个周期后, 治疗间隔可延长至每28天一个周期。主要终点是根据实体瘤疗效评价标准(RECIST)评估的客观缓解率。次要终点为无进展生存期(PFS)、总生存期(OS)和联合用药的毒性。
结果.中位既往治疗数为2(每名患者的范围为1‐5)。患者接受伊沙匹隆联合贝伐珠单抗治疗的中位周期数为8(范围为2‐54)。中位随访时间为36.4个月(范围为23.5‐96.5)。19名患者(63.3%)的疾病稳定为最佳缓解。3名患者(10%)为部分缓解。中位PFS为8.3个月 [95%置信区间(CI), 4.9‐10.6], 中位OS为15.0个月(95%CI, 11.3‐28.8)。安全性评价的总周期数为289。3级或4级不良事件(发生率>5%)包括淋巴细胞减少症(16.7%)、高血压(6.7%)和白细胞减少症(6.7%)。
结论.伊沙匹隆与贝伐珠单抗联合给药的耐受性良好, 在二线或二线以上的mRCC治疗中具有中度效果, 但在未进行进一步临床开发的情况下, 不推荐将其作为治疗方案。在未来的研究中可以探索与这些药物联用的替代方案。
Discussion
The unproven hypothesis that angiogenesis is a key step in the development and metastasis of solid tumors [1], especially mRCC [2], led to the development of a large number of agents whose putative target was the vascular endothelial growth factor (VEGF) pathway. Several of these agent were shown to have modest activity in the therapy of mRCC and this led to their approval by regulatory agencies. However, given their limited activity, their use in combinations has been extensively explored. We studied the combination of ixabepilone, a microtubule‐targeting epothilone [3], [4], with bevacizumab, a monoclonal antibody that binds VEGF.
Preclinical data from several in vivo models including breast, kidney, lung, and colon cancers demonstrated increased activity of ixabepilone in combination with bevacizumab, suggesting a synergistic effect in both antitumor and antiangiogenic activities and the absence of overlapping toxicities [5]. More recently, randomized trials reported acceptable activities and toxicities of combined therapy in platinum/taxane‐resistant cervical‐uterine [6] and locally recurrent or metastatic breast cancer [7].
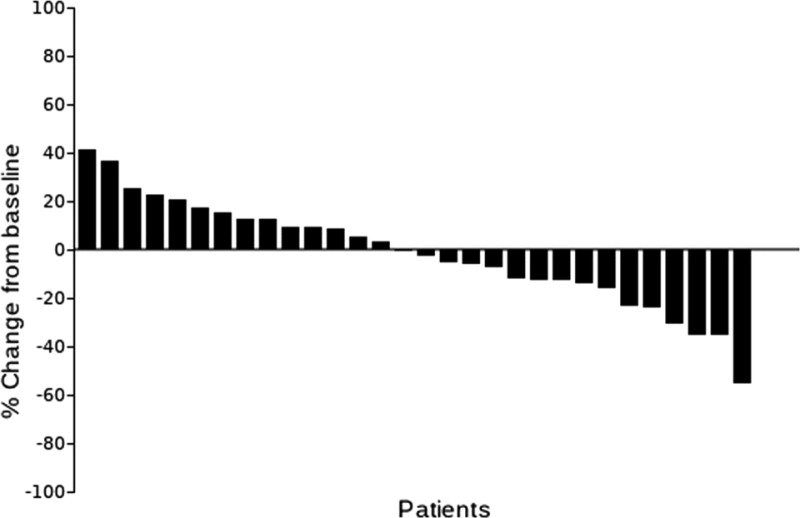
Best percentage change from baseline for target lesions per patient.
This investigation was designed as a single‐arm phase II multi‐center trial with a primary aim of determining the objective response rate of ixabepilone plus bevacizumab using RECIST criteria in patients with relapsed or refractory mRCC. We also evaluated PFS, OS, and toxicities of the combined therapy. The observed activity of the combined therapy was less than originally expected, considering results in an earlier phase II study of ixabepilone in renal cancer that demonstrated an objective response rate of 13% [16]. Regarding side effects, the tested combination was well tolerated without major side effects or deaths related to treatment.
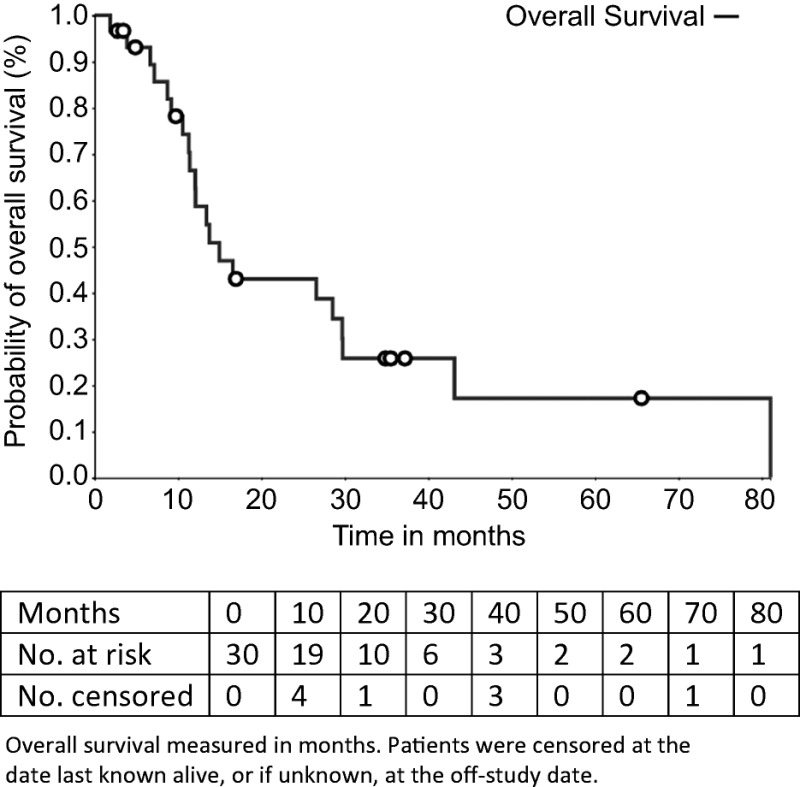
Despite the low response rate of 11.3%, the median PFS of 8.3 month and OS of 15 months compare favorably with putative antiangiogenic agents approved for mRCC in second‐line treatment before 2015 [8], [9], [10]. Furthermore, with a median number of two previous lines of treatment, a majority of patients were receiving this treatment in third line, making this combination potentially active in the third‐line setting. However, recent advances in immunotherapy for RCC restrict the potential scope of this combination.
Trial Information
- Disease
Renal cell carcinoma – clear cell
- Stage of Disease/Treatment
Metastatic/Advanced
- Prior Therapy
1 prior regimen
- Type of Study – 1
Phase II
- Type of Study – 2
Single arm
- Primary Endpoint
Overall response rate
- Secondary Endpoint
Overall survival
- Secondary Endpoint
Progression‐free survival
- Secondary Endpoint
Toxicity
- Investigator's Analysis
Active but results overtaken by other developments
Drug Information for Phase II Ixabepilone + Bevacizumab
- Drug 1
- Generic/Working name
Ixabepilone
- Trade name
Ixempra
- Company name
Bristol‐Myers Squibb
- Drug type
Microtubule inhibitor
- Drug class
Microtubule‐targeting agent
- Dose
6 milligrams (mg) per squared meter (m2)
- Route
IV
- Drug 2
- Generic/Working name
Bevacizumab
- Trade name
Avastin
- Company name
Genentech/Roche
- Drug type
Antibody
- Drug class
Angiogenesis ‐ VEGF
- Dose
15 milligrams (mg) per kilogram (kg)
- Route
IV
Patient Characteristics for Phase II Ixabepilone + Bevacizumab
- Number of patients, male
23
- Number of patients, female
7
- Stage
Metastatic or recurrent
- Age
Median (range): 62.3 (44.3–78.8)
- Number of prior systemic therapies
Median (range): 2 (1–5)
- Performance Status: ECOG
-
0 — 2
1 — 24
2 — 4
3 —
unknown —
- Cancer Types or Histologic Subtypes
Clear cell, 30
Primary Assessment Method for Phase II Ixabepilone + Bevacizumab
- Assessment
- Number of patients screened
40
- Number of patients enrolled
30
- Number of patients evaluable for toxicity
30
- Number of patients evaluated for efficacy
30
- Evaluation method
RECIST 1.1
- Response assessment CR
n = 0 (0%)
- Response assessment PR
n = 3 (10%)
- Response assessment SD
n = 19 (63.3%)
- Response assessment PD
n = 8 (26.7%)
- Response assessment OTHER
n = 0 (0%)
- (Median) duration assessments PFS
8.3 months, CI: 4.9–10.6
- (Median) duration assessments OS
15.0 months, CI: 11.3–29.8
- Kaplan‐Meier Time units
Months
Best percentage change from baseline for target lesions per patient.
Secondary Assessment Method for Phase II Ixabepilone + Bevacizumab (PFS)
- Assessment
- Number of patients evaluated for efficacy
30
- Kaplan‐Meier Time units
months
Secondary Assessment Method for Phase II Ixabepilone + Bevacizumab (OS)
- Assessment
- Number of patients evaluated for efficacy
30
- Kaplan‐Meier Time units
months
Adverse Events: Phase II Ixabepilone + Bevacizumab
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Active but results overtaken by other developments
Renal cell carcinoma is among the ten most frequently diagnosed cancers in the general population in the U.S. [2], with approximately 63,000 new cases and almost 14,000 deaths from RCC each year [12]. Given its refractory nature, mRCC remains a difficult problem with 5‐year survival rates of 8% [2].
Interest in the angiogenesis hypothesis, especially its putative role in RCC led to the development of innumerable “antiangiogenic agents” that sought to interdict signaling through the VEGF pathway. Despite the approval of several similar agents for the treatment of mRCC by regulatory agencies, efficacy was modest and short‐lived, in part due to the emergence of resistance [13]. This has provided the impetus to develop combination regimens using antiangiogenic agents in the hopes of improving therapeutic efficacy. For example, although initial studies with single‐agent bevacizumab in patients with mRCC demonstrated a significant increase in time to progression [14], its efficacy was not consider sufficient for use as a single agent. It was then explored and subsequently approved by regulatory agencies for the treatment of mRCC in combination with interferon alfa, based on the results of a phase III trial [15]. With this background, we embarked on a clinical trial exploring the activity of the combination of bevacizumab with ixabepilone. In preclinical studies, ixabepilone, a non‐taxane microtubule‐stabilizing agent, had been shown to be active against cancer cell lines intrinsically insensitive to taxanes as well as cell lines that had developed resistance. To date, the only regulatory approval for ixabepilone is in metastatic breast cancer as a monotherapy or in combination with capecitabine based on an open‐label phase III trial that enrolled 752 patients [16].
In mRCC, we initially explored the activity of ixabepilone monotherapy in previously untreated patients [16] using the same schedule of administration—6 mg/m2/day, for 5 consecutive days every 3 weeks—used in combination with bevacizumab in this trial. In the previous trial, the overall response rate was 13% with a median duration of response of 5.5 months and an OS of 19.25 months [16]. This regimen is different from that approved in breast cancer (40 mg/m2 every 3 weeks) and was chosen because of the lower rate of neurotoxicity. Another phase II trial with the 40 mg/m2 every 3 weeks was published with 12 patients and no objective responses [17]. Given that both ixabepilone and bevacizumab had demonstrated modest activity in mRCC, appeared not to have overlapping toxicities, and had shown encouraging activity in preclinical models, we chose to explore the combination of ixabepilone plus bevacizumab in mRCC. The results demonstrated the combination was well tolerated with modest activity.
In our view, the recent approval of cabozantinib [18] and especially nivolumab [19] for the therapy of mRCC in second line [2] make the further development of the tested combination very difficult. Accrual for this trial that began in 2009 was challenging and would be even more challenging in 2017.
Footnotes
ClinicalTrials.gov Identifier: NCT00923130
Sponsor(s): National Cancer Institute (NCI)
Principal Investigator: Tito Fojo
IRB Approved: Yes
Disclosures
The authors indicated no financial relationships.
References
- 1. Kerbel RS. Tumor angiogenesis. N Engl J Med 2008;358:2039–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal‐cell carcinoma. N Engl J Med. 2017;376:354–366. [DOI] [PubMed] [Google Scholar]
- 3. Dorff TB, Gross ME. The epothilones: New therapeutic agents for castration‐resistant prostate cancer. The Oncologist 2011;16:1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burotto M, Edgerly M, Poruchynsky M et al. Phase II clinical trial of ixabepilone in metastatic cervical carcinoma. The Oncologist 2015;20:725–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee FY, Covello KL, Castaneda S et al. Synergistic antitumor activity of ixabepilone (BMS‐247550) plus bevacizumab in multiple in vivo tumor models. Clin Cancer Res 2008;14:8123–8131. [DOI] [PubMed] [Google Scholar]
- 6. Roque DM, Ratner ES, Silasi DA et al. Weekly ixabepilone with or without biweekly bevacizumab in the treatment of recurrent or persistent uterine and ovarian/primary peritoneal/fallopian tube cancers: A retrospective review. Gynecol Oncol 2015;137:392–400. [DOI] [PubMed] [Google Scholar]
- 7. Rugo HS, Barry WT, Moreno‐Aspitia A et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin‐bound nab‐paclitaxel once per week or ixabepilone with bevacizumab as first‐line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance). J Clin Oncol 2015;33:2361–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang H, Menefee M, Edgerly M et al. A phase II clinical trial of ixabepilone (Ixempra; BMS‐247550; NSC 710428), an epothilone B analog, in patients with metastatic renal cell carcinoma. Clin Cancer Res 2010;16:1634–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Escudier B, Eisen T, Stadler WM et al. Sorafenib in advanced clear‐cell renal‐cell carcinoma. N Engl J Med 2007;356:125–134. [DOI] [PubMed] [Google Scholar]
- 10. Motzer RJ, Escudier B, Oudard S et al. Efficacy of everolimus in advanced renal cell carcinoma: A double‐blind, randomised, placebo‐controlled phase III trial. Lancet 2008;372:449–456. [DOI] [PubMed] [Google Scholar]
- 11. Rini BI, Escudier B, Tomczak P et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet 2011;378:1931–1939. [DOI] [PubMed] [Google Scholar]
- 12. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 13. Zarrabi K, Fang C, Wu S. New treatment options for metastatic renal cell carcinoma with prior anti‐angiogenesis therapy. J Hematol Oncol 2017;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang, JC, Haworth L, Sherry RM et al. A randomized trial of bevacizumab, an anti‐vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003;349:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Escudier B, Pluzanska A, Koralewski P et al. Bevacizumab plus interferon alfa‐2a for treatment of metastatic renal cell carcinoma: A randomised, double‐blind phase III trial. Lancet 2007;370:2103–2111. [DOI] [PubMed] [Google Scholar]
- 16. Thomas ES, Gomez HL, Li RK et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol 2007;25:5210–5217. [DOI] [PubMed] [Google Scholar]
- 17. Posadas EM, Undevia S, Manchen E et al. A phase II study of ixabepilone (BMS‐247550) in metastatic renal‐cell carcinoma. Cancer Biol Ther 2007;6:490–493. [DOI] [PubMed] [Google Scholar]
- 18. Choueiri TK, Escudier B, Powles T. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open‐label, phase 3 trial. Lancet Oncol 2016;17:917–927. [DOI] [PubMed] [Google Scholar]
- 19. Motzer RJ, Escudier B, McDermott DF. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]