Abstract
Objective
Young patients with advanced interstitial lung disease (ILD) are potential candidates for cadaveric lung transplantation. This study aimed to examine clinical features, outcomes, and prognostic factors in Japanese ILD patients awaiting lung transplantation.
Methods
We investigated the clinical features and outcomes of 77 consecutive candidates with ILD who were referred to Kyoto University Hospital and subsequently actively listed for lung transplant in the Japan Organ Transplant Network between 2010 and 2014.
Results
Of the 77 candidates, 33 had idiopathic pulmonary fibrosis (IPF) and 15 had unclassifiable ILD. During the observational period, 23 patients (30%) received lung transplantations and 49 patients (64%) died before transplantation. Of the 33 patients with IPF, 13 (39%) had a family history of ILD and 13 (39%) had an “inconsistent with usual interstitial pneumonia pattern” on high-resolution computed tomography (HRCT). The median survival time from registration was 16.7 months, and mortality was similar among patients with IPF, unclassifiable ILD, and other ILDs. Using a multivariate stepwise Cox proportional hazards model, 6-min walking distance was shown to be an independent prognostic factor in candidates with ILD (per 10 m, hazard ratio (HR): 0.97; 95% confidence interval (CI): 0.95–0.99, p<0.01), while lower body mass index (HR: 0.83; 95% CI: 0.72–0.95, p < 0.01) independently contributed to mortality in patients with IPF.
Conclusions
Japanese patients with ILD awaiting transplantation had very poor outcomes regardless of their specific diagnosis. A substantial percentage of IPF patients had an atypical HRCT pattern. 6-min walking distance in ILD patients and body mass index in IPF patients were independent predictors of mortality.
Introduction
Interstitial lung disease (ILD) is difficult to cure and its prognosis is often poor. In particular, idiopathic pulmonary fibrosis (IPF) has very poor outcome with a median survival of 2–3 years from the time of diagnosis [1]. Meanwhile, patients with other ILDs, such as idiopathic nonspecific interstitial pneumonia (NSIP), connective tissue disease-associated ILD (CTD-ILD), and chronic hypersensitivity pneumonitis (CHP), sometimes have progressive disease, although their prognosis is usually more favorable than that of IPF [2–4]. Moreover, a substantial number of patients with ILD cannot be definitively classified as a specific ILD subtype, and are assigned as “unclassifiable ILD” [5]. A previous study showed that unclassifiable ILD has a prognosis that is intermediate between IPF and non-IPF ILDs [6].
Lung transplantation is the only effective therapy in patients with advanced ILD refractory to medical treatment. In Japan, cadaveric lung transplantation candidates must be younger than 60 years old at the time of registration on the Japan Organ Transplant Network (JOTN) waiting list because of a severe donor shortage [7]. As the number of cadaveric lung transplantation in Japan is limited, the average waiting time is more than 800 days [7, 8]. Moreover, lung allocation is performed based on accrued time on the waiting list, and there is no rule to prioritize patients with more rapidly progressing diseases such as IPF. Therefore, that Japanese lung transplant candidates with ILD may have poor outcome.
Hence, it is important to elucidate the prognostic factors and outcomes of lung transplant candidates with ILD in Japan. Recently, the simple scoring (GAP index), which consists of gender (G), age (A), and lung physiology variables (P), has been reported to predict mortality in patients with IPF [9]. However, prognostic factors of young (<60 years old) patients with ILD have not been elucidated.
We hypothesized that young lung transplant candidates with ILD have distinct clinical features and prognostic factors, and aimed to investigate the clinical features and outcomes in lung transplant candidates with ILD at our hospital.
Materials and methods
Study population
This study cohort comprised 77 consecutive lung transplant candidates with ILD who were referred to Kyoto University Hospital and subsequently actively listed for lung transplant in JOTN between April 2010 and July 2014. These candidates were selected based on the international guidelines [10]. IPF was diagnosed by surgical lung biopsy or high-resolution computed tomography (HRCT) based on the guidelines [1, 11]. Other idiopathic interstitial pneumonias (IIPs) were diagnosed as previously described [12–14], and CHP was diagnosed according to the established criteria [15]. We defined unclassifiable ILD as patients without a specific ILD diagnosis following multidisciplinary review of clinical, radiological, and pathological data [5]. This study was approved by the Ethics Committee of Kyoto University (E1355). Written informed consent was obtained from all subjects.
Data collection
Clinical data were extracted from the lung transplantation registration database and medical records at our institute. Pulmonary function tests were performed using the CHESTAC system (Chest M.I. Inc., Tokyo, Japan), and diffusing capacity of the lung for carbon monoxide (DLCO) was determined using the single-breath technique. The GAP stage was defined using the method previously reported by Ley et al [9]. The 6-min walk test (6MWT) was conducted according to the American Thoracic Society guidelines [16]. Supplemental oxygen flow during 6MWT was recorded. Pulmonary hypertension was defined as mean pulmonary artery pressure ≥25 mmHg by right heart catheterization or by estimated systolic pulmonary artery pressure ≥40 mmHg by echocardiography in six patients without right heart catheterization data. The HRCT findings in 33 patients with IPF were classified as usual interstitial pneumonia (UIP) pattern, possible UIP pattern, or “inconsistent with UIP pattern” per the guidelines [1]. We observed patients from the date of registration at JOTN to the date of last contact, transplantation, or death.
Statistical analysis
Statistical analyses were performed using JMP version 10 (SAS Institute Inc., Cary, NC, USA) and R version 3.3.1 (R Studio, Boston, MA, USA). Results of continuous variables are presented as mean ± standard deviation. Differences in continuous and categorical variables among the three groups were assessed by analysis of variance, followed by the Tukey-Kramer post-hoc test and the chi-square test, respectively. To identify factors predictive of mortality, we first used a conventional Cox proportional hazards model (treating lung transplants as censoring events). Next, we used Fine and Gray subdistribution hazards models while treating transplantation as a competing risk [17]. In the multivariate Cox proportional hazards model analysis, a stepwise variable-selecting procedure was performed, and parameters with p < 0.1 in univariate analysis were exclusively entered. The proportional hazards assumption was assessed using Schoenfeld residuals. Survival curves were generated using the Kaplan–Meier method, and survival rates between subgroups were compared using the log-rank test. All analyses were considered statistically significant for p < 0.05.
Results
Diagnoses and outcomes
The diagnoses and outcomes of 77 lung transplant candidates with ILD are shown in Table 1. Of these patients, 53 (69%) were diagnosed with IIP and 17 (22%) and 7 (9%) were diagnosed with CTD-ILD and CHP, respectively. Of the 53 patients with IIP, 33 had IPF and 15 of the 20 patients with non-IPF IIP were diagnosed with unclassifiable ILD. During the observation period (median: 14.4 months, range: 0.3–48.3 months), 23 patients (30%) received lung transplantations (cadaveric lung transplantation (n = 20) and living-donor lobar lung transplantation (n = 3)), while 49 patients (64%) died before transplantation. Among the remaining 5 patients, only one patient was still awaiting transplantation, while 4 patients were removed from our institutional list due to stable disease (n = 2), contraindications (n = 1), and change in institution (n = 1). Among the 23 patients who received lung transplantations, 90-day survival was 100% (23/23), and 1-year survival was 94% (16/17).
Table 1. Diagnoses and outcomes of lung transplantation candidates with interstitial lung disease (n = 77).
| Diagnosis | |
| Idiopathic interstitial pneumonia | 53 (69%) |
| Idiopathic pulmonary fibrosis | 33 (43%) |
| Nonspecific interstitial pneumonia | 4 (5%) |
| Pleuroparenchymal fibroelastosis | 1 (1%) |
| Unclassifiable ILD | 15 (19%) |
| Connective tissue disease-associated ILD | 17 (22%) |
| Chronic hypersensitivity pneumonitis | 7 (9%) |
| Outcomes | |
| Death | 49 (64%) |
| Transplantation | 23 (30%) |
| Awaiting | 1 (1%) |
| Removal from the list | 4 (5%) |
Data are number of cases (percentage). ILD, interstitial lung disease.
Clinical characteristics and physiologic/laboratory data
The clinical characteristics of 77 candidates with ILD are shown in Table 2. Mean age was 49.0 ± 9.0 years, 48 patients (62%) were male, and 53 patients (69%) had received long-term oxygen therapy. Patients were classified into three groups: IPF (n = 33), unclassifiable ILD (n = 15), and other ILDs (n = 29), which included idiopathic NSIP (n = 4), idiopathic pleuroparenchymal fibroelastosis (n = 1), CTD-ILD (n = 17), and CHP (n = 7). Patients with IPF had a higher mean age than those with other ILDs, as well as a higher rate of male gender compared with the other two groups. Notably, 13 patients (39%) with IPF had a family history of ILD, which was significantly more common than in patients with other ILDs.
Table 2. Characteristics/Physiologic and laboratory data of lung transplantation candidates with interstitial lung disease (n = 77).
| All (n = 77) |
IPF (n = 33) |
Unclassifiable ILD (n = 15) |
Other ILDs (n = 29) |
|
|---|---|---|---|---|
| Characteristics | ||||
| Age, years | 49.0 ± 9.0 | 52.7 ± 7.4 | 47.3 ± 10.4 | 45.7 ± 8.7* |
| Male gender | 48 (62%) | 28 (85%) | 5 (33%)* | 15 (52%)* |
| ever smoker | 45 (59%) | 24 (73%) | 7 (47%) | 14 (50%) |
| BMI, kg/m2 | 21.2 ± 4.2 | 22.4 ± 3.8 | 20.2 ± 4.1 | 20.3 ± 4.5 |
| Family history of ILD | 17 (22%) | 13 (39%) | 3 (20%) | 1 (4%)* |
| Long term oxygen therapy | 53 (69%) | 19 (58%) | 10 (67%) | 24 (83%) |
| History of acute exacerbation | 13 (17%) | 4 (12%) | 1 (7%) | 8 (29%) |
| History of pneumothorax | 22 (29%) | 5 (15%) | 5 (36%) | 12 (41%)* |
| Physiologic/laboratory data | ||||
| %FVC, %† | 48.5 ± 15.8 | 54.6 ± 14.3 | 40.7 ± 13.3* | 45.2 ± 16.6 |
| >75 | 4 (6%) | 3 (9%) | 0 (2%) | 1 (4%) |
| 50–75 | 28 (39%) | 16 (50%) | 4 (27%) | 8 (33%) |
| <50 | 39 (55%) | 13 (41%) | 11 (73%) | 15 (63%) |
| %DLCO, %‡ | 26.2 ± 11.6 | 29.6 ± 11.5 | 24.5 ± 11.6 | 20.5 ± 10.1* |
| >55 | 1 (1%) | 1 (3%) | 0 (0%) | 0 (0%) |
| 36–55 | 9 (13%) | 7 (22%) | 1 (6%) | 1 (4%) |
| ≤35 | 40 (56%) | 20 (63%) | 7 (47%) | 13 (54%) |
| Could not perform | 21 (30%) | 4 (12%) | 7 (47%) | 10 (42%) |
| 6MWD, m | 343 ± 165 | 436 ± 125 | 309 ± 160* | 254 ± 156* |
| ≥250 | 53 (69%) | 30 (91%) | 8 (53%) | 15 (52%) |
| <250 | 24 (31%) | 3 (9%) | 7 (47%) | 14 (48%) |
| Oxygen supplementation at 6MWT | 47 (61%) | 16 (48%) | 9 (60%) | 22 (76%) |
| Oxygen flow at 6MWT, L/min | 2.2 ± 2.4 | 1.4 ± 1.6 | 2.1 ± 1.9 | 3.2 ± 3.0* |
| GAP stage I/II/III†, n (%) | 15/50/6 (21/70/8) |
9/23/0 (28/72/0) |
2/11/2 (13/73/13) |
4/16/4 (17/67/17) |
| LDH, mg/dL | 236 ± 61 | 234 ± 56 | 213 ± 42 | 248 ± 72 |
| KL-6, U/mL | 1633 ± 1458 | 1380 ± 823 | 1443 ± 1003 | 1956 ± 2075 |
Data are number of cases (percentage) or mean ± standard deviation. IPF, idiopathic pulmonary fibrosis; ILD, interstitial lung disease; BMI, body mass index; %FVC, percent predicted forced vital capacity; %DLCO, percent predicted diffusing capacity of the lung for carbon monoxide; 6MWD, 6-min walking distance; 6MWT, the 6-min walk test; GAP stage, gender-age-physiology stage; LDH, lactate dehydrogenase; KL-6, Krebs von den Lungen-6. Other ILDs: idiopathic nonspecific interstitial pneumonia (n = 4), pleuroparenchymal fibroelastosis (n = 1), connective tissue disease-associated ILD (n = 17), and chronic hypersensitivity pneumonitis (n = 7).
*p < 0.05 vs. IPF group.
†n = 71
‡n = 50.
In addition, we investigated the HRCT pattern of 33 candidates with IPF. Among them, 16 patients (49%) showed a UIP pattern on HRCT and 4 patients showed a possible UIP pattern. However, the remaining 13 (39%) patients had an “inconsistent with UIP pattern”, although surgical lung biopsies showed a UIP pattern.
Physiologic and laboratory data from candidates with ILD are shown in Table 2. Six patients could not undergo pulmonary function testing because of the existence of pneumothorax, but pulmonary function data were available in the remaining 71 patients. Of these patients, 39 (55%) had a forced vital capacity (FVC) of <50% of the predicted value. Most candidates had a DLCO of <35% of the predicted value or were unable to perform the DLCO test due to low vital capacity or difficulty holding their breath. Most patients were GAP stages I and II due to their young age despite their pulmonary functions being extremely poor. The pulmonary function in patients with IPF was relatively preserved compared to the other two groups, though the distribution of GAP stage was not significantly different between groups.
Survival analysis
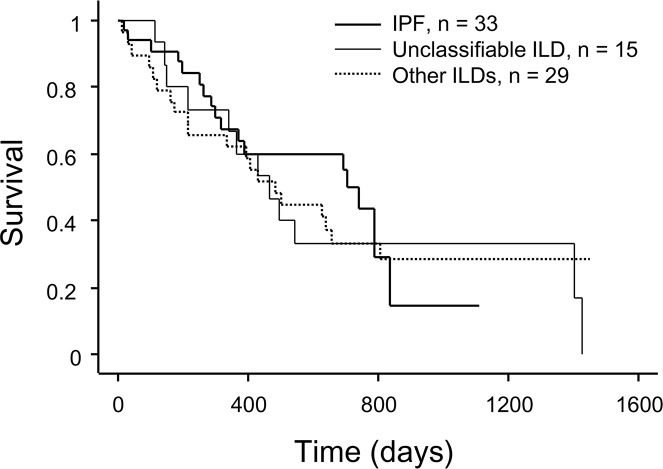
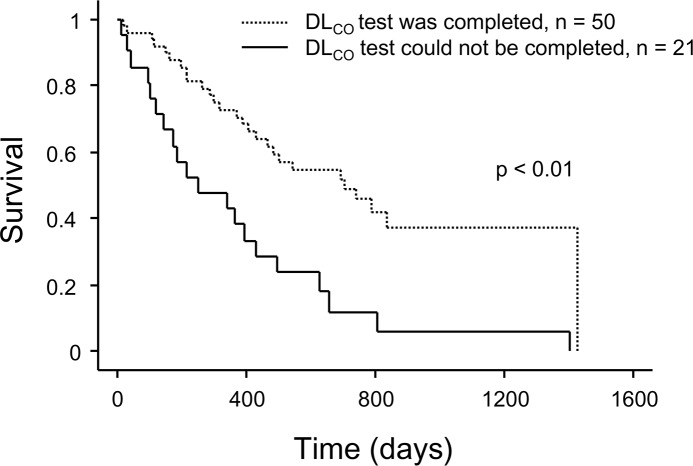
Last, we performed survival analysis of the 77 candidates with ILD. The median survival time from registration to JOTN was 16.7 months. In a univariate Cox proportional hazards analysis, body mass index (BMI) (hazards ratio (HR) of 0.94 for each 1 kg/m2 increase in BMI; 95% confidence interval (CI): 0.88–0.996; p = 0.04), percent predicted FVC (%FVC) (HR: 0.78 for every 10% increase; 95% CI: 0.64–0.96, p = 0.02), percent predicted DLCO (%DLCO) (HR: 0.60 for every 10% increase; 95% CI: 0.40–0.90, p = 0.01), the 6-min walking distance (6MWD) (HR: 0.97 for every 10 m increase; 95% CI: 0.95–0.99, p < 0.01), oxygen flow ≥2L/min at 6MWT (HR: 2.33; 95% CI: 1.28–4.25, p < 0.01), and GAP stage III (HR: 2.55; 95% CI: 1.07–6.04, p = 0.03) were significantly associated with mortality; however, DLCO data were missing for 27 patients (Table 3). Kaplan–Meier survival curves showed that the mortality rate did not differ significantly among the three groups (Fig 1). We compared the mortality rate in patients whose DLCO data were missing due to respiratory limitations (n = 21) and patients with available DLCO data (n = 50), and showed that the patients without DLCO data had significantly shorter survival times than the group with DLCO data (p < 0.01, Fig 2).
Table 3. Cox proportional hazards model results for evaluating the risk of mortality in lung transplant candidates with interstitial lung disease (n = 77).
| Univariate analysis | Hazards ratio | 95%CI | p-value | ||
| Age, years | 1.02 | 0.99 | – | 1.05 | 0.27 |
| Male gender | 0.82 | 0.46 | – | 1.46 | 0.49 |
| IPF diagnosis | 0.82 | 0.45 | – | 1.50 | 0.52 |
| BMI, kg/m2 | 0.94 | 0.88 | – | 0.996 | 0.04 |
| Ever smoker | 0.93 | 0.52 | – | 1.66 | 0.81 |
| Pulmonary hypertension | 1.16 | 0.60 | – | 2.23 | 0.66 |
| History of acute exacerbation | 1.39 | 0.69 | – | 2.81 | 0.36 |
| History of pneumothorax | 1.26 | 0.69 | – | 2.31 | 0.45 |
| %FVC, per 10%* | 0.78 | 0.64 | – | 0.96 | 0.02 |
| %DLCO, per 10%† | 0.60 | 0.40 | – | 0.90 | 0.01 |
| 6MWD, per 10m | 0.97 | 0.95 | – | 0.99 | < 0.01 |
| Oxygen flow ≥2L/min at 6MWT | 2.33 | 1.28 | – | 4.25 | < 0.01 |
| GAP stage III | 2.55 | 1.07 | – | 6.04 | 0.03 |
| Multivariate analysis | Hazards ratio | 95%CI | p-value | ||
| Model 1 | |||||
| BMI, kg/m2 | – | – | – | ||
| %FVC, per 10% | – | – | – | ||
| 6MWD, per 10m | 0.97 | 0.95 | – | 0.99 | < 0.01 |
| Oxygen flow ≥2L/min at 6MWT | – | – | – | ||
| Model 2 | |||||
| BMI, kg/m2 | – | – | – | ||
| 6MWD, per 10m | 0.97 | 0.95 | – | 0.99 | < 0.01 |
| Oxygen flow ≥2L/min at 6MWT | – | – | – | ||
| GAP stage III | – | – | – | ||
CI, confidence interval; IPF, idiopathic pulmonary fibrosis; BMI, body mass index; ILD, interstitial lung disease; %FVC, percent predicted forced vital capacity; %DLCO, percent predicted diffusing capacity of the lung for carbon monoxide; 6MWD, 6-min walking distance; 6MWT, the 6-min walk test; GAP stage, gender-age-physiology stage.
*n = 71
†n = 50.
Fig 1. Kaplan−Meier survival curves for candidates with interstitial lung disease (ILD) grouped by diagnosis; idiopathic pulmonary fibrosis (n = 33), unclassifiable ILD (n = 15), and other ILDs (n = 29).
Other ILDs: idiopathic nonspecific interstitial pneumonia (n = 4), pleuroparenchymal fibroelastosis (n = 1), connective tissue disease-associated ILD (n = 17), and chronic hypersensitivity pneumonitis (n = 7).
Fig 2. Kaplan−Meier survival curves for candidates with interstitial lung disease grouped based on the ability to perform the DLCO test.
p < 0.01 by log-rank test. DLCO, diffusing capacity of the lung for carbon monoxide.
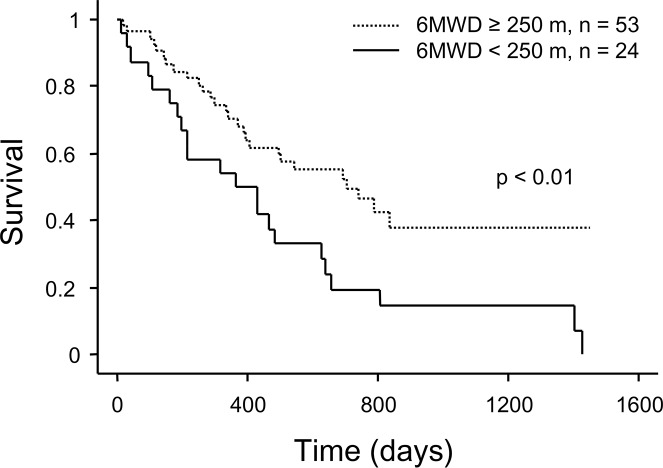
In multivariate stepwise analyses, we excluded %DLCO as a variable due to the considerable number of patients with missing data. We demonstrated that 6MWD independently contributed to mortality when selecting BMI, %FVC, 6MWD, and oxygen flow ≥2L/min at 6MWT (HR: 0.97 for every 10 m increase; 95% CI: 0.95–0.99, p < 0.01; model 1), or BMI, GAP stage III, 6MWD, and oxygen flow ≥2L/min at 6MWT (HR: 0.97 for every 10 m increase; 95% CI: 0.95–0.99, p < 0.01; model 2; Table 3). Validity of the proportional hazards assumption was confirmed using Schoenfeld residuals in multivariate analyses. To address the possible bias arising from censoring due to transplantation, we also performed Fine and Gray subdistribution hazards models while treating transplantation as a competing risk. In multiple stepwise analyses, 6MWD also independently contributed to mortality (HR: 0.97 for each 10 m increase; 95% CI: 0.95–0.99, p < 0.01, S1 Table). The cut-off value for 6MWD was set at 250 m based on the international consensus report [18]. Kaplan–Meier survival curves showed that patients with a 6MWD less than 250 m had significantly shorter survival times than those with a 6MWD ≥ 250 m (p < 0.01, Fig 3). Among the 24 patients with a 6MWD < 250 m, 22 (92%) died before transplantation, while 22 patients (42%) in the group with a 6MWD ≥ 250 m (n = 53) received a transplant.
Fig 3. Kaplan−Meier survival curves for candidates with interstitial lung disease grouped based on 6MWD.
p < 0.01 by log-rank test. 6MWD, 6-min walking distance.
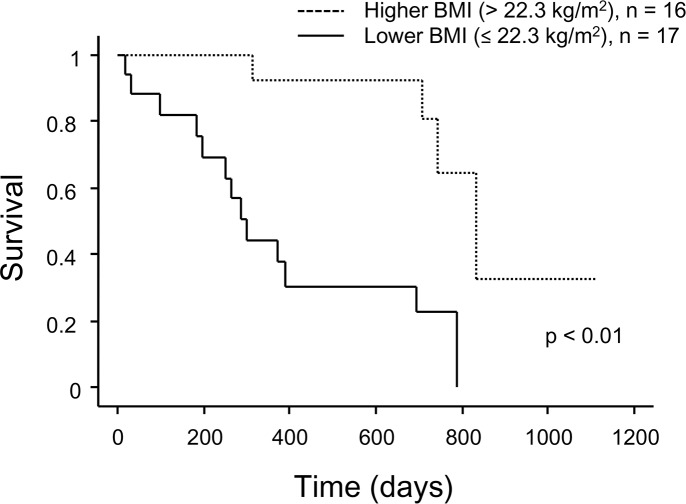
Additionally, survival analysis was performed in 33 candidates with IPF. Using the stepwise multivariate Cox proportional hazards model, BMI (HR: 0.83; 95% CI: 0.72–0.95, p < 0.01) and a history of acute exacerbation (AE) (HR: 4.50; 95% CI: 1.15–17.66, p = 0.03) independently contributed to increased mortality (Table 4). A univariate Fine and Gray subdistribution hazards model showed that a history of AE was not significantly associated with mortality. However, BMI also independently contributed to mortality in multiple stepwise Fine and Gray subdistribution hazards models (HR: 0.83; 95% CI: 0.75–0.91, p < 0.01, S2 Table). When patients with IPF were classified based on the median BMI value (22.3 kg/m2), patients with a lower BMI had significantly shorter survival times than those with a higher BMI (p < 0.01, Fig 4). In the lower BMI subgroup (n = 17), 13 (76%) patients died before transplantation and only 4 (24%) patients received a transplant, while 11 (69%) patients received a transplant in the higher BMI group (n = 16).
Table 4. Cox proportional hazards model results for evaluating the risk of mortality in patients with idiopathic pulmonary fibrosis (n = 33).
| Univariate analysis | Hazards ratio | 95%CI | p-value | ||
| Age, years | 1.01 | 0.95 | – | 1.07 | 0.75 |
| Male gender | 0.38 | 0.12 | – | 1.20 | 0.099 |
| BMI, kg/m2 | 0.85 | 0.74 | – | 0.97 | 0.01 |
| Ever smoker | 0.96 | 0.34 | – | 2.68 | 0.94 |
| Pulmonary hypertension | 0.91 | 0.20 | – | 4.06 | 0.90 |
| History of acute exacerbation | 3.38 | 0.91 | – | 12.58 | 0.07 |
| History of pneumothorax | 1.94 | 0.61 | – | 6.15 | 0.26 |
| %FVC, per 10%* | 0.78 | 0.57 | – | 1.08 | 0.14 |
| %DLCO, per 10%† | 0.48 | 0.27 | – | 0.84 | 0.01 |
| 6MWD, per 10m | 0.96 | 0.93 | – | 1.00 | 0.08 |
| Oxygen flow ≥2L/min at 6MWT | 2.13 | 0.79 | – | 5.77 | 0.14 |
| GAP stage II or III | 2.70 | 0.76 | – | 9.66 | 0.13 |
| Multivariate analysis | Hazards ratio | 95%CI | p-value | ||
| Male gender | – | – | – | ||
| BMI, kg/m2 | 0.83 | 0.72 | – | 0.95 | < 0.01 |
| History of acute exacerbation | 4.50 | 1.15 | – | 17.66 | 0.03 |
| 6MWD, m | – | – | – | ||
CI, confidence interval; BMI, body mass index; ILD, interstitial lung disease; %FVC, percent predicted forced vital capacity; %DLCO, percent predicted diffusing capacity of the lung for carbon monoxide; 6MWD, 6-min walking distance; 6MWT, the 6-min walk test; GAP stage, gender-age-physiology stage.
*n = 32
†n = 28.
Fig 4. Kaplan−Meier survival curves for candidates with idiopathic pulmonary fibrosis grouped based on BMI.
p < 0.01 by log-rank test. BMI, body mass index.
Discussion
In the present study, we showed that 30% of lung transplant candidates with ILD received lung transplantation, while 64% died before transplantation in Japan. Among all candidates with ILD, 6MWD was independently associated with survival, while BMI was independently associated with survival in 33 patients with IPF. To the best of our knowledge, this is the first study to present prognostic factors in Japanese lung transplantation candidates with ILD.
Our study demonstrated two important findings regarding the prognosis for lung transplant candidates with ILD in Japan. First, these patients had very poor prognosis and the mortalities were similar among patients with IPF, unclassifiable ILD, and other ILDs. Our study results were different from previous reports, partly due to the lower frequency of IPF patients with severely impaired pulmonary function or exercise capacity compared with unclassifiable ILD and other ILDs at the time of registration (Table 2).
Second, 6MWD was independently associated with survival. The link between 6MWD and survival has been reported previously in patients with IPF and ILD [19–21]. 6MWT is a practical and simple test that requires no exercise equipment or advanced training for technicians [16]. In our study, 6MWT was performed by all participants, though most had severe pulmonary impairment, while the pulmonary function test was not performed by patients with complicated pneumothorax. Additionally, 6MWT is a reliable and reproducible test [22]. We set the cut-off value at 250 m for 6MWD based on the international consensus report [18], and showed that most of patients with a 6MWD of less than 250 m died before transplantation. Although a 6MWD below 250 m is one of the factors to consider for lung transplants in ILD patients [18], surviving until transplantation under the current time-based allocation system in Japan may be a challenge for these patients.
We considered that %DLCO was an inappropriate predictive factor for prognosis since DLCO data were unattainable in approximately a quarter of the participants. However, patients who could not complete the DLCO test had significantly worse outcome than those who completed the DLCO test, suggesting that DLCO is a prognostic factor in patients with ILD [23–26]. It is, however, a major problem that the DLCO test cannot be performed according to the standard approach when patients have an FVC of less than 1 L or cannot hold their breath for 10 s due to respiratory limitations [27]. 6MWD was strongly correlated with %DLCO in our study (r = 0.768, p < 0.001), and this correlation has been previously reported [28, 29]. 6MWD may be the most useful prognostic factor since it indirectly represents the lung diffusion capacity.
Among patients with IPF, an independent prognostic factor was low BMI, not 6MWD. This result is partly because pulmonary function and exercise tolerance were maintained relatively well in patients with IPF. Indeed only 3 patients (9%) had a 6MWD less than 250 m (Table 2). Nevertheless, the mortality of IPF patients was similar to that of patients with unclassifiable ILD and other ILDs. Thus, patients with IPF may need to be registered on the waiting list for transplantation earlier than those with other types of ILD. Low BMI was reported to be associated with shorter survival in US and Asian IPF cohorts [30, 31], although the cut-off level of BMI differed between studies. Nadrous et al. investigated the outcome in IPF patients younger than 50 years, and found that these patients had a similarly poor prognosis as older patients [32]. However, no prognostic factors had been identified in younger patients with IPF. Low BMI may be a unique prognostic factor in young and advanced patients with IPF.
Like the US ILD and IIP candidates listed for lung transplantation in previous studies, the most frequent diagnosis in our study was IPF. However, our study participants were younger and had a lower BMI than those in earlier studies, mainly due to the different ethnicity and upper age limit for transplant candidates [20, 33]. Therefore, the Japanese candidates for lung transplant may have specific clinical characteristics and prognostic factors which differ from the US and European cohorts, although 6MWD is one of the previously reported prognostic factors for IPF and ILD transplant candidates [19, 20].
In this study, we discovered intriguing characteristics present in young patients with advanced ILD. First, approximately 20% of candidates had unclassifiable ILD and approximately 40% with IPF had an “inconsistent with UIP pattern” by HRCT. Young patients with IIP may have a lower frequency of typical UIP pattern than elderly patients, by both HRCT and histology. Second, approximately 40% of patients with IPF had a family history of ILD. A previous report showed that patients with familial IIP, who showed a histological UIP pattern, had shorter survival times and younger average age at death [34]. Patients with familial IIP may frequently be included in the group of young and advanced patients with IPF.
There are some limitations of our study. First, the sample size was small. Although the present study was a single-center study, our hospital is the highest volume center for lung transplantation in Japan and one of the institutes that specialize in the care of ILD. Second, there were no longitudinal physiologic and laboratory data in our study. However, it was difficult to collect longitudinal data because most of the patients had severe respiratory impairments and poor outcomes.
In conclusion, our study demonstrated that Japanese patients with ILD on the waiting list for transplantation had very poor outcomes regardless of their specific diagnosis, and that 6MWD and BMI were independent predictors of mortality in patients with ILD and IPF, respectively. We may need to establish a rule to allocate the limited donor lungs based on prognostic factors, similar to the lung allocation system in the US, to save more patients with ILD.
Supporting information
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by grants from the Scientific Research/Research on intractable diseases, the Practical Research Project for Rare Intractable Diseases from Japan Agency for Medical Research and Development, AMED no. 15ek0109079h0001 (to Tomohiro Handa), and the Japan Society for the Promotion of Science no. 17K09612 (to Tomohiro Handa). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An Official ATS/ERS/JRS/ALAT Statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011; 183: 788–824. doi: 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nunes H, Schubel K, Piver D, Magois E, Feuillet S, Uzunhan Y, et al. Nonspecific interstitial pneumonia: survival is influenced by the underlying cause. Eur Respir J. 2015; 45: 746–755. doi: 10.1183/09031936.00148613 [DOI] [PubMed] [Google Scholar]
- 3.Fischer A, Du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012; 380: 689–698. doi: 10.1016/S0140-6736(12)61079-4 [DOI] [PubMed] [Google Scholar]
- 4.Pérez ERF, Swigris JJ, Forssén AV, Tourin O, Solomon JJ, Huie TJ, et al. Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest. 2013; 144: 1644–1651. doi: 10.1378/chest.12-2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skolnik K, Ryerson CJ. Unclassifiable interstitial lung disease: a review. Respirology. 2016; 21: 51–56. [DOI] [PubMed] [Google Scholar]
- 6.Ryerson CJ, Urbania TH, Richeldi L, Mooney JJ, Lee JS, Jones KD, et al. Prevalence and prognosis of unclassifiable interstitial lung disease. Eur Respir J. 2013; 42: 750–757. doi: 10.1183/09031936.00131912 [DOI] [PubMed] [Google Scholar]
- 7.Date H. Current status and problems of lung transplantation in Japan. J Thorac Dis. 2016; 8: S631–S636. doi: 10.21037/jtd.2016.06.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato M, Okada Y, Oto T, Minami M, Shiraishi T, Nagayasu T, et al. Registry of the Japanese Society of Lung and Heart–Lung Transplantation: official Japanese lung transplantation report, 2014. Gen Thorac Cardiovasc Surg. 2014; 62: 594–601. doi: 10.1007/s11748-014-0418-6 [DOI] [PubMed] [Google Scholar]
- 9.Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012; 156: 684–695. doi: 10.7326/0003-4819-156-10-201205150-00004 [DOI] [PubMed] [Google Scholar]
- 10.Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ, et al. International guidelines for the selection of lung transplant candidates: 2006 update-a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006; 25: 745–755. doi: 10.1016/j.healun.2006.03.011 [DOI] [PubMed] [Google Scholar]
- 11.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care med. 2000; 161: 646–664. doi: 10.1164/ajrccm.161.2.ats3-00 [DOI] [PubMed] [Google Scholar]
- 12.Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013; 188: 733–748. doi: 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travis WD, Hunninghake G, King TE Jr, Lynch DA, Colby TV, Galvin JR, et al. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am J Respir Crit Care Med. 2008; 177: 1338–1347. doi: 10.1164/rccm.200611-1685OC [DOI] [PubMed] [Google Scholar]
- 14.Reddy TL, Tominaga M, Hansell DM, Von Der Thusen J, Rassl D, Parfrey H, et al. Pleuroparenchymal fibroelastosis: a spectrum of histopathological and imaging phenotypes. Eur Respir J. 2012; 40: 377–385. doi: 10.1183/09031936.00165111 [DOI] [PubMed] [Google Scholar]
- 15.Schuyler M, Cormier Y. The diagnosis of hypersensitivity pneumonitis. Chest. 1997; 111: 534–535. [DOI] [PubMed] [Google Scholar]
- 16.Crapo RO, Casaburi R, Coates AL, Enright PL, MacIntyre NR, McKay RT, et al. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002; 166: 111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999; 94: 496–509. [Google Scholar]
- 18.Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, et al. A consensus document for the selection of lung transplant candidates: 2014—an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015; 34: 1–15. doi: 10.1016/j.healun.2014.06.014 [DOI] [PubMed] [Google Scholar]
- 19.Du Bois RM, Albera C, Bradford WZ, Costabel U, Leff JA, Noble PW, et al. 6-minute walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2014; 43: 1421–1429. doi: 10.1183/09031936.00131813 [DOI] [PubMed] [Google Scholar]
- 20.Lederer DJ, Arcasoy SM, Wilt JS, D'Ovidio F, Sonett JR, Kawut SM. Six-minute-walk distance predicts waiting list survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006; 174: 659–664. doi: 10.1164/rccm.200604-520OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawut SM, O'Shea MK, Bartels MN, Wilt JS, Sonett JR, Arcasoy SM. Exercise testing determines survival in patients with diffuse parenchymal lung disease evaluated for lung transplantation. Respir Med. 2005; 99: 1431–1439. doi: 10.1016/j.rmed.2005.03.007 [DOI] [PubMed] [Google Scholar]
- 22.Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, Hill CJ, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014; 44: 1447–1478. doi: 10.1183/09031936.00150414 [DOI] [PubMed] [Google Scholar]
- 23.Hamada K, Nagai S, Tanaka S, Handa T, Shigematsu M, Nagao T, et al. Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest. 2007; 131: 650–656. doi: 10.1378/chest.06-1466 [DOI] [PubMed] [Google Scholar]
- 24.Shin KM, Lee KS, Chung MP, Han J, Bae YA, Kim TS, et al. Prognostic determinants among clinical, thin-section CT, and histopathologic findings for fibrotic idiopathic interstitial pneumonias: tertiary hospital study. Radiology. 2008; 249: 328–337. doi: 10.1148/radiol.2483071378 [DOI] [PubMed] [Google Scholar]
- 25.Song JW, Lee HK, Lee CK, Chae EJ, Jang SJ, Colby TV, et al. Clinical course and outcome of rheumatoid arthritis-related usual interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis. 2013; 30: 103–112. [PubMed] [Google Scholar]
- 26.Winstone TA, Assayag D, Wilcox PG, Dunne JV, Hague CJ, Leipsic J, et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest. 2014; 146: 422–436. doi: 10.1378/chest.13-2626 [DOI] [PubMed] [Google Scholar]
- 27.MacIntyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CPM, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005; 26: 720–735. doi: 10.1183/09031936.05.00034905 [DOI] [PubMed] [Google Scholar]
- 28.Eaton T, Young P, Milne D, Wells AU. Six-minute walk, maximal exercise tests: reproducibility in fibrotic interstitial pneumonia. Am J Respir Crit Care Med. 2005; 171: 1150–1157. doi: 10.1164/rccm.200405-578OC [DOI] [PubMed] [Google Scholar]
- 29.Rasekaba T, Lee AL, Naughton MT, Williams TJ, Holland AE. The six-minute walk test: a useful metric for the cardiopulmonary patient. Intern Med J. 2009; 39: 495–501. doi: 10.1111/j.1445-5994.2008.01880.x [DOI] [PubMed] [Google Scholar]
- 30.Alakhras M, Decker PA, Nadrous HF, Collazo-Clavell M, Ryu JH. Body mass index and mortality in patients with idiopathic pulmonary fibrosis. Chest. 2007; 131: 1448–1453. doi: 10.1378/chest.06-2784 [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Lee JH, Ryu YJ, Chang JH. Clinical predictors of survival in idiopathic pulmonary fibrosis. Tuberc Respir Dis. 2012; 73: 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nadrous HF, Myers JL, Decker PA, Ryu JH. Idiopathic pulmonary fibrosis in patients younger than 50 years. Mayo Clin Proc. 2005; 80: 37–40. doi: 10.1016/S0025-6196(11)62955-8 [DOI] [PubMed] [Google Scholar]
- 33.Timmer SJ, Karamzadeh AM, Yung GL, Kriett J, Jamieson SW, Smith CM. Predicting survival of lung transplantation candidates with idiopathic interstitial pneumonia: does PaO2 predict survival? Chest. 2002; 122: 779–784. [DOI] [PubMed] [Google Scholar]
- 34.Leslie KO, Cool CD, Sporn TA, Curran-Everett D, Steele MP, Brown KK, et al. Familial idiopathic interstitial pneumonia histopathology and survival in 30 patients. Arch Pathol Lab Med. 2012; 136: 1366–1376. doi: 10.5858/arpa.2011-0627-OAI [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.