Abstract
The recently released Recommended Dietary Allowance of vitamin C for women, 75 mg daily, was based on data for men. We now report results of a depletion–repletion study with healthy young women hospitalized for 186 +/− 28 days, using vitamin C doses of 30–2,500 mg daily. The relationship between dose and steady-state plasma concentration was sigmoidal. Only doses above 100 mg were beyond the linear portion of the curve. Plasma and circulating cells saturated at 400 mg daily, with urinary elimination of higher doses. Biomarkers of endogenous oxidant stress, plasma and urine F2-isoprostanes, and urine levels of a major metabolite of F2-isoprostanes were unchanged by vitamin C at all doses, suggesting this vitamin does not alter endogenous lipid peroxidation in healthy young women. By using Food and Nutrition Board guidelines, the data indicate that the Recommended Dietary Allowance for young women should be increased to 90 mg daily.
In April 2000, Recommended Dietary Allowances (RDAs) for vitamin C (ascorbic acid) were released by the Food and Nutrition Board, U.S. National Academy of Sciences (1). The recommendations are for the U.S. and Canada. On the basis of new Food and Nutrition Board guidelines, the RDAs were calculated from estimated average requirement (EAR) values, those estimated to meet the nutrient requirement of half the individuals in a group (2). The EAR for vitamin C was selected as 80% saturation of neutrophils with little urinary loss (1). For men, an EAR of 75 mg daily was determined from depletion–repletion data (3). On the basis of this value, the RDA for men was increased from 60 to 90 mg daily (1). Because similar data were not available for women, an EAR had to be extrapolated on the basis of body weight differences between sexes, and an RDA of 75 mg daily was derived (1).
On the basis of depletion–repletion data for men (3), recommended daily vitamin C intake was increased to 100 mg in Germany, Switzerland, Austria, and Japan, and to 110 mg in France (4–6). Because of absence of data for women, vitamin C intake in these countries was recommended as the same for both sexes.
We now present data that can be used to calculate a new RDA specifically for young women (1, 3). By using an inpatient depletion–repletion design, vitamin C in healthy young women subjects was measured at steady state for each of seven doses in plasma, circulating cells, and urine. Also measured were effects of vitamin C concentrations on lipid peroxidation in vivo (7).
Experimental Methods
Study Design.
The study (DK92–0032) was approved by the Institutional Review Board, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. Written informed consent was obtained from enrolled women.
Study design and diet, subject recruitment, vitamin C measurements, and calculation of steady state are described in detail (3, 8). Women selected by thorough preadmission examination were hospitalized on an endocrinology–metabolism ward for control of nutrient intake. Subjects were usually hospitalized two at a time, sharing a standard hospital room.
Subjects consumed a vitamin C-deficient diet, providing <5 mg daily, for the entire hospitalization (8). Personal food preferences were chosen from 14 different daily menus with >300 items. At least 234 menu items contained no vitamin C. Of the 56 items containing vitamin C (0.1–2.4 mg), 5 were not prepared or prepackaged as individual portions. These items were measured to a specified gram weight when served. Menus were selected 1 day in advance by each subject and scanned by computer to generate a nutrient limit report for vitamin C. When the 5.0-mg daily maximum for vitamin C was exceeded, selections were revised. Meals were delivered to the nursing unit and on completion returned to the kitchen for observational and weighed measurements. By these means, vitamin C intake from foods was determined at every meal for every subject and was less than 5 mg daily. Food content and dietary intakes were also monitored for 16 other nutrients, energy, protein, fat, and saturated fat. Vitamin and mineral deficiencies were prevented by supplement administration, so that only vitamin C intake was restricted.
Subjects selected were in good health and had no prior history of smoking, regular use of medications or dietary supplements, or habitual alcohol consumption. During the study, subjects did not consume alcohol, cigarettes, or regular medications. The only vitamin and mineral supplements consumed were those given with the deficiency diet.
On hospitalization, consumption of the study diet caused vitamin C depletion. At plasma concentrations ≤8 μM, subjects were depleted, without signs of scurvy. Neutrophils were isolated to measure vitamin C content (9), plasma samples were collected for F2-isoprostanes as a biomarker of in vivo lipid oxidation (7), and 36-h bioavailability sampling for vitamin C, 15 mg, was performed. Twenty-four-hour urine samples were collected to measure excreted vitamin C, creatinine, F2-isoprostanes, F2-isoprostane-M (a major urinary metabolite of F2-isoprostanes), and other metabolites. Vitamin C, 15 mg twice daily, was then administered until subjects achieved steady state for this dose (30 mg daily). Thirty-six-hour bioavailability sampling for this daily dose was performed, and subjects underwent apheresis (cell separation) to obtain monocytes, lymphocytes, and platelets for analyses of vitamin C content (3, 10, 11). After twenty-four-hour urine samples were collected and samples obtained for neutrophils and plasma F2-isoprostanes, the vitamin C dose was increased and the sequence repeated at the new dose. This study design allowed subjects to receive in succession daily vitamin C doses of 30, 60, 100, 200, 400, 1,000, and 2,500 mg, with bioavailability sampling for vitamin C doses of 15, 30, 50, 100, 200, 500, and 1,250 mg. Vitamin C in water, pH adjusted to 6.5, was administered as half the dose twice daily in the fasting state, at least 2 hours before breakfast and dinner. Pure vitamin C powder was a gift from Takeda Vitamin and Food, Wilmington, NC. Batches prepared for clinical use were routinely assayed for purity and stability, as described (3).
Fifteen healthy women were studied as inpatients. Women were nonsmokers, ages 19–27, with Body Mass Index of 22.3 ± 2.7 kg/m2, height 162.8 ± 6.1 cm, and weight 59.1 ± 8.7 kg (mean ± SD). Data were not available for all subjects at all doses because of inability of some subjects to remain hospitalized for the entire time required, i.v. access limitations, or inadvertent nursing errors or sample loss. No effect of menses on pharmacokinetics data was apparent. Plasma F2-isoprostanes were available only for seven subjects because of the specific collection method required.
Assays.
All vitamin C measurements used HPLC with coulometric electrochemical detection, as explained previously (3), including conditions for sample processing, storage, intraassay stability, deproteinization, sensitivity, and specificity (3, 12, 13). Vitamin C samples were analyzed in triplicate, SD < 5% of the mean. Plasma data represent fasting morning samples.
For measurement of plasma F2-isoprostanes, blood obtained from fasting subjects at steady state was slowly drawn into Ca-EDTA glass tubes and immediately centrifuged to obtain plasma, which was immediately frozen in liquid nitrogen until F2-isoprostanes were analyzed as described (7). F2-isoprostane-M was measured in aliquots from 24-h urine collections at steady state for each dose by gas chromatography/negative ion chemical ionization mass spectrometry (14, 15). Urine F2-isoprostanes were analyzed as described (7).
Results
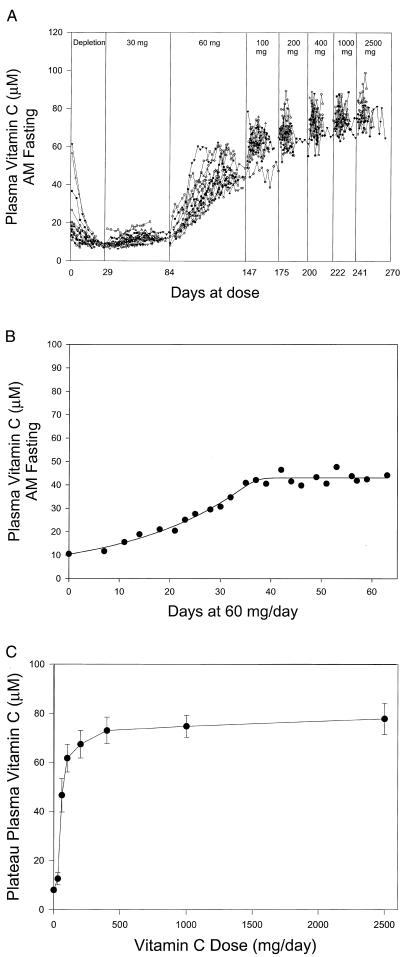
To obtain data over the dose range, subjects were hospitalized for 186 +/− 28 days. Plasma vitamin C concentrations at all doses are shown (Fig. 1A). Subjects required different amounts of time to achieve steady state at doses <100 mg daily. Gaps are displayed between doses, because some subjects remained at a dose longer than others. Every subject achieved steady state at each dose before the dose was increased. Steady-state plasma concentration was defined as the mean of at least 6 samples over at least 7 days with less than 10% standard deviation. The first sample included in the steady-state calculation was >90% of the mean (3). An example of a steady-state plasma concentration is shown (Fig. 1B) in one subject for 60 mg, administered as 30 mg twice daily. Eight-five percent of steady-state plateau values were based on seven or more plasma samples obtained over more than 1 week (Fig. 1 A and B).
Figure 1.
Vitamin C plasma concentration as a function of dose in women. See Experimental Methods for details. (A) Vitamin C concentrations as function of days at dose. Doses are indicated (Top). Each symbol represents a different subject. There is a 1-day gap between all doses for bioavailability sampling. Doses through 200 mg daily were received by 15 subjects, through 1,000 mg daily by 13 subjects, and through 2,500 mg by 10 subjects. (B) Steady-state vitamin C concentrations in plasma. Data show determination of steady state for one subject at 60 mg daily. (C) Steady-state vitamin C concentrations in plasma for subjects (in A) as a function of all doses, mean ± SD.
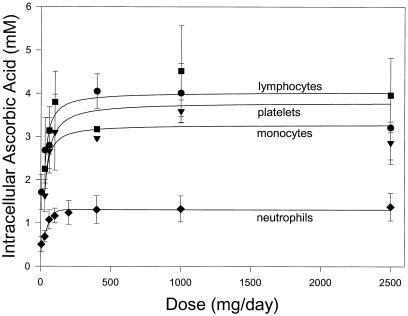
Plasma and tissue steady-state concentrations of vitamin C for each dose in every available subject were determined and displayed as a function of dose. There was a sigmoid relationship between dose and plasma concentration, with a linear relationship at doses 30–100 mg daily, and plasma saturation occurred between 200 and 400 mg daily (Fig. 1C). The first dose beyond the linear portion of the curve was more than 100 mg daily, producing a plasma concentration similar to Vmax of the human sodium-dependent vitamin C tissue transporter (SVCT2) (16–18). Vitamin C concentrations were determined at steady state over the dose range in neutrophils, monocytes, lymphocytes, and platelets (Fig. 2; Table 1). As for plasma, most cells saturated between 200 and 400 mg daily.
Figure 2.
Intracellular vitamin C concentrations (millimolar) in circulating cells as function of dose. Neutrophils (⧫), monocytes (▾), platelets (●), and lymphocytes (■) were isolated when steady state was achieved for each dose. For neutrophils, samples were available from 13 subjects at doses 0–200 mg daily, from 11 subjects at doses 400 and 1,000 mg daily, and from 10 subjects at 2,500 mg daily. For lymphocytes, monocytes, and platelets, samples were available from 13 subjects at 30 mg daily, from 12 subjects at 60 mg daily, from 6 subjects at 100 mg daily, from 2 subjects at 400 and 1,000 mg daily, and from 9 subjects at 2,500 mg daily. Data are mean values ± SD.
Table 1.
Intracellular ascorbic acid concentrations, mM, in neutrophils as a function of dose
| Subject | Vitamin C dose, mg/day
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 5 | 30 | 60 | 100 | 200 | 400 | 1,000 | 2,500 | |
| 1 | 0.39 | 0.90 | 0.97 | 1.05 | 1.03 | 1.10 | 1.12 | 1.27 |
| 2 | 0.50 | 0.66 | 0.82 | 0.91 | 0.93 | 1.07 | 1.10 | 1.21 |
| 3 | 0.58 | 0.79 | 1.22 | 1.09 | 1.24 | 1.37 | 1.23 | NA |
| 4 | 0.61 | 0.61 | 1.02 | 1.11 | 1.15 | 1.21 | 1.20 | NA |
| 5 | 0.72 | 0.79 | 1.30 | 1.46 | 1.60 | 1.61 | 1.81 | 1.79 |
| 6 | 0.80 | 0.86 | 1.67 | 1.52 | 1.82 | 2.04 | 1.80 | 2.03 |
| 7 | 0.37 | 0.51 | 0.96 | 1.00 | 1.03 | 1.07 | 1.00 | 1.04 |
| 8 | 0.55 | 0.61 | 1.19 | 1.22 | 1.04 | 1.47 | 1.68 | 1.56 |
| 9 | 0.73 | 0.75 | 1.01 | 1.20 | 1.45 | 1.41 | 1.46 | 1.22 |
| 10 | 0.49 | 0.77 | 0.94 | 1.13 | 1.22 | 0.96 | 1.09 | 1.36 |
| 11 | 0.23 | 0.63 | 0.96 | 1.25 | 1.02 | NA | NA | NA |
| 12 | 0.22 | 0.58 | 0.98 | 1.03 | 1.46 | 1.08 | 1.11 | 1.19 |
| 13 | 0.44 | 0.49 | 0.96 | 1.19 | 1.08 | NA | NA | 1.10 |
| Median | 0.50 | 0.66 | 0.98 | 1.13 | 1.15 | 1.21 | 1.20 | 1.25 |
| Mean | 0.51 | 0.69 | 1.08 | 1.17 | 1.24 | 1.31 | 1.33 | 1.38 |
| SD | 0.18 | 0.13 | 0.22 | 0.17 | 0.27 | 0.32 | 0.30 | 0.32 |
NA, not available.
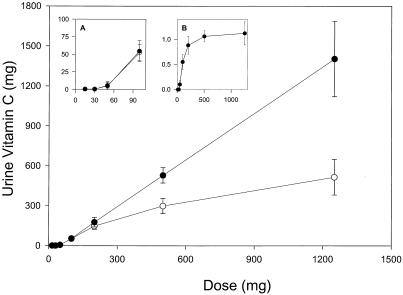
Vitamin C urinary excretion was measured at steady state for each dose (Fig. 3). The excretion threshold was between 60 and 100 mg daily. With i.v. administration, the entire dose was excreted at the 500- and 1,250-mg doses, and fractional excretion was ≈1.0. Bioavailability, or gastrointestinal absorption, presumably declined at vitamin C doses above 200 mg (3, 19), which may explain why at oral doses of 500 mg and 1,250 mg, urine excretion was less than that of the corresponding i.v. dose. These data indicate that renal reabsorption and excretion are partially responsible for tight control of vitamin C plasma concentrations (19).
Figure 3.
Urinary vitamin C excretion as a function of single vitamin C doses at steady state. Vitamin C excretion over 24 h was determined after administration of single doses given either orally (○) or intravenously (●). Data indicate mean values ± SD. (Inset A) Vitamin C excretion for single oral (○) or intravenous (●) doses of 15–100 mg. x axis indicates dose, and y axis indicates amount (milligrams) excreted in urine. (Inset B) Fractional excretion (the fraction of the dose excreted) after i.v. administration of single doses of vitamin C. x axis indicates dose, and y axis indicates fractional excretion (vitamin C excreted in urine in milligrams divided by the vitamin C dose in milligrams). Data from oral and i.v. administration were available from 11 subjects at doses 15, 30, 50, and 200 mg, from 10 subjects at 100 mg, from 8 subjects at 500 mg, and from 9 subjects at 1,250 mg.
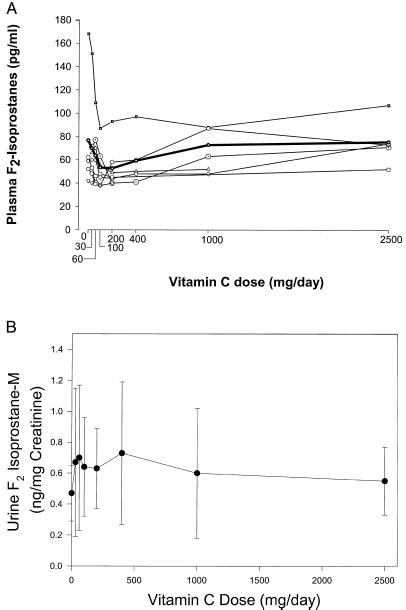
Vitamin C inhibits lipid peroxidation in vitro (20). At steady state for each vitamin C dose, plasma F2-isoprostanes were measured as a biomarker of endogenous lipid peroxidation in vivo (7). No significant relationship between vitamin C dose and plasma F2-isoprostanes was apparent (P1 = 0.15 by analysis of variance; Fig. 4A), although there was a possible inverse relationship for some individual subjects. Plasma samples were available from only seven subjects, and analysis of variance might have decreased power for detecting trend with this limited sample number (21). Therefore, as a second measure of lipid peroxidation in vivo, we quantified a major human urinary metabolite of the F2-isoprostanes, 2, 3-dinor-5, 6-dihydro-15 F2t-isoprostane (F2-isoprostane-M), using a highly precise and accurate mass spectrometric assay (14, 15). Analogous to the quantification of endogenous production of other eicosanoids, measurement of urinary metabolites of F2-isoprostanes likely provides the most reliable index of systemic production of these compounds (22, 23). F2-isoprostane-M was measured in 24-h urine samples from all available subjects at steady state for each vitamin C dose. There was no significant relationship between vitamin C dose and urine F2-isoprostane-M (P1 = 0.40 by analysis of variance; Fig. 4B). As an additional measure of lipid peroxidation in vivo, we quantified urine F2-isoprostanes and found no relationship between this compound and the vitamin C dose (data not shown). Taken together, the findings suggest that vitamin C does not affect isoprostane formation in vivo in young healthy women.
Figure 4.
(A) Plasma F2-isoprostanes from all available subjects as a function of vitamin C doses at steady state. Plasma F2-isoprostanes were available in seven subjects. All available data are shown; each symbol represents a different subject, and the bold line/symbol indicate mean F2-isoprostanes. (B) Urine F2-isoprostane-M as a function of vitamin C dose at steady state. Samples at doses 0–200 mg daily were available from 15 subjects, at doses 400 and 1,000 mg daily from 13 subjects, and at 2,500 mg daily from 10 subjects. Data indicate mean values ± SD.
Discussion
The data in this paper describe, to our knowledge for the first time, the relationship between vitamin C doses and steady-state concentrations in healthy young women, by using a dose range of 30–2,500 mg. In plasma, there was a steep sigmoidal relationship between vitamin C doses and resulting concentrations for doses of 30–100 mg daily, with an approximate 5-fold increase in plasma concentration over this dose range. At doses of 200 mg daily and higher, there was little change in plasma concentrations, with saturation between 200 and 400 mg daily. Circulating neutrophils, monocytes, and lymphocytes contained 0.5–4.0 mM concentrations of vitamin C and also saturated between 200 and 400 mg daily. At doses of 400 mg daily and higher, vitamin C plasma concentrations did not increase, in part because of increasing vitamin C excretion in urine. In contrast to in vitro observations (20), in vivo F2-isoprostanes in plasma and urine and a urine F2-isoprostanes metabolite were unchanged at all vitamin C doses.
On the basis of criteria for ideal vitamin C intake (24), these data provide evidence that the recent RDA of 75 mg for young women should be increased. This RDA is on the steep linear portion of the sigmoid dose curve for plasma, where small dose changes cause large changes in plasma concentration. The first dose beyond the linear portion of the sigmoid dose curve is above 100 mg daily, yielding a plasma concentration at which the vitamin C transporter SVCT2 should function at or near Vmax (16–18). There are no certain health benefits for vitamin C alone except to prevent scurvy, but foods containing vitamin C confer benefit, specifically fruits and vegetables. Increased fruit and vegetable intake is associated with decreased mortality (25). Five or more daily servings are associated with decreased cancer incidence and provide ≈210 mg of vitamin C daily (24). Consistent with these observations, data here show that daily vitamin C intakes of 100–200 mg produce near saturation of plasma and tissues. Vitamin C, 200 mg daily, from fruits and vegetables might be needed in place of 100 mg of pure vitamin C, as it is possible that bioavailability from foods is less than that from pure vitamin C (24, 26).
A new RDA for young women can be calculated from these data, by using Food and Nutrition Board guidelines and on the basis of determination of an EAR. By using the same criteria as for men, we calculate the EAR for young women to be 60 mg daily. This dose produces a median of ≈80% vitamin C saturation of neutrophils, or 0.98 mM, compared with 1.3 mM at saturation (Table 1) (1). These values, virtually identical to those selected for men, have the advantage of being based on a measured standard deviation (20.4%) (Table 1) rather than an assumed one (1). At the 60-mg daily dose, there is also minimal urine excretion (Fig. 3). The RDA = EAR + (EAR × 2 SDs expressed as percent of EAR) (1, 2), or 85 mg. Because some patients at 60 mg are below the urine threshold for vitamin C excretion, the RDA can be rounded up to 90 mg for young women.
On the basis of these calculations, RDAs for men and young women become equal (1). However, identical RDAs are unexpected given sex differences in lean body mass, total body water, and body size. A possible explanation is that the RDA for men was underestimated. The RDA for men was determined by using an assumed standard deviation of 10% for their EAR. On the basis of Food and Nutrition Board curve fitting calculations using the data, a standard deviation of 19.4% was determined (1, 3). By using this standard deviation rather than 10%, the RDA for men becomes EAR + (EAR × 2 SDs) = EAR + 38.8% EAR = 75 mg + 29.1 mg = 104.1 mg. By examining neutrophil data for both men and young women, it is seen that an assumed standard deviation of 10% may be too low.
The data show there is tight control of vitamin C concentrations in healthy young women, as in men. Over the narrow dose range of 30–100 mg, vitamin C concentrations increased substantially in both plasma and cells. At these doses, interindividual differences in time to reach steady state may have been because of differences in absorption, distribution, body size, catabolism, or reutilization (vitamin C recycling) (27, 28). At higher doses, plasma and cell concentrations were changed minimally, presumably because of urine excretion of the absorbed dose and saturation of the cell transporter SVCT2. Compared with men (3), some data for young women are different. By repeated measures analysis of variance, young women had higher plasma (P2 < 0.01) vitamin C concentrations than men at some doses. These differences were seen at doses of 30–100 mg daily but disappeared at higher doses. One explanation for this finding is that body size and composition are determinants of distribution volume. At doses below saturation, women would have lower volumes of distribution and hence higher circulating concentrations. The data here are from young women. It is unknown whether age or illness alters the dose–concentration relationships, and why vitamin C is so tightly controlled. Tight control could maximize biological benefits while preventing possible oxidative damage at higher concentrations (29), although the data here show that isoprostane formation in healthy young women is unaffected by vitamin C.
Dietary antioxidants have become increasingly linked to human health and disease (30). Studies of vitamin C dose–concentration relationships and functional consequences are needed in patients with diabetes, hypertension, hyperlipidemias, renal failure, and chronic heart disease, as well as in smokers, the elderly, and those at risk for infection.
Acknowledgments
We are grateful to Dr. Robert Wesley for rigorous statistical analyses and extensive constructive comments, and to the 8 West nursing and dietary staffs (Clinical Center, National Institutes of Health) for their dedication. This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (Z01 DK 54506, DK48831, and DK26657), the National Cancer Institute (CA77839), the National Institute of General Medical Science (GM15431), and by a Burroughs Wellcome Clinical Scientist Award in Translational Research (J.D.M.).
Abbreviations
- RDA
Recommended Dietary Allowance
- EAR
estimated average requirement
References
- 1.Food and Nutrition Board and Panel on Dietary Antioxidants and Related Compounds. Vitamin C. 2000;5:95–185. [Google Scholar]
- 2.Yates A A, Schlicker S A, Suitor C W. J Am Diet Assoc. 1998;98:699–706. doi: 10.1016/S0002-8223(98)00160-6. [DOI] [PubMed] [Google Scholar]
- 3.Levine M, Conry-Cantilena C, Wang Y, Welch R W, Washko P W, Dhariwal K R, Park J B, Lazarev A, Graumlich J, King J, Cantilena L R. Proc Natl Acad Sci USA. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deutsche Gesellschaft für Ernährung (German Society of Nutrition); Österreichische Gesellschaft für Ernährung (Austrian Society of Nutrition); Schweizerische Gesellschaft für Ernährungsforschung (Swiss Society of Nutrition) & Schweizerische Vereinigung für Ernährung (Swiss Unit of Nutrition) Referenzwerte für die Nahrstoffzefuhr (Recommended Values for Nutritional Intake) (Umschau, Frankfurt), pp. 137–144. 2000. [Google Scholar]
- 5.Health and Nutrition Information Society. Recommended Dietary Allowances (Dietary Reference Intakes) Tokyo: Daiichi Shuppan; 2000. pp. 112–127. [Google Scholar]
- 6.Birlouez-Aragon L, Fieux B, Potier de Courcy G, Hercberg C. Apports Nutritionnels Conseillés pour la Population Française. 3rd Ed. London: Editions TEC and Doc; 2000. pp. 215–230. [Google Scholar]
- 7.Morrow J D, Roberts L J. Methods Enzymol. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 8.King J, Wang Y, Welch R W, Dhariwal K R, Conry-Cantilena C, Levine M. Am J Clin Nutr. 1997;65:1434–1440. doi: 10.1093/ajcn/65.5.1434. [DOI] [PubMed] [Google Scholar]
- 9.Washko P W, Rotrosen D, Levine M. J Biol Chem. 1989;264:18996–19002. [PubMed] [Google Scholar]
- 10.Bergsten P, Amitai G, Kehrl J, Dhariwal K R, Klein H G, Levine M. J Biol Chem. 1990;265:2584–2587. [PubMed] [Google Scholar]
- 11.Sloand E M, Yu M, Klein H G. Transfusion. 1996;36:955–959. doi: 10.1046/j.1537-2995.1996.36111297091737.x. [DOI] [PubMed] [Google Scholar]
- 12.Dhariwal K R, Hartzell W O, Levine M. Am J ClinNutr. 1991;54:712–716. doi: 10.1093/ajcn/54.4.712. [DOI] [PubMed] [Google Scholar]
- 13.Washko P W, Welch R W, Dhariwal K R, Wang Y, Levine M. Anal Biochem. 1992;204:1–14. doi: 10.1016/0003-2697(92)90131-p. [DOI] [PubMed] [Google Scholar]
- 14.Morrow J D, Zackert W E, Yang J P, Kurhts E H, Callewaert D, Dworski R, Kanai K, Taber D, Moore K, Oates J A, Roberts L J. Anal Biochem. 1999;269:326–331. doi: 10.1006/abio.1999.4008. [DOI] [PubMed] [Google Scholar]
- 15.Burke A, Lawson J A, Meagher E A, Rokach J, FitzGerald G A. J Biol Chem. 2000;275:2499–2504. doi: 10.1074/jbc.275.4.2499. [DOI] [PubMed] [Google Scholar]
- 16.Daruwala R, Song J, Koh W S, Rumsey S C, Levine M. FEBS Lett. 1999;460:480–484. doi: 10.1016/s0014-5793(99)01393-9. [DOI] [PubMed] [Google Scholar]
- 17.Tsukaguchi H, Tokui T, Mackenzie B, Berger U V, Chen X-Z, Wang Y, Brubaker R F, Hediger M A. Nature (London) 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 18.Rajan D P, Huang W, Dutta B, Devoe L D, Leibach F H, Ganapathy V, Prasad P D. Biochem Biophys Res Commun. 1999;262:762–768. doi: 10.1006/bbrc.1999.1272. [DOI] [PubMed] [Google Scholar]
- 19.Graumlich J F, Ludden T M, Conry-Cantilena C, Cantilena L R, Jr, Wang Y, Levine M. Pharm Res. 1997;14:1133–1139. doi: 10.1023/a:1012186203165. [DOI] [PubMed] [Google Scholar]
- 20.Lynch S M, Morrow J D, Roberts L J, Frei B. J Clin Invest. 1994;93:998–1004. doi: 10.1172/JCI117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollander M, Wolfe D A. Non-parametric Statistical Methods. New York: Wiley; 1999. pp. 415–426. [Google Scholar]
- 22.Catella F, Nowak J, FitzGerald G A. Am J Med. 1986;81:23–29. doi: 10.1016/0002-9343(86)90905-8. [DOI] [PubMed] [Google Scholar]
- 23.Frolich J C, Wilson T W, Sweetman B J, Smigel M, Nies A S, Carr K, Watson J T, Oates J A. J Clin Invest. 1975;55:763–770. doi: 10.1172/JCI107987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine M, Rumsey S C, Daruwala R C, Park J B, Wang Y. J Am Med Assoc. 1999;281:1415–1423. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- 25.Khaw K T, Bingham S, Welch A, Luben R, Wareham N, Oakes S, Day N. Lancet. 2001;357:657–663. doi: 10.1016/s0140-6736(00)04128-3. [DOI] [PubMed] [Google Scholar]
- 26.Park J B, Levine M. J Nutr. 2000;130:1297–1302. doi: 10.1093/jn/130.5.1297. [DOI] [PubMed] [Google Scholar]
- 27.Rumsey S C, Levine M. J Nutr Biochem. 1998;9:116–130. [Google Scholar]
- 28.Rumsey S C, Welch R W, Garraffo H M, Ge P, Lu S F, Crossman A T, Kirk K L, Levine M. J Biol Chem. 1999;274:23215–23222. doi: 10.1074/jbc.274.33.23215. [DOI] [PubMed] [Google Scholar]
- 29.Halliwell B. Trends Biochem Sci. 1999;24:255–259. doi: 10.1016/s0968-0004(99)01418-8. [DOI] [PubMed] [Google Scholar]
- 30.Ames B N. Toxicol Lett. 1998;102–103:5–18. doi: 10.1016/s0378-4274(98)00269-0. [DOI] [PubMed] [Google Scholar]