Abstract
Background
Repetitive transcranial magnetic stimulation (TMS) is an FDA-approved antidepressant treatment but little is known of its mechanism of action. Specifically, downstream effects of TMS remain to be elucidated.
Objective/Hypothesis
To identify brain structural changes from TMS treatment of a treatment resistant depressive episode through an exploratory analysis
Methods
27 subjects in a DSM-IV current major depressive episode and on a stable medication regimen, had a 3T magnetic resonance T1 structural scan before and after five weeks of standard TMS treatment to the left dorsolateral prefrontal cortex. 27 healthy volunteer (HVs) subjects had the same brain MRI acquisition. Voxel-based morphometry was performed using high dimensional non-linear diffusomorphic anatomical registration (DARTEL).
Results
Six clusters of grey matter volume (GMV) that were lower in pre-treatment MRI’s of depressed subjects than in HV’s. GMV in four of these regions increased in MDD after TMS treatment by 3.5% to 11.2%. The four brain regions that changed with treatment were centered in the left anterior cingulate cortex, the left insula, the left superior temporal gyrus and the right angular gyrus. Increases in the anterior cingulate GMV with TMS correlated with improvement in depression severity.
Conclusions
To our knowledge, this is the first study of brain structural changes during TMS treatment of depression. The affected brain areas are involved in cognitive appraisal, decision-making and subjective experience of emotion. These effects may have potential relevance for the antidepressant action of TMS.
Keywords: Transcranial Magnetic Stimulation, Antidepressive Agents, Depressive Disorder, Magnetic Resonance Imaging, Gray Matter, Anterior Cingulate Cortex
Introduction
Major Depressive Episodes are a major cause of disability worldwide and associated with an increased risk for suicide as well as medical comorbidity [1, 2]. Depression is common, affecting about 5.4–8.9 percent of the U.S. population [3].
Antidepressant medication and psychotherapy are the first lines of treatment, and are effective for many patients [4]. However, response rates in individual medication treatment trials are only about 50%, and 10–30% of people will not achieve remission, even with multiple trials [5]. Antidepressant medication side effects can also reduce patient adherence.
Repetitive transcranial magnetic stimulation (TMS) was approved in 2007 by the Food and Drug Administration (FDA) for the treatment of major depressive disorder [6–9]. TMS induces an electrical current in brain tissue that is strongest immediately beneath the surface of the skull where an inducer coil is placed. Stimulation at the left dorsolateral prefrontal cortex (DLPFC) is the FDA-approved antidepressant intervention, and is based on the observation that, in depression, this structure has lower resting metabolism [10–12] and impaired functional connectivity [13–15] and that left prefrontal cortical strokes increase the risk of depression [16]. The mechanism of action of TMS is largely unknown but it is hypothesized that it alters the functional connectivity and morphology of neural structures that process emotion.
Here, we examined whether TMS can induce anatomical changes in brain regions that were found to be abnormal in a treatment-resistant major depressive episode. We hypothesized that TMS would induce structural changes in these brain regions that are in the opposite direction to that of the disease effect, and that the degree of morphological change would correlate with treatment outcome.
To our knowledge this is the first quantitative MRI study of cortical morphology in depressed subjects before and after TMS antidepressant treatment. One previous study of the volume of the hippocampus and surrounding structures reported a correlation between increase in amygdala volume with antidepressant treatment response to TMS. The same study found that baseline hippocampal volume was lower in non-responders to treatment [17]. Previous studies comparing treatment resistant depression to healthy volunteers found decreases in cortical volume in a broad region of the left parietal and temporal cortex, as well as several regions of the frontal lobe and the rostral anterior cingulate cortex [18].
Materials and Methods
Subjects
27 depressed subjects meeting DSM-IV-Text Revision criteria for a current major depressive episode (MDE) without psychotic features participated in the study. 25 subjects had a diagnosis of major depressive disorder and 2 had a diagnosis of bipolar II disorder. Diagnosis was based on clinical interview and consultations with patients’ psychiatrists and family members. Subjects also met criteria for treatment resistance, defined as a failure to respond to at least two previous antidepressant trials at adequate doses for 8 weeks during the current episode. 27 healthy volunteer (HV) subjects without known psychiatric diagnoses were included.
Inclusion and exclusion criteria have been reported elsewhere [10, 13]. Briefly, subjects were between the ages of 18–70 yrs and did not have active medical conditions. Depressed subjects were permitted to continue taking psychotropic medications and receive psychotherapy, but could not change doses of medications or either goals or frequency of psychotherapy during the rTMS course. They were excluded if they had prior exposure to TMS, current MDE with more than three years duration, scored < 16 on the 24-item Hamilton Depressive Rating Scale [19], had co-morbid borderline personality disorder, pregnancy, suicidal intent, substance use disorder within the past three years, current psychosis or history of spontaneous seizures. The Institutional Review Board of Weill Cornell Medical College approved the study, and it was in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). All subjects provided written informed consent. The Columbia collaborators worked with de-identified data.
MRI acquisition
MRI scans were obtained within 7 days prior to starting the first session of TMS and within 3 days after finishing the final treatment of TMS. High-resolution T1-weighted anatomical scans were obtained with an 8-channel phase array head coil (3.0 Tesla Signa Excite; General Electric Co., Fairfield, Connecticut) using a three dimensional spoiled gradient echo sequence with TR/TE/FA of 9 ms / 3.5 ms / 13°, acquisition matrix of 256 × 256, voxel size 1 × 1 × 1 mm, 176 contiguous axial slices covering the entire brain. All scans were performed on the same scanner and no hardware or software upgrade was carried out on the scanner during the study. The scan protocols were identical at baseline and follow-up for all subjects.
Clinical Treatment
37.5 min (3000 pulses) of 10-Hz facilitatory TMS was delivered over the left dorsolateral prefrontal cortex daily, Monday through Friday, for five weeks, or 25 days of treatment (NeuroStar TMS Therapy System; Neuronetics, Inc., Malvern, Pennsylvania). The magnetic stimulation coil was positioned on the scalp using the Beam F3 method based on surface distances between nasion, inion, tragus and vertex as landmarks [20]. The average strength of the pulse was 87.9% (SD 14.4) of the individual resting motor threshold, although the target strength was 120% of motor threshold. The discrepancy between these values reflects the fact that several subjects were unable to tolerate 120% intensity due to scalp discomfort. Depressed subjects were assessed with the 24-item Hamilton Depression Rating Scale (HAM-D) at baseline and 1 to 3 days after completing treatment.
Image Processing
The data for each subject were first visually inspected for scanner artifacts and gross anatomical abnormalities. N3 bias field correction and skull stripping of the T1 images were performed using Advanced Normalization Tools (ANTS, www.picsl.upenn.edu/ANTS) [21]. Preprocessing for voxel-based morphometry (VBM) was performed using SPM8 software (Welcome Trust Center for Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) in Matlab R2012a (MathWorks, Natick, MA, USA). First, the images were segmented into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) probability maps by the unified segmentation [22]. Next, the Diffeomorphic Anatomical Registration through Eponentiated Lie algebra (DARTEL)[23] toolbox was used that performed the registration, normalization and modulation of segmented GM and WM maps. Finally, the modulated GM maps were smoothed using an 8-mm FWHM kernel.
Statistical Analysis
Student’s t-test was used to calculate statistical differences of continuous data, and Fisher’s exact test was used to calculate differences in categorical data. All statistical tests were two sided.
A two-sample t-test with unequal variance was performed in SPM8 using the smoothed GM maps of HV and pre-treatment MDD scans with age, sex and Total Intracranial Volume (TIV) were used as covariates of no interest. The analysis was designed to detect any effect of diagnosis (HV vs. pre-treatment MRI scans) in GMV in the brain. The statistical parametric maps were thresholded at FWE corrected p value of <0.05 with an extent threshold of 500 contiguous voxels. Mean GMV was calculated for statistically significant clusters in each subject in order to perform analyses of possible confounds. Mean GMV values in these regions were calculated in the post-treatment MRI scans to determine changes with TMS treatment in these regions. Mean GMV was also calculated for two spheres of 3 cm and 5 cm diameter centered at the stimulation site [20].
A paired t-test was performed in SPM8 using smoothed GM maps of pre-treatment and post-treatment MDD subjects to determine clusters that changed with TMS treatment.. In addition, a simple regression analysis was performed in SPM8 between change in GMV between pre-treatment and post-treatment MDD scans and the change in HAMD scores to identify clusters of significant correlation. For both analyses, no nuisance regressors were included, while the statistical parametric maps were thresholded at FWE corrected p value of <0.05 with an extent threshold of 500 contiguous voxels.
Results
Clinical Variables
Two thirds (18/27, 67%) of depressed subjects were female. Average age was 41.5 yrs. (SD 16.0). 15/27 (55.6%) of HV subjects were female. Average age was 39.2 yrs. (SD 16.7). Depressed and HV subject groups did not differ in age (p=0.99) or sex (p=0.99). The average number of lifetime trials of antidepressants for depressed subjects was 4.4 (SD 2.0) and average number of previous mood stabilizer or atypical antipsychotic trials was 0.85 (SD 0.36). 16 depressed subjects (59.3%) were taking SSRI, SNRI or monoamine oxidase antidepressants, and 12 subjects (44.4%) were taking benzodiazepines during the TMS treatment. Five depressed subjects were on anticonvulsant mood stabilizers and one of those subjects was also on lithium. Six depressed subjects were on “atypical” antipsychotics and one was on a “typical” antipsychotic. Mean baseline 24-item HAM-D score was 27.3 (SD 6.5) and the average final 24-item HAM-D score was 18.1 (SD 7.8) for depressed subjects. 9/27 (33%) of subjects were responders to the TMS treatment (>50% reduction in HAM-D score).
Imaging Results
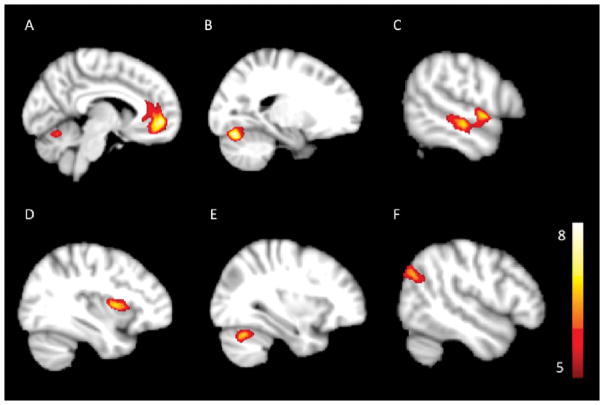
Whole brain analysis of smoothed GM maps from pre-treatment depressed subjects and HVs identified six clusters of lower grey matter volume (GMV) with age, sex and total intracranial volume as covariates (Table 1, Figure 1). Post hoc, analysis revealed that the GMV differences were not correlated with age of the subjects but did differ between sexes in three clusters. No significant clusters of higher GMV were identified.
Table 1.
Results of whole brain voxel based morphometry analysis of MRI scans of pre-treatment depressed subjects (n=27) and healthy volunteers (n=27) with effects of possible covariates. Cluster Size: Number of voxels. X, Y and Z: MNI coordinates of maximum voxel of cluster.
| Annotation | Size | X | Y | Z | Correlation with Age | Association with Sex |
|---|---|---|---|---|---|---|
| A: Left medial frontal gyrus; anterior cingulate; frontal medial orbital | 2362 | −9 | 50 | −5 | R=0.12, p=0.551 | P=0.030 |
| B: Left lingual gyrus, fusiform gyrus | 1367 | −18 | −73 | −21 | R=0.02, p=0.921 | P=0.022 |
| C: Left Middle Temporal Gyrus; Superior Temporal Gyrus | 1207 | −58 | −19 | −8 | R=0.17, p=0.397 | P=0.281 |
| D: Left insula; Frontal inferior opercularis; precentral gyrus | 847 | −34 | 5 | 6 | R=0.02, p=0.921 | P=0.022 |
| E: Right cerebellum posterior lobe | 638 | 28 | −64 | −26 | R=0.08, p=0.692 | P=0.328 |
| F: Right angular gyrus; right middle temporal gyrus; Right superior temporal gyrus | 511 | 54 | −63 | 25 | R=0.34, p=0.082 | P=0.22 |
Figure 1.
Regions of significant Gray Matter Volume (GMV) changes when depressed pre-treatment MRI scans are compared to HV’s, overlaid on standard space T1 image. The extent of increase in GMV is provided by the color-coded t-values. The color scale represents the t-statistics, with colored regions surviving the PFWE < 0.05 and minimum cluster of 500 voxels.
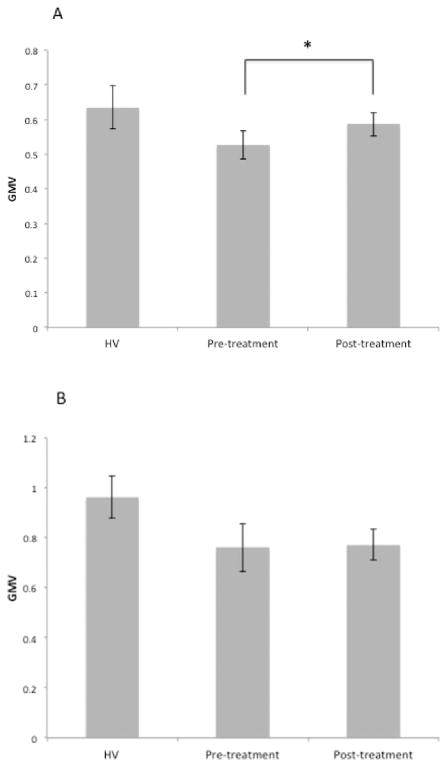
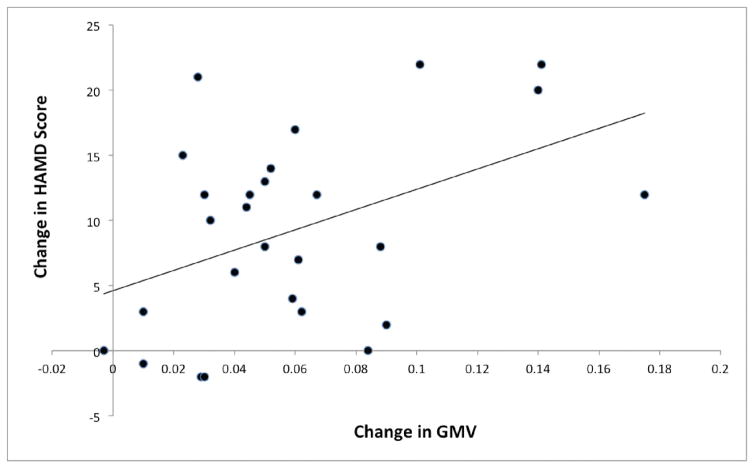
Mean GMV in four of the six above clusters were greater in post-treatment scans than pre-treatment scans, and those clusters were centered in the anterior cingulate, the left middle temporal gyrus, the left insula and the right angular gyrus (Table 2, Figure 3). Mean GMV in two other clusters were not different between post-treatment and pre-treatment scans, and they were centered on the lingual gyrus and right cerebellum (Table 2, Figure 2). Post hoc analyses were performed to identify whether the GMV increases from TMS were correlated with decrease in HAM-D score. The left anterior cingulate cortex GMV change did correlate with the antidepressant effect (Table 2, Figure 3), although this was not corrected for multiple comparisons. The GMV change in other brain regions did not correlate with the antidepressant effect..
Table 2.
Mean GMV change with TMS treatment. Regions of interest were determined based on the clusters found lower in pre-treatment MRI scans of depressed subjects compared to HV. % GMV change with TMS: % increase in GMV of post-treatment MRI scans when compared to pre-treatment MRI scans. Significance: Statistical significance of difference in GMV between pre-treatment MRI scans and post-treatment MRI scans. Correlations of GMV change with treatment to the change in HAMD score, age, and total brain volume (TBV). Differences in change in GMV between male and female subjects.
| Annotation | % GMV change with rTMS | Significance | Correlation with change HAMD | Correlation with Age | Association with Sex | Correlation with TIV |
|---|---|---|---|---|---|---|
| A: Left medial frontal gyrus; anterior cingulate; frontal medial orbital | 11.2 | 9.5 e-4 | R=0.44, p=0.022 | R=−0.36, p=0.065 | P=0.697 | R=0.03, p=0.882 |
| B: Lingual gyrus, left fusiform gyrus | 3.1 | 0.09 | R=−0.02, p=0.921 | R=−0.08, p=0.692 | P=0.379 | R=−0.12, p=0.551 |
| C: Left Middle Temporal Gyrus; Superior Temporal Gyrus | 3.9 | 8.0 e-4 | R=0.13, p=0.518 | R=−0.12, p=0.551 | P=0.139 | R=0.02, p=0.921 |
| D: Left insula; Frontal inferior opercularis; precentral gyrus | 3.5 | 2.6 e-3 | R=0.09, p=0.655 | R=−0.15, p=0.455 | P=0.472 | R=0.04, p=0.843 |
| E: Right cerebellum posterior lobe | 1.5 | 0.58 | R=−0.17, p=0.397 | R=−0.15, p=0.455 | P=0.607 | R=−0.27, p=0.173 |
| F: Right angular gyrus; right middle temporal gyrus; Right superior temporal gyrus | 3.9 | 8.6 e-4 | R=0.23, p=0.248 | R=−0.09, p=0.655 | P=0.296 | R=0.08, p=0.692 |
Figure 3.
Mean GMV increased in the anterior cingulate cortex cluster with TMS treatment (A) but did not increase in the cerebellum cluster (B). Mean GMV from the HV subject MRI’s are included as a comparison. * p<0.05
Figure 2.
Correlation between the increase in mean grey matter volume in the anterior cingulate cluster with improvement in depression severity.
A whole brain analysis to identify regions of correlation between change in GMV and change in HAM-D score found no significant cluster effects. A whole brain analysis on smoothed GM maps comparing pre-treatment and post-treatment MDD scans found no significant clusters of GMV changes. Neither the 3 cm (p=0.44) nor the 5 cm (p=0.33) diameter region of interest that was centered at the stimulation site increased in volume with TMS treatment.
Discussion
This study reports selective reversal of structural grey matter deficits found in treatment-resistant depression by a course of TMS treatment. Increases in GMV in the anterior cingulate cortex correlated with treatment improvement, suggesting a relationship to antidepressant mechanism. The areas of cerebral cortex change include those that are associated with the cognitive regulation and appraisal of emotions, including anterior cingulate (ACC) and an area involved with subjective experience emotions, the insula. To our knowledge, this is the first study to quantitatively investigate structural changes to the cerebral cortex in a major depressive episode treated by TMS. One previous study found structural brain differences in healthy volunteers after five days of TMS to the superior temporal cortex using voxel-based morphometry (VBM), indicating that TMS can induce morphological changes [24].
Smaller ACC volume has previously reported in MDD compared with healthy volunteers [25, 26]. Our results replicate this finding. This deficit has been found in first episode, treatment naïve adolescent depression [27, 28], and in individuals at risk for MDD based on family history [10, 29], indicating that low ACC volume can be detected early in the disorder’s pathogenesis and may be a familial biological trait. Lower ACC volume has also been found in subjects with traumatic brain injury with comorbid depression, consistent with its presence in depression of an environmental etiology [30].
TMS over the left dorsolateral prefrontal cortex has been reported to increase baseline brain metabolic activity and blood flow in the ACC in healthy volunteers [31–35] and subjects with MDD [36, 37]. These studies have been consistent across different methods using either positron emission tomography or single photon emission computed tomography and different radiotracers. TMS over the left DLPFC also modulated pooled glutamate and glutamine levels in the ACC in healthy volunteers [38]. Our results extend these metabolic findings to demonstrate structural changes with treatment. TMS therefore potentially has neuroplastic effects in this region as opposed to transient metabolic effects. MRI data does not provide information about the cellular processes that caused the morphological changes. However, increased cortex volume is thought to be primarily due to increased synaptogenesis and gliogenesis [39].
The anterior cingulate cortex (ACC) is associated with salience assessment of emotional or motivational information [40, 41]. It is involved with conflict monitoring [42, 43], processing emotional cues and recall of emotional events [44, 45]. The medial prefrontal cortex is involved with reward learning, decision-making and memory [40, 46]. Damage to the region has been associated with inability to make choices that meet an individual’s goals and needs due to problems with value computations [47]. These psychological functions have relevance to the symptoms of depression, and may explain why the change in structure in this region was correlated with change in depression severity. Several studies have shown that TMS at the left DLPFC in healthy volunteers increase their ability to cognitively assess emotional cues [48, 49]. This effect is consistent with this change in the anterior cingulate cortex.
TMS also reversed depression related GMV decreases in the anterior insula, although this change was not correlated with antidepressant treatment outcome. The anterior insula becomes actively engaged during emotional awareness, or conscious experience of emotion [50, 51]. A recent meta-analysis reported a hypoactive cluster in subjects with MDD in the anterior insula and rostral ACC during tasks involved with affectively biased information processing [52], consistent with our result in the depressed sample. The insula is involved with creating an interoceptive image of one’s physiological state and processing that information in regards to a subjective awareness of feelings. This emotional function relates to the anhedonia of depression.
Our result is consistent with a previous study of treatment resistant depression that showed extensive volumetric decreases in the temporal neocortex when compared to healthy volunteers [18]. Our study extends that result by indicating that TMS treatment reverses this deficit. The cluster in the temporal lobe on the right extends into the angular gyrus. Several previous papers have reported differences in the resting state activity in the angular gyrus in major depressive disorder [53, 54] and that these differences may relate to response to antidepressant treatment [55].
Our results are consistent with predictions of the neurotrophic hypothesis of antidepressant mechanism of action. Monoaminergic-based antidepressant treatment has been found to increase neurotrophic factor release in the brain and cause increased neurogenesis downstream from their effects on monoamine neurotransmission [56, 57]. We found no decreases in volume in the brain after TMS, consistent with neurotrophic effects on synapse formation and dendritic arborization in anterior cingulate and insular cortex. Several studies have identified increases in hippocampal or dorsolateral prefrontal cortical volume after antidepressant medication treatment indicating a different pattern of trophic effects across antidepressant modalities [58, 59]. Two small studies reported increases in serum BDNF level in depressed subjects after TMS treatment, consistent with this mechanism [60, 61]. Our data cannot determine, though, whether the volumetric changes with TMS were due to a neurotrophic effect, a change in activity due to a direct neurostimulation, or a downstream neural consequence of being less depressed.
Our results contrast with a recent study of structural volumetric changes of the cortex in major depressive disorder before and after electroconvulsive therapy (ECT) [62]. In that study, ECT was found to increase hippocampal volume, an effect that we did not find with TMS. ECT also induced greater volume in the anterior cingulate cortex in that study, although centered in the subgenual region that did not fall within the anterior cingulate region we found to increase with TMS. These differing effects may be due to differences in the antidepressant mechanisms underlying ECT and TMS.
Our study is limited by modest sample size and the absence of a sham-treated control arm. We therefore cannot determine if these changes were due to a placebo effect. Focusing our analysis of TMS treatment effects to only regions that were different between depressed and healthy volunteer subjects holds the risk of a type 2 statistical error due to a regression towards the mean. This work therefore requires replication. No neuropsychological measures such as performance on cognitive or emotional tasks were performed on the subjects. It therefore cannot be determined from these data if the volumetric changes are correlated with behavioral or cognitive changes that occur with treatment and are required for an antidepressant response. Previous studies have identified changes in executive functions, primarily selective and sustained attention with TMS to the left dorsolateral prefrontal cortex [63]. Future studies should aim to link the neurocognitive effects of TMS in MDD with structural and functional brain changes.
Highlights.
First quantitative study of brain volume changes with transcranial magnetic stimulation in a major depressive episode
Structural 3T magnetic resonance imaging obtained before and after treatment
Several regions that were lower in volume with a major depressive episode increased in volume with treatment.
Increases in the anterior cingulate cortex volume with treatment correlated with improvement in depression severity
Acknowledgments
This work was supported by grants from the Brain and Behavior Research Foundation (National Alliance for Research on Schizophrenia and Depression Young Investigator Award) to MJD and by funds from the Department of Psychiatry at Weill Cornell Medical College. ML was supported by a grant from the National Institutes of Health (K23MH104688) and CL was supported by grants from the National Institutes of Health (K99 MH097822) and the DeWitt Wallace Reader’s Digest Foundation at Weill Cornell. Dr. Mann has received royalties for commercial use of C-SSRS rating scale from the Research Foundation for Mental Hygeine. No other author has financial conflicts of interest to report. We thank Harry Rubin-Falcone for his help in revision of the manuscript.
Footnotes
Meeting Presentation: Presented at 2015 Annual Meeting of the Society of Biological Psychiatry
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The World Health Report 2002:Reducing Risks, Promoting Healthy Life. World Health Organization; Geneva, Switzerland: 2002. [DOI] [PubMed] [Google Scholar]
- 2.Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. J Affect Disord. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- 3.Narrow WE, Rae DS, Robins LN, Regier DA. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry. 2002;59:115–123. doi: 10.1001/archpsyc.59.2.115. [DOI] [PubMed] [Google Scholar]
- 4.Mann JJ. The medical management of depression. N Engl J Med. 2005;353:1819–1834. doi: 10.1056/NEJMra050730. [DOI] [PubMed] [Google Scholar]
- 5.Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry. 1998;55:694–700. doi: 10.1001/archpsyc.55.8.694. [DOI] [PubMed] [Google Scholar]
- 6.Connolly KR, Helmer A, Cristancho MA, Cristancho P, O’Reardon JP. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73:e567–573. doi: 10.4088/JCP.11m07413. [DOI] [PubMed] [Google Scholar]
- 7.George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, Hallett M, Post RM. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6:1853–1856. doi: 10.1097/00001756-199510020-00008. [DOI] [PubMed] [Google Scholar]
- 8.O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, Demitrack MA, George MS, Sackeim HA. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Pascual-Leone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–237. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- 10.Weiduschat N, Dubin MJ. Prefrontal cortical blood flow predicts response of depression to rTMS. J Affect Disord. 2013;150:699–702. doi: 10.1016/j.jad.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald PB, Oxley TJ, Laird AR, Kulkarni J, Egan GF, Daskalakis ZJ. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148:33–45. doi: 10.1016/j.pscychresns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behavioural brain research. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, Voss HU, Casey BJ, Etkin A, Dubin MJ. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76:517–526. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimoda K, Robinson RG. The relationship between poststroke depression and lesion location in long-term follow-up. Biol Psychiatry. 1999;45:187–192. doi: 10.1016/s0006-3223(98)00178-4. [DOI] [PubMed] [Google Scholar]
- 17.Furtado CP, Hoy KE, Maller JJ, Savage G, Daskalakis ZJ, Fitzgerald PB. An investigation of medial temporal lobe changes and cognition following antidepressant response: a prospective rTMS study. Brain stimulation. 2013;6:346–354. doi: 10.1016/j.brs.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Shah PJ, Ebmeier KP, Glabus MF, Goodwin GM. Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatry. 1998;172:527–532. doi: 10.1192/bjp.172.6.527. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mir-Moghtadaei A, Caballero R, Fried P, Fox MD, Lee K, Giacobbe P, Daskalakis ZJ, Blumberger DM, Downar J. Concordance Between BeamF3 and MRI-neuronavigated Target Sites for Repetitive Transcranial Magnetic Stimulation of the Left Dorsolateral Prefrontal Cortex. Brain stimulation. 2015;8:965–973. doi: 10.1016/j.brs.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 24.May A, Hajak G, Ganssbauer S, Steffens T, Langguth B, Kleinjung T, Eichhammer P. Structural brain alterations following 5 days of intervention: dynamic aspects of neuroplasticity. Cereb Cortex. 2007;17:205–210. doi: 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- 25.Bora E, Harrison BJ, Davey CG, Yucel M, Pantelis C. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol Med. 2012;42:671–681. doi: 10.1017/S0033291711001668. [DOI] [PubMed] [Google Scholar]
- 26.van Tol MJ, van der Wee NJ, van den Heuvel OA, Nielen MM, Demenescu LR, Aleman A, Renken R, van Buchem MA, Zitman FG, Veltman DJ. Regional brain volume in depression and anxiety disorders. Arch Gen Psychiatry. 2010;67:1002–1011. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- 27.Han KM, Choi S, Jung J, Na KS, Yoon HK, Lee MS, Ham BJ. Cortical thickness, cortical and subcortical volume, and white matter integrity in patients with their first episode of major depression. J Affect Disord. 2014;155:42–48. doi: 10.1016/j.jad.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 28.Pannekoek JN, van der Werff SJ, van den Bulk BG, van Lang ND, Rombouts SA, van Buchem MA, Vermeiren RR, van der Wee NJ. Reduced anterior cingulate gray matter volume in treatment-naive clinically depressed adolescents. NeuroImage Clinical. 2014;4:336–342. doi: 10.1016/j.nicl.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson BS, Warner V, Bansal R, Zhu H, Hao X, Liu J, Durkin K, Adams PB, Wickramaratne P, Weissman MM. Cortical thinning in persons at increased familial risk for major depression. Proc Natl Acad Sci U S A. 2009;106:6273–6278. doi: 10.1073/pnas.0805311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maller JJ, Thomson RH, Pannek K, Bailey N, Lewis PM, Fitzgerald PB. Volumetrics relate to the development of depression after traumatic brain injury. Behavioural brain research. 2014;271:147–153. doi: 10.1016/j.bbr.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 31.Paus T, Castro-Alamancos MA, Petrides M. Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci. 2001;14:1405–1411. doi: 10.1046/j.0953-816x.2001.01757.x. [DOI] [PubMed] [Google Scholar]
- 32.Barrett J, Della-Maggiore V, Chouinard PA, Paus T. Mechanisms of action underlying the effect of repetitive transcranial magnetic stimulation on mood: behavioral and brain imaging studies. Neuropsychopharmacology. 2004;29:1172–1189. doi: 10.1038/sj.npp.1300411. [DOI] [PubMed] [Google Scholar]
- 33.Knoch D, Treyer V, Regard M, Muri RM, Buck A, Weber B. Lateralized and frequency-dependent effects of prefrontal rTMS on regional cerebral blood flow. Neuroimage. 2006;31:641–648. doi: 10.1016/j.neuroimage.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 34.Ohnishi T, Matsuda H, Imabayashi E, Okabe S, Takano H, Arai N, Ugawa Y. rCBF changes elicited by rTMS over DLPFC in humans. Supplements to Clinical neurophysiology. 2004;57:715–720. doi: 10.1016/s1567-424x(09)70412-x. [DOI] [PubMed] [Google Scholar]
- 35.Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PloS one. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng XM. Regional cerebral blood flow changes in drug-resistant depressed patients following treatment with transcranial magnetic stimulation: a statistical parametric mapping analysis. Psychiatry Res. 2000;100:75–80. doi: 10.1016/s0925-4927(00)00073-1. [DOI] [PubMed] [Google Scholar]
- 37.Teneback CC, Nahas Z, Speer AM, Molloy M, Stallings LE, Spicer KM, Risch SC, George MS. Changes in prefrontal cortex and paralimbic activity in depression following two weeks of daily left prefrontal TMS. J Neuropsychiatry Clin Neurosci. 1999;11:426–435. doi: 10.1176/jnp.11.4.426. [DOI] [PubMed] [Google Scholar]
- 38.Michael N, Gosling M, Reutemann M, Kersting A, Heindel W, Arolt V, Pfleiderer B. Metabolic changes after repetitive transcranial magnetic stimulation (rTMS) of the left prefrontal cortex: a sham-controlled proton magnetic resonance spectroscopy (1H MRS) study of healthy brain. Eur J Neurosci. 2003;17:2462–2468. doi: 10.1046/j.1460-9568.2003.02683.x. [DOI] [PubMed] [Google Scholar]
- 39.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nature neuroscience. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry. 2011;168:968–978. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- 41.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 42.Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 43.Liston C, Matalon S, Hare TA, Davidson MC, Casey BJ. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron. 2006;50:643–653. doi: 10.1016/j.neuron.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 44.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 45.Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectr. 2004;9:258–266. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- 46.Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Kennerley SW, Walton ME. Decision making and reward in frontal cortex: complementary evidence from neurophysiological and neuropsychological studies. Behavioral neuroscience. 2011;125:297–317. doi: 10.1037/a0023575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balconi M, Ferrari C. rTMS stimulation on left DLPFC affects emotional cue retrieval as a function of anxiety level and gender. Depression and anxiety. 2012;29:976–982. doi: 10.1002/da.21968. [DOI] [PubMed] [Google Scholar]
- 49.Balconi M, Canavesio Y. High-frequency rTMS on DLPFC increases prosocial attitude in case of decision to support people. Social neuroscience. 2014;9:82–93. doi: 10.1080/17470919.2013.861361. [DOI] [PubMed] [Google Scholar]
- 50.Gasquoine PG. Contributions of the insula to cognition and emotion. Neuropsychology review. 2014;24:77–87. doi: 10.1007/s11065-014-9246-9. [DOI] [PubMed] [Google Scholar]
- 51.Gu X, Hof PR, Friston KJ, Fan J. Anterior insular cortex and emotional awareness. The Journal of comparative neurology. 2013;521:3371–3388. doi: 10.1002/cne.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage. 2012;61:677–685. doi: 10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 53.de Kwaasteniet BP, Rive MM, Ruhe HG, Schene AH, Veltman DJ, Fellinger L, van Wingen GA, Denys D. Decreased Resting-State Connectivity between Neurocognitive Networks in Treatment Resistant Depression. Frontiers in psychiatry. 2015;6:28. doi: 10.3389/fpsyt.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen T, Qiu M, Li C, Zhang J, Wu Z, Wang B, Jiang K, Peng D. Altered spontaneous neural activity in first-episode, unmedicated patients with major depressive disorder. Neuroreport. 2014;25:1302–1307. doi: 10.1097/WNR.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 55.Shen Y, Yao J, Jiang X, Zhang L, Xu L, Feng R, Cai L, Liu J, Wang J, Chen W. Sub-hubs of baseline functional brain networks are related to early improvement following two-week pharmacological therapy for major depressive disorder. Hum Brain Mapp. 2015;36:2915–2927. doi: 10.1002/hbm.22817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, Arango V. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry. 2012;72:562–571. doi: 10.1016/j.biopsych.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith R, Chen K, Baxter L, Fort C, Lane RD. Antidepressant effects of sertraline associated with volume increases in dorsolateral prefrontal cortex. J Affect Disord. 2013;146:414–419. doi: 10.1016/j.jad.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 59.Schermuly I, Wolf D, Lieb K, Stoeter P, Fellgiebel A. State dependent posterior hippocampal volume increases in patients with major depressive disorder. J Affect Disord. 2011;135:405–409. doi: 10.1016/j.jad.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 60.Yukimasa T, Yoshimura R, Tamagawa A, Uozumi T, Shinkai K, Ueda N, Tsuji S, Nakamura J. High-frequency repetitive transcranial magnetic stimulation improves refractory depression by influencing catecholamine and brain-derived neurotrophic factors. Pharmacopsychiatry. 2006;39:52–59. doi: 10.1055/s-2006-931542. [DOI] [PubMed] [Google Scholar]
- 61.Zanardini R, Gazzoli A, Ventriglia M, Perez J, Bignotti S, Rossini PM, Gennarelli M, Bocchio-Chiavetto L. Effect of repetitive transcranial magnetic stimulation on serum brain derived neurotrophic factor in drug resistant depressed patients. J Affect Disord. 2006;91:83–86. doi: 10.1016/j.jad.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 62.Dukart J, Regen F, Kherif F, Colla M, Bajbouj M, Heuser I, Frackowiak RS, Draganski B. Electroconvulsive therapy-induced brain plasticity determines therapeutic outcome in mood disorders. Proc Natl Acad Sci U S A. 2014;111:1156–1161. doi: 10.1073/pnas.1321399111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guse B, Falkai P, Wobrock T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J Neural Transm. 2010;117:105–122. doi: 10.1007/s00702-009-0333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]