Abstract
The configuration of synaptic circuits underlies their ability to process and store information. Research on dendritic spines has revealed that their structural and functional alterations are clustered along the parent dendrite. Here we review the evidence supporting such notion of clustered synaptic plasticity, discuss its functional implications and possible contributing factors, and suggest potential strategies to deal with open challenges.
Keywords: Dendritic spine, Synapse, Structural dynamics, Clustered plasticity, Neural circuit
1. Prologue
The mammalian brain is arguably the most complex system known to humans. Its complexity derives not only from the myriad of constituent neurons, but moreover from the immense variety of possible ways in which they may interconnect. Synapses are the sites where neurons connect and communicate with each other to form functional circuits. Such circuits typically comprise of multiple types of neurons with distinct morphology, connectivity, and functional properties. Each neuron in the circuit in turn works as a signal processing unit, integrating diverse synaptic inputs to generate its responses.
2. The dendritic spine and its distribution
The majority of excitatory synapses reside at dendritic spines, minuscule protrusions emanating from dendrites. A typical spine consists of a head (diameter ~1 μm) and a thin neck (diameter ~0.1 μm) of variable lengths (0.1–2 μm) (Harris and Kater, 1994). It contains the postsynaptic molecular apparatus for synaptic transmission and plasticity (Bourne and Harris, 2008; Sheng and Hoogenraad, 2007; Sheng and Kim, 2011), and acts as a functional compartment for intracellular signaling (Nishiyama and Yasuda, 2015; Shepherd, 1996; Yuste and Denk, 1995).
The density of spines along dendrites varies among different types of neurons (Nimchinsky et al., 2002); it is not even uniform across all dendritic branches of the same neuron. In the rabbit cerebral cortex, apical dendritic shafts of pyramidal neurons exhibit more than twice the spine density on basal dendrites (Globus and Scheibel, 1966, 1967). Indeed, the general pattern that apical dendritic shafts have a higher spine density than apical tufts, oblique branches, or basal dendrites seems to hold for different cortical areas in rat (Feldman and Dowd, 1975; Parnavelas et al., 1973), monkey (Kemper et al., 1973), and human (Marin-Padilla, 1967). Other studies have shown different, but nevertheless non-uniform, spine density distributions on striatal medium spiny neurons (Kemp and Powell, 1971; Wilson and Groves, 1980), hippocampal CA1 pyramidal neurons (Druckmann et al., 2014), and cortical pyramidal neurons in mice (Valverde and Ruiz-Marcos, 1969) and cats (Mungai, 1967). Finally, there is also evidence that spines are not uniformly distributed along a single dendritic segment, and that the extent of clustering correlates with spine morphology (Yadav et al., 2012).
3. Clustered structural dynamics and functional plasticity of dendritic spines
Combining meticulous histological examination with unsurpassed imagination, Ramón y Cajal intimated the idea that dendritic spines could grow with activity and retract during inactivity, and speculated that the dynamism in the axo-dendritic connections underlies learning in an experience-dependent manner (Ramón y Cajal, 1911). Validation of this visionary idea came a century later. In late 1990s, the advent of two-photon microscopy (Denk et al., 1990) and the generation of transgenic mice expressing fluorescent proteins in selected subsets of neurons (Feng et al., 2000) enabled observation of dendritic spines in living animals over time. A series of experiments demonstrated the continual emergence, morphological alteration, and retraction of dendritic spines in different brain regions (Chen et al., 2014; Fu and Zuo, 2011; Holtmaat and Svoboda, 2009). Such structural changes are tightly regulated during development, with higher spine turnover (growth and elimination) rate in adolescence than in adulthood (Holtmaat et al., 2005; Zuo et al., 2005a). In addition, sensory experiences and learning profoundly impact spine turnover in various brain regions (Attardo et al., 2015; Fu et al., 2012; Hofer et al., 2009; Holtmaat et al., 2006; Jung and Herms, 2014; Lai et al., 2012; Miquelajauregui et al., 2015; Trachtenberg et al., 2002; Xu et al., 2009; Yang et al., 2009; Zuo et al., 2005b). Recently, a novel photoactivatable optoprobe (AsPaRac1) enabled manipulation of specific subsets of spines in vivo, and showed that shrinkage of task-specific spines disrupts performance of the learned task (Hayashi-Takagi et al., 2015). The rate of spine turnover is also altered in some pathological states. Stroke causes rapid loss of spines in the infarct area, the majority of which can be restored by reperfusion within an hour; in the peri-infarct area spine turnover increases during the recovery period (Brown and Murphy, 2008; Brown et al., 2008; Murphy et al., 2008; Zhang et al., 2005; Zhang and Murphy, 2007). In Alzheimer’s disease model mice, there is pronounced spine loss in the vicinity of amyloid plaques (Spires et al., 2005; Tsai et al., 2004). The Fragile X syndrome mice exhibit increased spine turnover and immature spine morphology (Cruz-Martin et al., 2010; Pan et al., 2010). In autism model mice generated by prenatal testosterone exposure, dendritic spines show abnormal morphology, density, and instability (Hatanaka et al., 2015). Abnormal spine turnover has also been reported in a mouse model of the MECP2 duplication syndrome, a childhood neurological disorder (Jiang et al., 2013).
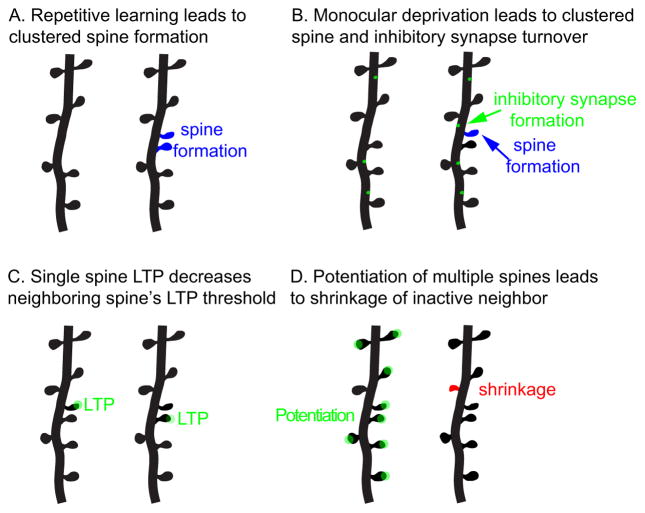
In vivo imaging studies also reveal interesting spatial rules of dendritic spine formation and elimination. Gan and his colleagues found that spine formation is branch-specific (Yang et al., 2014). They trained mice to run on an accelerating, rotating rod (“rotarod”), and found that the rates of spine formation on sibling dendritic branches (i.e., two branches split from the same parent branch) of layer (L) 5 pyramidal neurons in the primary motor cortex differ significantly after training. Another study (Fu et al., 2012) trained mice to reach through a slit to grasp a food pellet, which led to rapid spine formation in clusters, mainly as pairs of neighboring spines (see Fig. 1A). In such clusters, the second new spine could form in close vicinity to a stable spine. This contrasts with the situation under baseline conditions, in which new spines appear to avoid existing stable spines on the same dendritic branch, rather than emerging uniformly. Furthermore, Nedivi and colleagues extended such studies to include both spines and inhibitory synapses (marked by gephyrin) in the primary visual cortex (Chen et al., 2012). They found that during normal visual experience a significant fraction of turnover events of inhibitory synapses and dendritic spines occurred within 10 μm of each other. Monocular deprivation further increased the frequency of such events of clustered changes (see Fig. 1B).
Fig. 1.
Schematic illustration of clustered synaptic alterations under various experimental conditions. A. Repetitive learning of the same task leads to clustered dendritic spine formation. B. Monocular deprivation leads to clustered formation of dendritic spines and inhibitory synapses. C. LTP induced at a single spine decreases the threshold for LTP at its neighboring spine. D. Simultaneous potentiation of multiple dendritic spines in a cluster leads to the shrinkage of the inactive spine in the cluster.
The spatial correlation between spines extends beyond their formation and elimination. Morphological and functional alterations in spines also exhibit correlation either between neighboring spines or within a short spatial window. In acute mouse brain slices, pairing a train of glutamate uncaging stimuli with postsynaptic depolarization induces input-specific long-term potentiation (LTP) at individual dendritic spines of hippocampal pyramidal neurons. The LTP induction at one spine reduces the threshold for LTP induction at neighboring spines within a ~10 μm window (Harvey and Svoboda, 2007) (see Fig. 1C). Furthermore, sensory experience mediated by whiskering preferentially potentiates nearby dendritic spines on L2/3 neurons in the mouse barrel cortex via AMPA receptor insertion into the synaptic membrane (Makino and Malinow, 2011). This phenomenon is believed to help bind behaviorally relevant information on sub-compartments of dendrites. Another research used high-frequency glutamate uncaging to potentiate synapses of cultured hippocampal CA1 neurons (Oh et al., 2015). Interestingly, if multiple synapses on neighboring spines (≥6) are potentiated simultaneously, the inactive synapse within the cluster weakens and shrinks (see Fig. 1D). However, the structural potentiation can be decoupled from the heterosynaptic shrinkage of inactive synapses by inhibiting different signaling molecules. This study suggests that an activity-dependent shrinkage signal, rather than competition for local resources (see below), drives heterosynaptic structural shrinkage and functional weakening. Long-term depression (LTD) exhibits analogous spatial correlations. In organotypic hippocampal slice cultures, channelrhodopsin-mediated optogenetic stimulation of CA3 pyramidal neurons at 1 Hz leads to elimination of depressed CA3-CA1 synapses and their neighbors within an 8 μm stretch of dendrite in the days following LTD (Wiegert and Oertner, 2013).
4. Implications of clustered structural and functional plasticity
What do such spatial rules of synaptic dynamics and plasticity imply? On the postsynaptic side, clustering could be due to diffusion of intracellular signaling molecules. For example, after LTP induction, the activity of the small guanosine triphosphatase (GTPase) Ras spreads over ~10 μm along the dendrite and invades neighboring spines. This spread of signaling regulates local LTP threshold (Harvey et al., 2008). Similarly, activated RhoA (a Rho GTPase) diffuses out of the spine and spreads over ~5 μm of dendritic shaft (Murakoshi et al., 2011). Synaptic activity may also trigger molecular signaling involving calcineurin, inositol 1,4,5-trisphosphate receptors, and group I metabotropic glutamate receptors, which acts on neighboring spines as suggested by heterosynaptic structural and functional depression (Oh et al., 2015). An alternative idea is that spines may compete for limited cellular resources. In the developing barrel cortex, the postsynaptic density (PSD) of individual spines competes for rapidly diffusing PSD-95, a postsynaptic scaffolding protein that organizes glutamate receptors and associated signaling molecules (Gray et al., 2006). After rapid synaptogenesis by LTP induction, marked loss of small spines is accompanied and counterbalanced by enlargement of remaining spines, such that the total synaptic surface area per length of dendrite remains constant (Bourne and Harris, 2011). Recently one study suggests that the coordinated elimination of spines with the maturation of surviving neighbors is mediated by inter-spine competition for cadherin/catenin cell adhesion complexes (Bian et al., 2015).
The structured distribution of synapses on dendrites has profound impact on information processing in the postsynaptic neuron. Theoretical modeling and electrophysiological experiments have shown that adjacent synaptic inputs can sum nonlinearly (Govindarajan et al., 2006; Larkum and Nevian, 2008; London and Hausser, 2005). Nonlinear integration can render each dendritic compartment a separate summing unit, hence greatly increasing the information storage capacity of neurons (Poirazi and Mel, 2001). Such a coding scheme may allow a single neuron to behave as an abstract neural network (Poirazi et al., 2003).
On the presynaptic side, the observed synaptic clustering may reflect input sharing, i.e., two or more spines receiving synaptic inputs from the same presynaptic axon (Fiala et al., 2002; Kasthuri et al., 2015; Knott et al., 2006). Moreover, it may result from the availability of axonal processes. If a dendrite can only form synapses with axons that pass within a spine’s length, it is conceivable that the observed spine clustering is dictated by the geometric arrangement of local axons. Chklovskii and colleagues (Stepanyants et al., 2002) estimated the number of different synaptic connectivity patterns that are compatible with a given geometry, and found that the filling fraction (ratio between actual synapses and “potential” synapses) is much smaller than 1. This is compatible with the finding that synaptic dynamism occurs without major reorganization of dendritic or axonal arbors (Mizrahi and Katz, 2003; Trachtenberg et al., 2002). In addition, adjacent spines may receive locally convergent inputs from axons that originate from the same neuronal assembly and exhibit similar activity patterns (Kleindienst et al., 2011; Takahashi et al., 2012; Wilms and Hausser, 2015). In such a scenario, clustered plasticity at postsynaptic sites can emerge from shared input patterns due to activity-dependent plasticity rules.
5. Open questions and potential solutions
In the pursuit to understand the organizational principle of dendritic spines and its functional implications, many challenges await us. First, we need to clarify the definition of synaptic clustering. Currently a number of metrics with different underlying assumptions (i.e., null hypotheses) exist in the literature. One heuristic approach, adopted in many studies on clustered plasticity, uses a fixed-length window. If plasticity at one synapse affects the alterations at other synapses within the window, the effect is considered as clustered. Other studies have used more sophisticated algorithms to examine the distribution of spines along a dendritic segment as a whole. Weaver and colleagues uses a hierarchical clustering algorithm to count the number of “clustered” spines, and compares that to a null model in which spines distribute uniformly and independently along the dendrite (Yadav et al., 2012). This null model is mathematically tractable, but does not take into account non-uniform baseline distribution. Another in vivo imaging study (Fu et al., 2012) focuses on the locations of newly formed spines. Its null model only assumes that new spines form uniformly and independently, while the locations of existing spines are taken as-is. Therefore, inhomogeneity in the baseline spine distribution is accommodated. These divergent definitions confound any attempt to compare and synthesize results from different studies. Nevertheless, as synaptic distribution along a dendritic segment at any particular time point is nothing but the result of the cumulative history of synapse formation and elimination, the spatial pattern of synapse turnover should leave its imprint on the observed synapse distribution.
The second challenge is to put spine clustering into the context of the overall organization of the parent neuron’s synaptic circuit. This will greatly facilitate the quest for synaptic engram of specific information (Hayashi-Takagi et al., 2015). Although the molecular mechanisms underlying spine clustering act locally, the functional consequence could depend on the location of the cluster in the dendritic arbor, given the nonlinear signal integration discussed above. However, due to the intrinsic limit of optical resolution (Svoboda and Yasuda, 2006) and imaging depth (Theer and Denk, 2006) in two-photon microscopy, most in vivo imaging studies focus on subsets of dendritic segments in the superficial layers of the cerebral cortex. Consequently, the position of these dendritic segments in the overall dendritic arbor is usually unknown. In principle, it is possible to reconstruct post hoc the whole parent dendritic arbor from fixed and sliced tissue and re-locate the imaged segments. Nevertheless, such reconstructions require extremely delicate tissue preparation and handling; completeness of the reconstructed arbor is hence difficult to achieve. The recent development of tissue clearing techniques (Miyawaki, 2015; Richardson and Lichtman, 2015) provides the prospect of addressing this issue by large-scale neuronal reconstruction in intact brains.
It is also challenging to identify the presynaptic axons that connect with the postsynaptic spines. This knowledge is crucial for understanding the organization of the synaptic circuit, as it will clarify the geometric constraints (see above) that induce synapse clustering, as well as the relationship between anatomical clustering and functional clustering (e.g., clustering of spines sharing similar inputs). The technical difficulty lies in establishing that a particular axonal process forms a bona fide synapse with the spine, rather than merely passing by or contacting it. An axodendritic touch does not necessarily indicate synaptic connection (Kalisman et al., 2005); even a touch between an axonal bouton and a spine only predicts the existence of an actual synapse with ~80% precision (Mishchenko et al., 2010). The gold standard to ascertain the presence of synapses remains electron microscopy (EM). Unfortunately, sample preparation for EM, especially for correlative light microscopy and EM (Knott et al., 2006), is a sophisticated and delicate process; imaging throughput tends to be low; and image analysis from electron micrographs is laborious and extremely time-consuming. Nevertheless, this situation is rapidly improving. The invention of serial block-face scanning EM (Denk and Horstmann, 2004), focused ion beam/scanning EM (Bosch et al., 2015; Hayworth et al., 2015; Knott et al., 2008; Maco et al., 2014), and automated tape-collecting ultramicrotome (ATUM) (Hayworth et al., 2014) is alleviating or circumventing the difficulties in cutting, collecting, and aligning long sequences of ultra-thin sections. The development of transmission EM camera array (Bock et al., 2011) and multi-beam scanning EM (Eberle et al., 2015) promise vast acceleration of data acquisition. Various endeavors are ongoing to automate neuronal tracing and synapse detection from large EM data sets (Helmstaedter, 2015; Helmstaedter and Mitra, 2012; Jain et al., 2010; Lu, 2011; Plaza et al., 2014). Alternatively, the recently developed mGRASP (mammalian GFP reconstitution across synaptic partners), which enables synapse identification under light microscopy, has been applied successfully to label hippocampal CA3-CA1 synapses (Druckmann et al., 2014).
6. Conclusion
Many studies suggest that structural and functional alterations of dendritic spines exhibit local clustering along the parent dendritic segment. Given the significance of dendritic spines in the functional synaptic circuit, understanding the meaning of such clustered plasticity will shed light on the experience-dependent, circuit-specific modification of the nervous system. Synergistic application of molecular, anatomical, physiological, behavioral, and computational methods will enable us to arrive at a comprehensive understanding of the mechanisms and implications of clustered spine alterations.
Acknowledgments
This work is supported by grants from California Blueprint for Research to Advance Innovation in Neuroscience (CalBRAIN) to J.L. and Y.Z.; from QB3-Calico, the National Institute of Mental Health (MH094449, MH104227), and the National Institute of Neurological Disorders and Stroke (NS078791) to Y.Z.
References
- Attardo A, Fitzgerald JE, Schnitzer MJ. Impermanence of dendritic spines in live adult CA1 hippocampus. Nature. 2015;523:592–596. doi: 10.1038/nature14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian WJ, Miao WY, He SJ, Qiu Z, Yu X. Coordinated spine pruning and maturation mediated by inter-spine competition for cadherin/catenin complexes. Cell. 2015;162:808–822. doi: 10.1016/j.cell.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Bock DD, Lee WC, Kerlin AM, Andermann ML, Hood G, Wetzel AW, Yurgenson S, Soucy ER, Kim HS, Reid RC. Network anatomy and in vivo physiology of visual cortical neurons. Nature. 2011;471:177–182. doi: 10.1038/nature09802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch C, Martinez A, Masachs N, Teixeira CM, Fernaud I, Ulloa F, Perez-Martinez E, Lois C, Comella JX, DeFelipe J, et al. FIB/SEM technology and high-throughput 3D reconstruction of dendritic spines and synapses in GFP-labeled adult-generated neurons. Front Neuroanat. 2015;9:60. doi: 10.3389/fnana.2015.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP. Hippocampus. 2011;21:354–373. doi: 10.1002/hipo.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Murphy TH. Livin’ on the edge: imaging dendritic spine turnover in the peri-infarct zone during ischemic stroke and recovery. Neuroscientist. 2008;14:139–146. doi: 10.1177/1073858407309854. [DOI] [PubMed] [Google Scholar]
- Brown CE, Wong C, Murphy TH. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke. 2008;39:1286–1291. doi: 10.1161/STROKEAHA.107.498238. [DOI] [PubMed] [Google Scholar]
- Chen JL, Villa KL, Cha JW, So PT, Kubota Y, Nedivi E. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron. 2012;74:361–373. doi: 10.1016/j.neuron.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Lu J, Zuo Y. Spatiotemporal dynamics of dendritic spines in the living brain. Front Neuroanat. 2014;8:28. doi: 10.3389/fnana.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Martin A, Crespo M, Portera-Cailliau C. Delayed stabilization of dendritic spines in fragile X mice. J Neurosci. 2010;30:7793–7803. doi: 10.1523/JNEUROSCI.0577-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Horstmann H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2004;2:e329. doi: 10.1371/journal.pbio.0020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- Druckmann S, Feng L, Lee B, Yook C, Zhao T, Magee JC, Kim J. Structured synaptic connectivity between hippocampal regions. Neuron. 2014;81:629–640. doi: 10.1016/j.neuron.2013.11.026. [DOI] [PubMed] [Google Scholar]
- Eberle AL, Mikula S, Schalek R, Lichtman J, Knothe Tate ML, Zeidler D. High-resolution, high-throughput imaging with a multibeam scanning electron microscope. J Microsc. 2015;259:114–120. doi: 10.1111/jmi.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman ML, Dowd C. Loss of dendritic spines in aging cerebral cortex. Anat Embryol (Berl) 1975;148:279–301. doi: 10.1007/BF00319848. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Allwardt B, Harris KM. Dendritic spines do not split during hippocampal LTP or maturation. Nat Neurosci. 2002;5:297–298. doi: 10.1038/nn830. [DOI] [PubMed] [Google Scholar]
- Fu M, Zuo Y. Experience-dependent structural plasticity in the cortex. Trends Neurosci. 2011;34:177–187. doi: 10.1016/j.tins.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Yu X, Lu J, Zuo Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 2012;483:92–95. doi: 10.1038/nature10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globus A, Scheibel AB. Loss of dendrite spines as an index of pre-synaptic terminal patterns. Nature. 1966;212:463–465. doi: 10.1038/212463a0. [DOI] [PubMed] [Google Scholar]
- Globus A, Scheibel AB. Synaptic loci on visual cortical neurons of the rabbit: the specific afferent radiation. Exp Neurol. 1967;18:116–131. doi: 10.1016/0014-4886(67)90093-3. [DOI] [PubMed] [Google Scholar]
- Govindarajan A, Kelleher RJ, Tonegawa S. A clustered plasticity model of long-term memory engrams. Nat Rev Neurosci. 2006;7:575–583. doi: 10.1038/nrn1937. [DOI] [PubMed] [Google Scholar]
- Gray NW, Weimer RM, Bureau I, Svoboda K. Rapid redistribution of synaptic PSD-95 in the neocortex in vivo. PLoS Biol. 2006;4:e370. doi: 10.1371/journal.pbio.0040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450:1195–1200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka Y, Wada K, Kabuta T. Abnormal instability excess density, and aberrant morphology of dendritic spines in prenatally testosterone-exposed mice. Neurochem Int. 2015;85–86:53–58. doi: 10.1016/j.neuint.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, Kuhlman B, Hahn KM, Kasai H. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature. 2015;525:333–338. doi: 10.1038/nature15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayworth KJ, Morgan JL, Schalek R, Berger DR, Hildebrand DG, Lichtman JW. Imaging ATUM ultrathin section libraries with WaferMapper: a multi-scale approach to EM reconstruction of neural circuits. Front Neural Circuits. 2014;8:68. doi: 10.3389/fncir.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayworth KJ, Xu CS, Lu Z, Knott GW, Fetter RD, Tapia JC, Lichtman JW, Hess HF. Ultrastructurally smooth thick partitioning and volume stitching for large-scale connectomics. Nat Methods. 2015;12:319–322. doi: 10.1038/nmeth.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter M, Mitra PP. Computational methods and challenges for large-scale circuit mapping. Curr Opin Neurobiol. 2012;22:162–169. doi: 10.1016/j.conb.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter M. The mutual inspirations of machine learning and neuroscience. Neuron. 2015;86:25–28. doi: 10.1016/j.neuron.2015.03.031. [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. Experience leaves a lasting structural trace in cortical circuits. Nature. 2009;457:313–317. doi: 10.1038/nature07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- Jain V, Seung HS, Turaga SC. Machines that learn to segment images: a crucial technology for connectomics. Curr Opin Neurobiol. 2010;20:653–666. doi: 10.1016/j.conb.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Ash RT, Baker SA, Suter B, Ferguson A, Park J, Rudy J, Torsky SP, Chao HT, Zoghbi HY, et al. Dendritic arborization and spine dynamics are abnormal in the mouse model of MECP2 duplication syndrome. J Neurosci. 2013;33:19518–19533. doi: 10.1523/JNEUROSCI.1745-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CK, Herms J. Structural dynamics of dendritic spines are influenced by an environmental enrichment: an in vivo imaging study. Cereb Cortex. 2014;24:377–384. doi: 10.1093/cercor/bhs317. [DOI] [PubMed] [Google Scholar]
- Kalisman N, Silberberg G, Markram H. The neocortical microcircuit as a tabula rasa. Proc Natl Acad Sci U S A. 2005;102:880–885. doi: 10.1073/pnas.0407088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasthuri N, Hayworth KJ, Berger DR, Schalek RL, Conchello JA, Knowles-Barley S, Lee D, Vazquez-Reina A, Kaynig V, Jones TR, et al. Saturated reconstruction of a volume of neocortex. Cell. 2015;162:648–661. doi: 10.1016/j.cell.2015.06.054. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The structure of the caudate nucleus of the cat: light and electron microscopy. Philos Trans R Soc Lond B Biol Sci. 1971;262:383–401. doi: 10.1098/rstb.1971.0102. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Caveness WF, Yakovlev PI. The neuronographic and metric study of the dendritic arbours of neurons in the motor cortex of Macaca mulatta at birth and at 24 months of age. Brain. 1973;96:765–782. doi: 10.1093/brain/96.4.765. [DOI] [PubMed] [Google Scholar]
- Kleindienst T, Winnubst J, Roth-Alpermann C, Bonhoeffer T, Lohmann C. Activity-dependent clustering of functional synaptic inputs on developing hippocampal dendrites. Neuron. 2011;72:1012–1024. doi: 10.1016/j.neuron.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- Knott G, Marchman H, Wall D, Lich B. Serial section scanning electron microscopy of adult brain tissue using focused ion beam milling. J Neurosci. 2008;28:2959–2964. doi: 10.1523/JNEUROSCI.3189-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CS, Franke TF, Gan WB. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483:87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Nevian T. Synaptic clustering by dendritic signalling mechanisms. Curr Opin Neurobiol. 2008;18:321–331. doi: 10.1016/j.conb.2008.08.013. [DOI] [PubMed] [Google Scholar]
- London M, Hausser M. Dendritic computation. Annu Rev Neurosci. 2005;28:503–532. doi: 10.1146/annurev.neuro.28.061604.135703. [DOI] [PubMed] [Google Scholar]
- Lu J. Neuronal tracing for connectomic studies. Neuroinformatics. 2011;9:159–166. doi: 10.1007/s12021-011-9101-6. [DOI] [PubMed] [Google Scholar]
- Maco B, Holtmaat A, Jorstad A, Fua P, Knott GW. Correlative in vivo 2-photon imaging and focused ion beam scanning electron microscopy: 3D analysis of neuronal ultrastructure. Methods Cell Biol. 2014;124:339–361. doi: 10.1016/B978-0-12-801075-4.00016-1. [DOI] [PubMed] [Google Scholar]
- Makino H, Malinow R. Compartmentalized versus global synaptic plasticity on dendrites controlled by experience. Neuron. 2011;72:1001–1011. doi: 10.1016/j.neuron.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Padilla M. Number and distribution of the apical dendritic spines of the layer V pyramidal cells in man. J Comp Neurol. 1967;131:475–490. doi: 10.1002/cne.901310407. [DOI] [PubMed] [Google Scholar]
- Miquelajauregui A, Kribakaran S, Mostany R, Badaloni A, Consalez GG, Portera-Cailliau C. Layer 4 pyramidal neurons exhibit robust dendritic spine plasticity in vivo after input deprivation. J Neurosci. 2015;35:7287–7294. doi: 10.1523/JNEUROSCI.5215-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishchenko Y, Hu T, Spacek J, Mendenhall J, Harris KM, Chklovskii DB. Ultrastructural analysis of hippocampal neuropil from the connectomics perspective. Neuron. 2010;67:1009–1020. doi: 10.1016/j.neuron.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki A. Brain clearing for connectomics. Microscopy (Oxf) 2015;64:5–8. doi: 10.1093/jmicro/dfu108. [DOI] [PubMed] [Google Scholar]
- Mizrahi A, Katz LC. Dendritic stability in the adult olfactory bulb. Nat Neurosci. 2003;6:1201–1207. doi: 10.1038/nn1133. [DOI] [PubMed] [Google Scholar]
- Mungai JM. Dendritic patterns in the somatic sensory cortex of the cat. J Anat. 1967;101:403–418. [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Li P, Betts K, Liu R. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J Neurosci. 2008;28:1756–1772. doi: 10.1523/JNEUROSCI.5128-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Nishiyama J, Yasuda R. Biochemical computation for spine structural plasticity. Neuron. 2015;87:63–75. doi: 10.1016/j.neuron.2015.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh WC, Parajuli LK, Zito K. Heterosynaptic structural plasticity on local dendritic segments of hippocampal CA1 neurons. Cell Rep. 2015;10:162–169. doi: 10.1016/j.celrep.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Aldridge GM, Greenough WT, Gan WB. Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2010;107:17768–17773. doi: 10.1073/pnas.1012496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnavelas JG, Globus A, Kaups P. Continuous illumination from birth affects spine density of neurons in the visual cortex of the rat. Exp Neurol. 1973;40:742–747. doi: 10.1016/0014-4886(73)90108-8. [DOI] [PubMed] [Google Scholar]
- Plaza SM, Scheffer LK, Chklovskii DB. Toward large-scale connectome reconstructions. Curr Opin Neurobiol. 2014;25:201–210. doi: 10.1016/j.conb.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Poirazi P, Mel BW. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron. 2001;29:779–796. doi: 10.1016/s0896-6273(01)00252-5. [DOI] [PubMed] [Google Scholar]
- Poirazi P, Brannon T, Mel BW. Pyramidal neuron as two-layer neural network. Neuron. 2003;37:989–999. doi: 10.1016/s0896-6273(03)00149-1. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histologie du système nerveux de l’homme et des vertébrés. Paris: Maloine; 1911. [Google Scholar]
- Richardson DS, Lichtman JW. Clarifying tissue clearing. Cell. 2015;162:246–257. doi: 10.1016/j.cell.2015.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim E. The postsynaptic organization of synapses. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM. The dendritic spine: a multifunctional integrative unit. J Neurophysiol. 1996;75:2197–2210. doi: 10.1152/jn.1996.75.6.2197. [DOI] [PubMed] [Google Scholar]
- Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanyants A, Hof PR, Chklovskii DB. Geometry and structural plasticity of synaptic connectivity. Neuron. 2002;34:275–288. doi: 10.1016/s0896-6273(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Svoboda K, Yasuda R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron. 2006;50:823–839. doi: 10.1016/j.neuron.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kitamura K, Matsuo N, Mayford M, Kano M, Matsuki N, Ikegaya Y. Locally synchronized synaptic inputs. Science. 2012;335:353–356. doi: 10.1126/science.1210362. [DOI] [PubMed] [Google Scholar]
- Theer P, Denk W. On the fundamental imaging-depth limit in two-photon microscopy. J Opt Soc Am A: Opt Image Sci Vis. 2006;23:3139–3149. doi: 10.1364/josaa.23.003139. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7:1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- Valverde F, Ruiz-Marcos A. Dendritic spines in the visual cortex of the mouse: introduction to a mathematical model. Exp Brain Res. 1969;8:269–283. doi: 10.1007/BF00234253. [DOI] [PubMed] [Google Scholar]
- Wiegert JS, Oertner TG. Long-term depression triggers the selective elimination of weakly integrated synapses. Proc Natl Acad Sci U S A. 2013;110:E4510–4519. doi: 10.1073/pnas.1315926110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms CD, Hausser M. Reading out a spatiotemporal population code by imaging neighbouring parallel fibre axons in vivo. Nat Commun. 2015;6:6464. doi: 10.1038/ncomms7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J Comp Neurol. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A, Gao YZ, Rodriguez A, Dickstein DL, Wearne SL, Luebke JI, Hof PR, Weaver CM. Morphologic evidence for spatially clustered spines in apical dendrites of monkey neocortical pyramidal cells. J Comp Neurol. 2012;520:2888–2902. doi: 10.1002/cne.23070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Lai CS, Cichon J, Ma L, Li W, Gan WB. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344:1173–1178. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Denk W. Dendritic spines as basic functional units of neuronal integration. Nature. 1995;375:682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]
- Zhang S, Murphy TH. Imaging the impact of cortical microcirculation on synaptic structure and sensory-evoked hemodynamic responses in vivo. PLoS Biol. 2007;5:e119. doi: 10.1371/journal.pbio.0050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Boyd J, Delaney K, Murphy TH. Rapid reversible changes in dendritic spine structure in vivo gated by the degree of ischemia. J Neurosci. 2005;25:5333–5338. doi: 10.1523/JNEUROSCI.1085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005a;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005b;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]