Summary
The objective was to determine whether the depression comorbid with epilepsy could be predicted based on inherent premorbid patterns of monoaminergic transmission. In male Wistar rats, despair- and anhedonia-like behaviors were examined using forced swimming and taste preference tests respectively. Serotonergic raphe nucleus (RN)-prefrontal cortex (PFC), and dopaminergic ventral tegmental area (VTA)-nucleus accumbens (NAcc) pathways were interrogated by fast scan cyclic voltammetry (FSCV). The assays were performed before, and two months after pilocarpine status epilepticus (SE). In a subset of naïve rats, FSCV, coupled with the intensity-dependent stimulation paradigm, detected specific deviations in each pathway (6 rats for RN-PFC, and 7 rats for VTA-NAcc, with the overlap in 2, out of total 19 subjects), in the absence of behavioral impairments. During epilepsy, animals with preexisting deviations in RN-PFC invariably developed despair, and rats with deviations in VTA-NAcc- anhedonia. Serotonergic and dopaminergic pathways respectively showed signs of explicit deterioration. We suggest that epilepsy triggers decompensations in the already vulnerable depression-relevant neuronal circuits, which culminate in depression. The established connection between the identified specific signatures in monoamine transmission in naïve rats, and specific symptoms of epilepsy-associated depression may help understanding causes of comorbidity, and developing its early biomarkers.
Keywords: Epilepsy, depression, biomarkers, serotonin, dopamine
Introduction
Identification of early biomarkers of depression is a matter of high priority in general, but is especially acute in the context of epilepsy. Given high prevalence of epilepsy-associated depression (30–50% vs 16–18% for the stand-alone depressive disorder1), and its detrimental effects on the quality of life, the ability to identify patients at risk before the comorbidity emerges, would have apparent benefits.
The fact that depression, as common as it is, is not present in all epilepsy patients, suggests the existence of predisposing factors which, while being normally latent, facilitate decompensation in relevant neuronal circuits once epilepsy develops. Clinical and experimental studies employing genetic, imaging, behavioral, and electrographic methodologies have been successful in the biomarker identification2–5. Still, given multifactorial nature of depression, it is safe to assume that no single biomarker would offer the ultimate solution for the prediction of the disease.
Among mechanisms of depression, perturbations in monoamine transmission remain best established. All monoamines play complex and overlapping roles in regulating mood, and have been implicated in the disease 6–8. The present study stems from our observations that chronic epilepsy in rats, triggers perturbations in monoaminergic pathways and subsequent depressive-like impairments 9; 10. Here, we examined relationships between inherent patterns of monoaminergic transmission and the development of depressive-like behaviors upon the establishment of epilepsy. We focused on serotonergic projection from raphe nucleus (RN) into prefrontal cortex (PFC), and dopaminergic pathway from ventral tegmental area (VTA) into the nucleus accumbens (NAcc), the dysfunctions in which have been associated with depressive disorder 6; 7.
Methods
The experiments were performed in 19 Male Wistar rats, 2 months-old at the beginning of the study (Charles River, Wilmington, MA), and complied with policies of the National Institutes of Health and of the UCLA Office of Protection of Research Subjects. All experiments were performed in the blinded fashion.
Behavior was first studied in naïve rats. Anhedonia was examined using taste preference test (TPT)10. Over 24 hours, the animals were offered fluids from two equally accessible bottles, one filled with water, and another- with 0.1% saccharin. Twenty four hours later, the amounts of remaining water and saccharin were recorded. Anhedonic index was calculated by dividing the volume of consumed water by the total volume of consumed fluid, multiplied by 100. Normal animals strongly prefer saccharin, while anhedonia is evident is the lack of preference towards either of the drinks. One – three days after TPT, forced swimming test (FST) was used to assess the despair-like state9; 10. The animals were allowed to swim for 5 min in the water-filled tank. Cumulative duration of active swimming and of immobility were calculated off-line by reviewing pre-recorded videos. The increase in immobility is an indicator of despair.
Fast scan cyclic voltammetry (FSCV) in vivo was used for interrogating serotonergic RN-PFC and dopaminergic VTA-NAcc pathways9–11. The equipment included potentiostat with the built-in stimulator, two-electrode headstage, digital acquisition board, and computer with the acquisition/analysis software (Invilog, Kuopio, Finland). Carbon fiber electrode (CFE, 32-micron, Invilog) was calibrated in incremental concentrations of serotonin (5-HT) and dopamine (DA, Sigma, St. Louis, MO) to generate standard curves. For the 5-HT detection, ramp current applied to CFE consisted of a resting potential 0 V scanned to 1.2 V, −0.6 V and 0 V at 300 V/s; for the DA detection, ramp current consisted of a resting potential −0.4 V, scanned to 1.15 V and −400 V at 300 V/s 9; 11.
FSCV involved stereotaxic surgery 12 under isoflurane anesthesia. Dry Ag/AgCl reference electrode was placed on the nasal bone, CFE- in infralimbic PFC and stimulating electrode- in dorsal RN. After the acquisition from the RN-PFC pathway, CFE was moved to NAcc shell, and stimulating electrode- to VTA. Ramp current was applied to CFE simultaneously with electrical stimuli. Stimulations (1 ms square wave bipolar pulses, 40 Hz,1 s) were delivered every 2 minutes, with the stimulus intensity increasing from 0 to 400 μV, at 50 μV increments, to generate stimulus-response curves. The strength of neurotransmission was expressed as the amount of the transmitter released under CFE in response to standard electrical stimulus, by converting amplitudes to concentrations derived from the calibration curves. After FSCV, the wound was closed and the animal was release in the home cage.
Three weeks after FSCV, status epilepticus (SE) was induced by LiCl (127 mg/kg s.c.) and pilocarpine (40 mg/kg i.p.). Two and six hours after the SE onset, the animals received i.p. injections of diazepam (10 mg/kg) and phenytoin (50 mg/kg). The animals were under continuous video monitoring beginning from four weeks after SE, to confirm the presence of spontaneous generalized tonic-clonic seizures. Two months after SE, the animals underwent behavioral tests and FSCV, as described above.
Data were analyzed using Prism 6 software (GraphPad, San Diego, CA). Statistical tests and sample sizes are indicated in figure legends.
Results
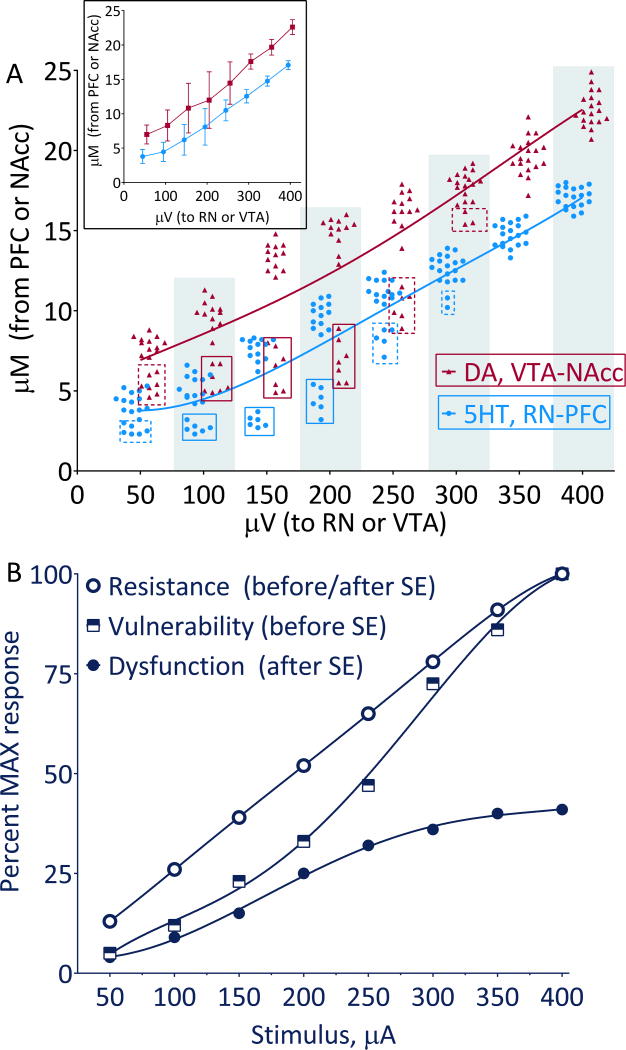
Before SE, monoamine release in both RN-PFC and VTA-NAcc pathways was stimulus-dependent (Fig. 1A). In the 100–200 μV range, the dispersion of the responses was most evident (Fig. 1A inset). The pattern best fitted sigmoidal 5th order polynomial model (Fig. 1A, B). Versus the fit, in the 100–200 μV range, 6 rats had clearly lower 5-HT, and 7 rats- lower DA responses (Fig 1A, solid boxes), as compared with the rest of the subjects, although the separation lingered on both sides of the range (Fig 1A, dashed boxes). As a working definition, these animals and response patterns are further referred to as “vulnerable”, and the rest of the animals and responses- as “resistant” vis-à-vis specific monoamine. Two rats had low responses in both pathways. Before SE, there were no differences in the taste preference and in the immobility among the animals of all categories (i.e. DA- resistant, DA-vulnerable, 5-HT- resistant, and 5-HT- vulnerable, Fig. 2A, D).
Figure 1. Patterns of monoamine transmission.
A. Plot of responses from individual animals (n=19) measured before SE in the RN-PFC and VTA-NAcc pathways. The amount of the released neurotransmitter (Y-axis) is plotted against stimulus intensity (X- axis). The data best fit the 5th order polynomial nonlinear models, which are shown by the color-matched lines. Inset shows Mean±SD. B. Fifth order polynomial nonlinear model built on the stimulus intensity-dependent responses detected by FSCV in the animals of different categories. In resistant subjects (the rest of animals on A), the pattern was nearly linear throughout the stimulation range. In vulnerable subjects, (indicated by solid boxes on A) the responses followed sigmoidal pattern, with less progressive increase occurring at lower stimulus intensities, and more linear- at higher intensities. After the induction of epilepsy and the development of depressive-like behaviors, in the vulnerable animals the response pattern was still sigmoidal, however it shifted downward and became saturated at 350 μV. Resistant animals, which developed no behavioral impairments after SE, retained the same response pattern as before SE.
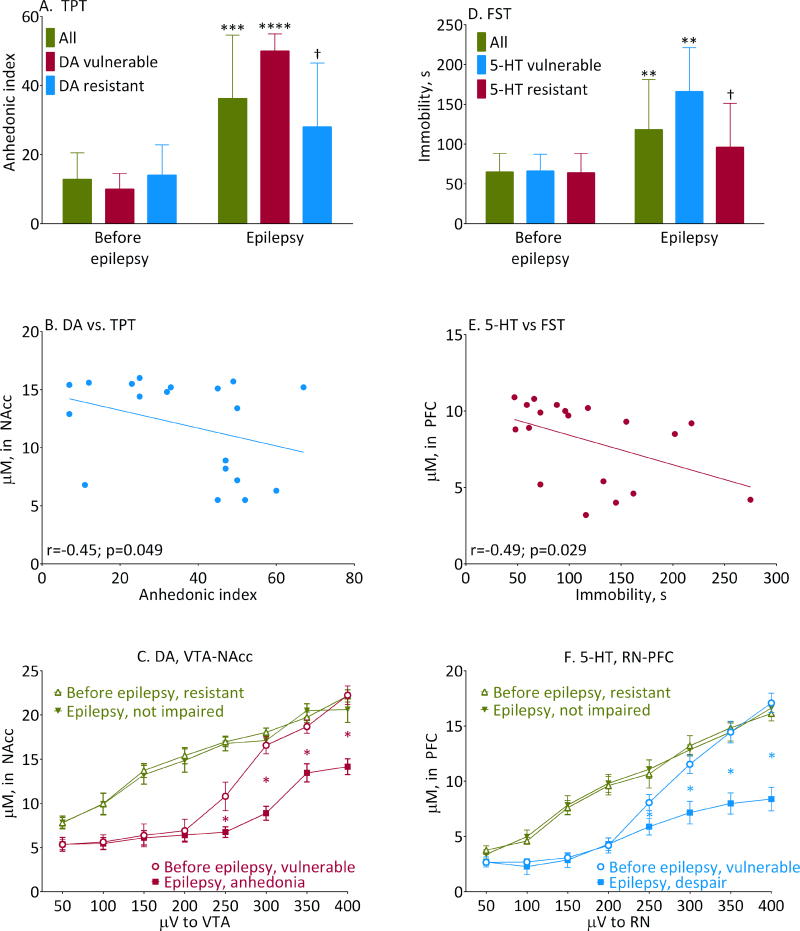
Figure 2. Performance in behavioral tests and its relationship with monoaminergic transmission.
A- behavior in the TPT and D- behavior in the FST before and after SE. Shown are Mean±SD, **-p<0.05; ***- p<0.01; ****0 p<0.001, Epilepsy vs. Before Epilepsy; †- p<0.05, normal vs. respective vulnerable (2-Way ANOVA+Tukey’s). Sample sizes: All n=19; 5-HT-vulnerable n=6; 5-HT-resistant n=13; DA-vulnerable n=7; DA-resistant n=12. FST interaction F (2, 70) = 3.1, p<0.05; effects of animal category F (2, 70) = 3.388, p<0.05; effects of epilepsy F (1, 70) = 28.58, p<0.0001. TPT interaction F (2, 70) = 5.420, p<0.01; effects of animal category F (2, 70) = 2.574, p<0.05; effects of epilepsy F (1, 70) = 62.44, p<0.0001. B and E- individual plots of dopaminergic and serotonergic responses to 200 μV stimulus prior to SE vs. behavior in TPT and FST after SE. At this stimulus intensity, lower inherent monoamine responses correlated with more severe behavioral abnormalities (Pearson correlation coefficients “r” are shown on respective panels). No such correlation was detected for other stimulus intensities. C- dopaminergic transmission in the VTA-NAcc pathway and F- serotonergic transmission in the RN-PFC measured by FSCV in resistant and vulnerable rats before and after the development of epilepsy and behavioral impairments. Shown are Mean± SD. *- p<0.05 (paired t-tests). Sample sizes: 5-HT vulnerable, n=6; 5-HT-resistant n=6; DA-vulnerable n=7; DA vulnerable n=5.
After SE, during the four-weeks observation, all animals presented with 1–5 spontaneous generalized seizures per week. No differences in seizure frequency were observed between 5-HT-resistant and 5-HT-vulnerable rats (minimal/median/maximal values over 4 weeks were 5/8/19 and 4/12.5/19 respectively, p=0.36, Mann-Whitney test). Likewise, DA-resistant and DA-vulnerable rats developed similar number of generalized seizures (5/9/19 and 4/10/17 respectively, p=0.81, Mann-Whitney test).
With the development of epilepsy, there was the overall increase of both anhedonic index, and immobility. No correlation was detected between the frequency of spontaneous seizures and the extent of either of the behavioral impairments (Pearson correlation, p>0.05 for all categories). The analysis on the subcategory level revealed that the overall increase of anhedonic index occurred at the expense of DA-vulnerable rats, while the overall increase of immobility- at the expense of 5-HT- vulnerable subjects (Fig. 2A, D). The difference in anhedonic index was significant between DA-vulnerable and DA-resistant animals; the difference in immobility was significant between 5-HT-vulnerable and 5-HT-resistant rats (Fig. 2A, D). At the same time, anhedonic index was similar between all 5-HT-vulnerable (35±19, n=6) and 5-HT-resistant rats (38±16, n= 13, p>0.05 unpaired t-test), and immobility- between all DA-vulnerable (105±47 s, n=7) and DA-resistant subjects (139±83 s, n=12, p>0.05). As a result, TPT and FST parameters did not correlate with each other either before SE (Pearson r=−0.1, p=0.68), or after SE (Pearson r=0.36, p=0.12).
At 200 μV stimulus intensity, there was statistically significant correlation between pre-SE response in VTA-NAcc and anhedonic index, as well as between pre-SE response in RN-PFC and immobility (Fig. 2 B, E). No such correlation was detected for other stimulus intensities (Pearson correlation, p>0.05).
After SE, on the individual level, all 5-HT-vulnerable rats invariably developed despair, and all DA-vulnerable rats invariably developed anhedonia. Congruently with the baseline FSCV, 2 rats had both behavioral deficits. Not all rats with 5-HT vulnerability developed anhedonia, and not all rats with DA vulnerability developed despair, whereby 3 animals in each category showed the mismatch.
With the establishment of epilepsy, all monoamine-vulnerable animals showed deterioration of neurotransmission in respective pathways, with sigmoidal pattern shifting further downward, and response saturating at 350 μV (Fig. 2C, F; Fig. 1B); as a working definition, this pattern is referred to as “dysfunction” (Fig. 1B).
Three rats developed despair and three rats- anhedonia, without pre-SE deviations in the RN-PFC and the VTA-NAcc pathways respectively. Five animals had none of pre-SE vulnerabilities, and developed no behavioral deficits after SE. The analysis of FSCV data in those DA-resistant and 5-HT-resistant rats, which developed no respective behavioral impairments during epilepsy revealed, that both before and after SE, there were identical linear stimulus-dependent increases in DA and 5-HT release (Fig. 2C, F; Fig 1B).
Discussion
Our main finding is the existence of inherent signatures of monoaminergic transmission, which may predict depressive-like behaviors upon the development of epilepsy. These predictive patterns appear to exist on the symptom level, whereby the RN-PFC vulnerability predetermines epilepsy-associated despair-like state, while the VTA-NAcc vulnerability – predetermines anhedonia.
Stimulus-dependent paradigm afforded discerning latent monoaminergic perturbations (i.e. patterns which did not translate into behavioral deficits in naïve rats) from their explicit dysfunctions (i.e. those associated with depressive-like behaviors in epilepsy). In naïve, behaviorally intact animals, the deviations were only present at lower stimulus intensities, while in animals with behavioral abnormalities, suppressed monoaminergic tone was present throughout the stimulation range.
The role of different monoamines in symptoms of depression is complex and overlapping. Nevertheless, the connection between dopaminergic perturbation and anhedonia, as well as between serotonergic perturbation and despair, appeared strong. These findings are generally congruent with predominant role of 5-HT in mood impairments 6, and in behavior in FST 13, and of DA- in anhedonia7, and in behavior in TPT14.
Causes of the observed patterns require an examination. Candidates include marginal upregulation of monoamine transporters, and/or autoreceptors (e.g. due to single nucleotide polymorphisms15), which become decompensated in epilepsy. Along with usefulness of such studies from mechanistic perspective, there is translational promise. FSCV is not clinically feasible, but PET of monoamine receptors and transporters is. If the suggested association is confirmed, our findings may have advantages, for example in preclinical drug trials, as the cost of FSCV is a fraction of costs of PET.
It appeared that inherent vulnerabilities in monoamine transmission had no predictive power towards the frequency of spontaneous generalized seizures. This was in line with earlier findings which failed to establish a correlation between depressive behaviors and spontaneous seizures 10. Limitations of the study should be acknowledged. To name few, the findings should be corroborated, as they are based on single series. Other mechanisms can be also liable: for example, impairments in noradrenergic8, glutamatergic16, GABAergic17 neurotransmission, and chronic inflammation18, all have been implicated in depression. Finally, the identification of vulnerable subjects occurred before SE, which was skewed from the biomarkers perspective. The limitation is model-related: behavioral and transmitter impairments are already evident 3 weeks post-SE, but at earlier time points the animals are not yet physically fit for surgery and behavioral tests. Therefore, the system offers no clear latent post-insult window.
In summary, we suggest that epilepsy triggers decompensations in the already vulnerable relevant neuronal circuits, which culminate in depression. Even with the discussed limitations in mind, the identified specific signatures of monoamine transmission, which predict epilepsy-associated depression, may help in understanding causes of comorbidity, and in developing its early biomarkers.
Acknowledgments
This work was supported by research grant R01NS065783 (NIH/NINDS) to A.M. and by the postdoctoral fellowships 237168 and 264551 from National Council of Science and Technology (CONACYT, Mexico) to JSMM.
Footnotes
Conflict of interest. The authors report no conflicts of interest.
Ethical Publication Statement. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Kanner AM. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanisms, and treatment. Biol Psychiatry. 2003;54:388–398. doi: 10.1016/s0006-3223(03)00469-4. [DOI] [PubMed] [Google Scholar]
- 2.Jentsch MC, Van Buel EM, Bosker FJ, et al. Biomarker approaches in major depressive disorder evaluated in the context of current hypotheses. Biomark Med. 2015;9:277–297. doi: 10.2217/bmm.14.114. [DOI] [PubMed] [Google Scholar]
- 3.Olbrich S, van Dinteren R, Arns M. Personalized Medicine: Review and Perspectives of Promising Baseline EEG Biomarkers in Major Depressive Disorder and Attention Deficit Hyperactivity Disorder. Neuropsychobiology. 2015;72:229–240. doi: 10.1159/000437435. [DOI] [PubMed] [Google Scholar]
- 4.Woods AG, Iosifescu DV, Darie CC. Biomarkers in major depressive disorder: the role of mass spectrometry. Adv Exp Med Biol. 2014;806:545–560. doi: 10.1007/978-3-319-06068-2_27. [DOI] [PubMed] [Google Scholar]
- 5.Becker C, Bouvier E, Ghestem A, et al. Predicting and treating stress-induced vulnerability to epilepsy and depression. Ann Neurol. 2015;78:128–136. doi: 10.1002/ana.24414. [DOI] [PubMed] [Google Scholar]
- 6.Albert PR, Vahid-Ansari F, Luckhart C. Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci. 2014;8:199. doi: 10.3389/fnbeh.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorwood P. Neurobiological mechanisms of anhedonia. Dialogues Clin Neurosci. 2008;10:291–299. doi: 10.31887/DCNS.2008.10.3/pgorwood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ressler KJ, Nemeroff CB. Role of norepinephrine in the pathophysiology and treatment of mood disorders. Biol Psychiatry. 1999;46:1219–1233. doi: 10.1016/s0006-3223(99)00127-4. [DOI] [PubMed] [Google Scholar]
- 9.Kumar U, Medel-Matus JS, Redwine HM, et al. Effects of selective serotonin and norepinephrine reuptake inhibitors on depressive- and impulsive-like behaviors and on monoamine transmission in experimental temporal lobe epilepsy. Epilepsia. 2016;57:506–515. doi: 10.1111/epi.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazarati A, Siddarth P, Baldwin RA, et al. Depression after status epilepticus: behavioural and biochemical deficits and effects of fluoxetine. Brain. 2008;131:2071–2083. doi: 10.1093/brain/awn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wightman RM, Heien ML, Wassum KM, et al. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007;26:2046–2054. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- 12.Paxinos G, Watson C. The Rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 1986. [Google Scholar]
- 13.Warden MR, Selimbeyoglu A, Mirzabekov JJ, et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tye KM, Mirzabekov JJ, Warden MR, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binder EB, Holsboer F. Pharmacogenomics and antidepressant drugs. Ann Med. 2006;38:82–94. doi: 10.1080/07853890600551045. [DOI] [PubMed] [Google Scholar]
- 16.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brambilla P, Perez J, Barale F, et al. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721–737. 715. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- 18.Young JJ, Bruno D, Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J Affect Disord. 2014;169:15–20. doi: 10.1016/j.jad.2014.07.032. [DOI] [PubMed] [Google Scholar]